Abstract
Parents of many species provision their young, and the extent of parental provisioning constitutes a major component of the offspring’s social environment. Thus, a change in parental provisioning can alter selection on offspring, resulting in the coevolution of parental and offspring traits. Although this reasoning is central to our evolutionary understanding of family life, there is little direct evidence that selection by parents causes evolutionary change in their offspring. Here we use experimental evolution to examine how populations of burying beetles adapt to a change in posthatching parental provisioning. We measured the performance of larvae descended from lab populations that had been maintained with and without posthatching parental care (Full Care and No Care populations). We found that adaptation to the absence of posthatching care led to rapid and consistent changes in larval survival in the absence of care. Specifically, larvae from No Care populations had higher survival in the absence of care than larvae from Full Care populations. Other measures of larval performance, such as the ability of larvae to consume a breeding carcass and larval mass at dispersal, did not differ between the Full Care and No Care populations. Nevertheless, our results show that populations can adapt rapidly to a change in the extent of parental care and that experimental evolution can be used to study such adaptation.
Keywords: parental care, experimental evolution, coadaptation, burying beetle, Nicrophorus vespilloides, interacting phenotypes
Introduction
All animals provision their young in some form. This provisioning may come as yolk deposited in eggs, nutrients transferred between mothers and embryos via a placenta, or resources provided to dependent young after birth or hatching. Understanding why animals vary in the mode by which they provision their young and the amount of resources they provide has been a major focus of evolutionary and behavioral ecology (Clutton-Brock 1991; Roff 1992, 2002). Much of this work has considered parental provisioning as an adult life-history trait that is likely to experience different strengths or forms of selection in different environments (Smith and Fretwell 1974; Roff 1992, 2002). If levels of parental provisioning are heritable, then selection for different levels of parental provisioning in different populations can result in divergence in traits such as egg size or offspring size at birth (e.g., Schwarzkopf et al. 1999; Czesak and Fox 2003; Heath et al. 2003; Fischer et al. 2006). Indeed, several studies have found associations between environmental variables and egg size or size at birth, suggesting that parental provisioning is often locally adapted (e.g., Reznick and Endler 1982; Johnston and Leggett 2002; Heath et al. 2003: Schrader and Travis 2012).
Parental provisioning is not only an important adult life-history trait. In animals with parental care it also constitutes an important part of the offspring’s social environment (Wolf et al. 1998). Therefore, a change in parental provisioning may alter the form or strength of selection on offspring phenotype, resulting in the further evolution of traits expressed in offspring (Kirkpatrick and Lande 1989; Moore et al. 1997; Wolf et al. 1998; Lock et al. 2004). That offspring will adapt to variation in parental provisioning is a central assumption of models of both parent-offspring conflict and coadaptation. For example, parent-offspring conflict is predicted to favor adaptations in offspring that allow them to extract more care from parents than is optimal for parents to provide, and models of coadaptation predict that selection on offspring will result in the joint evolution of traits expressed in parents (e.g., supply of resources) and offspring (e.g., demand for resources) that interact to influence offspring fitness (Trivers 1974; Wolf and Brodie 1998; Wolf 2000; Hinde et al. 2010). Support for this assumption comes mainly from quantitative genetic estimates of social epistasis or genetic correlations between traits involved in parental supply of resources and offspring demand for these resources (reviewed in Kölliker et al. 2012). The presence of such correlations suggests that selection has favored specific combinations of these traits (i.e., that there has been correlational selection on parent-offspring interactions). In most cases, however, it is not known how these genetic correlations became established, and explanations for the forces that generated them are inferred from the direction of the correlation (Agrawal et al. 2001; Lock et al. 2004; Kölliker et al. 2005; reviewed in Kölliker et al. 2012). For example, Agrawal et al. (2001) found a negative genetic correlation between parental provisioning and offspring begging in burrower bugs (Sehirus cinctus), and the direction of this correlation is consistent with predictions of parent-offspring coadaptation theory that assume the presence of stabilizing selection on offspring phenotype (Wolf and Brodie 1998; Agrawal et al. 2001). In short, previous work suggests that traits involved in parent-offspring interactions should coevolve, and some studies have uncovered genetic correlations between traits expressed in parents and offspring consistent with such coevolution. However, no studies have measured whether a change in parental behavior causes an evolved change in traits expressed by the offspring.
Here we describe an experiment in which we examine directly how populations of burying beetles Nicrophorus vespilloides adapt to a change in parental provisioning. Like all species in the genus, N. vespilloides breeds on the carcasses of small vertebrates. Upon encountering a carcass, parents mate and prepare the carcass for their young to feed on. Carcass preparation involves shaving the fur or feathers from the carcass, rolling it into a ball, and smearing the surface of the carcass with anal exudates that delay decomposition (Scott 1998). The eggs, which are laid near the carcass, hatch, and the larvae migrate to the carcass, where they feed. Nicrophorus vespilloides larvae exhibit begging behaviors, and parents respond to these behaviors by regurgitating predigested carrion that larvae consume. Posthatching parental care is facultative in N. vespilloides. Although larvae are able to complete development with no posthatching care, measures of breeding success and larval performance are typically higher when parents are allowed to provision larvae than when they are not (Eggert et al. 1998).
We took advantage of the facultative nature of posthatching care in this species to ask how populations adapt to the complete removal of posthatching care. To do this, we used experimental evolution. This approach involves establishing replicate experimental populations, exposing these populations to different environments for many generations, and then comparing traits between populations that have evolved in these different environments. Experimental evolution has been used to study how populations adapt to changes in environmental variables, such as predation risk (e.g., Reznick et al. 1997), as well as social aspects of the environment, such as the mating system (e.g., Hollis and Kawecki 2014), but it has not yet been used to study the evolution of parent-offspring interactions. We allowed N. vespilloides populations to evolve for several generations with and without posthatching parental care (Full Care and No Care populations, respectively). Then, for each experimental population, we measured offspring performance with and without posthatching parental care. We predicted that adaptation to the absence of posthatching parental care would reduce larval dependence on care such that, in the absence of parental care, larvae from No Care populations would perform better than larvae from Full Care populations.
Methods
Establishment and Maintenance of Experimental Populations
The experimental populations used in this study were created as part of a larger experiment designed to test whether posthatching parental care influences the response to selection on body size (B. J. M. Jarrett, M. Schrader, and R. M. Kilner, unpublished manuscript). For the purposes of this study, we ignore selection on body size and focus on four experimental populations that differed in the presence of posthatching parental care. A complete description of the protocols used to establish and maintain these populations, as well as data on differences in body size between the populations, is provided in the appendix, available online.
The experimental populations were descended from beetles collected in 2012 from two wild populations (Byron’s Pool and Wicken Fen) in Cambridgeshire, United Kingdom. These populations were interbred over the course of four generations, with 40 pairs breeding in each generation. We then interbred this population with a laboratory population for a single generation (breeding 160 pairs) to create a large, genetically diverse stock population. From this stock population, we created two populations that were maintained without posthatching parental care (No Care 1 and 2) and two populations that were maintained with full parental care (Full Care 1 and 2).
Each No Care (NC) population was initiated by breeding 60 pairs of beetles from the stock population. We placed each pair in a box with commercially bought compost and a thawed mouse carcass (8–14 g) and then placed these boxes in a dark cupboard to simulate underground conditions. We removed both parents from the breeding box 53 h after pairing. This is enough time for females to complete egg laying and carcass preparation but is before eggs begin to hatch (Smiseth et al. 2006; Boncoraglio and Kilner 2012). After removing both parents, we returned the box to the cupboard, where it remained until larval dispersal (8 days after pairing). On the eighth day, we counted and removed all of the larvae from each breeding box, weighed each brood, and then calculated the average mass of larvae in each brood (total brood mass/brood size). We measured brood size and brood mass 8 days after pairing because by this time larvae have nearly always left the carcass (even in cases where some flesh remains) and are wandering in the soil. Furthermore, in our population, leaving the larvae for longer increases that probability that one of the parents will eat some of the wandering larvae.
After measuring brood size and larval mass, we placed the larvae in 25-celled eclosion boxes (box dimensions, length × width × depth: 10 cm × 10 cm × 1.8 cm), with one larva in each cell (individual cell dimensions: 2 cm × 2 cm × 1.8 cm), covered them with damp peat, and left them to pupate for 17 days. Most individuals had eclosed by 17 days. However, those that had not were allowed more time to pupate (usually an additional day). After eclosion, we sexed and photographed each beetle and then placed individual beetles in plastic boxes (box dimensions, length × width × depth: 12 cm × 8 cm × 2 cm) with damp compost and a small amount of ground beef. Adult beetles remained in these boxes for 2 weeks and were fed ground beef twice per week. Two weeks after eclosion, we bred beetles from each population as described above. The number of pairs bred in each generation varied. In No Care 1 (NC1), we bred between 64 and 120 pairs per generation (mean = 110.75), and in No Care 2 (NC2), we bred between 39 and 120 pairs per generation (mean = 82.7)
The Full Care (FC) populations were each initiated by breeding 40 pairs of beetles and were treated in exactly the same way as the NC populations, except that we allowed parents to remain with their larvae until larval dispersal. In the Full Care 1 population (FC1), we bred 80 pairs per generation, and in Full Care 2 (FC2), we bred between 60 and 80 pairs per generation (mean = 75.7).
Block 1
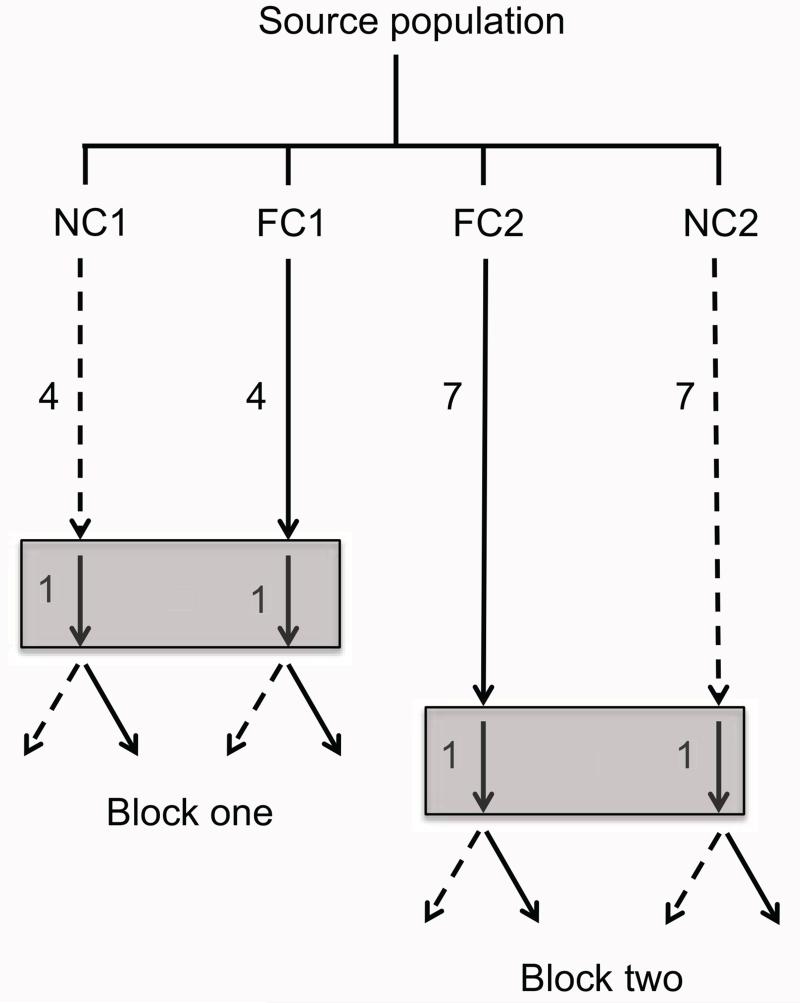
We maintained NC1 and FC1 for four generations as described above. We then passed both populations through a fifth generation in which larvae were reared with full parental care, following the protocol for the Full Care populations (fig. 1). This was done to minimize differences between lines in transgenerational effects of care. The resulting offspring were used as the parents in the sixth generation.
Figure 1.
Simplified schematic of the experimental populations and design. Dashed and solid lines represent populations that had been propagated without (No Care [NC]) and with (Full Care [FC]) posthatching parental care, respectively. Numbers to the left of each arrow are the number of generations that each population had been maintained without or with posthatching parental care. For example, NC1 had been propagated for four generations with no posthatching parental care. All populations were passed through a single generation with full parental care to minimize differences between lines in transgenerational effects (indicated by solid lines enclosed in gray rectangles). The resulting offspring were then bred with or without posthatching parental care (solid and dashed arrows, respectively). For each pair we measured breeding success, brood size at dispersal, mean larval mass, and carcass consumption. A complete description of the establishment and maintenance of the experimental populations can be found in the appendix, available online.
In the sixth generation, we bred beetles from the NC1 and FC1 populations with and without posthatching parental care (see fig. 1). To do this, we randomly paired unrelated adult males and females from within each experimental population (n = 47 pairs from the NC1 population; n = 69 pairs from the FC1 population). In the NC1 population, we bred 22 pairs without posthatching parental care and 25 pairs with posthatching parental care. In the FC1 population, we bred 41 pairs without posthatching parental care and 28 pairs with posthatching parental care. Eight days after pairs were bred, we recorded several measures of larval performance. First, we recorded two proxies of larval survival: whether the breeding attempt produced at least one dispersing larva (breeding success, scored as a binary variable) and, for pairs that bred successfully, the number of larvae at dispersal (brood size). We then recorded two measures of larval performance: whether the larvae had consumed the entire breeding carcass (carcass consumption) and the average mass of dispersing larvae (larval mass). Carcass consumption was scored as a binary variable. If there was no flesh remaining on the carcass at the time of larval dispersal, we considered the carcass to be completely consumed; otherwise, we scored the carcass as unconsumed (as in Rauter and Moore 2002). The average mass of dispersing larvae was calculated as the total brood mass at dispersal divided by the number of larvae in the brood.
Block 2
We replicated the experiment described above, using NC2 and FC2 populations. These populations had been maintained for seven generations and were each passed through an eighth generation in which larvae were reared with full parental care (fig. 1). The resulting offspring were used as the parents in the ninth generation. In the ninth generation, we bred beetles from the NC2 and FC2 populations with and without posthatching parental care (fig. 1). Our methods were identical to those used in block 1, although sample sizes were different. In the NC2 population, we bred 50 pairs without posthatching parental care and 30 pairs with posthatching parental care. In the FC2 population, we bred 50 pairs without posthatching parental care and 30 pairs with posthatching parental care.
Analysis
Our experimental design was a 2 × 2 factorial with two population types (No Care and Full Care) and two levels of environment (No Care and Full Care). The entire experiment was replicated twice with independent No Care populations that had been evolving without parental care for different amounts of time (four generations in block 1 and seven generations in block 2). To account for this, we included block as an additional factor in all analyses. When interactions involving block were significant, we analyzed the data from each block separately.
We examined the effect of population, environment, block, and their interactions on breeding success, using two complementary analyses. We first scored each breeding attempt as being either a success or a failure and analyzed the data, using a general linear model (GLM) with a binomial error term. For this analysis, we scored pairs that laid eggs but did not produce any dispersing larvae as failures and pairs that had at least one dispersing larva as successes. Second, for pairs that bred successfully, we examined the effect of population, environment, block, and their interactions on the number of dispersing larvae (brood size), using a GLM with a Gaussian error term. Although brood size takes only integer values, we chose to use a Gaussian error term instead of a Poisson error term because the overall mean brood size was fairly high (14.19) and because the residuals from a Gaussian model and a quasi-Poisson model (the Poisson model was overdispersed) behaved similarly. We note, however, that using a quasi-Poisson error term did not qualitatively affect our results. Carcass mass did not influence brood size in our experiments, so we did not include it as a covariate in this analysis (linear regression of carcass mass on brood size in both blocks pooled: slope [SE] = 0.11 [0.46], R2 = 0.0004, F1,181 = 0.066, P = .80).
We restricted our analysis of carcass consumption to the No Care environment because parents also feed on the carcass, making it impossible to attribute carcass consumption to offspring when parents are also present (we note, however, that carcass consumption was >90% in all treatments where parents were allowed to remain with their larvae). We examined the effect of population (Full Care or No Care), brood size, block, and their interactions on carcass consumption, using a GLM with a binomial error term.
We intended to examine the effects of population, environment, and block on larval mass, using a GLM with brood size as a covariate. We originally included the interaction between brood size and environment (Full Care vs. No Care) in this analysis. This interaction was highly significant (P< .01) in both blocks of the experiment, indicating that the homogeneity-of-slopes assumption of the model was violated. Because the relationship between brood size and mean larval mass differs between the two parental-care environments, it is inappropriate to proceed with testing the significance of the main effects assuming a common slope. Below, we report the results of the analysis of larval mass excluding brood size as a covariate.
Results
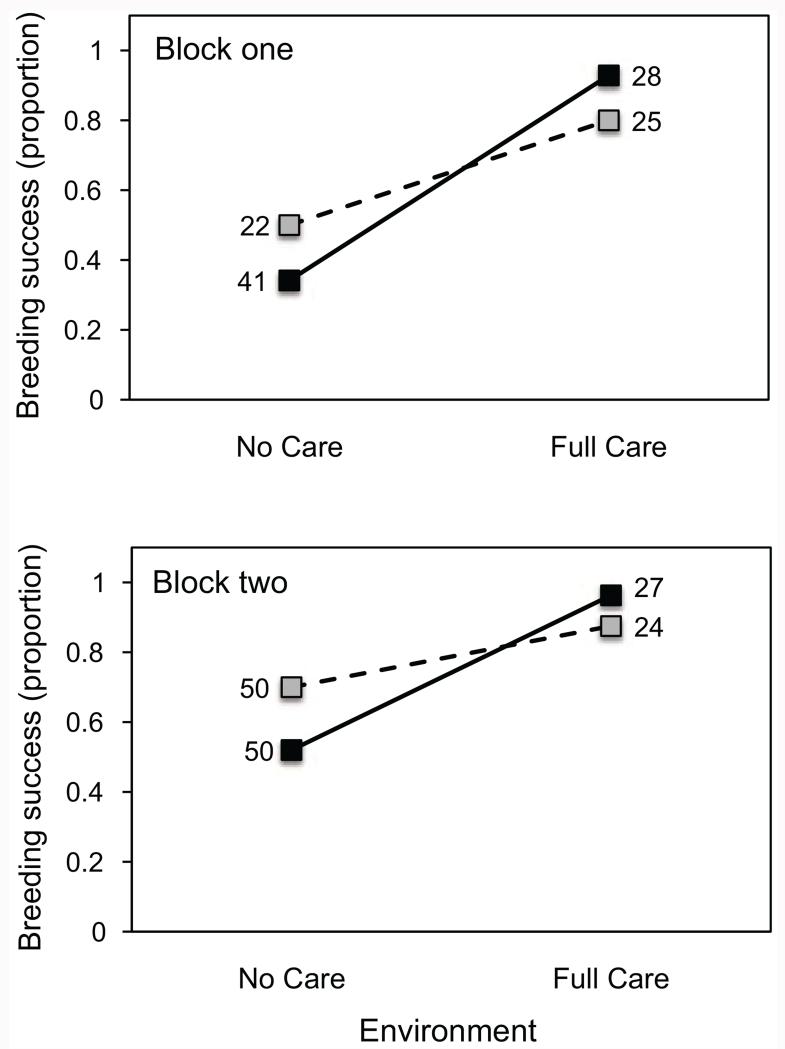
Breeding success, measured as the proportion of pairs producing at least one dispersing larva, was lower without posthatching parental care than with posthatching parental care. However, the magnitude of this difference varied between the No Care and Full Care populations (fig. 2; table 1). In the absence of posthatching care, the No Care populations had higher breeding success than the Full Care populations (50% vs. 34% in block 1, 70% vs. 52% in block 2). In the presence of posthatching care, breeding success was lower when the beetles were from the No Care populations than when they were from the Full Care populations (80% vs. 93% in block 1, 88% vs. 97% in block 2).
Figure 2.
Breeding success of beetles descended from No Care (gray squares, dashed lines) and Full Care (black squares, solid lines) populations in the absence or presence of posthatching care (X-axis). Breeding success is the proportion of breeding attempts producing at least one dispersing larva. Numbers next to each square are sample sizes. Data in the top panel are from block 1, and data in the bottom panel are from block 2.
Table 1.
Results of a general linear model of the effects of population (No Care or Full Care), treatment (No Care or Full Care), the population × treatment interaction, and experimental block on breeding success
| Factor | X 2 | P |
|---|---|---|
| Population | 2.9 | .08 |
| Treatment | 44.5 | < .00001 |
| Population × treatment | 6.50 | .011 |
| Block | 7.61 | .0075 |
Note: See figure 2 for sample sizes in each treatment.
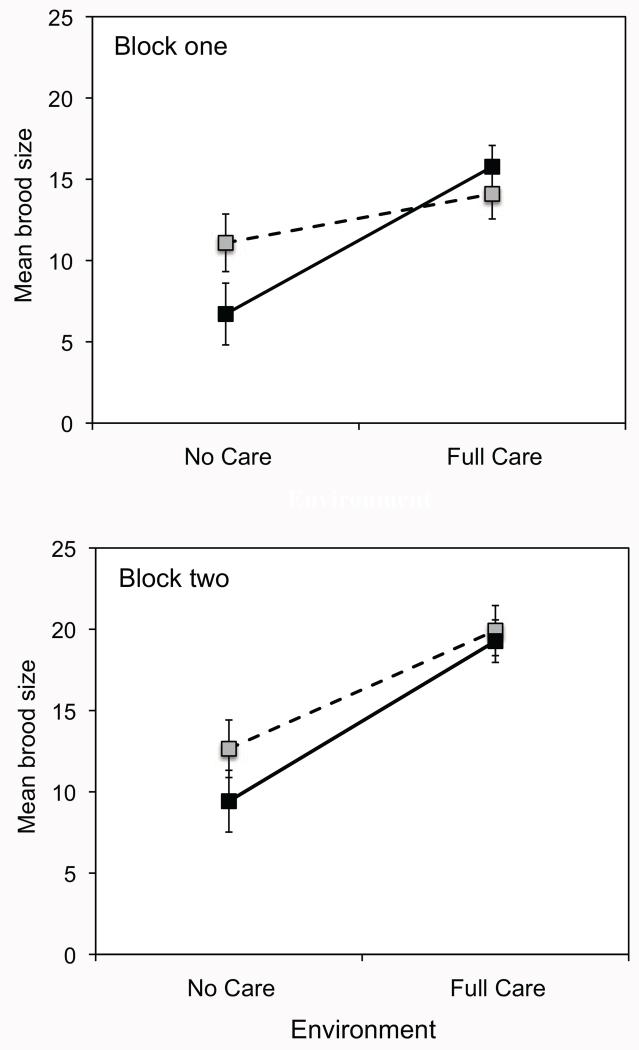
In both blocks of the experiment, posthatching parental care led to an approximately 60% increase in mean brood size at dispersal (pooling lines within each environment). The effect of parental care on average brood size did not differ between the No Care and Full Care populations (fig. 3; table 2).
Figure 3.
Mean brood size (±1 SEM) of beetle pairs descended from No Care (gray squares, dashed lines) and Full Care (black squares, solid lines) populations in the absence or presence of posthatching care (X-axis). Data in the top panel are from block 1, and data in the bottom panel are from block 2.
Table 2.
Results of ANOVAs of the effects of population, treatment, the population × treatment interaction, and experimental block on average brood size
| Factor | F | df | P |
|---|---|---|---|
| Population | .016 | 1, 177 | .89 |
| Treatment | 32.90 | 1, 177 | <.00001 |
| Population × treatment | 2.35 | 1, 177 | .13 |
| Block | 8.80 | 1, 177 | .0034 |
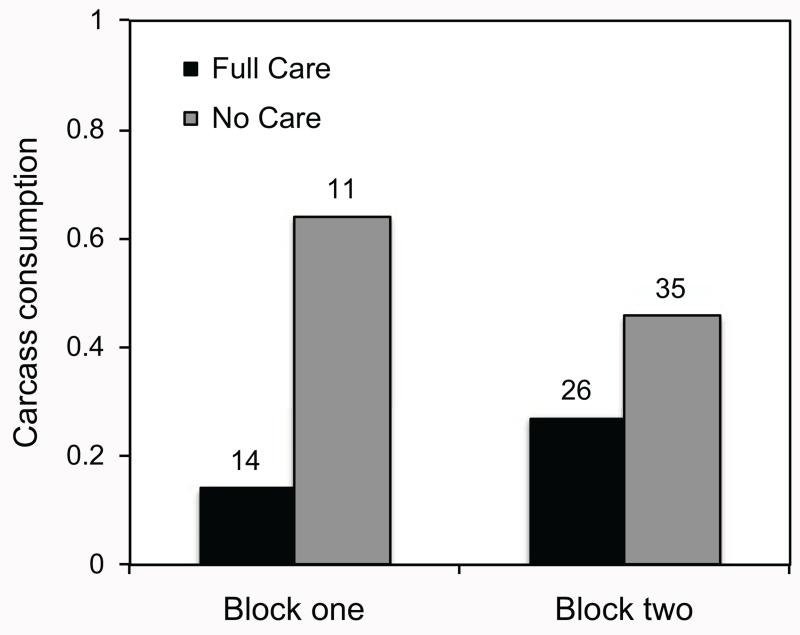
In both blocks of the experiment, the proportion of broods that successfully consumed the carcass appeared higher when the larvae were from the No Care populations than when they were from the Full Care populations (fig. 4). In block 1, carcass consumption was 4.6 times greater when beetles were descended from the No Care line than when they were from the Full Care line, and in block 2, carcass consumption was 1.7 times greater when the beetles were descended from the No Care line than when they were from the Full Care line (fig. 4). However, after the effect of brood size on carcass consumption was accounted for, there was no evidence for a difference between populations in carcass consumption (table 3).
Figure 4.
Proportion of successful breeding bouts without parental care in which the breeding carcass was completely consumed when beetle pairs were descended from No Care (gray bars) and Full Care (black bars) populations in each experimental block. The sample size is indicated over each bar.
Table 3.
Results of a general linear model of the effects of population, brood size, and block on carcass consumption
| Factor | X 2 | P |
|---|---|---|
| Population | 1.97 | .16 |
| Brood size | 57.80 | <.0001 |
| Block | 1.51 | .22 |
Note: See figure 4 for sample sizes in each treatment.
The analysis of larval mass was complicated by differences between the Full Care and No Care environments in the relationship between larval mass and brood size. In the Full Care environment, there was a negative relationship between average offspring size and brood size, while in the No Care environment, this relationship was shallower and hump shaped (fig. A2; figs. A1, A2 available online). We have observed the same relationship in other experiments and discuss its potential causes elsewhere (Schrader et al., forthcoming). However, because the relationship between brood size and mean larval mass differs between the Full Care and No Care environments, we report the results of the analysis of larval mass excluding brood size as a covariate.
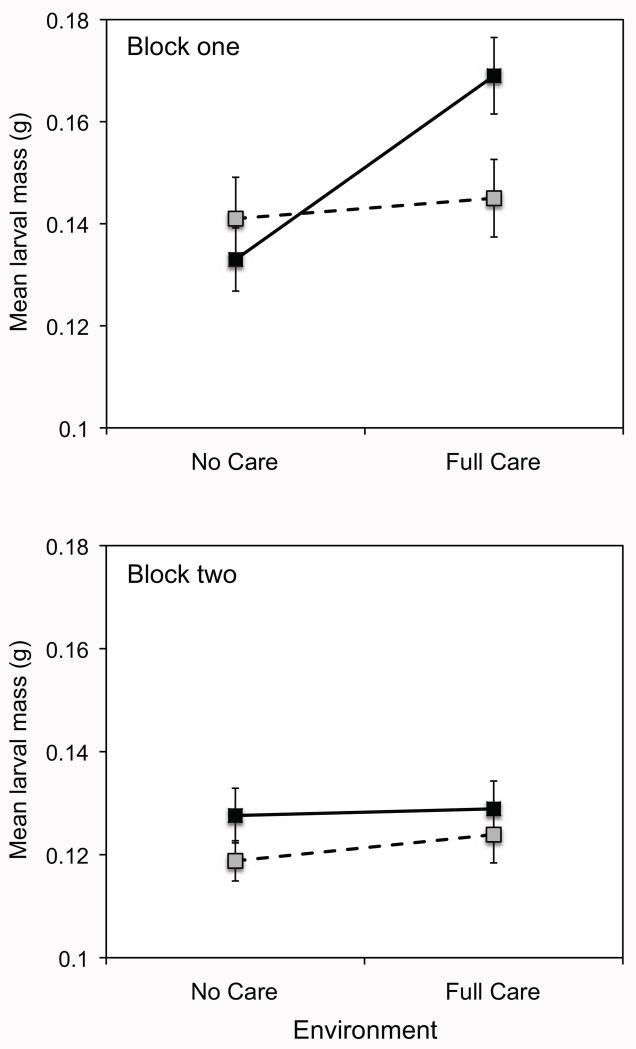
Initial analyses of the larval mass revealed a significant treatment × block interaction, so we analyzed each block separately. In block 1, mean larval mass was significantly influenced by carcass mass (linear regression of mean larval mass on carcass mass: slope ± SE = 0.008615 ± 0.00319, P = .0086, R2 = 0.086, n = 71), so we included carcass mass as a covariate in the analysis. After controlling for variation in carcass mass, we found a significant effect of environment and marginally significant effects of population and the population × environment interaction on mean larval mass (table 4). The presence of parental care increased larval mass; however, this effect was more pronounced for beetles from the Full Care population than for those from the No Care population (table 4; fig. 5).
Table 4.
Results of ANOVAs of the effects of population, treatment, and their interaction on average larval mass for the two blocks of the experiment considered separately
| Factor | F | df | P |
|---|---|---|---|
| Block 1: | |||
| Population | 3.018 | 1, 66 | .087 |
| Treatment | 8.39 | 1, 66 | .0051 |
| Population × treatment | 3.71 | 1, 66 | .058 |
| Carcass mass | 10.77 | 1, 66 | .0017 |
| Block 2: | |||
| Population | 2.19 | 1, 107 | .14 |
| Treatment | .45 | 1, 107 | .51 |
| Population × treatment | .14 | 1, 107 | .71 |
Note: Carcass mass was included as a covariate in block 1 of the experiment but was not included in the analysis of block 2.
Figure 5.
Mean larval mass (±1 SEM) of beetles descended from the No Care (gray squares, dashed lines) and Full Care (black squares, solid lines) populations in the absence or presence of posthatching care. Data in the top panel are from block 1, and data in the bottom panel are from block 2.
In block 2, the relationship between carcass mass and mean larval mass was marginally nonsignificant (linear regression of mean larval mass on carcass mass: slope ± SE = 0.003386 ± 0.00182, P = .066, R2 = 0.022, n = 110). Our results were qualitatively similar whether or not we included carcass mass as a covariate, and for simplicity we present the results excluding carcass mass. In contrast to block 1, there was no evidence that parental care increased mean larval mass, nor was there evidence that the Full Care and No Care populations differed in larval mass or the effects of parental care on larval mass (table 4; fig. 5).
Data underlying these results are deposited in the Dryad Data Repository: http://dx.doi.org/10.5061/dryad.rj370 (Schrader et al. 2015).
Discussion
Changes in parental provisioning are predicted to drive evolutionary changes in offspring; however, few studies have directly examined how offspring adapt to a change in a parental effect. In this experiment, we used experimental evolution to investigate how populations of Nicrophorus vespilloides evolve in response to the elimination of posthatching parental care. We found that breeding success in the absence of posthatching care was higher when the beetles had evolved in the No Care selection regime than when they had evolved in the Full Care selection regime. This observation is consistent with rapid adaptation to the absence of posthatching parental care. However, other components of larval performance related to resource use did not differ consistently between populations evolving with and without care.
Our results suggest that breeding success in the No Care populations has become less dependent on posthatching parental care, and we can envisage two routes through which this may have evolved. The first possibility involves the evolution of a trait expressed in the larvae. Eggert et al. (1998) found that the presence of parents during the first 12 h after larval hatching greatly increased larval survival and growth and suggested that this effect was due to parents chewing a hole in the carcass, thereby making it more accessible for the larvae. In our experiment, it may be that larvae from the No Care lines are better able to chew an opening in the breeding carcass themselves, whereas larvae from the Full Care lines are still dependent on their parents for this task. This change in the larvae could occur through either a behavioral (e.g., increased self-feeding) or a morphological (e.g., jaw structure) adaptation. We are currently examining these possibilities.
The second route involves evolutionary change in traits expressed in the parents. It is well known from earlier studies that parental investment in burying beetles involves activities before and after hatching (Scott 1998). Furthermore, there is individual variation in the relative magnitude of the pre- and posthatching components of parental care (Lock et al. 2004, 2007; Steiger 2013). By eliminating posthatching parental care in one of our experimental evolution treatments, it is possible that we selected for increased prehatching care. In other words, larvae from the No Care populations may be less dependent on posthatching care because their parents have invested more heavily in prehatching care.
There are two general ways this might happen. First, parents from the No Care populations may have invested more in carcass preparation than parents from the Full Care populations, possibly making it easier for larvae to penetrate and use the breeding carcass. Around the time of hatching, N. vespilloides parents create a feeding cavity in the carcass that larvae recruit to after hatching. Adaptation to the No Care selection regime could theoretically have selected parents that create this depression earlier than usual (before parental removal at 53 h after pairing). However, we think that such a shift is unlikely to explain our results, because we have never seen a feeding cavity (or the beginning of one) as early as 53 h after pairing. Another possibility is that selection in the No Care environment has favored parents that create small holes in the carcass (as opposed to the rather large feeding cavity) before parental removal. This could happen as a by-product of parents feeding on the carcass and neglecting to reseal the holes they create. We did not inspect the carcasses in this experiment for small holes. Nevertheless, some support for this idea comes from another experiment (Schrader et al., forthcoming). In that experiment, we removed parents at 53 h after pairing, manipulated brood sizes, and inspected carcasses at larval hatching (70 h after pairing). At 70 h after pairing, we found that 13.75% of prepared carcasses (11/80) did indeed have small holes visible. Although we cannot say with certainty that these holes were created by the parents, it is likely that at least some of them were. Nevertheless, this same experiment also found that larvae were able to penetrate the breeding carcass even if there was no preexisting hole. Determining whether adaptation to the No Care selection regime has involved a subtle change in carcass preparation will require more careful examination of carcasses.
A second possibility is that selection in the No Care populations has favored increased maternal investment into individual eggs. In many organisms, egg size or size at birth is positively correlated offspring performance (e.g., Sinervo 1990; Takakura 2004; Bashey 2006; Boivin and Gauvin 2009; Monteith et al. 2012; reviewed in Fox and Czesak 2000). In N. vespilloides, the relationship between egg size and larval performance depends on the presence of posthatching care (Monteith et al. 2012). When parents are allowed to provision larvae, there is no relationship between egg size and larval growth, but when posthatching parental care is removed, this relationship is positive. These results suggest that an increase in egg size can partially compensate for the absence of posthatching parental care. It is unclear, however, whether the benefits of increased egg size observed by Monteith et al. (2012) would be realized in our experiment. For example, Monteith et al. (2012) removed parents 72 h after pairing (around the time of larval hatching). By this time, parents have usually created a feeding cavity in the carcass. In our experiment, parents were removed well before they begin creating this cavity (as described above). In addition, Monteith et al.’s (2012) No Care treatment involved cutting a hole in the carcass with a razor blade immediately after removing the parents. This was done to facilitate larval entrance into the breeding carcass. Our No Care treatment did not involve cutting a hole in the carcass. Thus, larvae had to chew their way in on their own. Whether offspring from larger eggs are better at chewing their way into the carcass remains unknown.
We have suggested ways in which removing posthatching care may have led to the evolution of traits in either offspring or parents. However, we wish to stress that these hypotheses are not mutually exclusive. Adaptation to the absence of posthatching parental care may have involved the joint evolution of traits expressed in offspring and parents (i.e., coadaptation). For example, selection in the No Care populations may have favored parents that make small holes in the carcass and larvae that are able to exploit these holes effectively. Whatever traits may be involved, the rapid evolution of reduced offspring dependence on posthatching care in the No Care lines suggests that there is extensive genetic variation for traits affecting larval survival in the absence of posthatching care. It is possible that such variation persists cryptically in N. vespilloides because posthatching parental care typically shields it from natural selection.
Similar to a previous study (Eggert et al. 1998), we found that posthatching parental care positively affected the number of dispersing larvae. This effect did not differ between the Full Care and No Care lines, however. This suggests that adaptation to the No Care selection regime has not involved traits that regulate brood size after larvae become established on the carcass, either because selection at this stage has been weak or because there is little heritable variation for the larval performance at this stage. Our experiment does not allow us to distinguish between these two possibilities. Although the effect of posthatching parental care on brood size did not differ between the Full Care and No Care lines, we note that in the absence of care, the average brood size at dispersal in the No Care lines was 34%–65% higher than the average brood size in the Full Care lines (fig. 3). It is possible that further adaptation to the No Care selection regime will lead to greater divergence in brood size.
Although adaptation to the No Care selection regime reduced offspring dependence on parental care, it did not lead to consistent differences in the sensitivity of larval mass to posthatching care. In one block of the experiment, posthatching care increased larval mass, but only when the beetles were descended from the Full Care population. In the other block of the experiment, there was no effect of care on larval mass in beetles descended from either population. The absence of a consistent effect of posthatching care on larval mass is somewhat surprising, as previous studies of Nicrophorus beetles have found that posthatching care increases larval mass (Eggert et al. 1998; Rauter and Moore 2002; Monteith et al. 2012). However, these previous studies examined the effect of parental care under environmental conditions different from those in our experiment. For example, Eggert et al. (1998) and Rauter and Moore (2002) measured the impact of posthatching care in broods whose size had been standardized to minimize variation in larval mass caused by variation in brood size. We did not manipulate brood size and were unable to control for variation in brood size statistically because the presence or absence of posthatching care substantially changed the relationship between brood size and larval mass (see above). Second, although Monteith et al. (2012) measured the effect of care on larval growth without manipulating brood size, they used larger carcasses than we did, and their No Care treatment involved cutting a hole in the breeding carcass. Finally, all previous studies examining the impact of parental care on larval mass have removed care at larval hatching or very soon after. In contrast, our experiment involved removing parents well before larvae hatched.
Despite these methodological issues, the lack of divergence between lines in larval mass is not entirely surprising, for two reasons. First, the No Care and Full Care selection regimes probably did not involve consistent differential selection on larval mass. There was no selection on larval mass in the Full Care lines (mating was random with respect to adult size, which is positively correlated with larval mass). In the No Care lines, there was a history of selection for either large or small adults, but any history of this selection was likely erased by the protocol we used to create the experimental populations (see the appendix). Second, we know from previous studies that the total heritability of adult body size in N. vespilloides (which is strongly positively correlated with larval mass at dispersal) is very low, suggesting that a response to even very strong selection on body size is unlikely to result in evolutionary change over the short term (Head et al. 2012; M. Schrader, B. J. M. Jarrett, and R. M. Kilner, unpublished data).
Perhaps more important than the individual results described above, our study suggests that experimental evolution can be applied to the study of parental care. Much of what we know about the evolution of parent-offspring interactions comes from quantitative genetic estimates of correlations between parental and offspring traits (reviewed in Kölliker et al. 2012). While these studies have been tremendously valuable, they can be used only inferentially to understand how selection has shaped parent-offspring interactions. We suggest that combining quantitative genetic approaches with experimental evolution will provide more direct insights into how parent-offspring interactions coevolve in response to different selection regimes. For example, manipulating the presence of posthatching parental care over many generations might make it possible to study not only how offspring adapt to a change in parental care but also whether such adaptation changes the direction or magnitude of genetic correlations between parental and offspring behaviors. Similarly, it might be possible to manipulate the extent of parent-offspring conflict in experimental populations (e.g., through manipulating the mating system) and ask whether correlations between parental and offspring traits evolve in response to antagonistic selection. Insects with flexible patterns of parental care and rapid generation times, such as N. vespilloides, are ideal species for such experiments.
Supplementary Material
A burying-beetle mother (Nicrophorus vespilloides) feeds her young. Photograph by Tom Houslay.
Acknowledgments
We were supported by a Consolidator’s Grant from the European Research Council (310785 Baldwinian beetles). Research was funded by the Natural Environment Research Council of the United Kingdom (NE/H019731/1), the European Research Council, and the Department of Zoology at the University of Cambridge. We thank the associate editor and two anonymous reviewers for comments that greatly improved the quality of the article. We are also grateful to the other members of the burying-beetle group and K. McGhee for helpful discussions and to S. Aspinall and C. Swannack for help in the lab.
Literature Cited
- Agrawal AF, Brodie ED, III, Brown J. Parent-offspring coadaptation and the dual genetic control of maternal care. Science. 2001;292:1710–1712. doi: 10.1126/science.1059910. [DOI] [PubMed] [Google Scholar]
- Bashey F. Cross-generational environmental effects and the evolution of offspring size in the Trinidadian guppy Poecilia reticulata. Evolution. 2006;60:348–361. [PubMed] [Google Scholar]
- Boivin G, Gauvin MJ. Egg size affects larval performance in a coleopteran parasitoid. Ecological Entomology. 2009;34:240–245. [Google Scholar]
- Boncoraglio G, Kilner RM. Female burying beetles benefit from male desertion: sexual conflict and counter-adaptation over parental investment. PLoS One. 2012;7:e31713. doi: 10.1371/journal.pone.0031713. doi:10.1371/journal.pone.0031713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH. The evolution of parental care. Princeton University Press; Princeton, NJ: 1991. [Google Scholar]
- Czesak ME, Fox CW. Evolutionary ecology of egg size and number in a seed beetle: genetic trade-off differs between environments. Evolution. 2003;57:1121–1132. doi: 10.1111/j.0014-3820.2003.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Eggert AK, Reinking M, Müller JK. Parental care improves offspring survival and growth in burying beetles. Animal Behaviour. 1998;55:97–107. doi: 10.1006/anbe.1997.0588. [DOI] [PubMed] [Google Scholar]
- Fischer KA, Bot NM, Brakefield PM, Zwaan BJ. Do mothers producing large offspring have to sacrifice fecundity? Journal of Evolutionary Biology. 2006;19:380–391. doi: 10.1111/j.1420-9101.2005.01046.x. [DOI] [PubMed] [Google Scholar]
- Fox CW, Czesak ME. Evolutionary ecology of progeny size in arthropods. Annual Review of Entomology. 2000;45:341–369. doi: 10.1146/annurev.ento.45.1.341. [DOI] [PubMed] [Google Scholar]
- Head ML, Berry LK, Royle NJ, Moore AJ. Paternal care: direct and indirect genetic effects of fathers on offspring performance. Evolution. 2012;66:3570–3581. doi: 10.1111/j.1558-5646.2012.01699.x. [DOI] [PubMed] [Google Scholar]
- Heath DD, Heath JW, Bryden CA, Johnson RM, Fox CW. Rapid evolution of egg size in captive salmon. Science. 2003;299:1738–1740. doi: 10.1126/science.1079707. [DOI] [PubMed] [Google Scholar]
- Hinde CA, Johnstone RA, Kilner RM. Parent-offspring conflict and coadaptation. Science. 2010;327:1373–1376. doi: 10.1126/science.1186056. [DOI] [PubMed] [Google Scholar]
- Hollis B, Kawecki TJ. Male cognitive performance declines in the absence of sexual selection. Proceedings of the Royal Society B: Biological Sciences. 2014;281:20132873. doi: 10.1098/rspb.2013.2873. doi:10.1098/rspb.2013.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston TA, Leggett WC. Maternal and environmental gradients in the egg size of an iteroparous fish. Ecology. 2002;83:1777–1791. [Google Scholar]
- Kirkpatrick M, Lande R. The evolution of maternal characters. Evolution. 1989;43:485–503. doi: 10.1111/j.1558-5646.1989.tb04247.x. [DOI] [PubMed] [Google Scholar]
- Kölliker M, Brodie ED, III, Moore AJ. The coadaptation of parental supply and offspring demand. American Naturalist. 2005;166:506–516. doi: 10.1086/491687. [DOI] [PubMed] [Google Scholar]
- Kölliker M, Royle NJ, Smiseth PT. Parent-offspring coadaptation. In: Royle NJ, Smiseth PT, Kölliker M, editors. The evolution of parental care. Oxford University Press; Oxford: 2012. pp. 285–303. [Google Scholar]
- Lock JE, Smiseth PT, Moore AJ. Selection, inheritance, and the evolution of parent-offspring interactions. American Naturalist. 2004;164:13–24. doi: 10.1086/421444. [DOI] [PubMed] [Google Scholar]
- Lock JE, Smiseth PT, Moore PJ, Moore AJ. Coadaptation of prenatal and postnatal maternal effects. American Naturalist. 2007;170:709–718. doi: 10.1086/521963. [DOI] [PubMed] [Google Scholar]
- Monteith KM, Andrews C, Smiseth PT. Post-hatching parental care masks the effects of egg size on offspring fitness: a removal experiment on burying beetles. Journal of Evolutionary Biology. 2012;25:1815–1822. doi: 10.1111/j.1420-9101.2012.02567.x. [DOI] [PubMed] [Google Scholar]
- Moore AJ, Brodie ED, III, Wolf JB. Interacting phenotypes and the evolutionary process. I. Direct and indirect genetic effects of social interactions. Evolution. 1997;51:1352–1362. doi: 10.1111/j.1558-5646.1997.tb01458.x. [DOI] [PubMed] [Google Scholar]
- Rauter CM, Moore AJ. Quantitative genetics of growth and development time in the burying beetle Nicrophorus pustulatus in the presence and absence of post-hatching pareswntal care. Evolution. 2002;56:96–110. doi: 10.1111/j.0014-3820.2002.tb00852.x. [DOI] [PubMed] [Google Scholar]
- Reznick D, Endler JA. The impact of predation on life history evolution in Trinidadian guppies (Poecilia reticulata) Evolution. 1982;36:160–177. doi: 10.1111/j.1558-5646.1982.tb05021.x. [DOI] [PubMed] [Google Scholar]
- Reznick DN, Shaw FH, Rodd FH, Shaw RG. Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata) Science. 1997;275:1934–1937. doi: 10.1126/science.275.5308.1934. [DOI] [PubMed] [Google Scholar]
- Roff DA. The evolution of life histories: theory and analysis. Chapman & Hall; New York: 1992. [Google Scholar]
- Roff DA. Life history evolution. Sinauer; Sunderland, MA: 2002. [Google Scholar]
- Schrader M, Jarrett BJM, Kilner RM. Data from: Using experimental evolution to study adaptations for life within the family. American Naturalist, Dryad Data Repository. 2015 doi: 10.1086/680500. http://dx.doi.org/10.5061/dryad.rj370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M, Jarrett BJM, Kilner RM. Parental care masks a density-dependent shift from cooperation to competition among burying beetle larvae. Evolution. doi: 10.1111/evo.12615. Forthcoming. doi:10.1111/evo.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M, Travis J. Assessing the roles of population density and predation risk in the evolution of offspring size in populations of a placental fish. Ecology and Evolution. 2012;2:1480–1490. doi: 10.1002/ece3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzkopf L, Blows MW, Caley MJ. Life-history consequences of divergent selection on egg size in Drosophila melanogaster. American Naturalist. 1999;154:333–340. doi: 10.1086/303242. [DOI] [PubMed] [Google Scholar]
- Scott MP. The ecology and behavior of burying beetles. Annual Review of Entomology. 1998;43:595–618. doi: 10.1146/annurev.ento.43.1.595. [DOI] [PubMed] [Google Scholar]
- Sinervo B. The evolution of maternal investment in lizards: an experimental and comparative analysis of egg size and its effects on offspring performance. Evolution. 1990;44:279–294. doi: 10.1111/j.1558-5646.1990.tb05198.x. [DOI] [PubMed] [Google Scholar]
- Smiseth PT, Ward RJS, Moore AJ. Asynchronous hatching in Nicrophorus vespilloides, an insect in which parents provide food for their offspring. Functional Ecology. 2006;20:151–156. [Google Scholar]
- Smith CC, Fretwell SD. The optimal balance between size and number of offspring. American Naturalist. 1974;108:499–506. [Google Scholar]
- Steiger S. Bigger mothers are better mothers: disentangling size-related prenatal and postnatal maternal effects. Proceedings of the Royal Society B: Biological Sciences. 2013;280:1–9. doi: 10.1098/rspb.2013.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura K. Variation in egg size within and among generations of the bean weevil, Bruchidus dorsalis (Coleoptera): effects of host plant quality and paternal nutritional investment. Annals of the Entomological Society of America. 2004;97:346–352. [Google Scholar]
- Trivers RL. Parent-offspring conflict. American Zoologist. 1974;14:249–264. [Google Scholar]
- Wolf JB. Gene interactions from maternal effects. Evolution. 2000;54:1882–1898. doi: 10.1111/j.0014-3820.2000.tb01235.x. [DOI] [PubMed] [Google Scholar]
- Wolf JB, Brodie ED., III The coadaptation of parental and offspring characters. Evolution. 1998;52:299–308. doi: 10.1111/j.1558-5646.1998.tb01632.x. [DOI] [PubMed] [Google Scholar]
- Wolf JB, Brodie ED, III, Cheverud JM, Moore AJ, Wade MJ. Evolutionary consequences of indirect genetic effects. Trends in Ecology and Evolution. 1998;13:64–69. doi: 10.1016/s0169-5347(97)01233-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.