Abstract
Objective
Estrogen plus progestin therapy (EPT) in postmenopausal women increases breast cancer risk and mammographic density to a higher extent than does estrogen therapy (ET) alone. Data from the randomized placebo-controlled Postmenopausal Estrogen/Progestin Interventions (PEPI) trial showed that EPT-induced increases in serum estrone and estrone sulfate levels were positively correlated with increases in mammographic density. Here, after adjusting for serum estrone and estrone sulfate levels, we investigated the roles of post-treatment serum progestogen increase and of progesterone receptor gene (PGR) genetic variations on changes in mammographic density.
Methods
We measured percent mammographic density and serum progestogen levels in 280 PEPI participants randomized to EPT treatment. Analyses of genetic variations in PGR were limited to 260 white women for whom we successfully obtained PGR genotypes. We used linear regression analyses to determine how increase in progestogen levels and PGR genetic variations influenced mammographic density change following EPT.
Results
The increase in post-treatment serum progestogen level was positively associated with greater increases in mammographic density after adjustment for covariates (P-trend=0.044). Compared to women in the lowest quartile of serum progestogen, women in the highest quartile experienced a 3.5% greater increase in mammographic density (P=0.046). We did not find a strong indication that genetic variations in PGR were associated with mammographic density increase, or modified the association with serum progestogen, however confidence in these null findings is constrained by our small sample size.
Conclusions
Our results suggest that higher serum progestogen levels resulting from EPT treatment lead to greater increases in mammographic density.
Keywords: progestogen level, progesterone receptor gene, hormone therapy, mammographic density
Introduction
There is substantial epidemiological evidence that combined estrogen plus progestin therapy (EPT) increases the risk of breast cancer (1-6). The results from the Women's Health Initiative (WHI) trial showed that an EPT regimen consisting of conjugated equine estrogens (CEE) and medroxyprogesterone acetate (MPA) (7), but not estrogen therapy (ET) alone (8), increases breast cancer risk. Mammographic density is a strong risk factor for breast cancer, and has been supported as an early marker of breast cancer risk (9, 10). Results from both the WHI trial and the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial showed that EPT use was associated with a substantial mammographic density increase (11-14). However, there was large inter-individual variation in the mammographic density increase within EPT treatment arms. Therefore, it is important to identify factors that predict mammographic density increase in women who received EPT.
In PEPI, greater increases in mammographic density change among those who were randomized to EPT were positively associated with post-treatment (after 12 months) increases in serum estrone (E1) and estrone sulfate (E1S) levels (15, 16). An unanswered question is whether the increases in E1 and E1S were simply correlates of the increases in progestogen levels that accompanied EPT use. We therefore conducted a follow-up study to examine whether post-treatment increase in serum progestogen was associated with mammographic density increase in women randomized to EPT.
The effect of progesterone on breast cells is mediated through its binding to the progesterone receptor. While most studies have reported no association between genetic variations in the progesterone receptor gene (PGR) (17-21) and breast cancer risk, longitudinal data from the European Prospective Investigation into Cancer and Nutrition (EPIC) suggested that a genetic variation in PGR modified the effect of hormone therapy on mammographic density change (22). We therefore investigated the association between post-treatment (after 12 months) increase in serum progestogen levels, PGR polymorphisms, and mammographic density change using data from the PEPI trial participants who received EPT.
Methods
Parent study
Details of the PEPI trial study design have been published (11, 12). Briefly, the PEPI trial enrolled 875 postmenopausal women aged 45 to 64 years at 7 United States clinical centers between 1989 and 1991. Eligible participants were women who did not have any menstrual periods during the previous 12 months, had not used estrogen or progestin in the past 2 months, and had follicle-stimulating hormone levels of more than 40 mU/mL. Eligible participants were randomly assigned to receive one of the following treatments: placebo; conjugated equine estrogens 0.625 mg/d (CEE); CEE and medroxyprogesterone acetate (MPA) 10 mg on days 1 to 12 per 28-day cycle (CEE + MPA cyclic); CEE and MPA 2.5 mg/d (CEE + MPA continuous); or CEE and micronized progesterone (MP) 200 mg on days 1 to 12 per 28-day cycle (CEE + MP). The CEE and MPA or MP combination arms will be referred to as EPT arms in this manuscript. All study medications (including placebo) were taken each morning. Unused pills were counted at each visit. We collected information on demographics, medical history, physical activity, smoking, contraceptive and non-contraceptive estrogen and progestin use, as well as alcohol intake. Height and weight were measured with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as body weight in kilograms divided by the square of height in meters.
Mammographic Density Substudy
The PEPI Mammographic Density Study (PEPI-MDS) consisted of 580 of the original 875 PEPI participants for whom we were able to retrieve baseline mammograms (performed before randomization) and follow-up mammograms collected at 12 month follow-up visit (12). The PEPI parent study was approved by the institutional research review boards (IRB's) at each original PEPI study site, and informed consent was obtained from all participants. The PEPI Mammographic Density Study (PEPI-MDS) performed analysis of already collected data and specimens only; the University of California, Los Angeles (UCLA) and University of Southern California (USC) IRB's reviewed and approved the PEPI-MDS.
In a previous PEPI-MDS study, it has been shown that almost all increases in mammographic density following randomization to the hormone therapy occurred within the first year (11). We excluded 7 PEPI-MDS participants from the PEPI-MDS dataset because of breast implants, 2 because of mammographic technique, and 2 because of extreme projection differences between baseline and follow-up that precluded the ability to access accurately the change in percent density, leaving 569 women in the PEPI-MDS sample. Of the 569 women, the current study was conducted among 345 women who were randomized to one of the EPT arms (CEE+MPA cyclic; CEE+MPA continuous; CEE+MP).
The mammographic percent density measurements of this study have been described in detail (12, 16). Mammographic percent density was assessed by one of the authors (GU) on scanned images using the USC Madena method, a validated computer-assisted, quantitative technique (23). The reader was blinded to treatment, study visit, and which mammograms belonged to the same patient. We estimated change in mammographic percent density as an absolute change in mammographic percent density (ie, the mammographic percent density at 12 month follow-up minus mammographic percent density at baseline).
Serum progestogen level measurements
Progestogen assays were done using stored samples from PEPI; all samples were stored at -80 °C and had never been thawed. PEPI collected fasting blood samples between 7AM and 10AM before start of treatment at baseline and annually. Participants did not take study medications the morning of the blood draw. Serum levels of steroid hormones including progesterone, estrone (E1), and estrone sulfate (E1S) at baseline were measured by BR Hopper (University of California at San Diego, CA) using previously described radioimmunoassay (RIA) methods (24). Serum levels of E1S after 12 months of post-treatment follow-up were measured by one of the authors (FZS) using direct radioimmunoassay (DSL-5400, Diagnostic Systems Laboratories, Inc.), as described previously (25). Progesterone and medroxyprogesterone acetate (MPA) were measured by well-established, previously validated radioimmunoassay methods (26, 27) in the laboratory of FZS. Separate 0.5 ml aliquots of serum were taken for each assay, and approximately 1000 d.p.m. of the appropriate tritiated radioligands (3H-progesterone or 3HMPA) were added to the serum to follow procedural losses. The steroids were extracted with ethyl acetate:hexane (3:2) to remove conjugated steroids, and Celite column partition chromatography with ethylene glycol as stationary phase was used to eliminate potential interfering unconjugated metabolites from each analyte. Both steroids were eluted off the column with isooctane, and after evaporating the solvent, the residue was dissolved in assay buffer. For each sample, duplicate aliquots were taken for RIA and a single aliquot to determine the procedural loss, which was used to correct the RIA results. Each RIA utilizes a highly specific antiserum in conjunction with 20,000 d.p.m. of an iodinated derivate (progesterone RIA) or 10,000 d.p.m. of 3H-MPA (MPA RIA). After a 16 to 20-hour incubation period, antibody-bound progesterone is separated from unbound progesterone by use of a second antibody, whereas in the MPA RIA, the separation is achieved with dextran-coated charcoal. The sensitivities of the progesterone and MPA RIAs are 20 pg/ml and 50 pg/ml, respectively, and the interassay coefficients of variation, on average, ranged from 9-13% in both assays.
DNA extraction
DNA was extracted from baseline serum in a collaborative effort by 2 authors (WW, DVDB). We extracted DNA using QIAamp Blood Mini Kits (Qiagen, Hilden, Germany) according to the manufacturer's protocol with slight modifications. In brief, 500 μL serum was treated with 20 μL of protease and mixed with 500 μL of AL buffer, then incubated with 500 μL of ethanol. Six-hundred fifty microliter aliquots of the mixture were serially loaded onto the same QIAamp spin column. When all of the mixture was transferred and spun down, DNA was eluted twice with 30 μL of AE buffer and concentrated with a SpeedVac to a final volume of 5 μl, followed by whole genome amplification performed using REPLI-g midi kits (Qiagen, Hilden, Germany) according to the manufacturer's instructions. DNA concentration was determined by real-time polymerase chain reaction performed on the ABI PRISM 7900HT (AppliedBiosystems, Foster City, CA) using the TaqMan RNase P Detection kit (Applied Biosystems, Foster City, CA).
Tagging SNP selection and genotyping of PGR
We selected tagging SNPs in the PGR locus, 20kb upstream of 5′ untranslated region (UTR) to 10kb downstream of 3′ UTR, that tagged all of the common SNPs (minor allele frequency ≥5%) among the white population with R2>0.80. This selection was done using the Snagger (28) software and a custom database of the Hapmap CEU data build 36 (www.hapmap.org) merged with unique SNPs in the Affymetrix 500K panel. One of the tagging SNPs, rs474320, is in high linkage disequilibrium with the PROGINS allele (29). The PROGINS allele of the PGR has been associated with decreased stability of the PGR transcript in a breast cancer cell line (29). In addition, we also genotyped a potentially functional SNP, rs10895068 (+331 G/A) in the promoter region of PGR (30).
We genotyped the selected SNPs using an Illumina BeadLab System (San Diego, CA) with GoldenGate® Genotyping in the USC Genomics Center under the direction of DVDB. Briefly, samples were run in a 96-well format using the Illumina Sentrix Array technology, scanned on a BeadArray Reader, and analyzed using BeadStudio Software (v.3.0.9) with Genotyping Module (v.3.0.27) (Illumina). As a quality control, we included 42 duplicate samples and a set of 30 CEU HapMap Trios. After SNPs were automatically clustered using the BeadStudio software, we manually edited clusters to increase call rate, reduce genotype disagreement between replicate samples, and to reduce trio errors. Among the 32 SNPs genotyped, 23 SNPs had genotype frequencies that did not depart significantly from Hardy-Weinberg equilibrium (HWE) (P>0.01), and were analyzed in the current study (Supplementary Table 1).
Using MACH 1.0 (31), we imputed genotypes for 145 SNPs in the PGR locus using publicly available Hapmap genotype data in whites of European ancestry (genotype build 36). Among the 145 SNPs, we excluded 23 SNPs that were not in HWE with P<0.01, and 7 SNPs with MAF<0.01. None of these imputed SNPs were associated with mammographic density change, and therefore are not presented.
Derivation of the current analysis sample
Among the 345 women who were randomized to one of the EPT arms in the PEPI-MDS, we identified 8 women whose baseline progesterone levels were higher than 370 pg/ml. These values were extremely high for postmenopausal woman who were not using hormone therapy, and considered to be implausible physiologically. We therefore excluded these observations from the analyses of serum progestogen levels. We further excluded 57 women with missing serum progestogen measurement at 12 month follow-up (32 women in the CEE+MPA cyclic arm, 20 women in the CEE+MPA continuous arm, and 5 women in CEE+MP arm). The final dataset for serum progestogen analyses therefore included 280 women. The majority (n=245) were non-Hispanic whites.
For the analysis of genetic variations in the PGR locus, we restricted the analysis to the 306 non-Hispanic white women among the 345 women who were randomized to one of the EPT arms. This was because there were few minor allele carriers of women of other ethnic groups for most SNPs. After excluding individuals whose SNP call rates were <60%, 260 non-Hispanic white women were available for the genetic analyses. For the analysis of interaction between serum progestogen measures and the PGR genetic variations, the final dataset included 210 non-Hispanic white women who met the criteria for both analyses.
Statistical Analysis
We used linear regression to examine the association between the serum progestogen increase and the increase in the mammographic percent density from baseline to 12 month follow-up. Because the measured level of progestogen at 12 month follow-up (MPA or progesterone) was not directly comparable to the measured level of progesterone at baseline for all treatment arms, we modeled the increase in serum progestogen as follows: we included a categorical variable for the quartile of follow-up progestogen level (MPA or progesterone) adjusting for the baseline serum progesterone level. For analyses within each treatment group, the serum progestogen level at follow-up (MPA or progesterone) was categorized into quartiles according to the distribution within each treatment group. For analyses combining all treatment groups, we used these same treatment-specific quartile cutoff points. To evaluate the interaction between treatment regimen and serum progestogen level, we included a treatment group by serum progestogen level product term in the model and conducted a Wald's test.
The following covariates were included in the model: age, BMI (in kilograms per square meter), baseline mammographic percent density, serum E1 and/or E1S at 12 month follow-up, daily grams of alcohol (tertiles), cigarette smoking (current versus former or never), physical activity (tertiles), and the 12-month change in BMI. Considering that our previous data showed that serum E1 and E1S levels were correlated, and that serum E1S was a significant predictor of change in mammographic density when adjusted for E1 (but not vice versa), we kept E1S but not E1 in the final model (15). The results were similar regardless of whether E1 was included in the model. We also considered the randomization blocking variables (clinic site and hysterectomy status), serum E1 and E1S change from baseline to follow-up at 12 months (replacing follow-up levels), and type of progestin. Additional adjustment for these factors did not change the results, and therefore these factors are not included in the final model. We performed a sensitivity analyses excluding 15 participants who were considered as non-adherent to the study medication (i.e. returned >20% of the study medication) in the analyses of serum progesterone (n=15), and the results remained the same.
The primary analyses of PGR genetic variations were based on log-additive genetic models, which estimate the difference in the outcome variable (i.e. absolute change in the mammographic percent density) per copy of the minor allele of each polymorphism. We also estimated genotype-specific effects by comparing heterozygous carriers and homozygous carriers of minor allele with homozygous carriers of major allele. The analyses were performed within each treatment arm, as well as in all groups combined. We evaluated the interaction between treatment arm and the genetic variation by introducing product terms and conducting Wald's tests. To correct for multiple testing of the effects of the PGR SNPs, we calculated P values adjusted for multiple correlated tests (PACT) (32).
We also evaluated the interaction between serum progestogen level increase and PGR genetic variation on the mammographic density increase, by introducing product terms and conducting Wald's tests. To correct for multiple testing of the interaction between PGR SNPs and serum progestogen levels, we used Bonferroni adjustment. Analyses were conducted using SAS 9.2 (SAS Inc., NC) and STATA v10 software (Stata Corp, College Station, TX). All P values are two sided.
Results
PEPI-MDS participants randomized to EPT were similar to the analytic sample, except that the PEPI-MDS sample had, on average, a higher baseline progesterone level and slightly lower adherence to the treatment than the analytic sample. This was expected because the analytic sample excluded participants with implausibly high baseline progesterone (Table 1).
Table 1. Baseline characteristics of the PEPI Mammographic Density Study (PEPI-MDS) participants who were randomized to estrogen+progestin therapy, and the three analytic samples used in this study.
| PEPI-MDS participants who were randomized to EPT | Analytic samples for serum progestogen analyses | Analytic samples for PGR genetic analyses | Analytic samples for serum progestogen & PGR interaction | |
|---|---|---|---|---|
| Total N | 358 | 280 | 260 | 210 |
| Randomized to CEE+MPA cyclic | 114 | 74 | 88 | 57 |
| Randomized to CEE+MPA continuous | 124 | 98 | 90 | 76 |
| Randomized to CEE+MP | 120 | 108 | 82 | 77 |
| Non-white ethnicity, No. (%) | 41 (11.5%) | 35 (12.5%) | 0 | 0 |
| Age (mean±SD) | 56.1 ± 4.3 | 56.2 ± 4.1 | 56.5 ± 4.1 | 56.7 ± 3.9 |
| BMI (mean±SD) | 26.2 ± 4.5 | 26.2 ± 4.6 | 26.1 ± 4.5 | 26 ± 4.6 |
| Change in BMI | 0.09 ± 1.3 | 0.04 ± 1.2 | 0.1 ± 1.3 | 0.1 ± 1.3 |
| Baseline progesterone level (pg/ml; mean±SD) | 202.2 ± 259.6 | 172.9 ± 44.4 | 208.6 ± 287.4 | 173.1 ± 45.2 |
| Baseline mammographic density (%; mean±SD) | 24.1 ± 18.2 | 24.0 ± 18.2 | 24.5 ± 18.5 | 24.4 ± 18.5 |
| Parous women, No. (%) | 321 (89.7%) | 253 (90.4%) | 236 (90.8%) | 191 (91.0%) |
| Smoking status, No. (%) | ||||
| Current | 39 (10.9%) | 29 (10.4%) | 31 (11.9%) | 23 (11.0%) |
| Former | 130 (36.3%) | 101 (36.1%) | 101 (38.9%) | 80 (38.1%) |
| Never | 189 (52.8%) | 150 (53.6%) | 128 (49.2%) | 107 (51.0%) |
| Alcohol use, No. (%) | ||||
| > 5.43 (g/day) | 110 (30.7%) | 82 (29.3%) | 83 (31.9%) | 64 (30.5%) |
| > 0, = 5.43 (g/day) | 122 (34.1%) | 99 (35.4%) | 95 (36.5%) | 78 (37.1%) |
| None | 126 (35.2%) | 99 (35.4%) | 82 (31.5%) | 68 (32.4%) |
| Level of physical activity, No. (%) | ||||
| High | 116 (32.4%) | 91 (32.5%) | 86 (33.1%) | 68 (32.4%) |
| Medium | 123 (34.4%) | 103 (36.8%) | 92 (35.4%) | 81 (38.6%) |
| Low | 119 (33.2%) | 86 (30.7%) | 82 (31.5%) | 61 (29.1%) |
| Hysterectomy, No. (%) | 106 (29.6%) | 82 (29.3%) | 79 (30.4%) | 65 (31.0%) |
| Prior use of hormone therapy, No. (%) | 203 (56.7%) | 157 (56.1%) | 158 (60.8%) | 127 (60.5%) |
| Adherence to treatment assignment, No. (%) | 323 (90.2%) | 265 (94.6%) | 242 (93.1%) | 204 (97.1%) |
Abbreviations: CEE, conjugated equine estrogens; MPA, medroxyprogesterone acetate; MP, micronized progesterone
Increase in serum progestogen levels and increase in mammographic percent density
The post-treatment serum MPA level or progesterone level in each quartile category is summarized in Table 2. Since MPA and progesterone are different compounds, the measurement levels are not directly comparable across treatment arms.
Table 2. Serum levels of medroxyprogesterone acetate (MPA) and progesterone at 12 month follow-up according to treatment and progestogen quartile level.
| CEE + MPA Cyclic | CEE + MPA Continuous | CEE + MP | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Progestogen* Quartile | N | Median level of MPA (range) | N | Median level of MPA (range) | N | Median level of progesterone (range) |
| Q1 | 18 | 101 (48-167) pg/ml | 24 | 84 (43-124) pg/ml | 28 | 80 (51-145) pg/ml |
| Q2 | 18 | 290 (179-418) pg/ml | 24 | 146 (126-175) pg/ml | 27 | 224 (147-419) pg/ml |
| Q3 | 18 | 575 (434-657) pg/ml | 25 | 215 (176-264) pg/ml | 27 | 1306 (462-2156) pg/ml |
| Q4 | 20 | 967 (715-1857) pg/ml | 25 | 345 (269-515) pg/ml | 26 | 3197 (2187-6974) pg/ml |
Abbreviations: CEE, conjugated equine estrogens; MPA, medroxyprogesterone acetate; MP, micronized progesterone
Serum levels of MPA for treatment groups of CEE+MPA cyclic and CEE+MPA continuous. Serum levels of progesterone for treatment group of CEE+MP continuous.
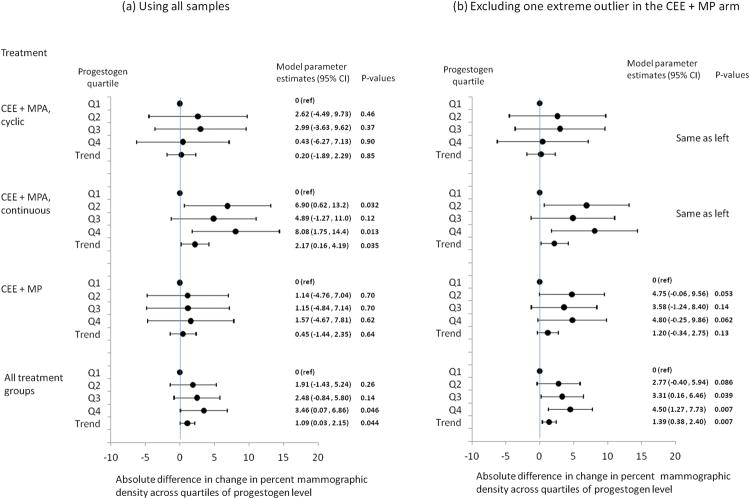
As presented in Figure 1A, the increase in serum level of progestogen was positively associated with change (increase) in mammographic percent density. This trend was most apparent in the CEE+MPA continuous combined treatment group: women with increases in serum MPA level in the highest quartile experienced about 8% greater increase in the mammographic percent density compared to women whose serum MPA levels were in the lowest quartile (P=0.013). In the CEE+MPA cyclic treatment group, this trend was also observed, although the trend was not observed in the fourth quartile. In the CEE+MP treatment group, the difference between progestogen quartiles appeared much smaller than in other treatment groups and the confidence intervals around each estimate were wide. However, when we tested for statistical heterogeneity across the treatment groups in the association between progestogen level increase and mammographic density increase, none of these tests were statistically significant. When all arms were combined, women with increases in serum progestogen in the highest quartile experienced about 3.5% greater increase in the mammographic percent density compared to women with increases in serum progestogen in the lowest quartile (P=0.046), and there was a statistically significant increasing trend per increasing quartile of serum progestogen increase (P=0.044). For all models, the E1S level at 12 months (or E1S change from baseline to 12 months) was statistically significantly associated with change in mammographic density after adjustment for change in progestogen level.
Figure 1.
Association between increase in mammographic percent density and increase in serum level of progestogen from baseline to follow-up (at 12 months) among women randomized to estrogen+progestin therapy (CEE+MPA cyclic, CEE+MPA continuous, or CEE+MP) in the PEPI-MDS. Model parameter estimates and p-values were obtained from linear regression models adjusted for baseline mammographic density, age, BMI, race, smoking, physical activity, alcohol drinking, parity, E1S level at follow-up, and change in BMI. Abbreviations: CEE, conjugated equine estrogens; MPA, medroxyprogesterone acetate; MP, micronized progesterone.
Regression diagnostics identified an extreme outlier and influential data point in the CEE+MP group. While this participant's post-treatment E1 and E1S levels belonged to the highest quartiles, her post-treatment progesterone level belonged to the lowest quartile and did not increase at all over her baseline level. We conducted an additional analysis excluding this participant. As shown in Figure 1B, after excluding this participant, the increase in mammographic density across increase in progesterone levels became stronger in the MP arm (1.20% increase in mammographic density change per increasing quartile of progesterone, 95% CI=-0.34 - 2.75, P for trend = 0.13). When all arms were combined, women whose serum progestogen increase was in the highest quartile experienced 4.50% greater increase (95% CI=1.27 - 7.73, P=0.007) in the mammographic percent density compared to women in the lowest quartile (P for trend=0.007).
PGR polymorphisms and changes in mammographic percent density after EPT treatment
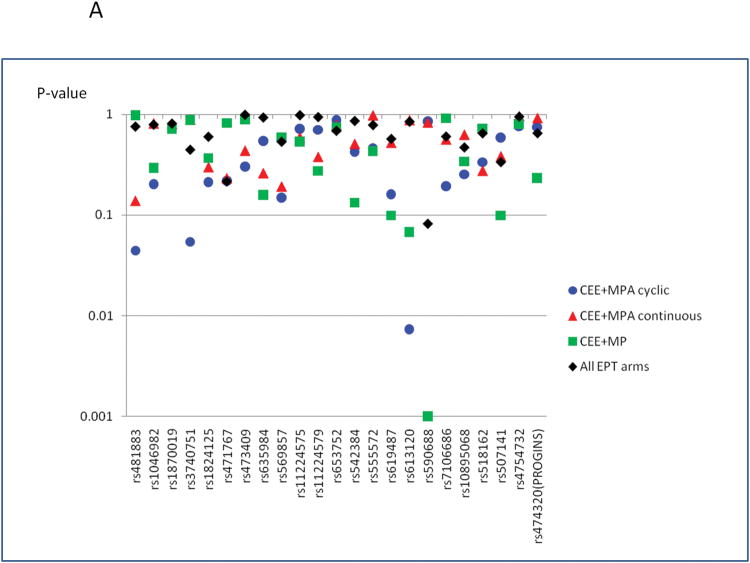
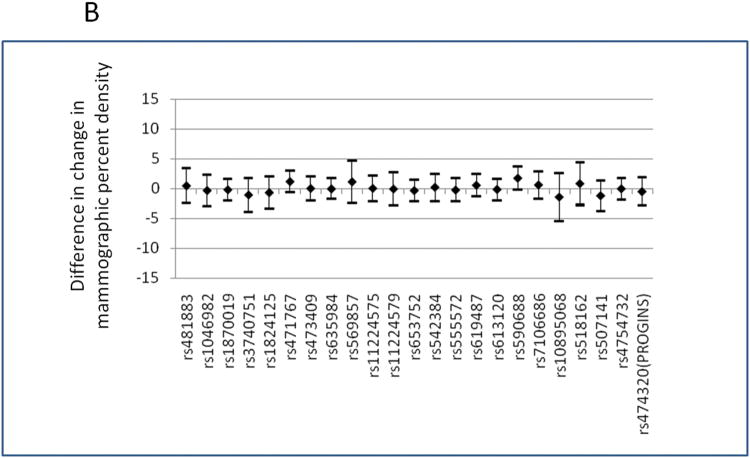
The list of SNPs that were investigated is presented in Supplementary Table 1. PGR genetic variations were not associated with change in mammographic percent density when all progestogen-containing treatment groups were combined (Figure 2 and Supplementary Table 2). There was no significant association between PGR genetic variations and baseline mammographic density either, although this was not the primary aim of this study (data not shown). When the analyses were stratified by treatment group, several SNPs showed nominal associations with change in mammographic percent density: rs481883 and rs613120 in the CEE+MPA cyclic group and rs590688 in the CEE+MP group (Figure 2 (a) and Supplementary Table 2). The statistical test for interaction between treatment arm and these SNPs were nominally significant for rs613120 and rs590688 (P for interaction=0.005, and 0.008, respectively). When we corrected the P values for multiple testing, the association of rs590688 in CEE+MP group remained statistically significant (P for trend=0.001; PACT for trend=0.023), and the change in mammographic percent density in this treatment group was higher among carriers of the minor allele (C allele) of rs590688, by 6.97% per minor allele (C allele). Two potentially functional SNPs, rs10895068 (+331 G/A) and rs474320 (marker of the PROGINS allele), were not associated with mammographic density change following EPT treatment. When we excluded the 8 women whose baseline progesterone levels were higher than 370 pg/ml, the results did not change (data not shown). When we performed a sensitivity analysis excluding the extreme outlier identified from the serum progestogen analyses, the results remained similar, although the magnitude of the associations with rs590688 in the CEE+MP group became slightly less: change in mammographic density was higher by 4.86% per minor allele (P for trend=0.009).
Figure 2.
(A) Summary of the associations between change in mammographic percent density and SNPs in the PGR locus, based on log-additive model. Y axis represent P values, pictured on log-scale. SNPs are presented on X axis as their physical location along the chromosome 11. Models were adjusted for age and BMI. P values presented are before adjusting for multiple testing. When we corrected the P values for multiple testing, the association of rs590688 in CEE+MP group remained statistically significant (P for trend=0.001; PACT for trend=0.023)
(B) Estimated mean differences (regression coefficients) in change in mammographic percent density and 95% confidence intervals per variant allele of each SNP in PGR locus, in women who were randomized to CEE + MPA (either cyclic or continuous) or CEE + MP. The models were adjusted for age and BMI. SNPs are presented on X axis as their physical location along the chromosome 11.
Interaction between serum progestogen level and PGR polymorphisms on changes in mammographic percent density after EPT treatment
Out of the 23 PGR SNPs tested, the P value for interaction testing was statistically significant only for rs473409 (P for interaction =0.024) and rs474320 (marker of the PROGINS allele; P for interaction=0.011) (Supplementary Table 3), unadjusted for multiple testing. For both SNPs, the positive association between serum progestogen level and change in mammographic density was limited to women who carried at least one minor allele. However, the P for interaction was not statistically significant after multiple testing using Bonferroni adjustment. There was no evidence of interaction for other PGR SNPs including a potentially functional SNP rs10895068 (+331 G/A). When we excluded the extreme outlier identified from the progestogen analysis, the interaction results became attenuated or did not change for all SNPs including the above mentioned SNPs.
Discussion
In data from this randomized placebo-controlled clinical trial, we observed that post-treatment increase in serum progesterone or MPA was associated with mammographic density increase among women who were randomized to receive EPT, with higher serum progestogen being associated with higher mammographic density. We did not find strong evidence supporting an association between genetic variations in the PGR locus and change in mammographic density.
Mammographic density is a proxy measure of the amount of stroma and epithelium relative to the amount of fat tissue in the breast (33). Mammographic density is one of the strongest known predictors of breast cancer risk (10, 34) and could affect the accuracy of screening mammography (35). Women with greater than 75% mammographic density have a risk of breast cancer 4-5 times higher than women of the same age with little or no density (<25%) (9).
Increases in mammographic density have been shown to predict a higher risk of breast cancer (36) (37) and, conversely, declines in mammographic density have been related to reductions in breast cancer risk (38). Two longitudinal studies found that an increase in mammographic density over time was associated with increased breast cancer risk in women overall (36) and among women who were not using hormone therapy (37). Conversely, in a nested case-control study within the first International Breast Cancer Intervention Study, tamoxifen-induced reduction of breast cancer risk was limited to women who experienced a substantial decrease in mammographic density (38). Thus these studies with serial mammograms suggest that hormone-induced changes in mammographic density may be associated with parallel changes in breast cancer risk.
HT-associated breast proliferation is higher with EPT than with ET in macaques (39) and in humans (40), implicating the progestin component of HT in breast cell proliferation (Reviewed by Lange 2008 (41)). Results from the PEPI and WHI trials showed that CEE+MPA or CEE + MP use was associated with higher mammographic density increase than ET use (11-14). The PEPI-MDS reported that ∼20% of women in the EPT arms experienced a one step increase in BIRADS grade, which represents 14-18% increase in density (11). Although most women did not experience this enormous change, some increase was observed in a larger number of women (11). Data from the PEPI-MDS further showed that post-treatment changes in serum E1 or E1S level were associated with larger increases in mammographic percent density in the EPT arms (15, 16). These observations raised the question of whether the degree of change in serum progestogen among women taking EPT would predict the amount of increase in mammographic density. The current study newly finds that women with higher serum progestogen levels after treatment experienced higher mammographic density change, independently of increases in levels of serum E1S and E1. Although MPA and MP have substantially different characteristics as a progestogen, the question we have addressed is the relative difference in increase in mammographic density according to the level of progestogen increase following each specific treatment. In other words, in the combined analyses of EPT arms, we are not directly comparing MPA and MP levels, but rather we analyzed the quartile levels of progestogen derived in each treatment arm.
We found no evidence that the promoter polymorphism (+331 G/A, rs10895068), the PROGINS polymorphism (rs474320) nor the tagging SNPs in the PGR locus modified the mammographic density change following EPT. Our observation of no effect of the PROGINS polymorphism agrees with results from the only existing longitudinal epidemiologic study of mammographic density in EPT users based on a Dutch and an English cohort of EPIC (22) and with findings from a large collaborative analysis (17). Similarly, our null result regarding the +331 G/A polymorphism is consistent with cross-sectional data from Nurses' Health Study (NHS) (42). However, the EPIC study unexpectedly suggested that the variant allele (A allelle) of the +331 G/A, shown to increase transcription and translation of PGR (30, 43), was associated with less mammographic density increase after hormone therapy. Difference in the type of HT between our study and the EPIC study may explain the inconsistent findings: type of HT (ET versus EPT) was not distinguished in the EPIC study, and the composition of EPT in the Europe can differ substantially from the EPT used in the PEPI study. Alternatively, it is also possible that the unexpected finding in the EPIC (i.e. to the opposite direction from the expected results based on the functional evidence) was due to chance.
Several PGR SNPs were associated with greater mammographic density change in specific EPT groups in PEPI. For example, the association with rs590688 in the CEE+MP arm was statistically significant after adjusting for multiple testing. Because the affinity of the progesterone receptor for specific progestins may be unequal (44), the effects of genetic variation in the PGR locus on change in mammographic density may differ according to type of progestogen. However, given the small sample size within specific EPT groups, this subgroup-specific association is likely to be due to chance.
That certain PGR genetic polymorphisms (rs473409 and rs474320) modified the association between serum progestogen increase and mammographic density increase should be interpreted cautiously, because the P values for interaction were significant only before correcting for multiple testing. However, it is possible that the serum levels of progestogen achieved following EPT treatment may be an important predictor of change in mammographic density only if women carry variant alleles of these PGR SNPs.
One limitation of this study was that we only had stored serum samples available to us, limiting the amount of cellular material available from which to obtain DNA. As a consequence, we had relatively low genotyping success rates despite meticulous laboratory procedures. Although this resulted in exclusion of considerable proportion of the participants, it is unlikely that this non-differential loss generated a systematic bias. Another limitation is that this trial only used regimens based on CEE with MP or MPA as the progestin. We had enough sample size to detect the effect of PGR SNPs with MAF frequency above 0.10, having 80% power to detect 2.4% (for SNPs with MAF of 0.45) to 3.9% (for SNPs with MAF of 0.10) difference in post-treatment increase in mammographic density per minor allele of PGR SNPs. However, we had limited power for certain SNPs such as rs10895068 with MAF of 0.054, having 80% power to detect 5.2% difference in mammographic density increase per minor allele. Therefore, our observation of no association with PGR SNPs with low MAF, such as +331 G/A (rs10895068), could be due to the small sample size of this study. We had limited power to detect the interaction between PGR SNPs and serum progestogen levels on post-treatment increase in mammographic density: with 80% power to detect 4.7% to 10.4% of difference in mammographic density associated with the interaction effect, depending on the MAF of each SNP. In future studies, it will be important to identify genetic and non-genetic determinants of serum levels of progestogen and increases in mammographic density in women who use EPT.
Conclusions
Our study demonstrated that higher post-treatment increases in serum progestogen levels were associated with greater mammographic density increases among women randomized to receive EPT. However, we did not find a strong indication that genetic variation in the PGR was associated with mammographic density increase, nor that it modified the association of mammographic density increase with change in serum progestogen. Combined with our previous findings on E1 and E1S (15, 16), these results linking increases in serum progestogen level with increase in mammographic density help explain inter-individual responses of mammographic density increases to menopausal hormone therapy. In summary, we find that among women taking EPT, the resulting serum levels of estrogens and progestins are independently related to increases in mammographic density. These results, if confirmed in other studies, would add further support to the current clinical advice: women who need HT for symptoms should use the lowest dose that affords control.
Supplementary Material
Acknowledgments
Funding: The PEPI Mammographic Density Study was funded by the National Institutes of Health, Department of Health and Human Services National Cancer Institute grant number [R01CA77708]. This work was also supported by National Institutes of Health (NIH) [NIH/NIEHS T32 ES013678 to L.C.].
List of Abbreviations
- PEPI-MDS
Postmenopausal Estrogen/Progestin Interventions Mammographic Density Study
- EPT
estrogen and progestin therapy
- ET
estrogen therapy
- E1
estrone
- E1S
estrone sulfate
- PGR
progesterone receptor gene
- WHI
Women's Health Initiative
- CEE
conjugated equine estrogen
- MPA
medroxyprogesterone acetate
- EPIC
European Prospective Investigation into Cancer and Nutrition
- MP
micronized progesterone
- BMI
body mass index
- RIA
radioimmunoassay
Footnotes
Competing Interests: None
References
- 1.Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52, 705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1997;350:1047–59. [PubMed] [Google Scholar]
- 2.Ross RK, Paganini-Hill A, Wan PC, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst. 2000;92:328–32. doi: 10.1093/jnci/92.4.328. [DOI] [PubMed] [Google Scholar]
- 3.Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogenprogestin replacement therapy and breast cancer risk. Jama. 2000;283:485–91. doi: 10.1001/jama.283.4.485. [DOI] [PubMed] [Google Scholar]
- 4.Olsson HL, Ingvar C, Bladstrom A. Hormone replacement therapy containing progestins and given continuously increases breast carcinoma risk in Sweden. Cancer. 2003;97:1387–92. doi: 10.1002/cncr.11205. [DOI] [PubMed] [Google Scholar]
- 5.Weiss LK, Burkman RT, Cushing-Haugen K, et al. Hormone replacement therapy regimens and breast cancer risk. Obstet Gynecol. 2002;100:1148–58. doi: 10.1016/s0029-7844(02)02502-4. [DOI] [PubMed] [Google Scholar]
- 6.Reeves GK, Beral V, Green J, Gathani T, Bull D. Hormonal therapy for menopause and breast-cancer risk by histological type: a cohort study and meta-analysis. The lancet oncology. 2006;7:910–8. doi: 10.1016/S1470-2045(06)70911-1. [DOI] [PubMed] [Google Scholar]
- 7.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Stefanick ML, Anderson GL, Margolis KL, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. Jama. 2006;295:1647–57. doi: 10.1001/jama.295.14.1647. [DOI] [PubMed] [Google Scholar]
- 9.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–69. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 10.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–36. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 11.Greendale GA, Reboussin BA, Sie A, et al. Effects of estrogen and estrogen-progestin on mammographic parenchymal density. Postmenopausal Estrogen/Progestin Interventions (PEPI) Investigators. Ann Intern Med. 1999;130:262–9. doi: 10.7326/0003-4819-130-4_part_1-199902160-00003. [DOI] [PubMed] [Google Scholar]
- 12.Greendale GA, Reboussin BA, Slone S, Wasilauskas C, Pike MC, Ursin G. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst. 2003;95:30–7. doi: 10.1093/jnci/95.1.30. [DOI] [PubMed] [Google Scholar]
- 13.McTiernan A, Chlebowski RT, Martin C, et al. Conjugated Equine Estrogen Influence on Mammographic Density in Postmenopausal Women in a Substudy of the Women's Health Initiative Randomized Trial. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.21.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McTiernan A, Martin CF, Peck JD, et al. Estrogen-plus-progestin use and mammographic density in postmenopausal women: Women's Health Initiative randomized trial. J Natl Cancer Inst. 2005;97:1366–76. doi: 10.1093/jnci/dji279. [DOI] [PubMed] [Google Scholar]
- 15.Crandall CJ, Guan M, Laughlin GA, et al. Increases in serum estrone sulfate level are associated with increased mammographic density during menopausal hormone therapy. Cancer Epidemiol Biomarkers Prev. 2008;17:1674–81. doi: 10.1158/1055-9965.EPI-07-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ursin G, Palla SL, Reboussin BA, et al. Post-treatment change in serum estrone predicts mammographic percent density changes in women who received combination estrogen and progestin in the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. J Clin Oncol. 2004;22:2842–8. doi: 10.1200/JCO.2004.03.120. [DOI] [PubMed] [Google Scholar]
- 17.Gaudet MM, Milne RL, Cox A, et al. Five polymorphisms and breast cancer risk: results from the Breast Cancer Association Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18:1610–6. doi: 10.1158/1055-9965.EPI-08-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feigelson HS, Rodriguez C, Jacobs EJ, Diver WR, Thun MJ, Calle EE. No association between the progesterone receptor gene +331G/A polymorphism and breast cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1084–5. [PubMed] [Google Scholar]
- 19.Johnatty SE, Spurdle AB, Beesley J, et al. Progesterone receptor polymorphisms and risk of breast cancer: results from two Australian breast cancer studies. Breast Cancer Res Treat. 2008;109:91–9. doi: 10.1007/s10549-007-9627-3. [DOI] [PubMed] [Google Scholar]
- 20.Pearce CL, Hirschhorn JN, Wu AH, et al. Clarifying the PROGINS allele association in ovarian and breast cancer risk: a haplotype-based analysis. J Natl Cancer Inst. 2005;97:51–9. doi: 10.1093/jnci/dji007. [DOI] [PubMed] [Google Scholar]
- 21.Pooley KA, Healey CS, Smith PL, et al. Association of the progesterone receptor gene with breast cancer risk: a single-nucleotide polymorphism tagging approach. Cancer Epidemiol Biomarkers Prev. 2006;15:675–82. doi: 10.1158/1055-9965.EPI-05-0679. [DOI] [PubMed] [Google Scholar]
- 22.van Duijnhoven FJ, Peeters PH, Warren RM, et al. Influence of estrogen receptor alpha and progesterone receptor polymorphisms on the effects of hormone therapy on mammographic density. Cancer Epidemiol Biomarkers Prev. 2006;15:462–7. doi: 10.1158/1055-9965.EPI-05-0754. [DOI] [PubMed] [Google Scholar]
- 23.Ursin G, Astrahan MA, Salane M, et al. The detection of changes in mammographic densities. Cancer Epidemiol Biomarkers Prev. 1998;7:43–7. [PubMed] [Google Scholar]
- 24.Anderson DC, Hopper BR, Lasley BL, Yen SS. A simple method for the assay of eight steroids in small volumes of plasma. Steroids. 1976;28:179–96. doi: 10.1016/0039-128x(76)90108-2. [DOI] [PubMed] [Google Scholar]
- 25.Slater CC, Hodis HN, Mack WJ, Shoupe D, Paulson RJ, Stanczyk FZ. Markedly elevated levels of estrone sulfate after long-term oral, but not transdermal, administration of estradiol in postmenopausal women. Menopause. 2001;8:200–3. doi: 10.1097/00042192-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Hiroi M, Stanczyk FZ, Goebelsmann U, Brenner PF, Lumkin ME, Mishell DR., Jr Radioimmunoassay of serum medroxyprogesterone acetate (Provera) in women following oral and intravaginal administration. Steroids. 1975;26:373–86. doi: 10.1016/0039-128x(75)90082-3. [DOI] [PubMed] [Google Scholar]
- 27.Scott JZ, Stanczyk FZ, Goebelsmann U, Mishell DR., Jr A double-antibody radioimmunoassay for serum progesterone using progesterone-3-(O-carboxymethyl) oximino-[125I]-iodo-histamine as radioligand. Steroids. 1978;31:393–405. doi: 10.1016/0039-128x(78)90052-1. [DOI] [PubMed] [Google Scholar]
- 28.Edlund CK, Lee WH, Li D, Van Den Berg DJ, Conti DV. Snagger: a user-friendly program for incorporating additional information for tagSNP selection. BMC Bioinformatics. 2008;9:174. doi: 10.1186/1471-2105-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romano A, Delvoux B, Fischer DC, Groothuis P. The PROGINS polymorphism of the human progesterone receptor diminishes the response to progesterone. J Mol Endocrinol. 2007;38:331–50. doi: 10.1677/jme.1.02170. [DOI] [PubMed] [Google Scholar]
- 30.De Vivo I, Huggins GS, Hankinson SE, et al. A functional polymorphism in the promoter of the progesterone receptor gene associated with endometrial cancer risk. Proc Natl Acad Sci U S A. 2002;99:12263–8. doi: 10.1073/pnas.192172299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Abecasis GR. Mach 1.0: Rapid Haplotype Reconstruction and Missing Genotype Inference. Am J Hum Genet. 2006;S79:2290. [Google Scholar]
- 32.Conneely KN, Boehnke M. So Many Correlated Tests, So Little Time! Rapid Adjustment of P Values for Multiple Correlated Tests. Am J Hum Genet. 2007;81 doi: 10.1086/522036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo YP, Martin LJ, Hanna W, et al. Growth factors and stromal matrix proteins associated with mammographic densities. Cancer Epidemiol Biomarkers Prev. 2001;10:243–8. [PubMed] [Google Scholar]
- 34.Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37:2486–92. doi: 10.1002/1097-0142(197605)37:5<2486::aid-cncr2820370542>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138:168–75. doi: 10.7326/0003-4819-138-3-200302040-00008. [DOI] [PubMed] [Google Scholar]
- 36.van Gils CH, Hendriks JH, Holland R, et al. Changes in mammographic breast density and concomitant changes in breast cancer risk. Eur J Cancer Prev. 1999;8:509–15. doi: 10.1097/00008469-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Kerlikowske K, Ichikawa L, Miglioretti DL, et al. Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J Natl Cancer Inst. 2007;99:386–95. doi: 10.1093/jnci/djk066. [DOI] [PubMed] [Google Scholar]
- 38.Cuzick J, Warwick J, Pinney E, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103:744–52. doi: 10.1093/jnci/djr079. [DOI] [PubMed] [Google Scholar]
- 39.Cline JM, Soderqvist G, von Schoultz E, Skoog L, von Schoultz B. Effects of conjugated estrogens, medroxyprogesterone acetate, and tamoxifen on the mammary glands of macaques. Breast Cancer Res Treat. 1998;48:221–9. doi: 10.1023/a:1005984932268. [DOI] [PubMed] [Google Scholar]
- 40.Hofseth LJ, Raafat AM, Osuch JR, Pathak DR, Slomski CA, Haslam SZ. Hormone replacement therapy with estrogen or estrogen plus medroxyprogesterone acetate is associated with increased epithelial proliferation in the normal postmenopausal breast. J Clin Endocrinol Metab. 1999;84:4559–65. doi: 10.1210/jcem.84.12.6194. [DOI] [PubMed] [Google Scholar]
- 41.Lange CA. Challenges to defining a role for progesterone in breast cancer. Steroids. 2008;73:914–21. doi: 10.1016/j.steroids.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kotsopoulos J, Tworoger SS, De Vivo I, et al. +331G/A variant in the progesterone receptor gene, postmenopausal hormone use and risk of breast cancer. Int J Cancer. 2009;125:1685–91. doi: 10.1002/ijc.24477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Vivo I, Hankinson SE, Colditz GA, Hunter DJ. A functional polymorphism in the progesterone receptor gene is associated with an increase in breast cancer risk. Cancer Res. 2003;63:5236–8. [PubMed] [Google Scholar]
- 44.Skouby SO, Jespersen J. Progestins in HRT: sufferance or desire? Maturitas. 2009;62:371–5. doi: 10.1016/j.maturitas.2008.12.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.