Abstract
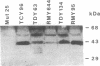
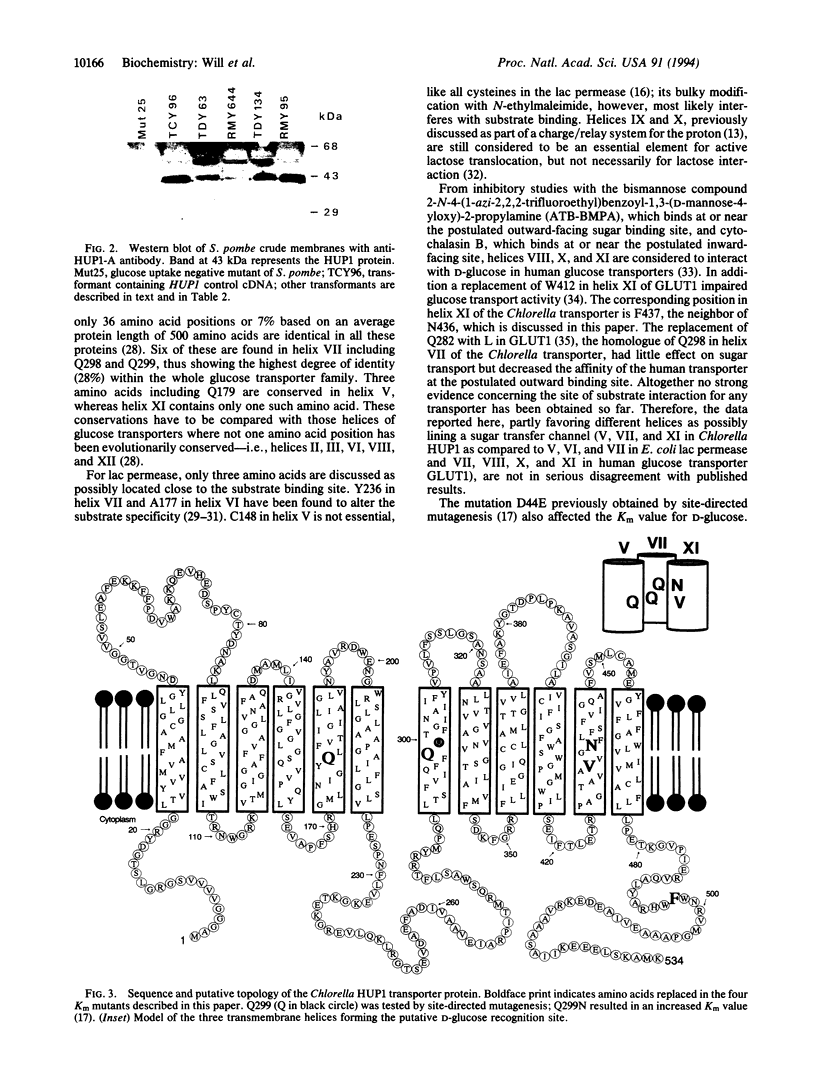
The HUP1 gene codes for the monosaccharide/H+ cotransporter protein of Chlorella kessleri. The gene is functionally expressed in Schizosaccharomyces pombe. This heterologous system has been used to screen for Km mutants of the Chlorella symporter. Since S. pombe transformed with HUP1 cDNA showed a 1000-fold increase in sensitivity toward the toxic sugar analogue 2-deoxyglucose, we screened for transformants with a decreased 2-deoxyglucose sensitivity. The transformants were produced with HUP1 cDNA randomly mutagenized by PCR. From 73 transformants with decreased 2-deoxyglucose sensitivity, four mutants with increased Km values for D-glucose were obtained. The amino acid exchanges responsible for the increased Km values are located in the center of the putative transmembrane helices V (Q179E), VII (Q298R), and XI (V433L/N436Y). Q179N and Q299N had previously been shown by directed mutagenesis to affect the Km value of the transporter for D-glucose. The drastic mutational changes Q298R and N436Y gave rise to very high Km values; however, the corresponding conservative amino acid changes Q298N or N436Q obtained by directed mutagenesis also result in Km values increased by a factor of 10 or 20, respectively. The data therefore support the proposal that at least helices V, VII, and XI may line the sugar translocation path and determine its specificity. These results are discussed in relation to other sugar transporters and to the interaction of the yeast hexokinase B with D-glucose as known from published crystal structures.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. M., Stenkamp R. E., McDonald R. C., Steitz T. A. A refined model of the sugar binding site of yeast hexokinase B. J Mol Biol. 1978 Aug 5;123(2):207–219. doi: 10.1016/0022-2836(78)90321-2. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Burant C. F., Takeda J., Gould G. W. Structure and function of mammalian facilitative sugar transporters. J Biol Chem. 1993 Sep 15;268(26):19161–19164. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brooker R. J., Wilson T. H. Isolation and nucleotide sequencing of lactose carrier mutants that transport maltose. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3959–3963. doi: 10.1073/pnas.82.12.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel D. E., Gronenborn B., Müller-Hill B. Sequence of the lactose permease gene. Nature. 1980 Feb 7;283(5747):541–545. doi: 10.1038/283541a0. [DOI] [PubMed] [Google Scholar]
- Calamia J., Manoil C. lac permease of Escherichia coli: topology and sequence elements promoting membrane insertion. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4937–4941. doi: 10.1073/pnas.87.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspari T., Stadler R., Sauer N., Tanner W. Structure/function relationship of the Chlorella glucose/H+ symporter. J Biol Chem. 1994 Feb 4;269(5):3498–3502. [PubMed] [Google Scholar]
- Collins J. C., Permuth S. F., Brooker R. J. Isolation and characterization of lactose permease mutants with an enhanced recognition of maltose and diminished recognition of cellobiose. J Biol Chem. 1989 Sep 5;264(25):14698–14703. [PubMed] [Google Scholar]
- Goswitz V. C., Brooker R. J. Isolation of lactose permease mutants which recognize arabinose. Membr Biochem. 1993 Jan-Mar;10(1):61–70. doi: 10.3109/09687689309150253. [DOI] [PubMed] [Google Scholar]
- Hashiramoto M., Kadowaki T., Clark A. E., Muraoka A., Momomura K., Sakura H., Tobe K., Akanuma Y., Yazaki Y., Holman G. D. Site-directed mutagenesis of GLUT1 in helix 7 residue 282 results in perturbation of exofacial ligand binding. J Biol Chem. 1992 Sep 5;267(25):17502–17507. [PubMed] [Google Scholar]
- Kaback H. R. Molecular biology of active transport: from membrane to molecule to mechanism. Harvey Lect. 1987;83:77–105. [PubMed] [Google Scholar]
- Katagiri H., Asano T., Shibasaki Y., Lin J. L., Tsukuda K., Ishihara H., Akanuma Y., Takaku F., Oka Y. Substitution of leucine for tryptophan 412 does not abolish cytochalasin B labeling but markedly decreases the intrinsic activity of GLUT1 glucose transporter. J Biol Chem. 1991 Apr 25;266(12):7769–7773. [PubMed] [Google Scholar]
- Komor E., Haass D., Komor B., Tanner W. The active hexose-uptake system of Chlorella vulgaris. Km-values for 6-deoxyglucose influx and efflux and their contribution to sugar accumulation. Eur J Biochem. 1973 Nov 1;39(1):193–200. doi: 10.1111/j.1432-1033.1973.tb03117.x. [DOI] [PubMed] [Google Scholar]
- Komor E. Proton-coupled hexose transport in Chlorella vulgaris. FEBS Lett. 1973 Dec 15;38(1):16–18. doi: 10.1016/0014-5793(73)80501-0. [DOI] [PubMed] [Google Scholar]
- Komor E., Tanner W. Can energy generated by sugar efflux be used for ATP synthesis in Chlorella? Nature. 1974 Apr 5;248(448):511–512. doi: 10.1038/248511a0. [DOI] [PubMed] [Google Scholar]
- Komor E., Tanner W. The determination of the membrane ptoential of Chlorella vulgaris. Evidence for electrogenic sugar transport. Eur J Biochem. 1976 Nov 1;70(1):197–204. doi: 10.1111/j.1432-1033.1976.tb10970.x. [DOI] [PubMed] [Google Scholar]
- Komor E., Tanner W. The hexose-proton cotransport system of chlorella. pH-dependent change in Km values and translocation constants of the uptake system. J Gen Physiol. 1974 Nov;64(5):568–581. doi: 10.1085/jgp.64.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E., Tanner W. The hexose-proton symport system of Chlorella vulgaris. Specificity, stoichiometry and energetics of sugar-induced proton uptake. Eur J Biochem. 1974 May 2;44(1):219–223. doi: 10.1111/j.1432-1033.1974.tb03476.x. [DOI] [PubMed] [Google Scholar]
- Lehle L., Schwarz R. T. Formation of dolichol monophosphate 2-deoxy-D-glucose and its interference with the glycosylation of mannoproteins in yeast. Eur J Biochem. 1976 Aug 1;67(1):239–245. doi: 10.1111/j.1432-1033.1976.tb10655.x. [DOI] [PubMed] [Google Scholar]
- Marger M. D., Saier M. H., Jr A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem Sci. 1993 Jan;18(1):13–20. doi: 10.1016/0968-0004(93)90081-w. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Quiocho F. A. Carbohydrate-binding proteins: tertiary structures and protein-sugar interactions. Annu Rev Biochem. 1986;55:287–315. doi: 10.1146/annurev.bi.55.070186.001443. [DOI] [PubMed] [Google Scholar]
- Robzyk K., Kassir Y. A simple and highly efficient procedure for rescuing autonomous plasmids from yeast. Nucleic Acids Res. 1992 Jul 25;20(14):3790–3790. doi: 10.1093/nar/20.14.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P., Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986 Apr 11;45(1):145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- Sahin-Tóth M., Kaback H. R. Cysteine scanning mutagenesis of putative transmembrane helices IX and X in the lactose permease of Escherichia coli. Protein Sci. 1993 Jun;2(6):1024–1033. doi: 10.1002/pro.5560020615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer N., Caspari T., Klebl F., Tanner W. Functional expression of the Chlorella hexose transporter in Schizosaccharomyces pombe. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7949–7952. doi: 10.1073/pnas.87.20.7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer N., Tanner W. The hexose carrier from Chlorella. cDNA cloning of a eucaryotic H+-cotransporter. FEBS Lett. 1989 Dec 18;259(1):43–46. doi: 10.1016/0014-5793(89)81489-9. [DOI] [PubMed] [Google Scholar]
- Tanner W. Light-driven active uptake of 3-O-methylglucose via an inducible hexose uptake system of Chlorella. Biochem Biophys Res Commun. 1969 Jul 23;36(2):278–283. doi: 10.1016/0006-291x(69)90326-x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van Iwaarden P. R., Pastore J. C., Konings W. N., Kaback H. R. Construction of a functional lactose permease devoid of cysteine residues. Biochemistry. 1991 Oct 8;30(40):9595–9600. doi: 10.1021/bi00104a005. [DOI] [PubMed] [Google Scholar]