Abstract
Class V myosins play a pivotal role in organelle distribution. In the budding yeast, Myo2p, a class V myosin, is essential for mitochondrial distribution. We identified MMR1 as a high-dose suppressor of the myo2 mitochondrial defect and that Mmr1p resides restrictively on the bud-localizing mitochondria and forms a complex with Myo2p tail. Mmr1p loss delayed mitochondrial transfer to buds and completely abolished mitochondrial distribution in the absence of Ypt11p, which promotes mitochondrial distribution by complex formation with Myo2p tail. The myo2-573 mutation, which causes a mitochondrial distribution defect and inactivates the Mmr1p function, reduced association between Myo2p and Mmr1p and depolarized Mmr1p localization on mitochondria. These strongly suggest that Mmr1p is a key mitochondrial component of the link between Myo2p and mitochondria for Myo2p-dependent mitochondrial distribution. Genetical analysis revealed that the Mmr1p–Myo2p pathway is independent of the Ypt11p–Myo2p pathway, suggesting that an essential system for mitochondrial distribution is composed of two independent Myo2p pathways.
Keywords: mitochondria, myosin, organelle distribution, yeast
Introduction
Proper organelle distribution is critical for the maintenance of cellular function and proliferation. The cytoskeleton and the motor proteins that move along it play essential roles in the distribution of various organelles. Among motor proteins, class V myosins are conserved in evolution and participate in the distribution of cellular components. For example, in mammalian melanocytes, myosin Va distributes melanosome (Wu et al, 1997). The budding yeast has two class V myosins, Myo2p and Myo4p, which move along actin cables, an actin structure running from the growing bud to the mother cell along the bud–mother axis (Reck-Peterson et al, 2000; Bretscher, 2003). Myo4p transports some of the mRNAs into the bud to establish their asymmetrical distribution (Munchow et al, 1999; Takizawa et al, 2000). Myo2p is essential for cell growth and is responsible for transport of several organelles into the growing bud, such as secretory vesicles for polarized growth (Johnston et al, 1991; Govindan et al, 1995; Schott et al, 2002), vacuoles (Catlett and Weisman, 2000), peroxisomes (Hoepfner et al, 2001), late-Golgi elements (Rossanese et al, 2001), and positions Kar9p to the bud for orientating the spindles through cytoplasmic microtubules (Yin et al, 2000). In addition, a particular myo2 mutant allele (myo2-573), inducing a defect in mitochondrial distribution, indicates the essential role of Myo2p in mitochondrial inheritance (Itoh et al, 2002).
The established mechanism for distribution of cellular components by class V myosins is that they interact with the cargo at the C-terminal tail domain and transport it along the actin cytoskeleton using the N-terminal motor domain. Cargo-specific myosin receptors act as the link between the myosin tail and cargo, transported selectively. Myosin receptors have been identified as protein complexes: Rab27 GTPase–melanophilin/Slac2-a for melanosome transport (Hume et al, 2001; Fukuda et al, 2002; Wu et al, 2002), She3p–She2p for mRNA transport by Myo4p (Bohl et al, 2000; Long et al, 2000; Takizawa and Vale, 2000), and Vac8p–Vac17p on vacuoles for Myo2p (Ishikawa et al, 2003). Kar9p–Bim1p acts similarly in orientating cytoplasmic microtubules by Myo2p (Bretscher, 2003).
Inheritance of mitochondria, which cannot be synthesized de novo from other membrane structures, is essential for the cell. In yeast, mitochondria form a tubular structure with a network morphology (Yaffe, 1999). During inheritance of mitochondria, a tubule, emerging in the network, is oriented along the mother–bud axis and inserted into the bud at an early stage of bud formation. The transported mitochondria are then fixed near the bud cortex. Insertion of mitochondria continues throughout the cell cycle, distributing almost the same amount of mitochondria to the daughter cell as in the mother cell (Simon et al, 1997). Actin function is essential for mitochondrial motility and inheritance in the budding yeast. Although involvement of myosin functions and actin cables was not detected in motility production, loss of actin cables disrupts the polarized movement of mitochondria and their distribution (Drubin et al, 1993; Simon et al, 1995, 1997; Hermann et al, 1997; Boldogh et al, 2001). This actin cable dependency suggests involvement of a myosin in the process. Actually, the myo2-573 mutation induces a defect in mitochondrial distribution without affecting polarized organization of actin cytoskeleton or other Myo2p functions, revealing that Myo2p is the myosin that is essential for mitochondrial distribution (Itoh et al, 2002).
Previously, we identified Ypt11p, a rab-type GTPase, as a factor that promotes the Myo2p function for mitochondrial distribution and forms a complex with the Myo2p C-terminal tail domain (Itoh et al, 2002). Loss of Ypt11p partially delays transfer of the mitochondrial tubule into the emerging bud. Overexpression of YPT11 causes abnormal accumulation of mitochondria in the bud, which in turn is suppressed by myo2-338 mutation. This mutation abolishes the interaction between Ypt11p and Myo2p. These observations indicate that Ypt11p interacts with the C-terminal tail domain of Myo2p to promote mitochondrial distribution. By analogy to the role of the rab-type GTPase Rab27a in melanosome transport, the possibility that Ypt11p constitutes the Myo2p receptor on mitochondria has been argued. However, Ypt11p localizes in a Myo2p-dependent manner at the growing cortex and colocalization with mitochondria is unlikely. Moreover, loss of Ypt11p causes a limited defect in mitochondrial distribution and does not affect cell growth. Therefore, Ypt11p is not, at least, a critical component of the Myo2p receptor on mitochondria. Originally, the myo2-573 mutation, causing mitochondrial defects, was identified as synthetically lethal with ypt11Δ (Itoh et al, 2002). This genetic interaction suggested that yeast cells may have a system for distributing mitochondria, which involves Myo2p, but not via the Myo2p–Ypt11p pathway. These features of Ypt11p reveal the complexity of the Myo2p-dependent distribution system and further identification of factors in this system will be crucial for clarifying it.
In this report, we identify MMR1 (mitochondrial Myo2p receptor-related 1), showing the features characteristic of the myosin receptors on cargo. This strongly suggests that Myo2p distributes mitochondria through the scheme where the myosin recognizes and interacts with the myosin receptor on the cargo. At the same time, the difference between Mmr1p and Ypt11p in localization and function revealed that the Ypt11p system is different from a typical myosin receptor-mediated one.
Results
Identification of Mmr1p
To identify genes required for Myo2p-dependent distribution of mitochondria, we screened for high-dose suppressors of the temperature sensitivity of myo2-573 cells. We reasoned that because the myo2-573 mutation caused a specific defect in the distribution of mitochondria, but not in other Myo2p functions, such a screen might lead to isolation of genes for Myo2p-dependent mitochondrial distribution. A yeast genomic library on a high-copy-number plasmid was introduced into myo2-573 cells and among about 100 000 transformants two independent Ts+ clones were isolated. One clone contained a DNA fragment encompassing YLR190w. The DNA fragment with YLR190w and without adjacent genes on a high-copy-number plasmid was sufficient for the Ts+ phenotype (Figure 1A). Therefore, we concluded that YLR190w is responsible for the suppression and we designated as MMR1. Mmr1p is a protein of 491 amino acids (aa) with a coiled-coil domain (294–384 aa region) and does not show similarity to any reported proteins in higher eukaryotes.
Figure 1.
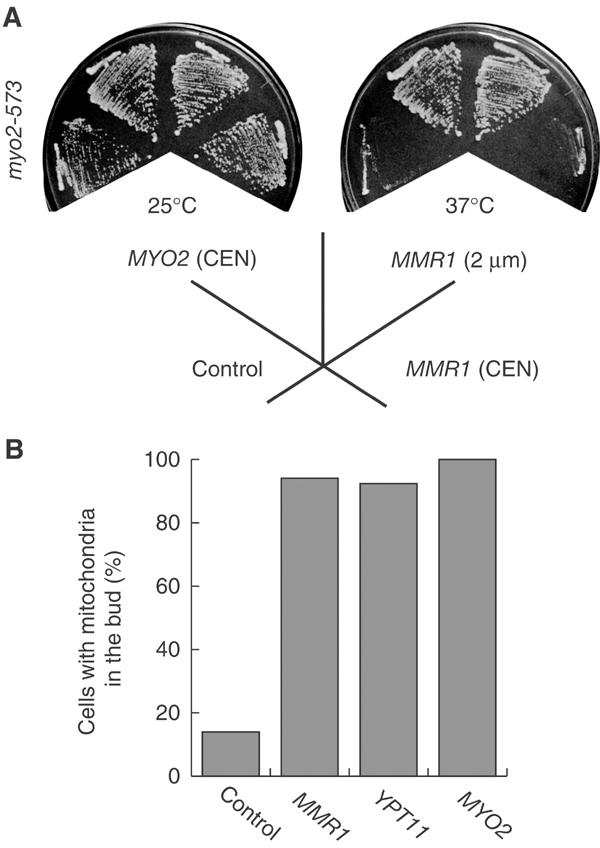
MMR1 suppression of the myo2-573 defect. (A) Suppression of Ts−. myo2-573 cells (strain yTO016) with YEplac195, a control vector (control), with a low-copy-number plasmid carrying MYO2 (MYO2 (CEN)), with pK051, a high-copy-number plasmid carrying MMR1 (MMR1 (2 μm)), or with pK052, a low-copy-number plasmid carrying MMR1 (MMR1 (CEN)), were streaked on SC plates lacking uracil and incubated at the indicated temperature for 2 days. (B) Suppression of mitochondrial defect. myo2-573 cells with YEplac195 (control), with pK051, a high-copy-number plasmid carrying MMR1 (MMR1), with pK008 (Itoh et al, 2002), a high-copy-number plasmid carrying YPT11 (YPT11), or with a low-copy-number plasmid carrying MYO2 (MYO2) were grown to mid-log phase in SC medium lacking uracil at 25°C. Cells were stained with DASPMI and over 200 budded cells were observed.
A high dose of MMR1 suppressed not only the temperature-sensitive growth defect of myo2-573 cells but also the defect in mitochondrial distribution in these cells. Only 14% of myo2-573 cells with a control plasmid contained mitochondria in the bud, whereas 94% of myo2-573 budded cells, carrying either MMR1 or YPT11 on a high-copy-number plasmid, transferred mitochondria into the bud (Figure 1B). This result suggests that Mmr1p plays a positive role in mitochondrial distribution to the bud.
Mmr1p localization
Localization of Mmr1p was determined in MMR1HA cells where the wild-type MMR1 ORF was replaced with a functional HA-tagged version. Mmr1pHA was detected as a dot at the bud tip or cable-like structure in the bud (Figure 2Aa, c, and g). DAPI staining of DNA (Figure 2Ab and d) revealed that localization of Mmr1pHA overlapped with strings of mitochondrial DNA (Figure 2Ae). Localization of Mmr1pHA also completely overlapped with mitochondria in the bud (Figure 2Af and g, merged in h). Mmr1p was not localized in all regions of the mitochondria, but was preferentially localized to mitochondria in the bud.
Figure 2.

Mmr1p localization. (A) (a–e) MMR1HA cells (a–e, strain yTO053) were grown to mid-log phase, fixed, and stained using anti-HA antibodies visualized by FITC (a and c, green) and with DAPI for DNA (b and d, red). (e) Merging (c) and (d). (f–h) MMR1HA cells with mitochondria-targeted GFP were grown to mid-log phase, fixed, and stained using anti-HA antibodies visualized with Alexa 546. (f) Mitochondria (green); (g) Mmr1pHA (red); and (h) merged. Scale bar: 5 μm. (B) Cell fractionation. Lysate of MMR1HA cells was fractionated by differential centrifugation. Equal amounts of cell equivalents of each fraction were analyzed by Western blotting. Total: total cell lysate; P13: the P13 pellet fraction; P100: the P100 pellet fraction; S100: the S100 supernatant fraction. Pgk1p, 3-phosphoglycerate kinase (a cytosolic marker), was enriched in the S100 fraction. (C) Fractionation of P13 fraction. P13 fraction (lane 1) from MMR1HA cells was loaded on top of a three-step sucrose gradient (60, 32, 23, and 15% sucrose) and centrifuged. Equal amounts of cell equivalents of each fraction (fraction from 32/60% interface as purified mitochondrial fraction (lane 2) and fraction from 15 and 23% phases as residual membrane fraction (lane 3)) were analyzed by Western blotting. (D) Treatment of salt and alkali. Cell lysates from MMR1HA cells without (NT) or with 0.5 M NaCl (salt) or 0.1 M sodium carbonate (alkali) were centrifuged to separate soluble fraction (S) from pellet fraction (P), which were analyzed by Western blotting. (E) Proteinase K treatment. Cell lysates from MMR1HA cells, without (lane 1) or with (lane 2) proteinase K treatment, were centrifuged and the resultant pellets were analyzed by Western blotting.
Mmr1p localization was subsequently characterized by cell fractionation. The P13 fraction contained almost all mitochondrial membranes (see Por1p, a mitochondrial outer membrane protein, in Figure 2B) in addition to membranes from the endoplasmic reticulum (ER; see Dpm1p, an ER marker protein, in Figure 2B), vacuoles, and endosomes. Consistent with the immunofluorescence results, most Mmr1pHA was detected in the P13 fraction (Figure 2B). By sucrose density gradient centrifugation of the P13 fraction, mitochondria are purified separately from the other membranes (Meisinger et al, 2000). Mmr1pHA was recovered with Por1p in the mitochondrial fraction, whereas Dpm1p was enriched in the fraction for residual membranes, indicating mitochondrial localization of Mmr1p (Figure 2C). Peripheral membrane proteins are extracted into soluble fraction, partly by treatment of cell lysate with 0.5 M NaCl and mostly by treatment with sodium carbonate, without disrupting membrane integrity (Kaiser et al, 2002; e.g. 69 kDa subunit of vacuolar H+-ATPase; see Figure 2D, lower panel). Part of Mmr1pHA was extracted into soluble fraction by the NaCl treatment and most of Mmr1pHA was extracted by sodium carbonate treatment, whereas both treatments failed to extract Por1p (Figure 2D). Proteinase K treatment of cell lysate degrades proteins except those that are fully integrated into membranes, such as Por1p (Pfaller and Neupert, 1987; see Figure 2E, lower panel), and those surrounded by membranes. After such treatment, Mmr1pHA was no longer detected (Figure 2E, lane 2). These results indicate that Mmr1p is not a protein surrounded by mitochondrial membranes and Mmr1p localizes on the cytoplasmic side of the mitochondrial membrane.
Mmr1p forms a complex with Myo2p
The genetic interactions between MYO2 and MMR1 suggest that Mmr1p acts with Myo2p. To manifest this possibility, we performed immunoprecipitation analysis using lysates from MMR1HA cells with replacement of MYO2 with the Flag-tagged version of MYO2. Using anti-Flag antibodies, Mmr1p was co-immunoprecipitated with Flag-tagged Myo2p, indicating that Mmr1p forms a complex with Myo2p in cells (Figure 3A, lane 3).
Figure 3.
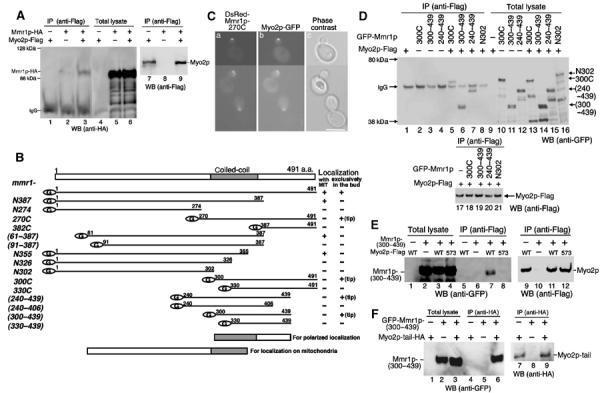
Co-immunoprecipitation of Mmr1p with Myo2p. (A) Cell lysate from MYO2-Flag cells (lanes 1, 4, and 7), MMR1HA cells (lanes 2, 5, and 8), and MYO2-Flag MMR1HA cells (lanes 3, 6, and 9) were precipitated using anti-Flag antibodies. Immunoprecipitants (lanes 1–3) and total cell lysate (1/50 volume of input for immunoprecipitation; lanes 4–6) were analyzed using anti-HA antibodies (upper panel). Immunoprecipitants (1/20 volume of samples for lanes 1–3) were analyzed using anti-Flag antibodies (lower panel, lanes 7–9). Migration of Mmr1p-HA in SDS–PAGE is slower than that expected from its calculated molecular weight for unknown reasons. (B) Schematic diagram of truncated Mmr1p. Each version of Mmr1p was produced as a GFP fusion at its N-terminus under the control of the GAL1 promoter in mmr1Δ cells and its localization was observed during culturing in SCGal. Localization with MIT: GFP signals were with rhodamine B-stained mitochondria (+), or not (−). Localization exclusively in the bud: GFP signals were on bud-residing mitochondria (+), at bud tip (+ (tip)), or not (−). (C) Colocalization of Mmr1p-270C with Myo2p. MYO2GFP cells, where MYO2 is replaced with the GFP-tagged version of MYO2 (MYO2GFP; Itoh et al, 2002), carrying pDsRed-Mmr1-270C, were cultured in SC, shifted to SCRGD, and harvested after 12 h from the shift. (a) Ds-Red signal (Mmr1p-270C); (b) GFP signal (Myo2p); and (c) phase contrast. Scale bar: 5 μm. (D) Co-immunoprecipitation of C-terminal domain of Mmr1p. Cell lysates from mmr1Δ cells (lanes 2–4 and 10–12) or mmr1Δ MYO2-Flag cells (lanes 1, 5–9, and 13–21), without (lanes 1 and 9) or with a plasmid for the GFP-fused Mmr1p (Mmr1p-300C (lanes 2, 5 10, 13, and 18), Mmr1p-(300–439) (lanes 3, 6, 11, 14, and 19), Mmr1p-(240–439) (lanes 4, 7, 12, 15, and 20), or Mmr1p-N302 (lanes 8, 16, and 21)), were immunoprecipitated using anti-Flag antibodies. Immunoprecipitants (lanes 1–8) and total cell lysates (1/50 volume of input for immunoprecipitation, lanes 9–16) were analyzed using anti-GFP antibodies (upper panel). Immunoprecipitants (lanes17–21, 1/5 volume of those for lanes 1 and 5–8) were analyzed using anti-Flag antibodies (lower panel). (E) myo2-573 effect on co-immunoprecipitation. Cell lysates from mmr1Δ cells (lanes 2, 6, and 10), mmr1Δ MYO2-Flag cells (lanes 1, 3, 5, 7, 9, and 11), mmr1Δ myo2-573-Flag cells (lanes 4, 8, and 12), without (lanes 1, 5, and 9) or with a plasmid for GFP-tagged Mmr1p-(300–439) (lanes 2–4, 6–8, and 10–12), were immunoprecipitated using anti-Flag antibodies. Immunoprecipitants (lanes 5–8) and total cell lysates (1/20 volume of input for immunoprecipitation, lanes 1–4) were analyzed using anti-GFP antibodies (left panel). Immunoprecipitants (lanes 9–12, 1/3 volume of those for lanes 1–4) were analyzed using anti-Flag antibodies (right panel). (F) Co-immunoprecipitation with Myo2p tail. Cell lysates from mmr1Δ cells, with (lanes 1, 3, 4, 6, 7, and 9) or without (lanes 2, 5, and 8) a plasmid for HA-tagged Myo2p C-terminal tail domain, and with (lanes 2, 3, 5, 6, 8, and 9) or without (lanes 1, 4, and 7) a plasmid for GFP-tagged Mmr1p-(300–439), were immunoprecipitated using anti-HA antibodies (16B12). Total cell lysates (1/200 volume of input for immunoprecipitation, lanes 1–3) and immunoprecipitants (lanes 4–9) were analyzed using anti-GFP (left panel) and anti-HA (16B12, right panel) antibodies.
To map the Mmr1p domains for mitochondrial localization and the Myo2p association, we constructed truncated versions of Mmr1p, fused with GFP at the N-terminus (Figure 3B). The entire truncated versions of Mmr1p lost Mmr1p activity, neither accumulated mitochondria in the bud, when overproduced, nor suppressed the mmr1Δ growth defect, when combined with ypt11Δ (described below). Using the GFP tag, we observed localization of the truncated Mmr1p. The exclusive localization on bud-residing mitochondria, observed in the full-length Mmr1p (see NC in Supplementary material 2), was lost in any of the truncated Mmr1p. Some of them were diffused in the cytosol (e.g. Mmr1p-N274, and -382C; see Supplementary material 2), whereas others showed restrictive localization in cells. Some versions of the Mmr1p with the deletion of C-terminus, such as Mmr1p-N387, Mmr1p-(61–387), and Mmr1p-N355, still localized as dots on mitochondria, although not in a polarized manner (see N387 in Supplementary material 2 as typical localization of this group). In contrast, Mmr1p-270C, Mmr1p-300C, Mmr1p-(240–439), and Mmr1p-(300–439), truncated versions with the deletion of N-terminus, abolished mitochondrial localization and localized on the growing bud cortex, such as the tip of the bud, showing similar localization of Myo2p (see 270C in Supplementary material 2 as typical localization of this group). Indeed, 100% (n=120) of the signals of Myo2p, tagged with GFP, were fully colocalized with Mmr1p-270C, tagged with Ds-Red (Figure 3C).
Co-immunoprecipitation assay revealed that Mmr1p-300C, Mmr1p-(300–439), and Mmr1p-(270–439),which showed polarized localization similar to that of Myo2p, were co-immunoprecipitated with Myo2p (Figure 3D, lanes 5–7), whereas Mmr1p-N302 was not (Figure 3D, lane 8). These results indicate that Myo2p associates with the Mmr1p region between 300 and 439 aa (Myo2p-associating domain). Therefore, the bud tip localization strongly suggests that the truncated Mmr1p, which retains the Myo2p-associating domain but loses the affinity for mitochondria, is recruited exclusively to Myo2p through its Myo2p-associating activity.
The myo2-573 mutation resides at the myo2 region for C-terminal tail domain and disturbs mitochondrial inheritance. We examined the effect of the myo2-573 mutation on the Myo2p–Mmr1p association. Whereas wild-type Myo2p co-immunoprecipitated Mmr1p-(300–439) (Figure 3E, lane 7), Myo2-573p did not (Figure 3E, lane 8), showing that the myo2-573 mutation abolishes the Myo2p–Mmr1p association. In addition, Mmr1p-(300–439) was co-immunoprecipitated with the Myo2p tail domain (Figure 3F, lane 6). These results indicate that the C-terminal tail domain of Myo2p associates with Mmr1p and suggests that the association is important for mitochondrial inheritance.
Mmr1p plays a positive role in transferring mitochondria into the bud
Disruption of MMR1 was not lethal and reduced growth rate slightly. In mmr1Δ cells, actin cables were organized normally. Vacuolar, ER, and nuclear morphologies were normal and those organelles were correctly distributed to the bud (data not shown). Mitochondria exhibited a normal network morphology, similar to that observed in wild-type cells. However, the transfer of mitochondria to the bud was delayed. About 37 % (n=200) of mmr1Δ cells with a small bud, whose diameter was one-third of that of the mother cell, failed to transfer a tubule of mitochondria to the bud, whereas most (about 89%, n=200) of the small-budded wild-type cells transferred mitochondria into the bud (Figure 4A). Because most large-budded mmr1Δ cells contained mitochondria in the bud, loss of Mmr1p delays the transfer of mitochondria into the emerging bud but does not induce a defect in mitochondrial inheritance. This indicates that, although Mmr1 is not essential for mitochondrial transfer into the bud, Mmr1p plays a positive role in the process.
Figure 4.
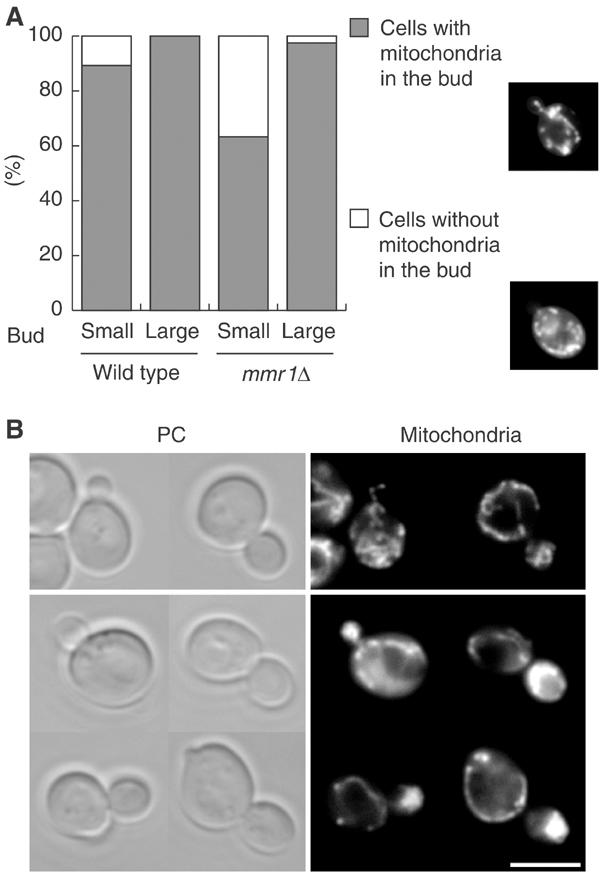
MMR1 effect on mitochondrial distribution to the bud. (A) mmr1Δ phenotype. Wild-type and mmr1Δ cells carrying mitochondria-targeted GFP were grown to mid-log phase in SC. Over 200 small-budded and large-budded cells were counted (n>200). Percentages of cells with (dark gray) or without (open) mitochondria in the bud are shown. Typical images of small-budded cells with (upper panel) and without (lower panel) mitochondria in the bud are shown on the right. (B) Accumulation of mitochondria in Mmr1p-overproducing buds. Wild-type cells without (upper panel) and with (lower panel) pGAL1-MMR1 were grown to mid-log phase in SCRaf, shifted to SCGal, harvested after 2 h from the shift, and stained with DASPMI. PC: phase contrast; mitochondria: DASPMI staining. Scale bar: 5 μm.
The phenotype, induced by overexpression of MMR1, also indicates the positive role of Mmr1p in the transfer of mitochondria into the bud. Mmr1p was overproduced from pGAL1-MMR1, MMR1 under the control of the GAL1 promoter. After 2 h induction of the MMR1 overexpression, about 80% of budded pGAL1-MMR1 cells accumulated mitochondria abnormally in the bud (Figure 4B).
Synthetic defect of mmr1Δ with ypt11Δ in mitochondrial distribution
Ypt11p is a positive factor for Myo2p-dependent distribution of mitochondria. Similar to MMR1, deletion of YPT11 delays transfer of mitochondria into the bud and overexpression of YPT11 accumulates mitochondria in the bud (Itoh et al, 2002). We, therefore, characterized the relationship between the Ypt11p and Mmr1p functions. Tetrad analysis of a cross between ypt11Δ and mmr1Δ revealed that mmr1Δ is synthetically lethal with ypt11Δ. Following 3 days of incubation at 25°C, the mmr1Δ ypt11Δ spores were unable to form colonies, whereas mmr1Δ spore clones and ypt11Δ spore clones grew well (Supplementary material 3A). After prolonged incubation, several such mmr1Δ ypt11Δ spores formed microcolonies, which were difficult to repropagate.
The lethality of mmr1Δ ypt11Δ cells was rescued by introduction and expression of pGAL1-MMR1. In SCRGD or YPRGD galactose-containing medium, which induces a moderate production of Mmr1p from pGAL1-MMR1 (Figure 5A, upper), mmr1Δ ypt11Δ pGAL1-MMR1 cells (yTO055 cells) grew but did not in SC or YPD medium, which represses the expression of pGAL1-MMR1 (Figure 5A, lower). The yTO055 cells, grown in SCRGD medium, contained mitochondria with normal tubular morphology and appeared to distribute mitochondria normally to the bud (Figure 5B, upper). After the shift to SC medium, the proportion of the cells, carrying mitochondria in the bud, was gradually reduced (Figure 5Ca). After 3 h from the shift, mitochondria (Figure 5Bd and Ca) were not detected in most of the buds. Actin organization was not affected during the Mmr1p depletion (Figure 5Cb; Supplementary material 3B-d). The ER morphology, visualized by Sec63pGFP (Prinz et al, 2000), was not changed significantly in the Mmr1p-depleted yTO055 cells (Supplementary material 3C). The polarized delivery of secretory vesicles, monitored by polarized distribution of Sec2pGFP (Elkind et al, 2000), was not affected during the Mmr1p depletion (Figure 5Cc). Vacuole inheritance was normal in yTO055 cells 3 h after a shift to SC medium (data not shown). During the Mmr1p depletion, Myo2p was localized at the growing cortex, showing the polarized localization as observed in wild-type cells (Figure 5Cd). These results indicate that simultaneous loss of both Ypt11p and Mmr1p specifically impairs distribution of mitochondria and the growth arrest of mmr1Δ ypt11Δ cells results from this defect.
Figure 5.
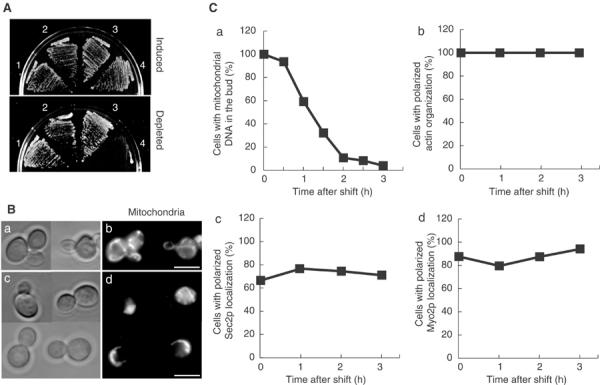
Synthetic phenotypes of ypt11Δ with Mmr1p depletion. (A) Growth defect of Mmr1p-depleted cells. Wild-type cells with pGAL1-MMR1 (1), ypt11Δ pGAL1-MMR1 cells (2), mmr1Δ pGAL1-MMR1 cells (3), and ypt11Δ mmr1Δ pGAL1-MMR1 cells (4, strain yTO055) were streaked on YPRGD (upper panel) or YPD plates (lower panel) and incubated at 25°C for 3 days. (B) Mitochondrial distribution in Mmr1p-depleted yTO055 cells. yTO055 cells were grown to mid-log phase in SCRGD and shifted to SC. Zero (a, b) and 3 h (c, d) after the shift, cells were stained with DASPMI. Phase contrast (a, c) and the DASPMI staining (b, d). (C) Diachronic observation of yTO055 cells during Mmr1p depletion. yTO055 cells were grown to mid-log phase in SCRGD and shifted to SC. At various times after the shift (indicated with squares), cells were harvested, fixed, and stained with DAPI for mitochondrial DNA for monitoring mitochondrial distribution (a), and with rhodamine–phalloidin (b). For observation of Sec2p localization, yTO055 cells with the replacement of SEC2 with SEC2GFP for GFP-tagged Sec2p (Itoh et al, 2002) were used (c). For observation of Myo2p localization, yTO055 cells with MYO2GFP were used (d).
Mmr1p and Ypt11p act independently
Because both Ypt11p and Mmr1p are the Myo2p-related factors for mitochondrial distribution, we examined whether Mmr1p requires Ypt11p for mitochondrial distribution or not, using the phenotype of overexpressed MMR1, accumulating mitochondria in the bud (Supplementary material 4). The populations of the cells that accumulated mitochondria in the bud were affected significantly, although it was neither by the deletion of YPT11 (79% in wild-type cells versus 81% in ypt11Δ cells, when MMR1 was overexpressed by the GAL1 promoter) nor by the deletion of MMR1 (84% in wild-type cells versus 74% in mmr1Δ cells, when YPT11 was overexpressed by the GAL1 promoter). These results strongly suggest that Mmr1p and Ypt11p mediate mitochondrial transfer to the bud independently of each other.
Genetic evidence showing that Mmr1p acts with Myo2p for mitochondrial distribution
We previously reported two mutant myo2 alleles (myo2-338 and myo2-573), displaying genetic interactions with YPT11 (Itoh et al, 2002). The myo2-338 mutation disrupts the Ypt11p–Myo2p interaction and abolishes the Ypt11p–Myo2p pathway for mitochondrial distribution. Consistent with the fact that ypt11Δ is not critical for mitochondrial distribution, the myo2-338 mutation is also not. In contrast to myo2-338, the myo2-573 mutation induces the mitochondrial distribution defect and is lethal with ypt11Δ. These phenotypes suggest the presence of another Myo2p-mediated pathway, which can act independently of the Ypt11p–Myo2p pathway and is essential for mitochondrial distribution in the absence of Ypt11p; that is, mitochondria in myo2-338 cells are distributed by the Ypt11p-independent pathway and the myo2-573 mutation mostly abolishes the Ypt11p-independent pathway, making the Ypt11p–Myo2p pathway essential. The genetic and biochemical features of Mmr1p strongly suggest that Mmr1p is a key factor of the Ypt11p-independent Myo2p pathway. To examine this model further, we investigated the effect of myo2-338 and myo2-573 mutations with mmr1Δ. In case Mmr1p is a key and specific factor of the Ypt11p-independent pathway, myo2-338 should be synthetically lethal with mmr1Δ and the effect of mmr1Δ should be negligible in myo2-573 cells. The results of the analysis revealed that it is the case. Through tetrad analysis, myo2-338 mmr1Δ spore clones did not form colonies, whereas myo2-573 mmr1Δ spore clones form colonies and grew as well as myo2-573 cells (data not shown). This phenotype was analyzed further by replacing MMR1 with the galactose-dependent mmr1 allele (pGAL1-MMR1:mmr1). In SC medium, which represses the MMR1 expression, the myo2-338 pGAL1-MMR1:mmr1 cells did not grow, whereas myo2-573 pGAL1-MMR1:mmr1 cells grew (Figure 6A). After 3 h from the shift to SC medium at 25°C, 91% (n=210) of budded myo2-338 pGAL1-MMR1:mmr1 cells failed to transfer mitochondria into the bud, whereas in SCRGD medium, which induces the MMR1 expression, 95% (n=232) of budded myo2-338 pGAL1-MMR1:mmr1 cells distributed mitochondria to the bud normally (Figure 6B, right). The mmr1 deletion did not enhance the defect in mitochondrial distribution of myo2-573 cells (Figure 6B, left). Therefore, these results consistently support that Mmr1p acts with Myo2p, independently of Ypt11p, and are the genetically evidence showing that an essential system for mitochondrial distribution is composed of two independent Myo2p pathways, Mmr1p–Myo2p and Ypt11p–Myo2p pathways.
Figure 6.
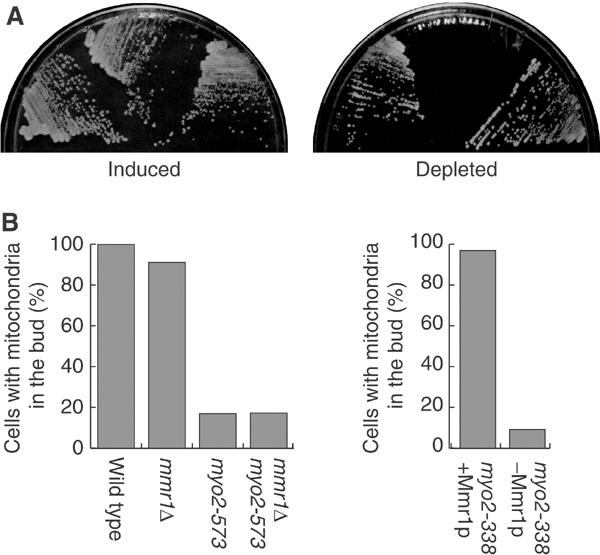
Effect of Mmr1p loss on myo2 mutants. (A) pGAL1-MMR1:mmr1 (left sector), myo-338 pGAL1-MMR1:mmr1 (middle sector), and myo2-573 pGAL1-MMR1:mmr1 (right sector) cells were streaked on SCRGD (left) and SC (right) plates, and incubated at 25°C for 3 days. (B) Left: cells with the indicated genotype (siblings from the cross between mmr1Δ and myo2-573) were grown to mid-log phase at 25°C in SC. Right: myo2-338 pGAL1-MMR1:mmr1 cells were grown to mid-log phase in SCRGD (myo2-338+Mmr1p), or shifted to SC and incubated for 3 h (myo2-338−Mmr1p) at 25°C. Cells were stained with DASPMI, and budded cells were counted (n>200).
Mmr1p-dependent movement of mitochondria from the mother cell
The biochemical and genetic evidence implies that Mmr1p associates and acts with Myo2p for mitochondrial distribution. To gain more insight into how Mmr1p works, we observed Mmr1p-dependent movement of mitochondria by time-lapse experiment using mmr1Δ ypt11Δ pGAL1-MMR1 cells. After over 6 h from the shift to Scarf medium, which does not induce the pGAL1-MMR1 expression, Mmr1p in the cells was depleted and the cells ceased to distribute mitochondria into the bud (Figure 7, noninduced cell), as observed in Figure 5. Galactose was added into the culture medium for inducing the pGAL1-MMR1 expression and cells were observed microscopically. After 10 min from the addition of galactose, movement of mitochondria toward the bud was observed and a mitochondrial tubule was inserted into the bud and moved along the bud axis toward the bud tip (Figure 7, induced cell; two examples among 22 recorded cases). These sequential images are matched with the idea that Mmr1p guides the mitochondrial tubule to the bud tip.
Figure 7.
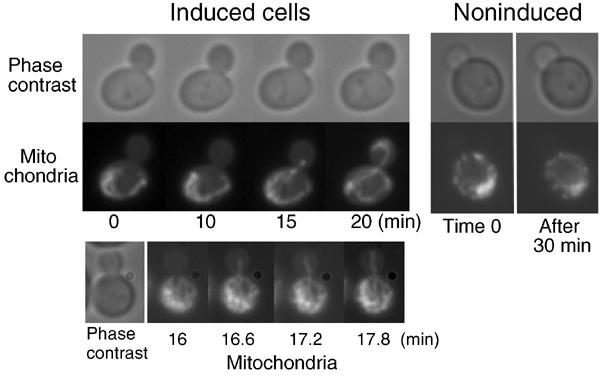
Time-lapse analysis. yTO055 cells carrying mitochondria-targeted GFP were grown in SCRaf for 6–8 h to deplete Mmr1p in the cells and the depletion was checked by the microscopic observation that none of the budded cells carried mitochondria in the bud. Mmr1p was induced by the addition of galactose to the medium (2% final concentration). Times after addition of galactose are indicated below (induced cells). For control, a cell without addition of galactose, observed for 30 min, is shown on the right (noninduced).
Polarized localization of Mmr1p requires Myo2p–Mmr1p association
We observed localization of Mmr1p in myo2-573 cells, where the Mmr1p–Myo2p pathway is inactivated. In contrast to wild-type cells (Figure 2) and myo2-338 cells (Figure 8A, lower) where Mmr1pHA localized preferentially on bud-residing mitochondria, Mmr1pHA in myo2-573 cells was depolarized, that is, detected in almost all regions of mitochondria, even when a part of the mitochondria was transported into the bud (Figure 8A, upper). Similar to this, the polarized localization of Mmr1p-270C, the truncated Mmr1p carrying the Myo2p-associating domain but losing the activity to reside on mitochondria, was impaired in myo2-573 cells. Ds-Red-tagged Mmr1p-270C were observed and cells with GFP signals were counted. As in wild-type cells (Figure 3C), in 100% (n=110) of the myo2-338 cells, polarized localization of Mmr1p-270C was observed and Mmr1p-270C was colocalized with Myo2-338p (Figure 8B, left). In contrast to this, although Myo2-573p was localized in a polarized manner, Myo2-573p was colocalized with Mmr1p-270C only in 31.6% (n=114) of the myo2-573 cells and in the rest Mmr1p-270C was diffused and not colocalized with Myo2-573p (Figure 8B, right). These results indicate that myo2-573 mutation drastically reduces association of Myo2p with Mmr1p in cells and impairs polarized localization of the Mmr1p-residing mitochondria.
Figure 8.

Mmr1p localization in myo2 mutants. (A) myo2-573 MMR1HA cells (upper panel) and myo2-338 MMR1HA cells (lower panel), carrying a plasmid for mitochondria-targeted GFP, were grown to mid-log phase in SC, fixed, and stained using anti-HA antibodies visualized with Alexa 546. (a) Mitochondria (green); (b) Mmr1pHA (red); and (c) merged. Scale bar: 5 μm. Rare myo2-573 cells, carrying mitochondria in the bud, were selected for the figure, to show that Mmr1p still delocalized even when mitochondria were distributed to the bud. (B) pDsRed-Mmr1-270C was introduced into myo2-338-GFP cells (a–c) and myo2-573-GFP cells (d–f), where MYO2 was replaced with the GFP-tagged version of myo2-338 and myo2-573, respectively (Itoh et al, 2002). Cells were cultured in SC, shifted to SCRGD, harvested after 12 h from the shift, and fixed. (a, d) Ds-Red signal; (b, e) GFP signal; and (c, f) phase contrast.
Discussion
While actin is essential for mitochondrial inheritance in the budding yeast, it has been unclear whether myosin is required for mitochondrial inheritance. In our previous report, we have provided evidence for the involvement of Myo2p in the process by isolation of the myo2-573 mutation, which induced a defect in mitochondrial inheritance specifically, and by identification of Ypt11p as its promoting factor (Itoh et al, 2002). However, further identification of factors is important to prove the Myo2p system for mitochondrial inheritance because of its complexity. Here, we reported identification of Mmr1p as a mitochondrial factor, acting with Myo2p for mitochondrial inheritance, and that Mmr1p is essential for the process in the absence of Ypt11p. This finding indicates that the complicated system, requiring Myo2p, Mmr1p, and Ypt11p, plays an essential role in mitochondrial inheritance and enables us to clarify the system.
Mmr1p is a key mitochondrial factor for Myo2p-dependent distribution of mitochondria
The established mechanism for distribution of cellular components by a class V myosin is that the C-terminal myosin tail interacts with the cargo through the myosin receptor, residing on the cargo, and the N-terminal motor transports the attaching cargo along the actin cytoskeleton. For the features described below, Mmr1p is highly likely to be a key mitochondrial factor, possibly one of the components of the mitochondrial Myo2p receptor, for constituting the link between Myo2p and mitochondria for Myo2p-dependent distribution of mitochondria. First, Mmr1p plays a positive role in mitochondrial distribution, that is, loss of Mmr1p delayed transfer of mitochondria into the emerging bud and overexpression caused accumulation of mitochondria in the bud (Figure 4). Second, Mmr1p has a Myo2p-associating domain, which forms a complex with the cargo-binding domain of Myo2p, and associates with Myo2p in cells (Figure 3). Third, genetic evidence indicates that Mmr1p requires Myo2p to mediate mitochondrial distribution (Figure 6). Fourth, the myo2-573 mutation, which cancels the Mmr1p function and induces defects in mitochondrial distribution, reduces the affinity of Myo2p for Mmr1p (Figures 3E and 8). Finally, localization of Mmr1p is restricted to the transferred tubules (Figure 2A) and this polarized localization is dependent on the affinity of Myo2p for Mmr1p (Figure 8B). It is a common feature among myosin V receptors that receptor molecules are distributed asymmetrically with the transported cargo in the cell, depending on the functions of myosin and the components of the receptor. These support a model whereby the Mmr1p-residing mitochondria are preferentially distributed to the bud by Myo2p activity, thereby establishing polarized distribution of Mmr1p on mitochondrial tubules.
All the reported myosin receptors are composed of a cargo-attaching protein and an adaptor protein that links the myosin with the cargo-attaching protein. Mmr1p does not display a significant homology to both the reported proteins, except for the coiled-coil domain, found in adaptor proteins. The coiled-coil domain of Mmr1p is encompassed by both domains for mitochondrial localization and for colocalization with Myo2p. Although these domains are overlapped, it is unlikely that Myo2p association competes with Mmr1p binding to mitochondria because, if competed, the Myo2p–Mmr1p complex would have been released from mitochondria and showed localization similar to Mmr1p-270C. Rather, it is likely that the coiled-coil domain is required for establishing the Mmr1p structure for cellular localization. The association between Myo2p and Mmr1p is detected not abundantly (Figure 3A) and we did not exclude the possibility of involvement of other factors in the Mmr1p–Myo2p association. It may be possible that the association requires some factor of a restricted amount and is highly regulated.
Different from the other myosin cargoes, motility of mitochondria is produced by a Myo2p-independent, actin-dependent system (Simon et al, 1995). Although the features of Mmr1p strongly suggest the myosin receptor-mediated distribution system in mitochondrial distribution, it is unlikely that the motor activity of Myo2p plays a main role in the movement of mitochondria from mother cell to the bud. It is reported that, although mitochondria do not usually move along actin cables, mitochondria partly associate with actin cables and actin cables are required for mitochondrial inheritance and for orientating their movement (Drubin et al, 1993; Hermann et al, 1997; Simon et al, 1997). Therefore, it is conceivable that the Myo2p–Mmr1p association plays a crucial role in guiding the mitochondrial tubule to the incipient bud by adjusting the mitochondrial movement along actin cables, rather than in transporting mitochondria along actin cables. The time-lapse experiments show the Mmr1p-dependent movement of the mitochondrial tubule toward the bud tip (Figure 7), which supports the idea that Myo2p guides the Mmr1p-residing mitochondrial tubule toward bud tips along actin cables.
Mmr1p acts in the Myo2p pathway, distinct from the Ypt11p–Myo2p pathway
Mmr1p resembles Ypt11p in function because both facilitate Myo2p function in mitochondrial distribution. Both form a complex with the tail domain of Myo2p, act as a high-dose suppressor of the myo2 defect in mitochondrial distribution, and cause accumulation of mitochondria in the bud when overproduced. As with loss of Ypt11p, loss of Mmr1p delays transfer of a tubule of mitochondria into the bud. These features may appear similar to those shared by sets of proteins, constituting a myosin receptor, such as the Rab27 GTPase and melanophilin on melanosome. However, the relationship between Ypt11p and Mmr1p is completely distinct from the above case.
Whereas loss of either Ypt11p or Mmr1p induced a partial delay in the transfer of mitochondria into the bud, simultaneous depletion of both proteins almost completely abolished mitochondrial distribution (Figure 5). Assuming that both Ypt11p and Mmr1p constitute a mitochondrial Myo2p receptor, this property is quite distinct from reported components constituting a myosin receptor. In the reported cases, lack of a component of the receptor disrupts the link between the myosin and the cargo, and abolishes myosin-dependent distribution completely. Genetic evidence also indicates that the assumption is unlikely. ypt11Δ is lethal in combination with myo2-573 but does not affect myo2-338 cells. In contrast, mmr1Δ is lethal in combination with myo2-338 but does not enhance the myo2-573 defect (Figure 6). These genetic interactions strongly suggest that Myo2p has two functional domains for mitochondrial distribution, one acting with Mmr1p and being destroyed by the myo2-573 mutation and the other acting with Ypt11p and being destroyed by the myo2-338 mutation. Consistent with this, the myo2-338 mutation disrupts the interaction with Ypt11p and the myo2-573 mutation impairs the Myo2p affinity for Mmr1p (Figure 3E). Moreover, the Mmr1p activity to accelerate mitochondrial transfer into the bud is independent of Ypt11p and vice versa (Supplementary material 4). These demonstrate that Myo2p has two independent partners for mitochondrial distribution, and that an essential system for mitochondrial distribution is composed of Ypt11p–Myo2p and Mmr1p–Myo2p pathways.
Depletion of both Mmr1p and Ypt11p abolished mitochondrial distribution and halted cell growth without affecting other Myo2p functions (Figure 5). This indicates that Mmr1p and Ypt11p are specific for Myo2p-mediated mitochondrial distribution and, at the same time, that mitochondrial distribution is the other essential Myo2p function for cell growth distinct from transport of secretory vesicles.
Our results suggest that the Mmr1p–Myo2p pathway affects mitochondria distribution to the bud through a myosin receptor-mediated mechanism. Thus, how does the Ypt11p–Myo2p pathway distribute mitochondria? At this moment, the role of Ypt11p is unclear. However, it is unlikely that Ypt11p constitutes another, Mmr1p-independent, Myo2p receptor on mitochondria and mediates the Myo2p-dependent distribution, because, different from the Mmr1p case, Ypt11p is not detected with mitochondria and localizes at the periphery of the cortex of the bud tip. Especially, in myo2-573 cells, where the Ypt11p pathway is critical, Ypt11p is still detected in a polarized manner in the bud tip, even when mitochondria are absent in the bud (Itoh et al, 2002). Cytological observations suggest that the transferred mitochondria are fixed at the periphery of the bud cortex (Simon et al, 1997). These observations led to an idea that Ypt11p affects interaction of the bud periphery with mitochondria for capture, which contributes to mitochondrial inheritance (Matsui, 2003). Dysfunction of the vacuole-specific myosin receptor completely abolishes vacuole inheritance, indicating that the single myosin receptor-mediated pathway is responsible (Ishikawa et al, 2003), which is distinct from the case of the dual path system of mitochondrial distribution by Myo2p. In the vacuole, a capture mechanism to fix the transported vacuole at a specific site in the bud is unlikely and it may result in a difference from the mitochondrial case. Clarification of the distribution mechanism, which accompanies capture in the destination site, is one of the interesting subjects on motor proteins and the finding of dual path system in mitochondrial distribution will provide an important clue for it.
In eukaryotic cells, mitochondria are an essential organelle for cell proliferation and keep their morphology changing dynamically. It is no doubt that a precise and highly regulated mechanism is involved in mitochondrial inheritance and requires cytoskeletal elements; however, the details of the mechanism still remain unclear. Our finding of specific factors of Myo2p-dependent distribution system of mitochondria indicates the importance of myosin in mitochondrial inheritance, which had not been established, and provides a new point of view to mitochondrial inheritance mechanism in higher eukaryotes. Precise analysis of the effect of motor proteins on local morphology and movement of mitochondria in yeast and higher eukaryotes will help to reveal the motor protein-dependent process of mitochondrial inheritance.
Materials and methods
Media, strains, and plasmids
Details of media, strains, plasmids, methods for cell culture, and construction of strains and plasmids are given in Supplementary material 1. Rich media (YPRGD and YPD) and synthetic complete media (initialed SC) were used. Sugar contents were varied for expression from the GAL1 promoter (SCRGD, YPRGD for moderate expression, SCGal for full induction, and SCRaf, YPD, and SC for noninduced condition). All yeast strains are in the YPH genetic background (Sikorski and Hieter, 1989). The mmr1Δ∷kanMX4DNA fragment, amplified from mmr1Δ∷kanMX4 cell DNA (EUROSCARF), was used to create the mmr1Δ strain. In MMR1HA cells, the MMR1 ORF under the control of its own promoter was replaced with the ORF, producing Mmr1p fused to HA tag at the C-terminus (Mmr1pHA). Mmr1pHA is functional, because the replacement of MMR1 with MMR1HA did not induce a delay in the distribution of mitochondria (data not shown) and was not lethal in ypt11Δ cells. The MMR1 ORF under the control of the GAL1 promoter (pGAL1-MMR1) was integrated at the ura3 locus. In pGAL1-MMR1:mmr1 cells, MMR1 was disrupted and replaced with MMR1 under the control of the GAL1 promoter. Mmr1p-270C fused to Ds-Red (Clontech) at the N-terminus was produced under the control of the GAL1 promoter on a low-copy-number plasmid (pDsRed-Mmr1-270C). In MYO2-Flag and myo2-573-Flag cells, MYO2 was replaced with the Flag-tagged version of MYO2 and myo2-573, respectively.
Morphological observation
Morphological observation was performed by standard procedures as described previously (Itoh et al, 2002). For rhodamine B staining of mitochondria, cells were grown to mid-log phase and observed for at least 10 min after the addition of rhodamine B (Molecular Probes) into the culture to a final concentration of 100 nM. Under these conditions, rhodamine B specifically stains mitochondria, as confirmed by the staining of cells carrying mitochondria-targeted GFP (data not shown). For indirect immunofluorescence staining, anti-HA mouse monoclonal antibodies (16B12, BAbCo), Alexa 546-conjugated anti-mouse IgG goat antibodies (Molecular Probe), and FITC-conjugated anti-mouse IgG goat antibodies (ICN) were used. Images were recorded by an Olympus IX70 with a SENSYS III cooled CCD camera using IP Lab software (Olympus Co., Tokyo, Japan).
Immunoprecipitation
Details of co-immunoprecipitation assay are described in Supplementary material 1. Cells producing tagged proteins were cultured to mid-log phase, harvested, disrupted with glass beads in lysis buffer (200 mM NaCl, 100 mM Tris–HCl pH 7.5, 1 mM EDTA, 5% glycerol, and protease inhibitors cocktail (Roche Diagnostics Corporation)), and centrifuged at 2600 g for 10 min after addition of about three volumes of buffer A (lysis buffer with 0.5% CHAPS). The resultant supernatant was used as cell lysate. For immunoprecipitation with anti-Flag antibodies, anti-Flag monoclonal antibodies-conjugated beads (Flag-beads, Sigma) were used. For immunoprecipitation with anti-HA antibodies, anti-HA antibodies were added to the lysate and after 30 min protein G beads were added. The cell lysates with beads were rotated for 1 h and recovered beads were washed five times with buffer A. Proteins were eluted from Flag-beads with 200 μg/ml of Flag peptide (Sigma) in buffer A. From protein G beads, proteins were eluted with 0.1 M glycine buffer (pH 3.0) and neutralized immediately by adding 1/20 volume of 1.5 M Tris–HCl (pH 8.8). Protein elutes were used as immunoprecipitants.
Cell fractionation
Fractionation by differential centrifugation and treatment of salt, sodium carbonate, and proteinase K were carried out as described (Kaiser et al, 2002). A three-step sucrose gradient was performed as described (Meisinger et al, 2000). Cells, grown to mid-log phase in YPD, were used. Anti-Dpm1p, anti-69 kDa subunit of vacuolar H+-ATPase, and anti-Por1p antibodies were from Molecular Probes. For proteinase K treatment, proteinase K (final concentration 20 μg/ml) was added to the cell lysates, prepared as described above. After 1 h on ice, PMSF was added to a final concentration of 2 mM and the digested lysates were centrifuged at 13 000 g for 10 min, washed once, and analyzed.
Supplementary Material
Supplemental Material 1
Supplemental Material 2
Supplemental Material 3
Supplemental Material 4
Acknowledgments
We thank R Matsui for time-lapse experiments, Drs S Nishikawa, T Endo, and T Rapoport for plasmids, A Watabe, T Araki, Y Saeki, and S Yosihda for helpful discussion and plasmids. The work was supported by grants from MEXT & JSPS.
References
- Bohl F, Kruse C, Frank AFerring D, Jansen RP (2000) She2p, a novel RNA-binding protein tethers ASH1 mRNA to the Myo4p myosin motor via She3p. EMBO J 19: 5514–5524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldogh IR, Yang HC, Nowakowski WD, Karmon SL, Hays LG, Yates JR III, Pon LA (2001) Arp2/3 complex and actin dynamics are required for actin-based mitochondrial motility in yeast. Proc Natl Acad Sci USA 98: 3162–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A (2003) Polarized growth and organelle segregation in yeast: the tracks, motors, and receptors. J Cell Biol 160: 811–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett NL, Weisman LS (2000) Divide and multiply: organelle partitioning in yeast. Curr Opin Cell Biol 12: 509–516 [DOI] [PubMed] [Google Scholar]
- Drubin DG, Jones HD, Wertman KF (1993) Actin structure and function: roles in mitochondrial organization and morphogenesis in budding yeast and identification of the phalloidin-binding site. Mol Biol Cell 4: 1277–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind NB, Walch-Solimena C, Novick PJ (2000) The role of the COOH terminus of Sec2p in the transport of post-Golgi vesicles. J Cell Biol 149: 95–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Kuroda TS, Mikoshiba K (2002) Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J Biol Chem 277: 12432–12436 [DOI] [PubMed] [Google Scholar]
- Govindan B, Bowser R, Novick P (1995) The role of Myo2, a yeast class V myosin, in vesicular transport. J Cell Biol 128: 1055–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann GJ, King EJ, Shaw JM (1997) The yeast gene, MDM20, is necessary for mitochondrial inheritance and organization of the actin cytoskeleton. J Cell Biol 137: 141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepfner D, van den Berg M, Philippsen P, Tabak HF, Hettema EH (2001) A role for Vps1p, actin, and the Myo2p motor in peroxisome abundance and inheritance in Saccharomyces cerevisiae. J Cell Biol 155: 979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume AN, Collinson LM, Rapak A, Gomes AQ, Hopkins CR, Seabra MC (2001) Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J Cell Biol 152: 795–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Catlett NL, Novak JL, Tang F, Nau JJ, Weisman LS (2003) Identification of an organelle-specific myosin V receptor. J Cell Biol 160: 887–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Watabe A, Toh-e A, Matsui Y (2002) Complex formation with Ypt11p, a rab-type small GTPase, is essential to facilitate the function of Myo2p, a class V myosin, in mitochondrial distribution in Saccharomyces cerevisiae. Mol Cell Biol 22: 7744–7757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GC, Prendergast JA, Singer RA (1991) The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J Cell Biol 113: 539–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser CA, Chen EJ, Losko S (2002) Subcellular fractionation of secretory organelles. Methods Enzymol 351: 325–338 [DOI] [PubMed] [Google Scholar]
- Long RM, Gu W, Lorimer E, Singer RH, Chartrand P (2000) She2p is a novel RNA-binding protein that recruits the Myo4p–She3p complex to ASH1 mRNA. EMBO J 19: 6592–6601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y (2003) Polarized distribution of intracellular components by class V myosins in Saccharomyces cerevisiae. Int Rev Cytol 229: 1–42 [DOI] [PubMed] [Google Scholar]
- Meisinger C, Sommer T, Pfanner N (2000) Purification of Saccharomyces cerevisiae mitochondria devoid of microsomal and cytosolic contaminations. Anal Biochem 287: 339–342 [DOI] [PubMed] [Google Scholar]
- Munchow S, Sauter C, Jansen RP (1999) Association of the class V myosin Myo4p with a localised messenger RNA in budding yeast depends on She proteins. J Cell Sci 112: 1511–1518 [DOI] [PubMed] [Google Scholar]
- Pfaller R, Neupert W (1987) High-affinity binding sites involved in the import of porin into mitochondria. EMBO J 6: 2635–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz WA, Grzyb L, Veenhuis M, Kahana JA, Silver PA, Rapoport TA (2000) Mutants affecting the structure of the cortical endoplasmic reticulum in Saccharomyces cerevisiae. J Cell Biol 150: 461–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck-Peterson SL, Provance DW Jr, Mooseker MS, Mercer JA (2000) Class V myosins. Biochim Biophys Acta 1496: 36–51 [DOI] [PubMed] [Google Scholar]
- Rossanese OW, Reinke CA, Bevis BJ, Hammond AT, Sears IB, O'Connor J, Glick BS (2001) A role for actin, Cdc1p, and Myo2p in the inheritance of late Golgi elements in Saccharomyces cerevisiae. J Cell Biol 153: 47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott DH, Collins RN, Bretscher A (2002) Secretory vesicle transport velocity in living cells depends on the myosin-V lever arm length. J Cell Biol 156: 35–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122: 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon VR, Karmon SL, Pon LA (1997) Mitochondrial inheritance: cell cycle and actin cable dependence of polarized mitochondrial movements in Saccharomyces cerevisiae. Cell Motil Cytoskeleton 37: 199–210 [DOI] [PubMed] [Google Scholar]
- Simon VR, Swayne TC, Pon LA (1995) Actin-dependent mitochondrial motility in mitotic yeast and cell-free systems: identification of a motor activity on the mitochondrial surface. J Cell Biol 130: 345–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa PA, DeRisi JL, Wilhelm JE, Vale RD (2000) Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science 290: 341–344 [DOI] [PubMed] [Google Scholar]
- Takizawa PA, Vale RD (2000) The myosin motor, Myo4p, binds Ash1 mRNA via the adapter protein, She3p. Proc Natl Acad Sci USA 97: 5273–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Bowers B, Wei Q, Kocher B, Hammer JA III (1997) Myosin V associates with melanosomes in mouse melanocytes: evidence that myosin V is an organelle motor. J Cell Sci 110: 847–859 [DOI] [PubMed] [Google Scholar]
- Wu XS, Rao K, Zhang H, Wang F, Sellers JR, Matesic LE, Copeland NG, Jenkins NA, Hammer JA III (2002) Identification of an organelle receptor for myosin-Va. Nat Cell Biol 4: 271–278 [DOI] [PubMed] [Google Scholar]
- Yaffe MP (1999) The machinery of mitochondrial inheritance and behavior. Science 283: 1493–1497 [DOI] [PubMed] [Google Scholar]
- Yin H, Pruyne D, Huffaker TC, Bretscher A (2000) Myosin V orientates the mitotic spindle in yeast. Nature 406: 1013–1015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material 1
Supplemental Material 2
Supplemental Material 3
Supplemental Material 4


