Abstract
Amphetamine (‘Speed’), methamphetamine (‘Ice’) and its congener 3,4-methylenedioxymethamphetamine (MDMA; ‘Ecstasy’) are illicit drugs abused worldwide for their euphoric and stimulant effects. Despite compelling evidence for chronic MDMA neurotoxicity in animal models, the physiological consequences of such toxicity in humans remain unclear. In addition, distinct differences in the metabolism and pharmacokinetics of MDMA between species and different strains of animals prevent the rationalisation of realistic human dose paradigms in animal studies. Here, we attempt to review amphetamine toxicity and in particular MDMA toxicity in the pathogenesis of exemplary human pathologies, independently of confounding environmental factors such as poly-drug use and drug purity.
Keywords: addiction; amphetamine; disease; 3,4-methylenedioxymethamphetamine (MDMA)
Introduction
Methamphetamine (METH), d-amphetamine (d-AMPH) and 3,4-methylenedioxymethamphetamine (MDMA; Figure 1) are illicit psychoactive drugs abused worldwide for their stimulant, euphoric and in the case of MDMA, emphathogenic/entactogenic effects. In contrast to MDMA, d-AMPH and METH are also available under medical prescription and are used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy and weight control (Sulzer et al., 2005; Berman et al., 2009). Although never marketed, MDMA is reported to have been used by US psychotherapists, prior to its classification as a schedule 1 drug in 1985 (Capela et al., 2009).
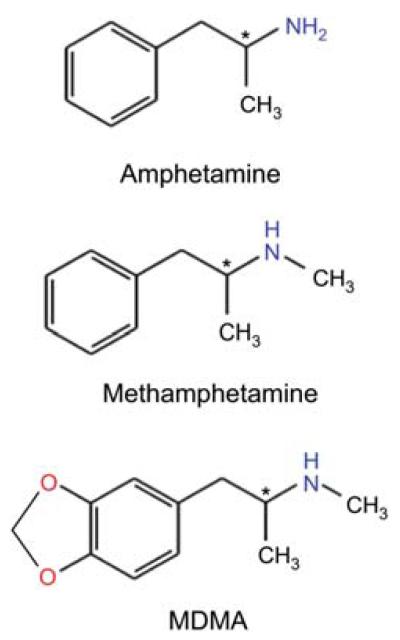
Figure 1.
Amphetamine and congeners.
The chemical structures of amphetamines and the congeners are shown as indicated (the chemical names in parentheses represent the IUPAC names): amphetamine [street name: ‘Speed’; d-form: (2S)-1-phenylpropan-2-amine], methamphetamine [street name: ‘crank’ or ‘Crystal meth’; d-form: (2S)-N-methyl-1-phenylpropan-2-amine], 3,4-methylenedioxymethamphetamine or MDMA [street name: ‘Ecstasy’; 1-(1,3-benzodioxol-5-yl)-N-methylpropan-2-amine].
Amphetamines are a subclass of psychostimulants characterised by the presence of a α-methylphenethyl-amine core structure. However, for the purposes of this review, the term amphetamines refers to METH, d-AMPH and the ring substituted amphetamine derivative MDMA. The chronic toxicological and pharmacological effects of each of these drugs have been well documented and much has been discovered with regard to the mechanisms of action and structure-function relationships of each compound. Amphetamines exert their acute effects both in the central nervous system (CNS) and in peripheral tissues. The acute clinical outcome is dependent upon the dose administered and typically includes positively prescribed subjective effects such as an increased state of arousal, euphoria, increased energy and talkativeness, but also negative emotions including anxiety, paranoia or auditory and visual hallucinations (Baylen and Rosenberg 2006; Cruickshank and Dyer, 2009). The peripheral effects of amphetamines are primarily mediated by its interaction with the noradrenaline transporter (NAT) and are associated with an increase in extracellular noradrenaline (NA) concentrations. These effects include increases in heart rate, blood pressure, respiration rate, body temperature, psychomotor activation and reduced appetite (Boenisch and Bruess, 2006; Cruickshank and Dyer, 2009). It is the sympathomimetic stimulating effect of amphetamines which renders them attractive as doping agents (Docherty, 2008).
Herein we will attempt to delineate the relationship which exists between amphetamines (in particular MDMA) and disease, and highlight areas of ignorance that would benefit from concerted research efforts. It is only now as the first generation of drug abusers begin to reach advanced age that we can truly begin to understand the long-term neuroadaptive and physiological consequences of amphetamine abuse in people.
The peripheral and central effects of amphetamines on the human nervous system are mediated primarily through their interaction with the presynaptic monoamine transporters for dopamine (DAT), serotonin (SERT) and noradrenaline (NAT). Presynaptic monoamine transporters are responsible for the reuptake of released neurotransmitter from the synaptic cleft and thus exert key dynamic spatial and temporal control over monoamine neurotransmission. As a consequence, the monoamine transporters act as important targets for multiple psychoactive agents in addition to amphetamines, such as cocaine and antidepressant drugs (Sitte and Freissmuth, 2010). All three transporters are members of the high affinity, neurotransmitter:sodium symporter (NSS) gene family of facilitated transporters (Saier, 2000). Their hydrophobic core is formed by 12 transmembrane helices. Their amino and carboxy termini are located within the cell and contain multiple consensus sites for protein kinase C (PKC), protein kinase A and Ca2+/calmodulin-dependent protein kinase (Vaughan, 2004). A crystal structure of a bacterial transporter LeuTAa has been available for several years (Yamashita et al., 2005). LeuTAa was originally thought to be a leucine transporter (hence its name), but it transports other substrates such as alanine more efficiently. LeuTAa is a bacterial homolog of the NSS and its structure has provided a template for educated guesses on the structure-function relationship in SLC6 family members (Forrest et al., 2008; Shi et al., 2008; Zhao et al., 2010).
Amphetamines act as competitive substrates at NAT, DAT and SERT. Therefore, the reuptake of endogenous neurotransmitters is reduced in their presence. Concomitantly and – more importantly – they also induce reverse transport of endogenous neurotransmitter and hence non-exocytotic neurotransmitter release (Sitte and Freissmuth, 2010). Such actions lead to a significant increase in the levels of extracellular serotonin (5-HT), NA and dopamine (DA) and are thought to account for the majority of acute amphetamine-induced behavioural and psychological effects (Green et al., 2003). Amphetamines also inhibit the action of vesicular monoamine transporters (VMATs) and monoamine oxidases (MAOs), although at significantly lower concentrations than that required for non-exocytotic neurotransmitter release. For example, MDMA inhibits MAO-A 10 times more potently than MAO-B, yet possesses an IC50 value of only 44 μm at MAO-A (Leonardi and Azmitia, 1994) and approximately 30 μm at VMAT2 (Partilla et al., 2006) when analysed in rat tissue in vitro, both of which are approximately 500-fold higher than its EC50 (0.074 μm) for 5-HT release in rat synaptosomes (Baumann et al., 2007). Furthermore, pharmacological doses (0.3–1 mg/kg) of MDMA, METH and d-AMPH do not block MAO activity when administered to male Sprague-Dawley rats in vivo (Zolkowska et al., 2006), whereas the MAO-A inhibitor clorgyline has been shown to synergistically enhance the effects of higher doses of MDMA (10–40 mg/kg) on serotonin mediated behaviour, body temperature and 5-HT release in rats (Hewton et al., 2007; Alves et al., 2009b). In contrast to MAO-A, both the gene silencing and inhibition of MAO-B protected against MDMA-induced 5-HT neurotoxicity and oxidative mitochondrial damage in rats (Sprague and Nichols, 1995; Falk et al., 2002; Hrometz et al., 2004; Alves et al., 2007), whereas 5-HT (but not DA depletions) are reduced in MAO-B deficient mice following chronic MDMA administration (Fornai et al., 2001). Thus, although MAOs clearly play a role in the mediation of chronic MDMA-induced neurotoxicity in rodents, the overall significance of MDMA-induced VMAT and MAO inhibition in acute central drug effects at pharmacological doses in vivo is as yet unclear.
The affinity for DAT, NAT and SERT differs among amphetamines: for instance, the addition of the 3,4-methylenedioxy group to the phenyl ring of MDMA significantly increases its affinity for SERT and NAT over DAT (Capela et al., 2009). The selectivity of METH, MDMA and d-AMPH for different monoamine transporters has also been associated with the specificity of their neurotoxic actions to the underlying monoaminergic neuronal systems. For example, METH has been reported to exert selective neurotoxicity in the dopaminergic nigrostriatal system, comparable to that of the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Subsequently, both MPTP and METH are used to produce animal models of Parkinson’s disease (PD) (Sonsalla et al., 1996; Harvey et al., 2000; Granado et al., 2010), even though an increased risk of PD in chronic human METH abusers has yet to be confirmed by epidemiological data (Guilarte, 2001; Chang et al., 2007). d-AMPH has also been reported to induce dopaminergic neurotoxicity in non-human primates when administered at doses equivalent to those used in the treatment of ADHD (Ricaurte et al., 2005). In contrast, MDMA is regarded as a selective serotonergic neurotoxin and has been associated with cognitive dysfunction in humans (Karlsen et al., 2008). All three drugs have addictive potential and can lead to varying degrees of drug dependence.
Acute MDMA toxicity and death in humans
MDMA abuse can lead to death. However, in comparison to the widespread use of MDMA, fatal intoxications are rare events (EMCDDA, 2009), the majority of which are accompanied by acute hyperthermia, hyponatramia, cardiovascular collapse and rhabdomyolysis (Lyles and Cadet, 2003; Hall and Henry, 2006). The direct mechanisms that underlie the toxic actions are difficult to elucidate because, in the majority of cases, MDMA is not the only drug involved. Acute MDMA toxicity in humans appears to be mainly due to the combined action of a serotonin syndrome and sympathomimetic effects (de la Torre et al., 2000). However, epileptic seizures have also been reported (Baylen and Rosenberg, 2006; Giorgi et al., 2006; Karlsen et al., 2008). A serotonin syndrome is the hallmark of acute MDMA toxicity in animals (Green et al., 2003).
Chronic MDMA neurotoxicity in animals
Few direct correlations exist between MDMA abuse and human disease states other than addiction. However, there are over 200 studies which demonstrate the acute biphasic effect of MDMA on serotonergic neurotransmission in rats and monkeys (Capela et al., 2009). Following the initial MDMA-induced release of 5-HT, the intracellular levels of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) are significantly reduced within 3–6 h. Recovery can occur within 24 h but the administration of multiple doses of MDMA, at ≥20 mg/kg in rats, can induce further decreases in serotonergic biomarkers and SERT surface levels that are still present 1 week later (Schmidt et al., 1986; Stone et al., 1986; Battaglia et al., 1987; Schmidt, 1987; Xie et al., 2006). Similar findings have been reported in monkeys but at lower doses, i.e., 2.5–5 mg/kg. This comparison indicates that monkeys are more susceptible to MDMA toxicity than rats (Capela et al., 2009, Green et al., 2009). Furthermore, in contrast to other rat strains such as the Sprague-Dawley or Wistar rats described above, significant decreases in serotonergic biomarkers have been reported in Dark Agouti rats following only single doses of 10–15 mg/kg MDMA (Capela et al., 2009).
MDMA also inhibits tryptophan hydroxylase activity, the rate limiting enzyme in 5-HT biosynthesis, in rats and reduces the expression of VMAT2 in baboons (Green et al., 2003). Numerous neuroanatomical and immunohistological studies have highlighted the axonal degeneration of 5-HT neurons following high doses of MDMA in rats and monkeys (Ricaurte et al., 1988; Green et al., 2003). Furthermore, abnormal reorganisation of 5-HT-projections and increased axonal ‘sprouting’ has also been reported in monkeys (Fischer et al., 1995; Hatzidimitriou et al., 1999). However, the selective nature of MDMA neurotoxicity in rats has recently been questioned (Schmued, 2003). Furthermore, in contrast to rats, MDMA primarily targets dopaminergic neurotransmission in mice (Itzhak et al., 2003; Granado et al., 2008) and has been shown to deplete both DA and its metabolites in numerous mouse studies (Capela et al., 2009). It is not clear why observations in rats and monkeys differ from those in mice, but species-dependent divergent oxidative metabolism is thought to be a contributing factor. Collectively, these findings indicate that MDMA has the potential to produce a distal axotomy of 5-HT neurons in rats and primates. Nonetheless, significant controversy exists regarding the ability of MDMA to induce ‘reactive gliosis’ in rats, or more specifically to increase glial fibrillary acidic protein expression (Pubill et al., 2003; Li et al., 2006, Chipana et al., 2008). Until a consensus can be reached regarding the ability of MDMA to induce the expression of this crucial neurotoxic CNS marker, the possibility that MDMA induced 5-HT depletions in rats are due to persistent adaptive changes in gene expression and/or protein function rather then neurotoxic damage must also be considered (Baumann et al., 2007). Furthermore, the majority of these studies were conducted using chronic drug administration paradigms (multiple i.p. or s.c. injections of 10 mg/kg or higher), as determined by ‘interspecies scaling’; an equation which estimates the equivalent dose rates between rodents and humans (Ricaurte and McCann, 2001). The relevance of the ‘allometric equation’ when extrapolating the results of MDMA animal studies to humans has recently been called into question (de la Torre and Farre, 2004; Baumann et al., 2007; Green et al., 2009) and is further complicated by both interspecies metabolic variations and the non-linear pharmacokinetics of MDMA metabolism in both humans and monkeys (de la Torre et al., 2000; Farre et al., 2004; Kolbrich et al., 2008; Green et al., 2009). The hepatic metabolism of MDMA is a crucial factor in the manifestation of MDMA neurotoxicity in rodents (de la Torre and Farre, 2004; Monks et al., 2004; Escobedo et al., 2005; Jones et al., 2005). This is also emphasised by the fact that the direct injection of MDMA into rat brain fails to reproduce the acute or long-term neurotoxic effects evident following peripheral administration (Esteban et al., 2001). Thus, neuroanatomical markers, dose, drug bioavailability and species-specific metabolic pathways must all be carefully assessed when interpreting the results of chronic toxicological animal data and more in-depth studies are required to further elucidate these areas. In spite of the obvious differences which exist in both species and even strain sensitivities to MDMA toxicity, the majority of animal data still suggests that MDMA is a serotonergic neurotoxin. However, the direct functional consequences of such neurotoxicity in humans have yet to be elucidated.
Chronic MDMA toxicity in humans: clinical studies
There are well-known limitations in extrapolating the results of animal studies to humans (de la Torre and Farre, 2004). Consequently, the actual neurotoxic potential of MDMA in humans remains a subject of much debate (Curran, 2000; Gouzoulis-Mayfrank and Daumann, 2006b; Baumann et al., 2007). Nonetheless, MDMA induces similar neurochemical, endocrine and behavioural effects in rats and humans at equivalent doses (Baumann et al., 2007). In addition, decreased levels of cerebrospinal fluid 5-HIAA and SERT binding sites have been reported in human MDMA users when compared to non-MDMA users (McCann et al., 1994, 1999; Semple et al., 1999; Reneman et al., 2001). Moreover, the magnitude of this decline was found to correlate with the extent of MDMA abuse (McCann et al., 1998). Findings from these early brain imaging studies have recently been corroborated by the employment of more stringent and sensitive parameters, thus confirming the interpretation that serotonergic neurotransmission is subject to long-lasting and subtle alterations following MDMA abuse in humans (Kish et al., 2010a,b). In line with its sympathomimetic affects, MDMA abuse has also been linked with myocardial damage and valvular heart disease (Baumann and Rothman 2009).
Numerous investigators have reported that memory, complex attention, decision-making and executive function are acutely impaired in MDMA users at low doses (Vollenweider et al., 2005; Kuypers and Ramaekers, 2007; Zakzanis et al., 2007; Karlsen et al., 2008). MDMA-induced reductions in SERT density have also been linked to impaired performance in short-term memory-dependent tasks such as complex calculations and semantic reasoning (McCann et al., 2008; Kish et al., 2010b). However, the study designs do not allow for the elucidation of cause-and-effect relationships. Two scenarios are conceivable: (i) frequent MDMA use causes impaired cognition and (ii) psychological difficulties associated with mild cognitive impairment also predispose to MDMA abuse. This question cannot be answered by comparing drug users and age-matched control individuals. However, MDMA impairs learning and memory formation in mice and increases phosphorylation of the fibrillary tangle protein TAU in the mouse hippocampus (Busceti et al., 2008). These observations support the conjecture that impaired memory is a consequence of MDMA consumption rather than that – vice versa – a cognitive impairment precedes MDMA consumption. Nevertheless, studies on the use of illicit drugs by people are difficult to interpret because the individual subjects typically buy impure drug preparations, typically consume several drugs, can suffer from pre-existing mental conditions and differ widely in their biographies (Gouzoulis-Mayfrank and Daumann, 2006a; Parrott, 2006; Soar et al., 2009). People also vary in their individual resilience; it is worth noting that a polymorphism in the serotonin transporter gene affects resilience/susceptibility to traumatic stress (Caspi et al., 2003). Thus, longitudinal studies are required to verify that MDMA does have an effect on human cognition independently of these confounding factors. In addition, it would be of major interest to determine whether a polymorphism in the promoter of the target gene of MDMA, i.e., SERT, affects susceptibility to the deleterious effects of MDMA. The currently available observations do not address these issues and they do not provide any conclusive evidence to support the conjecture that a promoter polymorphism renders people more likely to partake in MDMA consumption (Roiser et al., 2005; Martín-Santos et al., 2010).
A closer look into intracellular mechanisms: MDMA and mitochondrial dysfunction – a putative cause of neuropsychiatric disease?
Four key mechanisms have been identified that are thought to contribute to MDMA neurotoxicity and these include (1) hyperthermia, (2) MDMA metabolism, (3) oxidative stress, and (4) dysregulation of energy metabolism via mitochondrial dysfunction. A thorough review of each is beyond the scope of this review (more detailed information can be found in the following articles: Capela et al., 2009; Song et al., 2010; Yamamoto et al., 2010). Moreover, the role of MDMA-induced metabolic dysfunction is highlighted here in the context of neuropsychiatric disease.
Emerging evidence implicates both mitochondrial dysfunction and impaired metabolic fluxes in MDMA-induced neurotoxicity (Quinton and Yamamoto, 2006; Moon et al., 2008; Alves et al., 2009a; Pourahmad et al., 2010; Puerta et al., 2010). Mitochondria are the primary source of both cellular ATP and endogenous reactive oxygen species in the cell (Brown and Yamamoto, 2003). Oxidative stress and decreased energy production are hallmarks of acute MDMA exposure (Green et al., 2003). Hence, it seems probable that MDMA mediates at least some of its neurotoxic effects via mitochondrial dysfunction (Yamamoto and Raudensky, 2008; Puerta et al., 2010). The physiological consequences of MDMA-induced metabolic compromise are unclear. However, damage to the mitochondrial electron transport chain was recently proposed as an important factor in the pathogenesis of several neuropsychiatric disorders, including bipolar disorder, depression and schizophrenia (Rezin et al., 2009). There are several anecdotal reports of depressive symptoms, anxiety and psychiatric episodes following MDMA abuse (Schifano, 2000). In addition, long-lasting increases in anxiety-like behaviours have been documented in rats (Morton, 2005). However, there is much debate regarding the reliability of subjective MDMA user accounts, and several recent studies have questioned the role of polytoxicomanic drug intake, environmental stresses and preexisting mental disorders in the pathogenesis of ‘ecstasy’-induced depression and anxiety disorders (Durdle et al., 2008; Bedi et al., 2010; Pirona and Morgan, 2010; Scott et al., 2010). Thus, as yet it is unclear whether there is any direct correlation between MDMA and neuropsychobiological disease in the absence of polytoxicomanic drug use or confounding environmental factors.
Addictive potential of amphetamine abuse
An increase in synaptic mesocorticolimbic DA is believed to be the neurochemical correlate for the rewarding properties of drugs of abuse. This often initiates addiction in vulnerable individuals. The mesocorticolimbic DA system consists of dopaminergic neurons that reside in the ventral tegmental area (VTA) and project to various regions in the forebrain including the nucleus accumbens (NAc), prefrontal cortex and amygdala (Kauer and Malenka, 2007). Although all known drugs of abuse lead to an increase in mesocorticolimbic DA concentrations, the mechanisms that lead to DA release differ greatly.
The consumption of d-AMPH and METH results in a massive increase in extracellular DA within the NAc, far beyond that associated with its release in reward and motivation for naturally salient stimuli (Wise, 2004). In fact, because amphetamines lead to supraphysiological extracellular DA concentrations in the NAc, responses to natural rewarding stimuli are blunted. This desensitisation perturbs the normal physiological role of DA in reward and motivation through the induction of neuroplastic changes (Kalivas and Volkow, 2005; Kauer and Malenka, 2007). Psychostimulants such as d-AMPH and METH or the monoamine transporter blocker cocaine induce neurochemical sensitisation after repeated administration, i.e., a subsequent challenge with the drug leads to an increase in DAT-mediated DA efflux (Segal and Kuczenski, 1987; White and Kalivas, 1998). Amphetamines also increase locomotor activity, an effect which can be enhanced by the repeated administration of the drug. This hyperactivity is referred to as ‘behavioural sensitisation’ and is neurochemically correlated with an increase in striatal DA release. It can persist for several months following the last drug administration, thereby mimicking the sensitised states of human psychostimulant abusers (Paulson and Robinson, 1995; Pierce and Kalivas, 1997).
There is also substantial evidence to suggest that MDMA evokes drug seeking behaviour in animals, although to a lesser extent than either METH or d-AMPH. MDMA self-administration has been reported for mice (Trigo et al., 2007), rats (Ratzenboeck et al., 2001; Schenk, 2009) and non-human primates (Beardsley et al., 1986; Fantegrossi et al., 2002; Lile et al., 2005). MDMA can act as a reinforcer in both classical (conditioned place preference, CPP) and operant paradigms. However the rate of MDMA self-administration is consistently lower than either METH or cocaine in rhesus monkeys (Fantegrossi et al., 2002; Lile et al., 2005) and a high percentage of rats fail to partake in self-administration even after substantial training periods (Schenk, 2009). Likewise, lower doses of d-AMPH produce more consistent rates of drug induced self-administration than MDMA in rats and monkeys (Beardsley et al., 1986; Shin et al., 2008). Repeated MDMA exposure has also been reported to induce long-term behavioural and neurochemical sensitisation in rats (Kalivas et al., 1998; Ramos et al., 2005; Colussi-Mas and Schenk, 2008) and cross-sensitisation to the behavioural effects of cocaine (Schenk et al., 2008; Peraile et al., 2010) but not amphetamine in rats (Modi et al., 2006). Conversely, priming injections of racemic and (Sq)MDMA have recently been shown to reinstate extinguished d-AMPH- and MDMA-induced drug-seeking behaviour in rats (Schenk, 2009; McClung et al., 2010).
MDMA is clearly a reinforcer in animal studies, but the mechanism of MDMA-induced drug dependence is still under scrutiny. It appears to differ somewhat from that of AMPH (Degenhardt et al., 2010). As with amphetamines, the rewarding properties of MDMA are partially accounted for by drug-induced mesocorticolimbic DA release in rats and mice (Bankson and Yamamoto, 2004; Robledo et al., 2004b; Trigo et al., 2007; Tourino et al., 2008) and are attenuated by D1/D2 receptor antagonists in rats (Shin et al., 2008). Serotonergic neurotransmission and the endocannabinoid system are also implicated in the reinforcing effects of MDMA as MDMA self-administration is abolished in both SERT- and CB1-receptor-deficient mice (Trigo et al., 2007; Tourino et al., 2008) and attenuated by 5-HT2A antagonists in monkeys (Fantegrossi et al., 2002). However, in vivo microdialysis studies have shown that 5-HT stimulates DA release under both normal and drug-induced conditions via the activation of 5-HT1A, 5-HT1B, 5-HT2A, 5-HT3 and 5-HT4 receptors (Di Giovanni et al., 2002; Di Matteo et al., 2008), whereas 5-HT2 antagonists (Nash et al., 1990; Gudelsky et al., 1994; Schmidt et al., 1994) and SERT inhibitors suppress MDMA-induced DA release (Gudelsky and Nash, 1996; Koch and Galloway, 1997). Thus, the role of serotonergic neurotransmission in MDMA-induced drug dependence could be partly due to its indirect regulation of DA release. By contrast, the activation of the 5-HT2C receptor subtype has been shown to inhibit mesocortical DA function (Di Giovanni et al., 2002; Di Matteo et al., 2008). MDMA also stimulates GABA release in the VTA by 5-HT2C receptor activation. This counteracts MDMA-mediated DA efflux in the NAc (Bankson and Yamamoto, 2004). Accordingly, MDMA-induced behavioural sensitisation is antagonised by 5-HT2C activation in rats (Ramos et al., 2005). Thus, the net effect of 5-HT elevations on DA neurotransmission is dependent upon which serotonin receptors are activated within the circuitry. Furthermore, MDMA (but not METH or cocaine) activity in rat ventral striatal subregions can antagonise the reinforcing effects of DA in the medial olfactory tubercle (Shin et al., 2008). Thus, given the potential antagonistic effects of MDMA on mesolimbic DA release, it is not surprising that MDMA coadministration has also been shown to attenuate the reinforcing effects of both cocaine (Diller et al., 2007) and METH (Clemens et al., 2006) in rats.
In contrast to amphetamines, animals undergoing chronic MDMA administration experience limited withdrawal symptoms in response to acute serotonergic antagonism (Robledo et al., 2004a). These observations further question the relevance of animal experiments that rely on self-administration to gauge the addictive properties of MDMA. As outlined earlier, MDMA is not readily self-administered, indicating that its rewarding properties are modest in comparison to d-AMPH or cocaine. There have been numerous subjective accounts of MDMA withdrawal or ‘come-down’ in the days following MDMA ingestion in humans, but so far it has been difficult to separate these effects from either subacute drug effects or genuine dysphoria due to the removal of the drug (Degenhardt et al., 2010). Furthermore, and in contrast to rats, prolonged MDMA self-administration in monkeys results in a progressive reduction in MDMA responses. MDMA eventually fails to provide a reward that drives self-administration (Fantegrossi, 2007). This finding is in line with subjective reports of ‘MDMA tolerance’ experienced by frequent MDMA users (Green et al., 2003). MDMA tolerance has also been demonstrated in rats following acute high-dose administration and is believed to result from impaired 5-HT release and hyperthermia (Baumann et al., 2008; Jones et al., 2010).
Studies on ‘MDMA dependence’ in humans are often confounded by mixed sample populations of poly-drug users and typically rely on subjective user reports. Nonetheless, approximately 15% of routine MDMA users recently fit the diagnostic criteria for MDMA dependence according to the Diagnostic and Statistical Manual, fourth edition/DSMIV (Bruno et al., 2009). Comparable results have been reported following MDMA abuse in the United Kingdom (McCambridge et al., 2005). Furthermore, MDMA and d-AMPH were reported to have similar reinforcing effects in people during a controlled laboratory study (Tancer and Johanson, 2003). Thus, there is some epidemiological evidence to support the addictive potential of MDMA as described in animal studies, although to a much lesser extent than that of either METH or d-AMPH. MDMA-induced drug dependence is clearly a complex issue and many factors such as animal species/strain, receptor subtype, dose and duration of treatment must all be carefully considered when interpreting the results of animal studies. Further long-term studies are required to confirm the manifestation of MDMA dependence and recovery in humans in the absence of poly-drug use or environmental restrictions.
Amphetamine-induced dopamine efflux is partially dependent upon the specific activation of intracellular kinases (e.g., CamKIIα and PKCβ), glutamate receptors and l-type Ca2+ channels (Kantor et al., 2004; Licata et al., 2004; Fog et al., 2006; Chen et al., 2009; Singer et al., 2010). Pharmacological inhibition of CamKIIα or PKCβ in cells and rodents and gene knockout of PKCβ in mice reduces amphetamine-induced, DAT-mediated DA efflux (Fog et al., 2006; Chen et al., 2009). Furthermore, the pharmacological inhibition of CamKIIα or PKCβ (Pierce et al., 1998) and CamKIIα knockout (Licata et al., 2004) prevented sensitisation to cocaine in rodents; in line with these observations, herpes simplex-mediated transient overexpression of CamKIIα in the NAc shell enhanced d-AMPH-induced behavioural responses in rats (Loweth et al., 2010).
Taken together, these observations are consistent with a cellular model where amphetamine action in mesocorticolimbic dopaminergic neurons is the fundamental mechanism contributing to their reinforcing and addictive properties (Nestler, 2005; Kalivas, 2007). At the molecular level, the prime targets are the neurotransmitter transporters SERT and DAT which mediate amphetamine-induced non-exocytotic efflux (Sitte and Freissmuth, 2010).
d-AMPH and METH vary considerably in their toxic and addictive effects. Although d-AMPH has a higher affinity for DAT than METH (Howell and Kimmel, 2008), the latter is a more potent and also more perilous stimulant than d-AMPH. This could be as a result of their differing effects on cellular targets such as MAOs, mitochondrial electron transport chain complexes and their interactions with different signal transduction pathways. METH is more lipophilic than d-AMPH. Therefore, it readily enters the cell via diffusion in addition to DAT-dependent uptake. Furthermore, METH has been shown to release more DA and intracellular Ca2+ than d-AMPH at physiologic membrane potentials. These effects can be blocked by DAT inhibitors (Goodwin et al., 2009). This increased DA release perhaps provides an explanation for the enhanced abuse potential and the strong euphoric effects of acute METH exposure in humans.
Chronic METH abuse leads to the degeneration of monoaminergic terminals (Davidson et al., 2001; Krasnova and Cadet, 2009) and reduced DAT and DA levels in the striatum of mice, rats and monkeys (Anderson and Itzhak, 2006; Graham et al., 2008; Melega et al., 2008). Similar effects have been reported in people subjected to positron emission tomography (PET) (Volkow et al., 2001).
In contrast to MDMA, the metabolism of d-AMPH/METH does not appear to be significant in the manifestation of drug neurotoxicity. However, increases in DA metabolism following d-AMPH/METH-induced DA release have been implicated in the expression of amphetamine neurotoxicity, primarily through the production of oxidative stress (Krasnova and Cadet, 2009).
Many of these effects have been clarified and extended using animals rendered genetically deficient of a functional copy of DAT, NAT or SERT (‘gene knockout’ mice). DAT-knockout mice have an increase in extracellular DA concentrations which results in spontaneous hyperlocomotion (Giros et al., 1996). Interestingly, d-AMPH does not release additional DA in the striatum of DAT-knockout mice thus supporting the importance of DAT as a primary target for d-AMPH. Similarly, METH administration failed to induce either neurotoxicity or DA release in the striatum of DAT-deficient mice (Gainetdinov et al., 2002). Contrary to increasing locomotion in wild-type animals, d-AMPH inhibits hyperlocomotion in DAT-KO mice, but still increases the extracellular concentration of DA in the NAc. As outlined earlier, this could be the result of serotonergic modulation of dopaminergic neurons in the NAc because this effect occurs independently of DAT (Jones et al., 1998; Gainetdinov et al., 2002; Budygin et al., 2004). Serotonergic modulation can also explain why d-AMPH is still capable of inducing rewarding effects in these animals despite the absence of DAT (Budygin et al., 2004). By contrast, mice overexpressing DAT show markedly increased locomotor responses to d-AMPH and a 3-fold increase in DA release (Salahpour et al., 2008).
Although most of the research in sensitisation is focused on the dopaminergic system, new evidence is emerging to suggest that the noradrenergic and serotonergic systems might also be of fundamental importance (Tassin, 2008). Further support for this hypothesis came from observations that both cocaine and the SERT-specific antagonist fluoxetine were able to induce CPP in wild-type and DA-deficient mice (Hnasko et al., 2007). It is thus very probable that both DAT and SERT are important players in the mediation of drug-induced reward, as DAT/SERT-double knockout mice do not develop CPP in contrast to DAT-, SERT- or NAT single knockout mice (Sora et al., 2010).
Amphetamines and psychotic episodes
One of the prime findings in amphetamine abuse is the induction of psychotic episodes that are almost indistinguishable from the positive symptoms seen in schizophrenic patients (Ujike and Sato, 2004; Hermens et al., 2009). This supports the conjecture that there might be a link between amphetamine abuse and the psychopathic traits observed in schizophrenia. Although the aetiology of schizophrenia is very complex and comprises multiple genetic and environmental risk factors (Karam et al., 2010), psychostimulants such as d-AMPH or METH can increase the susceptibility of users to psychotic symptoms either during acute amphetamine abuse or during withdrawal (Ujike and Sato, 2004; Hermens et al., 2009). There are reports that DAT levels are not reduced in the striata of schizophrenic patients (Seeman and Niznik, 1990). However, others report that there is a reduction in DAT and tyrosine hydroxylase levels in the prefrontal cortex of postmortem specimens of schizophrenic subjects (Akil et al., 1999).
It has been recently shown that impulsive antisocial behaviours (a possible ‘negative symptom’ that can occur in schizophrenia) correlates with an increase in amphetamine-induced DA release in the NAc measured by [18F]fallypride PET and functional magnetic resonance imaging. These observations provide evidence for an association between substance abuse and psychopathic traits (Buckholtz et al., 2010). To obtain an animal model for the psychotic symptoms of schizophrenia, animals are subjected to amphetamine-induced sensitisation and observed during withdrawal periods after the sensitisation regimen (Paulson and Robinson, 1995; Peleg-Raibstein et al., 2009). Sensitised animals show an increase in subsequent amphetamine-induced DA release in the striatum and an increase in locomotor activity (Paulson and Robinson, 1995; Iwata et al., 1997). Thus, sensitisation is not only a model for addiction but also for psychosis (Gainetdinov et al., 2001). Abi-Dargham et al. (2009) have shown using [123I]IBZM (a DAT antagonist) single photon emission computed tomography that schizophrenic patients demonstrate a higher striatal DA release after challenge with d-AMPH and increased D2 receptor occupancy compared to control subjects (Abi-Dargham et al., 2009). Schizophrenia is a collection of presumably heterogeneous disease entities. Hence, many findings are controversial and difficult to reproduce. Nevertheless, at this stage it seems safe to conclude that there is sufficient evidence to substantiate the claim that DAT plays a role in the pathogenesis of schizophrenia.
Conclusions and future perspectives
Amphetamines are the second most commonly abused drugs in Europe after cannabis (EMCDDA, 2009) and the devastating effects of METH addiction are obvious in many parts of the world (Karila et al., 2010). All three drugs (METH, d-AMPH and MDMA) have been reported to induce psychotic episodes or ‘seizures’ in humans (Ujike and Sato, 2004; Karlsen et al., 2008). Furthermore, the loss of nigrostriatal dopaminergic neurons observed following repeated METH administration in animals has been associated with the pathogenesis of PD (Sonsalla et al., 1996; Harvey et al., 2000; Granado et al., 2010). These unintended (‘side’) effects should be carefully assessed when considering the long-term effects of amphetamine abuse on mental health and well-being. Conversely, both d-AMPH and METH are used in the treatment of ADHD, narcolepsy and obesity. Likewise, MDMA abuse has been implicated in both the origin and treatment of PD (Morton, 2005; Sotnikova et al., 2005). Moreover, MDMA has even been suggested as a therapeutic aid in post-traumatic stress disorder (Morton, 2005). Thus, investigations are continuing regarding the role of amphetamines in the pathogenesis (or indeed treatment) of CNS disease states. Long-term amphetamine administration has been shown to induce ample neurodegenerative side effects in animal models, thus rendering this the main cause for concern in humans following chronic amphetamine abuse. However, the results of animal behavioural and toxicity studies cannot be readily extrapolated to patients. This is particularly true for MDMA where major pharmacokinetic differences exist between commonly used laboratory animals. Thus, extensive longitudinal research in clinical settings is necessary to dissect confounding environmental factors such as polytoxicomanic drug use and pre-existing mental conditions from amphetamine-induced toxicity in the future. It is safe to predict that the availability of good data and reliable epidemiological evidence will also allow for a more temperate approach to the abuse and therapeutic use of amphetamines in the future.
Acknowledgments
This research is supported by a programme project grant of the Austrian Science Fund/FWF (SFB35 to H.H.S. and M.F.) and by a HRB/Marie Curie Postdoctoral Mobility fellowship to T.M.
References
- Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naive schizophrenic subjects. Biol. Psychiatry. 2009;65:1091–1093. doi: 10.1016/j.biopsych.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am. J. Psychiatry. 1999;156:1580–1589. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- Alves E, Summavielle T, Alves CJ, Gomes-da-Silva J, Barata JC, Fernandes E, Bastos Mde. L., Tavares MA, Carvalho F. Monoamine oxidase-B mediates ecstasy-induced neurotoxic effects to adolescent rat brain mitochondria. J. Neurosci. 2007;27:10203–10210. doi: 10.1523/JNEUROSCI.2645-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves E, Binienda Z, Carvalho F, Alves CJ, Fernandes E, de Lourdes, Bastos M, Tavares MA, Summavielle T. Acetyl-L-carnitine provides effective in vivo neuroprotection over 3,4-methylenedioximethamphetamine-induced mitochondrial neurotoxicity in the adolescent rat brain. Neuroscience. 2009a;158:514–523. doi: 10.1016/j.neuroscience.2008.10.041. [DOI] [PubMed] [Google Scholar]
- Alves E, Summavielle T, Alves CJ, Custodio JB, Fernandes E, de Lourdes Bastos M, Tavares MA, Carvalho F. Ecstasy-induced oxidative stress to adolescent rat brain mitochondria in vivo: influence of monoamine oxidase type A. Addict. Biol. 2009b;15:185–193. doi: 10.1111/j.1369-1600.2008.00143.x. [DOI] [PubMed] [Google Scholar]
- Anderson KL, Itzhak Y. Methamphetamine-induced selective dopaminergic neurotoxicity is accompanied by an increase in striatal nitrate in the mouse. Ann. N.Y. Acad. Sci. 2006;1074:225–233. doi: 10.1196/annals.1369.021. [DOI] [PubMed] [Google Scholar]
- Bankson MG, Yamamoto BK. Serotonin-GABA interactions modulate MDMA-induced mesolimbic dopamine release. J. Neurochem. 2004;91:852–859. doi: 10.1111/j.1471-4159.2004.02763.x. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Yeh SY, O’Hearn E, Molliver ME, Kuhar MJ, De Souza EB. 3,4-Methylenedioxymethamphetamine and 3,4-methylenedioxyamphetamine destroy serotonin terminals in rat brain: quantification of neurodegeneration by measurement of [3H]paroxetine-labeled serotonin uptake sites. J. Pharmacol. Exp. Ther. 1987;242:911–916. [PubMed] [Google Scholar]
- Baumann MH, Rothman RB. Neural and cardial toxicities associated with 3,4-methylenedioxymethamphetamine (MDMA) Int. Rev. Neurobiol. 2009;88:257–296. doi: 10.1016/S0074-7742(09)88010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats, a reappraisal of past and present findings. Psychopharmacology. 2007;189:407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Franken FH, Rutter JJ, Roth-man RB. Tolerance to 3,4-methylenedioxymethamphetamine in rats exposed to single high-dose binges. Neuroscience. 2008;152:773–784. doi: 10.1016/j.neuroscience.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylen CA, Rosenberg H. A review of the acute subjective effects of MDMA/ecstasy. Addiction. 2006;101:933–947. doi: 10.1111/j.1360-0443.2006.01423.x. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Balster RL, Harris LS. Self-administration of methylenedioxymethamphetamine (MDMA) by rhesus monkeys. Drug Alcohol Depend. 1986;18:149–157. doi: 10.1016/0376-8716(86)90047-5. [DOI] [PubMed] [Google Scholar]
- Bedi G, Van Dam NT, Redman J. Ecstasy (MDMA) and high prevalence psychiatric symptomatology, somatic anxiety symptoms are associated with polydrug, not ecstasy, use. J. Psychopharmacol. 2010;24:233–240. doi: 10.1177/0269881108097631. [DOI] [PubMed] [Google Scholar]
- Berman SM, Kuczenski R, McCracken JT, London ED. Potential adverse effects of amphetamine treatment on brain and behaviour: a review. Mol. Psychiatry. 2009;14:123–142. doi: 10.1038/mp.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boenisch H, Bruess M. The norepinephrine transporter in physiology and disease. In: Sitte HH, Freissmuth M, editors. Handbook of Experimental Pharmacology. Vol. 175. Springer Verlag; Heidelberg: 2006. pp. 485–524. [DOI] [PubMed] [Google Scholar]
- Brown JM, Yamamoto BK. Effects of amphetamines on mitochondrial function, role of free radicals and oxidative stress. Pharmacol. Ther. 2003;99:45–53. doi: 10.1016/s0163-7258(03)00052-4. [DOI] [PubMed] [Google Scholar]
- Bruno R, Matthews AJ, Topp L, Degenhardt L, Gomez R, Dunn M. Can the severity of dependence scale be usefully applied to ‘ecstasy’? Neuropsychobiology. 2009;60:137–147. doi: 10.1159/000253550. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, Ansari MS, Baldwin RM, Schwartz-man AN, Shelby ES, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat. Neurosci. 2010;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budygin EA, Brodie MS, Sotnikova TD, Mateo Y, John CE, Cyr M, Gainetdinov RR, Jones SR. Dissociation of rewarding and dopamine transporter-mediated properties of amphetamine. Proc. Natl. Acad. Sci. USA. 2004;101:7781–7786. doi: 10.1073/pnas.0401418101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busceti CL, Biagioni F, Riozzi B, Battaglia G, Storto M, Cinque C, Molinaro G, Gradini R, Caricasole A, Canudas AM, et al. Enhanced tau phosphorylation in the hippocampus of mice treated with 3,4-methylenedioxymethamphetamine (‘Ecstasy’) J. Neurosci. 2008;28:3234–3245. doi: 10.1523/JNEUROSCI.0159-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capela JP, Carmo H, Remiao F, Bastos ML, Meisel A, Carvalho F. Molecular and cellular mechanisms of ecstasy-induced neurotoxicity, an overview. Mol. Neurobiol. 2009;39:210–271. doi: 10.1007/s12035-009-8064-1. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, et al. Influence of life stress on depression, moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;1:16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Furman CA, Zhang M, Kim MN, Gereau RW, 4th, Leitges M, Gnegy ME. Protein kinase Cβ is a critical regulator of dopamine transporter trafficking and regulates the behavioral response to amphetamine in mice. J. Pharmacol. Exp. Ther. 2009;328:912–920. doi: 10.1124/jpet.108.147959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipana C, Torres I, Camarasa J, Pubill D, Escubedo E. Memantine protects against amphetamine derivatives-induced neurotoxic damages in rodents. Neuropharmacology. 2008;54:1254–1263. doi: 10.1016/j.neuropharm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Cornish JL, Hunt GE, McGregor IS. Intravenous methamphetamine self-administration in rats, effects of intravenous or intraperitoneal MDMA co-administration. Pharmacol. Biochem. Behav. 2006;85:454–463. doi: 10.1016/j.pbb.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Colussi-Mas J, Schenk S. Acute and sensitized response to 3,4-methylenedioxymethamphetamine in rats: different behavioral profiles reflected in different patterns of Fos expression. Eur. J. Neurosci. 2008;28:1895–1910. doi: 10.1111/j.1460-9568.2008.06467.x. [DOI] [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1084–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- Curran HV. Is MDMA (‘Ecstasy’) neurotoxic in humans? An overview of evidence and of methodological problems in research. Neuropsychobiology. 2000;42:34–41. doi: 10.1159/000026668. [DOI] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res. Brain Res. Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farre M. Neurotoxicity of MDMA (ecstasy), the limitations of scaling from animals to humans. Trends Pharmacol. Sci. 2004;25:505–508. doi: 10.1016/j.tips.2004.08.001. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farre M, Roset PN, Lopez CH, Mas M, Ortuno J, Menoyo E, Pizarro N, Segura J, Cami J. Pharmacology of MDMA in humans. Ann. N.Y. Acad. Sci. 2000;914:225–237. doi: 10.1111/j.1749-6632.2000.tb05199.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bruno R, Topp L. Is ecstasy a drug of dependence? Drug Alcohol Depend. 2010;107:1–10. doi: 10.1016/j.drugalcdep.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, Esposito E. Serotonin/dopamine interaction – focus on 5-HT2C receptor, a new target of psychotropic drugs. Indian J. Exp. Biol. 2002;40:1344–1352. [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Pierucci M, Esposito E. Serotonin control of central dopaminergic function, focus on in vivo microdialysis studies. Prog. Brain Res. 2008;172:7–44. doi: 10.1016/S0079-6123(08)00902-3. [DOI] [PubMed] [Google Scholar]
- Diller AJ, Rocha A, Cardon AL, Valles R, Wellman PJ, Nation JR. The effects of concurrent administration of +/−3,4-methylenedioxymethamphetamine and cocaine on conditioned place preference in the adult male rat. Pharmacol. Biochem. Behav. 2007;88:165–170. doi: 10.1016/j.pbb.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty JR. Pharmacology of stimulants prohibited by the world anti-doping agency (WADA) Br. J. Pharmacol. 2008;154:606–622. doi: 10.1038/bjp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durdle H, Lundahl LH, Johanson CE, Tancer M. Major depression, the relative contribution of gender, MDMA, and cannabis use. Depress Anxiety. 2008;25:241–247. doi: 10.1002/da.20297. [DOI] [PubMed] [Google Scholar]
- EMCDDA . Annual statistical report on state of drugs problem in Europe. European Monitoring Centre for Drugs and Drug Addiction; Publications Office of the European Union; Luxembourg: 2009. [Google Scholar]
- Escobedo I, O’Shea E, Orio L, Sanchez V, Segura M, de la Torre R, Farre M, Green AR, Colado MI. A comparative study on the acute and long-term effects of MDMA and 3,4-dihydroxymethamphetamine (HHMA) on brain monoamine levels after i.p. or striatal administration in mice. Br. J. Pharmacol. 2005;144:231–241. doi: 10.1038/sj.bjp.0706071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban B, O’Shea E, Camarero J, Sanchez V, Green AR, Colado MI. 3,4-Methylenedioxymethamphetamine induces monoamine release, but not toxicity, when administered centrally at a concentration occuring following a peripherally injected neurotoxic dose. Psychopharmacology. 2001;154:251–260. doi: 10.1007/s002130000645. [DOI] [PubMed] [Google Scholar]
- Falk EM, Cook VJ, Nichols DE, Sprague JE. An antisense oligonucleotide targeted at MAO-B attenuates rat striatal serotonergic neurotoxicity induced by MDMA. Pharmacol. Biochem. Behav. 2002;72:617–622. doi: 10.1016/s0091-3057(02)00728-1. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE. Reinforcing effects of methylenedioxy amphetamine congeners in rhesus monkey: are intravenous self-administration experiments relevant to MDMA neurotoxicity? Psychopharmacology (Berlin) 2007;189:471–482. doi: 10.1007/s00213-006-0320-8. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Ullrich T, Rice KC, Woods JH, Winger G. 3,4-Methylenedioxymethamphetamine (MDMA, ‘ecstasy’) and its stereoisomers as reinforcers in rhesus monkeys, serotonergic involvement. Psychopharmacology. 2002;161:356–364. doi: 10.1007/s00213-002-1021-6. [DOI] [PubMed] [Google Scholar]
- Farre M, de la Torre R, Mathina BO, Roset PN, Peiro AM, Torrens M, Ortuno J, Pujadas M, Cami J. Repeated doses administration of MDMA in humans: pharmacological effects and pharmacokinetics. Psychopharmacol. (Berl.) 2004;173:364–375. doi: 10.1007/s00213-004-1789-7. [DOI] [PubMed] [Google Scholar]
- Fischer C, Hatzidimitriou G, Wlos J, Katz J, Ricaurte G. Reorganization of ascending 5-HT axon projections in animals previously exposed to the recreational drug (+/−)3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) J. Neurosci. 1995;15:5476–5485. doi: 10.1523/JNEUROSCI.15-08-05476.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, Nikandrova Y, Bowton E, McMahon DG, Colbran RJ, et al. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Fornai F, Giorgi FS, Gesi M, Chen K, Alessri MG, Shi JC. Biochemical effects of the monoamine neurotoxins DSP-4 and MDMA in specific brain regions of MAO-B-deficient mice. Synapse. 2001;39:213–221. doi: 10.1002/1098-2396(20010301)39:3<213::AID-SYN1002>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Forrest LR, Zhang YW, Jacobs MT, Gesmonde J, Xie L, Honig BH, Rudnick G. Mechanism for alternating access in neurotransmitter transporters. Proc. Natl. Acad. Sci. USA. 2008;105:10338–10343. doi: 10.1073/pnas.0804659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Mohn AR, Caron MG. Genetic animal models, focus on schizophrenia. Trends Neurosci. 2001;24:527–533. doi: 10.1016/s0166-2236(00)01886-5. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Sotnikova TD, Caron MG. Monoamine transporter pharmacology and mutant mice. Trends Pharmacol. Sci. 2002;23:367–373. doi: 10.1016/s0165-6147(02)02044-8. [DOI] [PubMed] [Google Scholar]
- Giorgi FS, Lazzeri G, Natale G, Iudice A, Ruggieri S, Papa-relli A, Murri L, Fornai F. MDMA and seizures, a dangerous liaison? Ann. N.Y. Acad. Sci. 2006;1074:357–364. doi: 10.1196/annals.1369.035. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Goodwin JS, Larson GA, Swant J, Sen N, Javitch JA, Zahniser NR, De Felice LJ, Khoshbouei H. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J. Biol. Chem. 2009;284:2978–2989. doi: 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Daumann J. The confounding problem of polydrug use in recreational ecstasy/MDMA users: a brief overview. J. Psychopharmacol. 2006a;20:188–193. doi: 10.1177/0269881106059939. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Daumann J. Neurotoxicity of methylenedioxyamphetamines (MDMA; ecstasy) in humans, how strong is the evidence for persistent brain damage? Addiction. 2006b;101:348–361. doi: 10.1111/j.1360-0443.2006.01314.x. [DOI] [PubMed] [Google Scholar]
- Graham DL, Noailles PA, Cadet JL. Differential neurochemical consequences of an escalating dose-binge regimen followed by single-day multiple-dose methamphetamine challenges. J. Neurochem. 2008;105:1873–1885. doi: 10.1111/j.1471-4159.2008.05269.x. [DOI] [PubMed] [Google Scholar]
- Granado N, O’Shea E, Bove J, Vila M, Colado MI, Moratalla R. Persistent MDMA-induced dopaminergic neurotoxicity in the striatum and substantia nigra of mice. J. Neurochem. 2008;107:1102–1112. doi: 10.1111/j.1471-4159.2008.05705.x. [DOI] [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, O’Shea E, Vicario-Abejon C, Colado MI, Moratalla R. Selective vulnerability in striosomes and the nigrostriatal dopaminergic pathway after methamphetamine administration. Early loss of TH in striosomes after methamphetamine. Neurotox. Res. 2010;18:48–58. doi: 10.1007/s12640-009-9106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) Pharmacol. Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Green AR, Gabrielsson J, Marsden CA, Fone KC. MDMA, on the translation from rodent to human dosing. Psychopharmacology. 2009;204:375–378. doi: 10.1007/s00213-008-1453-8. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Nash JF. Carrier-mediated release of serotonin by 3,4-methylenedioxymethamphetamine: implications for serotonin-dopamine interactions. J. Neurochem. 1996;66:243–249. doi: 10.1046/j.1471-4159.1996.66010243.x. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Yamamoto BK, Nash JF. Potentiation of 3,4-methylenedioxymethamphetamine-induced dopamine release and serotonin neurotoxicity by 5-HT2 receptor agonists. Eur. J. Pharmacol. 1994;264:325–330. doi: 10.1016/0014-2999(94)90669-6. [DOI] [PubMed] [Google Scholar]
- Guilarte TR. Is methamphetamine abuse a risk factor in parkinsonism? Neurotoxicology. 2001;22:725–731. doi: 10.1016/s0161-813x(01)00046-8. [DOI] [PubMed] [Google Scholar]
- Hall AP, Henry JA. Acute toxic effects of ‘Ecstasy’ (MDMA) and related compounds, overview of pathophysiology and clinical management. Br. J. Anaesth. 2006;96:678–685. doi: 10.1093/bja/ael078. [DOI] [PubMed] [Google Scholar]
- Harvey DC, Lacan G, Melegan WP. Regional heterogeneity of dopaminergic deficits in vervet monkey striatum and substantia nigra after methamphetamine exposure. Exp. Brain Res. 2000;133:349–358. doi: 10.1007/s002210000386. [DOI] [PubMed] [Google Scholar]
- Hatzidimitriou G, McCann UD, Ricaurte GA. Altered serotonin innervation patterns in the forebrain of monkeys treated with (+/−)3,4-methylenedioxymethamphetamine seven years previously, factors influencing abnormal recovery. J. Neurosci. 1999;19:5096–5107. doi: 10.1523/JNEUROSCI.19-12-05096.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens DF, Lubman DI, Ward PB, Naismith SL, Hickie IB. Amphetamine psychosis: a model for studying the onset and course of psychosis. Med. J. Aust. 2009;190:S22–S25. doi: 10.5694/j.1326-5377.2009.tb02370.x. [DOI] [PubMed] [Google Scholar]
- Hewton R, Salem A, Irvine RJ. Potentiation of 3,4-methylenedioxymethamphetamine-induced 5-HT release in the rat substantia nigra by clorgyline, a monoamine oxidase A inhibitor. Clin. Exp. Pharmacol. Physiol. 2007;34:1051–1057. doi: 10.1111/j.1440-1681.2007.04734.x. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Sotak BN, Palmiter RD. Cocaine-conditioned place preference by dopamine-deficient mice is mediated by serotonin. J. Neurosci. 2007;27:12484–12488. doi: 10.1523/JNEUROSCI.3133-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochem. Pharmacol. 2008;75:196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Hrometz SL, Brown AW, Nichols DE, Sprague JE. 3,4-Methylenedioxymethamphetamine (MDMA, ecstasy)-mediated production of hydrogen peroxide in an in vitro model: the role of dopamine, the serotonin-reuptake transporter, and monoamine oxidase-B. Neurosci. Lett. 2004;26:56–59. doi: 10.1016/j.neulet.2004.05.075. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Ali SF, Achat CN, Anderson KL. Relevance of MDMA (‘ecstasy’)-induced neurotoxicity to long-lasting psychomotor stimulation in mice. Psychopharmacology. 2003;166:241–248. doi: 10.1007/s00213-002-1320-y. [DOI] [PubMed] [Google Scholar]
- Iwata SI, Hewlett GH, Ferrell ST, Kantor L, Gnegy ME. Enhanced dopamine release and phosphorylation of synapsin I and neuromodulin in striatal synaptosomes after repeated amphetamine. J. Pharmacol. Exp. Ther. 1997;283:1445–1452. [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J. Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DC, Duvauchelle C, Ikegami A, Olsen CM, Lau SS, de la Torre R, Monks TJ. Serotonergic neurotoxic metabolites of ecstasy identified in rat brain. J. Pharmacol. Exp. Ther. 2005;313:422–431. doi: 10.1124/jpet.104.077628. [DOI] [PubMed] [Google Scholar]
- Jones K, Brennan KA, Colussi-Mas J, Schenk S. Tolerance to 3,4-methylenedioxymethamphetamine is associated with impaired serotonin release. Addict. Biol. 2010;15:289–298. doi: 10.1111/j.1369-1600.2010.00217.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Cocaine and amphetamine-like psychostimulants, neurocircuitry and glutamate neuroplasticity. Dialogues Clin. Neurosci. 2007;9:389–397. doi: 10.31887/DCNS.2007.9.4/pkalivas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction, a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, White SR. MDMA elicits behavioral and neurochemical sensitization in rats. Neuropsychopharmacology. 1998;18:469–479. doi: 10.1016/S0893-133X(97)00195-4. [DOI] [PubMed] [Google Scholar]
- Kantor L, Zhang M, Guptaroy B, Park YH, Gnegy ME. Repeated amphetamine couples norepinephrine transporter and calcium channel activities in PC12 cells. J. Pharmacol. Exp. Ther. 2004;311:1044–1051. doi: 10.1124/jpet.104.071068. [DOI] [PubMed] [Google Scholar]
- Karam CS, Ballon JS, Bivens NM, Freyberg Z, Girgis RR, Lizardi-Ortiz JE, Markx S, Lieberman JA, Javitch JA. Signaling pathways in schizophrenia: emerging targets and therapeutic strategies. Trends Pharmacol. Sci. 2010;31:381–390. doi: 10.1016/j.tips.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. Br. J. Clin. Pharmacol. 2010;69:578–592. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen SN, Spigset O, Slordal L. The dark side of ecstasy, neuropsychiatric symptoms after exposure to 3,4-methylenedioxymethamphetamine. Basic Clin. Pharmacol. Toxicol. 2008;102:15–24. doi: 10.1111/j.1742-7843.2007.00159.x. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat. Rev. Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kish S, Fitzmaurice P, Chang L, Furukawa Y, Tong J. Low striatal serotonin transporter protein in a human polydrug MDMA (ecstasy) user, a case study. J. Psychopharmacol. 2010a;24:281–284. doi: 10.1177/0269881108097724. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Lerch J, Furukawa Y, Tong J, McCluskey T, Wilkins D, Houle S, Meyer J, Mundo E, Wilson AA, et al. Decreased cerebral cortical serotonin transporter binding in ecstasy users: a positron emission tomography/[11C]DASB and structural brain imaging study. Brain. 2010b;133:1779–1797. doi: 10.1093/brain/awq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Galloway MP. MDMA induced dopamine release in vivo: role of endogenous serotonin. J. Neural Transm. 1997;104:135–146. doi: 10.1007/BF01273176. [DOI] [PubMed] [Google Scholar]
- Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. Plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine after controlled oral administration to young adults. Ther. Drug Monit. 2008;30:320–332. doi: 10.1097/FTD.0b013e3181684fa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res. Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers KP, Ramaekers JG. Acute dose of MDMA (75 mg) impairs spatial memory for location but leaves contextual processing of visuospatial information unaffected. Psycho-pharmacology (Berlin) 2007;189:557–563. doi: 10.1007/s00213-006-0321-7. [DOI] [PubMed] [Google Scholar]
- Leonardi ET, Azmitia EC. MDMA (ecstasy) inhibition of MAO type A and type B, comparisons with fenfluramine and fluoxetine (Prozac) Neuropsychopharmacology. 1994;10:231–238. doi: 10.1038/npp.1994.26. [DOI] [PubMed] [Google Scholar]
- Li SX, Li J, Wang X, Peng ZG, Kuang WH, Huang MS. Long-term neurotoxic effects of MDMA results in cortical and hippocampal structural changes. Sheng Li Xue Bao. 2006;58:34–40. [PubMed] [Google Scholar]
- Licata SC, Schmidt HD, Pierce RC. Suppressing calcium/calmodulin-dependent protein kinase II activity in the ventral tegmental area enhances the acute behavioural response to cocaine but attenuates the initiation of cocaine-induced behavioural sensitization in rats. Eur. J. Neurosci. 2004;19:405–414. doi: 10.1111/j.0953-816x.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- Lile JA, Ross JT, Nader MA. A comparison of the reinforcing efficacy of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) with cocaine in rhesus monkeys. Drug Alcohol Depend. 2005;78:135–140. doi: 10.1016/j.drugalcdep.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Loweth JA, Singer BF, Baker LK, Wilke G, Inamine H, Bubula N, Alexander JK, Carlezon WA, Jr., Neve RL, Vezina P. Transient overexpression of α-Ca2+/calmodulin-dependent protein kinase II in the nucleus accumbens shell enhances behavioral responding to amphetamine. J. Neurosci. 2010;30:939–949. doi: 10.1523/JNEUROSCI.4383-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles J, Cadet JL. Methylenedioxymethamphetamine (MDMA, Ecstasy) neurotoxicity, cellular and molecular mechanisms. Brain Res. Brain Res. Rev. 2003;42:155–168. doi: 10.1016/s0165-0173(03)00173-5. [DOI] [PubMed] [Google Scholar]
- Martín-Santos R, Torrens M, Poudevida S, Langohr K, Cuyas E, Pacifici R, Farre M, Pichini S, de la Torre R. 5-HTTLPR polymorphism, mood disorders and MDMA use in a 3-year follow-up study. Addict. Biol. 2010;15:15–22. doi: 10.1111/j.1369-1600.2009.00180.x. [DOI] [PubMed] [Google Scholar]
- McCambridge J, Mitcheson L, Winstock A, Hunt N. Five-year trends in patterns of drug use among people who use stimulants in dance contexts in the United Kingdom. Addiction. 2005;100:1140–1149. doi: 10.1111/j.1360-0443.2005.001127.x. [DOI] [PubMed] [Google Scholar]
- McCann UD, Ridenour A, Shaham Y, Ricaurte GA. Serotonin neurotoxicity after (+/−)3,4-methylenedioxymethamphetamine (MDMA; ‘Ecstasy’), a controlled study in humans. Neuropsychopharmacology. 1994;10:129–138. doi: 10.1038/npp.1994.15. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. Positron emission tomographic evidence of toxic effect of MDMA (‘Ecstasy’) on brain serotonin neurons in human beings. Lancet. 1998;352:1433–1437. doi: 10.1016/s0140-6736(98)04329-3. [DOI] [PubMed] [Google Scholar]
- McCann UD, Mertl M, Eligulashvili V, Ricaurte GA. Cognitive performance in (+/−)3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) users, a controlled study. Psychopharmacology. 1999;143:417–425. doi: 10.1007/s002130050967. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Vranesic M, Palermo M, Mathews WB, Ravert HT, Dannals RF, Ricaurte GA. Positron emission tomographic studies of brain dopamine and serotonin transporters in abstinent (+/−)3,4-methylenedioxymethamphetamine (‘ecstasy’) users, relationship to cognitive performance. Psychopharmacology. 2008;200:439–450. doi: 10.1007/s00213-008-1218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung J, Fantegrossi W, Howell LL. Reinstatement of extinguished amphetamine self-administration by 3,4-methylenedioxymethamphetamine (MDMA) and its enantiomers in rhesus monkeys. Psychopharmacology. 2010;210:75–83. doi: 10.1007/s00213-010-1818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melega WP, Jorgensen MJ, Lacan G, Way BM, Pham J, Morton G, Cho AK, Fairbanks LA. Long-term methamphetamine administration in the vervet monkey models aspects of a human exposure: brain neurotoxicity and behavioral profiles. Neuropsychopharmacology. 2008;33:1441–1452. doi: 10.1038/sj.npp.1301502. [DOI] [PubMed] [Google Scholar]
- Modi GM, Yang PB, Swann AC, Dafny N. Chronic exposure to MDMA (Ecstasy) elicits behavioral sensitization in rats but fails to induce cross-sensitization to other psychostimulants. Behav. Brain Funct. 2006;2:1. doi: 10.1186/1744-9081-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks TJ, Jones DC, Bai F, Lau SS. The role of metabolism in 3,4-(+)-methylenedioxyamphetamine and 3,4-(+)-methylenedioxymethamphetamine (ecstasy) toxicity. Ther. Drug Monit. 2004;26:132–136. doi: 10.1097/00007691-200404000-00008. [DOI] [PubMed] [Google Scholar]
- Moon KH, Upreti VV, Yu LR, Lee IJ, Ye X, Eddington ND, Veenstra TD, Song BJ. Mechanism of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy)-mediated mitochondrial dysfunction in rat liver. Proteomics. 2008;8:3906–3918. doi: 10.1002/pmic.200800215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J. Ecstasy, pharmacology and neurotoxicity. Curr. Opin. Pharmacol. 2005;5:79–86. doi: 10.1016/j.coph.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Nash JF, Meltzer HY, Gudelsky GA. Effect of 3,4-methylenedioxymethamphetamine on 3,4-dihydroxyphenylalaxsnine accumulation in the striatum and nucleus accumbens. J. Neurochem. 1990;54:1062–1067. doi: 10.1111/j.1471-4159.1990.tb02358.x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat. Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Parrott AC. MDMA in humans, factors which affect the neuropsychobiological profiles of recreational ecstasy users, the integrative role of bioenergetic stress. J. Psychopharmacol. 2006;20:147–163. doi: 10.1177/0269881106063268. [DOI] [PubMed] [Google Scholar]
- Partilla JS, Dempsey AG, Nagpal AS, Blough BE, Baumann MH, Rothman RB. Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J. Pharmacol. Exp. Ther. 2006;319:237–246. doi: 10.1124/jpet.106.103622. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum, a microdialysis study in behaving rats. Synapse. 1995;19:56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Yee BK, Feldon J, Hauser J. The amphetamine sensitization model of schizophrenia, relevance beyond psychotic symptoms? Psychopharmacology. 2009;4:603–621. doi: 10.1007/s00213-009-1514-7. [DOI] [PubMed] [Google Scholar]
- Peraile I, Torres E, Mayado A, Izco M, Lopez-Jimenez A, Lopez-Moreno JA, Colado MI, O’Shea E. Dopamine transporter down-regulation following repeated cocaine, implications for 3,4-methylenedioxymethamphetamine-induced acute effects and long-term neurotoxicity in mice. Br. J. Pharmacol. 2010;159:201–211. doi: 10.1111/j.1476-5381.2009.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas RW. A circuitry model of the expression of behavioural sensitization to amphetamine-like psychostimulants. Brain Res. Brain Res. Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Quick EA, Reeder DC, Morgan ZR, Kalivas PW. Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. J. Pharmacol. Exp. Ther. 1998;286:1171–1176. [PubMed] [Google Scholar]
- Pirona A, Morgan MJ. An investigation of the suba-cute effects of ecstasy on neuropsychological performance, sleep and mood in regular ecstasy users. J. Psychopharmacol. 2010;24:175–185. doi: 10.1177/0269881109102780. [DOI] [PubMed] [Google Scholar]
- Pourahmad J, Eskandari MR, Nosrati M, Kobarfard F, Khajeamiri AR. Involvement of mitochondrial/lysosomal toxic cross-talk in ecstasy induced liver toxicity under hyperthermic condition. Eur. J. Pharmacol. 2010;643:162–169. doi: 10.1016/j.ejphar.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Pubill D, Canudas AM, Pallas M, Camins A, Camarasa J, Escubedo E. Differential glial response to meth-amphetamine- and methylendioxymethamphetamine-induced neurotoxicity. Naunyn-Schmiedebergs Arch. Pharmacol. 2003;367:490–499. doi: 10.1007/s00210-003-0747-y. [DOI] [PubMed] [Google Scholar]
- Puerta E, Hervias I, Goni-Allo B, Zhang SF, Jordan J, Starkov AA, Aguirre N. Methylenedioxymethamphetamine inhibits mitochondrial complex I activity in mice: a possible mechanism underlying neurotoxicity. Br. J. Pharmacol. 2010;160:233–245. doi: 10.1111/j.1476-5381.2010.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMA toxicity. AAPS J. 2006;8:E337–E347. doi: 10.1007/BF02854904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos M, Goni-Allo B, Aguirre N. Administration of SCH 23390 into the medial prefrontal cortex blocks the expression of MDMA-induced behavioral sensitization in rats: an effect mediated by 5-HT2C receptor stimulation and not by D1 receptor blockade. Neuropsychopharmacology. 2005;30:2180–2191. doi: 10.1038/sj.npp.1300735. [DOI] [PubMed] [Google Scholar]
- Ratzenboeck E, Saria A, Kriechbaum N, Zernig G. Reinforcing effects of MDMA (‘ecstasy’) in drug-naive and cocaine-trained rats. Pharmacology. 2001;62:138–144. doi: 10.1159/000056086. [DOI] [PubMed] [Google Scholar]
- Reneman L, Lavalaye J, Schmand B, de Wolff FA, van den Brink W, den Heeten GJ, Booij J. Cortical serotonin transporter density and verbal memory in individuals who stopped using 3,4-methylenedioxymethamphetamine (MDMA or ‘ecstasy’): preliminary findings. Arch. Gen. Psychiatry. 2001;58:901–906. doi: 10.1001/archpsyc.58.10.901. [DOI] [PubMed] [Google Scholar]
- Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL. Mitochondrial dysfunction and psychiatric disorders. Neurochem. Res. 2009;34:1021–1029. doi: 10.1007/s11064-008-9865-8. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, McCann UD. Assessing long-term effects of MDMA (Ecstasy) Lancet. 2001;358:1831–1832. doi: 10.1016/S0140-6736(01)06880-5. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Forno LS, Wilson MA, DeLanney LE, Irwin I, Molliver ME, Langston JW. (+/−)3,4-Methylenedioxymethamphetamine selectively damages central serotonergic neurons in nonhuman primates. J. Am. Med. Assoc. 1988;260:51–55. [PubMed] [Google Scholar]
- Ricaurte GA, Mechan AO, Yuan J, Hatzidimitriou G, Xie T, Mayne AH, McCann UD. Amphetamine treatment similar to that used in the treatment of adult attention-deficit/hyperactivity disorder damages dopaminergic nerve endings in the striatum of adult nonhuman primates. J. Pharmacol. Exp. Ther. 2005;315:91–98. doi: 10.1124/jpet.105.087916. [DOI] [PubMed] [Google Scholar]
- Robledo P, Balerio G, Berrendero F, Maldonado R. Study of the behavioural responses related to the potential addictive properties of MDMA in mice. Naunyn-Schmiedebergs Arch. Pharmacol. 2004a;369:338–349. doi: 10.1007/s00210-003-0862-9. [DOI] [PubMed] [Google Scholar]
- Robledo P, Mendizabal V, Ortuno J, de la Torre R, Kieffer BL, Maldonado R. The rewarding properties of MDMA are preserved in mice lacking mu-opioid receptors. Eur. J. Neurosci. 2004b;20:853–858. doi: 10.1111/j.1460-9568.2004.03532.x. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Cook LJ, Cooper JD, Rubinsztein DC, Sahakian BJ. Association of a functional polymorphism in the serotonin transporter gene with abnormal emotional processing in ecstasy users. Am. J. Psychiatry. 2005;162:609–612. doi: 10.1176/appi.ajp.162.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier MH., Jr. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 2000;64:354–411. doi: 10.1128/mmbr.64.2.354-411.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahpour A, Ramsey AJ, Medvedev IO, Kile B, Sotnikova TD, Holmstrand E, Ghisi V, Nicholls PJ, Wong L, Murphy K, et al. Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proc. Natl. Acad. Sci. USA. 2008;105:4405–4410. doi: 10.1073/pnas.0707646105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S. MDMA self-administration in laboratory animals, a summary of the literature and proposal for future research. Neuropsychobiology. 2009;60:130–136. doi: 10.1159/000253549. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hely L, Gittings D, Lake B, Daniela E. Effects of priming injections of MDMA and cocaine on reinstatement of MDMA- and cocaine-seeking in rats. Drug Alcohol Depend. 2008;96:249–255. doi: 10.1016/j.drugalcdep.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Schifano F. Potential human neurotoxicity of MDMA (‘Ecstasy’), subjective self-reports, evidence from an Italian drug addiction centre and clinical case studies. Neuropsychobiology. 2000;42:25–33. doi: 10.1159/000026667. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ. Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. J. Pharmacol. Exp. Ther. 1987;240:1–7. [PubMed] [Google Scholar]
- Schmidt CJ, Wu L, Lovenberg W. Methylenedioxymethamphetamine, a potentially neurotoxic amphetamine analogue. Eur. J. Pharmacol. 1986;124:175–178. doi: 10.1016/0014-2999(86)90140-8. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Sullivan CK, Fadayel GM. Blockade of striatal 5-hydroxytryptamine2 receptors reduces the increase in extracellular concentrations of dopamine produced by the amphetamine analogue 3,4-methylenedioxymethamphetamine. J. Neurochem. 1994;62:1382–1389. doi: 10.1046/j.1471-4159.1994.62041382.x. [DOI] [PubMed] [Google Scholar]
- Schmued LC. Demonstration and localization of neuronal degeneration in the rat forebrain following a single exposure to MDMA. Brain Res. 2003;974:127–133. doi: 10.1016/s0006-8993(03)02563-0. [DOI] [PubMed] [Google Scholar]
- Scott RM, Hides L, Allen JS, Burke R, Lubman DI. Depressive and anxiety symptomatology in ecstasy users, the relative contribution of genes, trauma, life stress and drug use. Psychopharmacology. 2010;209:25–36. doi: 10.1007/s00213-009-1763-5. [DOI] [PubMed] [Google Scholar]
- Seeman P, Niznik HB. Dopamine receptors and transporters in Parkinson’s disease and schizophrenia. FASEB J. 1990;4:2737–2744. doi: 10.1096/fasebj.4.10.2197154. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J. Pharmacol. Exp. Ther. 1987;242:917–926. [PubMed] [Google Scholar]
- Semple DM, Ebmeier KP, Glabus MF, O’Carroll RE, Johnstone EC. Reduced in vivo binding to the serotonin transporter in the cerebral cortex of MDMA (‘ecstasy’) users. Br. J. Psychiatry. 1999;175:63–69. doi: 10.1192/bjp.175.1.63. [DOI] [PubMed] [Google Scholar]
- Shi L, Quick M, Zhao Y, Weinstein H, Javitch JA. The mechanism of a neurotransmitter, sodium symporter – inward release of Na+ and substrate is triggered by substrate in a second binding site. Mol. Cell. 2008;30:667–677. doi: 10.1016/j.molcel.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R, Qin M, Liu ZH, Ikemoto S. Intracranial self-administration of MDMA into the ventral striatum of the rat, differential roles of the nucleus accumbens shell, core, and olfactory tubercle. Psychopharmacology. 2008;198:261–270. doi: 10.1007/s00213-008-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer BF, Loweth JA, Neve RL, Vezina P. Transient viral-mediated overexpression of α-calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell leads to long-lasting functional upregulation of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptors: dopamine type-1 receptor and protein kinase A dependence. Eur. J. Neurosci. 2010;31:1243–1251. doi: 10.1111/j.1460-9568.2010.07155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitte HH, Freissmuth M. The reverse operation of Na+/Cl−-coupled neurotransmitter transporters – why amphetamines take two to tango. J. Neurochem. 2010;112:340–355. doi: 10.1111/j.1471-4159.2009.06474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soar K, Parrott A, Turner J. Attributions for psychobiological changes in ecstasy/MDMA and other polydrug users. J. Psychopharmacol. 2009;23:745–758. doi: 10.1177/0269881108092594. [DOI] [PubMed] [Google Scholar]
- Song BJ, Moon KH, Upretti VV, Eddington ND, Lee IJ. Mechanisms of MDMA (ecstasy)-induced oxidative stress, mitochondrial dysfunction and organ damage. Curr. Pharm. Biotechnol. 2010;11:434–443. doi: 10.2174/138920110791591436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonsalla PK, Jochnowitz ND, Zeevalk GD, Oostveen JA, Hall ED. Treatment of mice with methamphetamine produces cell loss in the substantia nigra. Brain Res. 1996;738:172–175. doi: 10.1016/0006-8993(96)00995-x. [DOI] [PubMed] [Google Scholar]
- Sora I, Li B, Igari M, Hall FS, Ikeda K. Transgenic mice in the study of drug addiction and the effects of psychostimulant drugs. Ann. N.Y. Acad. Sci. 2010;1187:218–246. doi: 10.1111/j.1749-6632.2009.05276.x. [DOI] [PubMed] [Google Scholar]
- Sotnikova TD, Beaulieu JM, Barak LS, Wetsel WC, Caron MG, Gainetdinov RR. Dopamine-independent locomotor actions of amphetamines in a novel acute mouse model of Parkinson disease. PLoS Biol. 2005;3:e271. doi: 10.1371/journal.pbio.0030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague JE, Nichols DE. The monoamine oxidase-B inhibitor l-deprenyl protects against 3,4-methylenedioxymethamphetamine-induced lipid peroxidation and long-term serotonergic deficits. J. Pharmacol. Exp. Ther. 1995;273:667–673. [PubMed] [Google Scholar]
- Stone DM, Stahl DC, Hanson GR, Gibb JW. The effects of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) on monoaminergic systems in the rat brain. Eur. J. Pharmacol. 1986;128:41–48. doi: 10.1016/0014-2999(86)90555-8. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanism of neurotransmitter release by amphetamines, a review. Prog. Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Tancer M, Johanson CE. Reinforcing, subjective, and physiological effects of MDMA in humans, a comparison with d-amphetamine and mCPP. Drug Alcohol Depend. 2003;72:33–44. doi: 10.1016/s0376-8716(03)00172-8. [DOI] [PubMed] [Google Scholar]
- Tassin JP. Uncoupling between noradrenergic and serotonergic neurons as a molecular basis of stable changes in behavior induced by repeated drugs of abuse. Biochem. Pharmacol. 2008;75:85–97. doi: 10.1016/j.bcp.2007.06.038. [DOI] [PubMed] [Google Scholar]
- Tourino C, Ledent C, Maldonado R, Valverde O. CB1 cannabinoid receptor modulates 3,4-methylenedioxymethamphetamine acute responses and reinforcement. Biol. Psychiatry. 2008;63:1030–1038. doi: 10.1016/j.biopsych.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Trigo JM, Renoir T, Lanfumey L, Hamon M, Lesch KP, Robledo P, Maldonado R. 3,4-Methylenedioxymethamphetamine self-administration is abolished in serotonin transporter knockout mice. Biol. Psychiatry. 2007;62:669–679. doi: 10.1016/j.biopsych.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann. N.Y. Acad. Sci. 2004;1025:279–287. doi: 10.1196/annals.1316.035. [DOI] [PubMed] [Google Scholar]
- Vaughan RA. Phosphorylation and regulation of psychostimulant-sensitive neurotransmitter transporters. J. Pharmacol. Exp. Ther. 2004;310:1–7. doi: 10.1124/jpet.103.052423. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am. J. Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Liechti ME, Paulus MP. MDMA affects both error-rate dependent and independent aspects of decision-making in a two-choice prediction task. J. Psychopharmacol. 2005;19:366–374. doi: 10.1177/0269881105053287. [DOI] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Xie T, Tong L, McLane MW, Hatzidimitriou G, Yuan J, McCann U, Ricaurte G. Loss of serotonin transporter protein after MDMA and other ring-substituted amphetamines. Neuropsychopharmacology. 2006;31:2639–2651. doi: 10.1038/sj.npp.1301031. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Raudensky J. The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. J. Neuroimmune Pharmacol. 2008;3:203–217. doi: 10.1007/s11481-008-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: classical and emerging mechanisms. Ann. N.Y. Acad. Sci. 2010;1187:101–121. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Campbell Z, Jovanovski D. The neuropsychology of ecstasy (MDMA) use: a quantitative review. Hum. Psychopharmacol. 2007;22:427–435. doi: 10.1002/hup.873. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Terry D, Shi L, Weinstein H, Blanchard SC, Javitch JA. Single-molecule dynamics of gating in a neurotransmitter transporter homologue. Nature. 2010;465:188–193. doi: 10.1038/nature09057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolkowska D, Rothman RB, Baumann MH. Amphetamine analogs increase plasma serotonin: implications for cardiac and pulmonary disease. J. Pharmacol. Exp. Ther. 2006;318:604–610. doi: 10.1124/jpet.106.101618. [DOI] [PubMed] [Google Scholar]