Abstract
Reactivating the tumor suppressor p53 offers an attractive strategy for developing cancer therapy. We recently identified Inauhzin (INZ) as a novel non-genotoxic p53-activating compound. To develop INZ into a clinically applicable anticancer drug, we have initiated preclinical toxicity studies. Here, we report our study on determining the maximum tolerated dose (MTD) of INZ analog, Inauhzin-C (INZ (C)), following intraperitoneal (i.p.) administration (Phase A) and its toxicity following i.p. administration over a period of 5-day dosing plus 2-day recovery (Phase B) in CD-1 mice. The Phase A study showed that the MTD of INZ (C) is 200 mg/kg for female and 250 mg/kg for male, respectively. The Phase B study showed that the administration of INZ (C) via 5-day consecutive i.p. injection is tolerated by female CD-1 mice at all dose levels tested from 50 mg/kg to 120 mg/kg without significant changes in biochemical and pathological parameters in the animals. Together, these results indicate that our previously determined effective dose of INZ at 30–60 mg/kg via i.p. is quite safe to mice, and imply that this compound have the features worthy for further development into a clinically applicable drug.
Keywords: Inauhzin, Mouse, Toxicity study, Dose range finding, Maximum tolerated dose
1. Introduction
Over 10 million cases of cancer are diagnosed worldwide each year, leading to ∼6 million deaths. Despite numerous scientific breakthroughs and tremendous R&D investments in the pharmaceutical sector over the past 3 decades, cancer is still the leading cause of disease-related mortality and awaits further advances toward improved therapy [9]. Intensive effort has been focused on the tumor suppressor p53 pathway because nearly all cancers show defects in this pathway, >50% of which have mutations in the TP53 gene itself. Aberrations in p53 or its regulators are linked with increased resistance to current chemotherapy and hence poor prognosis [10], [11]. Thus, reactivation of the p53 pathway in cancer has served as one prime strategy for the development of novel cancer therapies. In an attempt to take this strategy, we discovered Inauhzin (INZ) as a novel non-genotoxic p53 activator [1]. Our previous studies revealed that INZ activates p53 by targeting SIRT1 in cancer cells and consequently suppresses tumor growth [1]. SIRT1, a NAD-dependent deacetylase, deacetylates p53 and facilitates its MDM2/MDMX-mediated degradation. Remarkably, INZ has no apparent detrimental effect on normal cells (data not shown). Also, INZ can sensitize the anti-cancer effect of cisplatin, doxorubicin, or Nutlin-3 (a MDM2 inhibitor) as tested in xenograft cancer models [2], [3]. Thus, this small molecule presents as a promising contender for a molecule-targeted anti-cancer therapy. We have been undertaking systematic chemical optimization of INZ by the analysis of structure–activity relationship (SAR) [4], and new target identification and validation [5]. We found that INZ indeed induced the expression of p53-dependent transcriptome [6], and surprisingly, we recently found that INZ can activate p53 by also targeting IMPDH2 [7], which is often highly expressed in human cancers. These studies demonstrate that INZ possesses an ability to target two oncoproteins, SIRT1 [1] and IMPDH2 [7]. This unique ability of INZ would offer an example for paradigm shifting from classical chemotherapy or a singular molecule targeted drug to dual or multi molecules-targeted therapy for human cancers.
In order to eventually develop INZ or its derivative into a clinically applicable form, we need to assess its drugability and toxicity in animals. Thus, this study as reported here was designed to determine the maximum tolerated dose (MTD) of INZ analog, INZ (C) (Compound 8 [4]), following intraperitoneal (i.p.) administration of ascending and/or descending doses (Phase A) and to evaluate its toxicity following i.p. administration over a period of 5-day dosing plus 2-day recovery (Phase B) in CD-1 mice. The results from these phase studies demonstrate that INZ (C) is quite safe within 100 mg/kg via i.p. to mice as tested, and suggest that this INZ derivative could serve as a lead compound for further preclinical analyses and development.
2. Materials and methods
2.1. Animals
Six to eight-week-old CD-1 mice were purchased from Shanghai SLAC Laboratory Animal Co. Ltd. Animals were randomly assigned to dose groups based body weight and/or sex using Microsoft Excel program. Prior to assignment to groups, the weight variation of the animals of each sex used did not exceed 20% of the mean weight. After assignment to groups the mean body weight for each group of each sex (both sexes for Phase A and single sex for Phase B) were not statistically different at the 5% probability level. This strain of mice has been historically used in safety evaluation studies and is recommended by appropriate regulatory agencies. In addition, mice were commonly used in the efficacy studies of cancer drugs.
Animals were group housed in solid bottom polycarbonate cages (3–5 animals/cage) and provided with pelleted food that is pre-irradiated via Co60 by the Supplier. The food was provided ad libitum unless otherwise specified. Animals were also provided with sterilized pure water ad libitum. No known contaminants were present in the diet or water at levels that might interfere with this study. Environmental controls for the animal room were set to maintain 20–25 °C, a relative humidity of 50–70%, a minimum of 10 air changes/h, and a 12-h light/12-h dark cycle. The light/dark cycle might be interrupted for study-related activities.
The numbers of animals, study design, and treatment of animals were reviewed, and approved by the ChemPartner Institutional Animal Care and Use Committee (IACUC). All procedures in this protocol are in compliance with the U.S. Department of Agriculture's (USDA) Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, the Office of Laboratory Animal Welfare and Laboratory Animal Administration ordinance issued by State Science and Technology Committee of the Peoples Republic of China (PR China), Act 2 and Laboratory Animal Administration regulations issued by Shanghai Laboratory Animal Administration Office, PR China.
The medical treatment necessary to prevent unacceptable pain and suffering, including euthanasia, is the responsibility of the attending Laboratory Animal Veterinarian. Discretionary medical treatment has been carried out based upon consensus agreement between the Study Director and the attending Laboratory Animal Veterinarian.
In the event of severe toxicity, in which decisions were to be made regarding treatment or euthanasia of study animal(s), the ChemPartner Veterinarian and the Study Director reserve the right for subsequent action.
2.2. Test and control articles
2.2.1. Test article
The test article, INZ (C), was synthesized in our lab. The purity of the article is greater than 99%. The synthesis route was described in [4].
2.2.2. Control article
The control article was composed of 5% DMAC + 7% Solutol HS15 + 88% Saline. The information of each component of the control article was described below:
| Control article | Manufacturer | Lot/batch no. |
|---|---|---|
| DMAC | Sigma | BCBK6482V |
| Solutol HS | BASF | 21466288KO |
| Saline | Zhejiang Tianrui | 113011102 |
2.3. Study design
12 naïve male and 12 naïve female CD-1 mice (for Phase A) and 24 naïve female CD-1 mice (for Phase B) were randomly assigned to the study. The animals received dose formulation containing INZ (C) and the control article (5% DMAC + 7% Solutol HS15 + 88% Saline) at various dosages via i.p. injection, q.d dosing for s single dose at each dose level (Phase A) and i.p. injection, q.d dosing for 5 consecutive days (Phase B). The location of the i.p. injection was lower left abdominal quadrant. The MTD in this study is defined as the highest dose that will be tolerated and not produce major life threatening toxicity for the study duration [12]. The starting dose level (Group 1) for DRF study has been selected based upon the findings of an efficacy study of the compound in SCID mice [1]. Detailed group design was listed in the following table:
Group designation and dose level.
| Phase A: dose range finding (DRF) study (escalating dose design) | ||||
|---|---|---|---|---|
| Group | No. of animals |
Dose level (mg/kg/day) | Dose concentration (mg/mL) | |
| Male | Female | |||
| Group 1 | 3 | 3 | 50 | 5 |
| Group 2 | 3 | 3 | 150a | 5 |
| Group 3 | 3 | 3 | 250a | 5 |
| Group 4 | 3 | 3 | 200a | 5 |
Dose level was determined based on the result after the previous dosing.
| Phase B: 5-day maximum tolerated dose study | |||
|---|---|---|---|
| Group | No. of animals | Dose level (mg/kg/day) | Dose concentration (mg/mL) |
| Female | |||
| 1 (control)a | 6 | 0 | 0 |
| 2 (low) | 6 | 30b | 1.25 |
| 3 (mid) | 6 | 60b | 2.5 |
| 4 (high) | 6 | 120b | 5 |
Group 1 received control article only.
Dose level was determined based on the result of Phase A.
The following parameters and end points were evaluated in this study: mortality, clinical signs, body weights, food consumptions, hematology and serum biochemistry parameters, post mortem evaluation included gross examination for all the animals at the terminal necropsy and histopathological examination of major organs in the control and high dose groups.
Hematology and clinical chemistry were tested at termination on Day 7 for each dose level. Animals were fasted for ∼4 h for scheduled blood collections.
The necropsy included an examination of the external features of the carcass; external body orifices; the abdominal, thoracic, and cranial cavities; organs/tissues defined in Suppl. Table 5 from 3 surviving animals were examined for morphological evaluations.
2.4. Termination
2.4.1. Unscheduled sacrifices and deaths
Animals exhibiting signs of acute pain or distress, which were unable to eat, walk, groom normally, or moribund, or have other signs of severe systemic toxicity were euthanized immediately.
Animals eventually found dead were refrigerated, if necessary, and necropsied at the earliest possible time. Animals sacrificed at an unscheduled interval were weighed, anesthetized and necropsied, and discarded after gross observations of tissues as specified in Suppl Table 5.
2.4.2. Scheduled sacrifice
2.4.2.1. Terminal sacrifice
Phase A: on Day 10 all surviving animals were euthanized and disposed of without necropsy.
Phase B: on Day 8, all surviving animals were fasted ∼4 h before termination, and then anesthetized with Phenobarbital, exsanguinated, and necropsied.
2.4.2.2. Postmortem procedures
Phase A: none.
Phase B: the necropsy includes an examination of the external features of the carcass; external body orifices; the abdominal, thoracic, and cranial cavities; organs/tissues defined in Table 6 from 3 surviving animals were subjected to morphological evaluations.
2.5. Statistical evaluation
No statistical evaluation was done for data collected from Phase A study. One-way analysis of variance (ANOVA) was used (if applicable) to analyze continuous clinical pathology values and body weight data (Phase B only). If the ANOVA were significant, Dunnett's t-test would be used for pairwise comparisons between treated and control groups. Group comparisons (Groups 2–4 versus Group 1) were evaluated at the 5.0%, two-tailed probability level.
3. Results
3.1. Phase A
In order to further develop INZ into a clinically applicable form, we started to perform a series of preclinical animal studies. Because our recent structure–activity relationship (SAR) study revealed that INZ (C) (Compound 8 [4]) is more effective than INZ in p53 activation [4] and better pharmacokinetics profile (data not shown), we decided to use this INZ analog as our lead compound for Phases A and B studies here. First, we determined the lethal dose or maximum tolerated dose of this compound by employing CD-1 mice. As shown in Table 1, mortality was observed in females (1 was found dead on Day 2 and 2 were found dead on Day 3) at 250 mg/kg only during this study phase (Suppl. Tables 1-1 and 1-2). Abnormal clinical signs, such as piloerection, decreased motor activity, discharge around eye(s), cold to touch, etc. were observed at 150 mg/kg and above for both sexes (Suppl. Tables 2-1 and 2-2). Also, a trend of increasing in mean body weight was observed at 50 mg/kg for both sexes, while a dose-dependent trend of mean body weight loss was observed at 150, 200 and 250 mg/kg for both sexes by comparing the mean body weight on Day 1 and 4 for all the 4 dose levels (data not shown).
Table 1.
Individual daily food consumption, female, Phase B.
| Group no. | Food weight (g) |
Total food consumption (g) | Mean individual food consumption (g) | |
|---|---|---|---|---|
| Day 1 | Day 5 | Day 1–Day 5 | ||
| Control 0 mg/kg |
201.1 | 141.53 | 59.57 | 1.99 |
| Low/♀ 30 mg/kg |
205.55 | 149.94 | 55.61 | 1.85 |
| Mid/♀ 60 mg/kg |
204.21 | 141 | 63.21 | 2.11 |
| High/♀ 120 mg/kg |
202.06 | 143.93 | 58.13 | 1.94 |
Unscheduled necropsy was conducted immediately after the animals were found dead (Suppl. Tables 3-1 and 3-2). Decreased size of spleen and liquid with large volume in the thoracic cavity was noticed. Other observations such as duodenum autolysis, liver discoloration were considered as degeneration of animal body post mortem; white substance in the abdominal cavity was considered as drug residue precipitated from the dose formulation after dosing. No evidence of dose accident was noticed.
Furthermore, scheduled necropsy was conducted in all the surviving animals at termination of each dose. White substance was observed on the surface of liver, spleen and diaphragm in the surviving animals from groups of 200 mg/kg and above, which was considered as drug residue precipitated from the dose formulation after dosing.
Taken together, the results obtained from the Phase A study show that the maximum tolerated dose of INZ (C) is 200 mg/kg for female and >250 mg/kg for male, respectively, based on the mortality and the clinical signs. Therefore, female mice are more sensitive than male mice to INZ (C) under the current study condition and were used in Phase B study as described below.
3.2. Phase B
Next, we used female mice for Phase B study here with different doses of INZ (C) from 30 mg/kg to 120 mg/kg per day via i.p. for five days. No mortality was observed to all the tested animals at all dose levels during the study phase. No abnormal clinical observations were noticed as well (Suppl. Tables 1-1 and 1-2 and data not shown). In addition, we have performed the following examinations and analyses, and found no apparent toxic effects of this compound on the animals as follows.
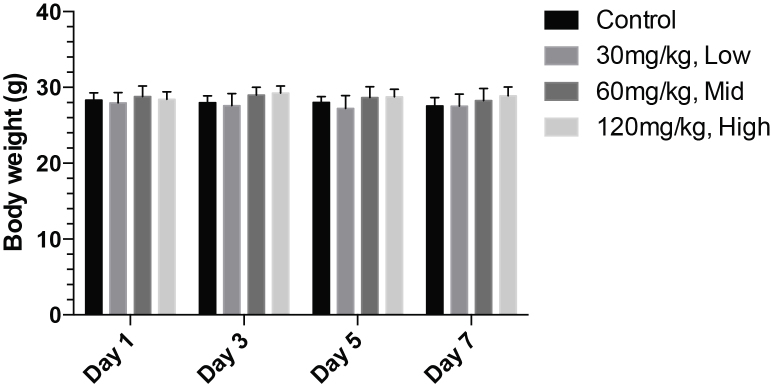
First, we found that the mean body weight of all the 3 treatment groups are comparable with the control group and fluctuated from day to day without clear trend of increasing or decreasing (Fig. 1). No statistic difference in body weight and body weight gain among the groups was detected by One-way ANOVA. Therefore, no treatment-related effects on body weight could be detected.
Fig. 1.
Mean body weight–time profile of INZ-C following by 5-day consecutive intraperitoneal injection at 30, 60 and 120 mg/kg and a 2-day recovery phase in female CD-1 mice (N = 6).
Also, the mean individual food consumption of all the 3 treatment groups was comparable with the control group and appeared not to be dose-dependent. Therefore, no treatment-related effects on mean individual food consumption could be detected either (Table 1).
Grossly examining the organs of the animals revealed some changes. For instance, although discoloration/enlargement of spleen and thin duodenum wall were observed sporadically in low and mid dose groups (Suppl. Table 4), in the high dose group, edge of liver lobes-blunt (6/6), spleen enlargement (4/6), adhesion (6/6) of organs (liver, spleen, stomach, kidney) with each other were extensively observed. Also white substance was observed extensively in the abdominal cavity in the high dose group, but not in low and mid dose groups, which was considered as drug residue. Therefore, the abnormal gross examination findings in the high dose group were considered non-systemic and correlated with the presentation of the drug residue, which probably was caused by the irritation of the INZ (C) formulations with the multiple i.p. injection.
Hematological analysis was also conducted. Dose-dependent increasing in absolute/relative neutrophil (1.2–1.6 (absolute) folds and 1.2–1.7 (relative) folds versus control group) and basophil (1.5–2.5 (absolute) folds and 1.0–1.9 (relative) folds versus control group) was noticed in treatment groups compared with the control group (Table 2). Also trend of increasing of relative reticulocyte (1.1–1.3 folds versus control group) was observed, which was considered as isolated incidence due to lack of dose-dependency and correlation with the changes of other erythrocytic parameters.
Table 2.
Hematology, female, Phase B.
| Group no. | Animal no. | WBC (×109/L) | RBC (×1012/L) | HGB (g/L) | HCT (%) | MCV (fL) | MCH (pg) | MCHC (g/L) | PLT (×109/L) | RET (%) | RET (×109 cells/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control/♀ | 21001 | 4.2 | 10.19 | 147 | 49 | 48.1 | 14.4 | 300 | 1094 | 5.32 | 541.9 |
| 0 mg/kg | 21002 | 3.56 | 10.2 | 149 | 49.5 | 48.6 | 14.6 | 300 | 797 | 5.55 | 565.8 |
| 21003 | 3.18 | 10.03 | 146 | 48.2 | 48 | 14.6 | 304 | 742 | 6.27 | 629.4 | |
| Mean | 3.65 | 10.14 | 147 | 48.9 | 48.2 | 14.5 | 301 | 878 | 5.71 | 579 | |
| SD | 0.52 | 0.1 | 2 | 0.7 | 0.3 | 0.1 | 2 | 189 | 0.5 | 45.2 | |
| Change (fold) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Inauhzin-C | 22001 | 4.01 | 8.96 | 136 | 43.9 | 49 | 15.2 | 310 | 1139 | 6.02 | 539.1 |
| Low/♀ | 22002 | 4.23 | 9.92 | 143 | 47.8 | 48.2 | 14.5 | 300 | 955 | 5.07 | 503.2 |
| 30 mg/kg | 22003 | 3.65 | 9.07 | 132 | 43.7 | 48.2 | 14.5 | 301 | 675 | 7.34 | 665.8 |
| Mean | 3.96 | 9.32 | 137 | 45.1 | 48.5 | 14.7 | 304 | 923 | 6.14 | 569.4 | |
| SD | 0.29 | 0.53 | 6 | 2.3 | 0.5 | 0.4 | 6 | 234 | 1.14 | 85.4 | |
| Change (fold) | 1.1 | 0.9 | 0.9 | 0.9 | 1 | 1 | 1 | 1.1 | 1.1 | 1 | |
| Inauhzin-C | 23001 | 3.19 | 9.35 | 141 | 46.7 | 50 | 15.1 | 302 | 665 | 6.11 | 570.9 |
| Mid/♀ | 23002 | 5.23 | 8.97 | 140 | 46.8 | 52.2 | 15.6 | 300 | 1182 | 10.08 | 904.3 |
| 60 mg/kg | 23003 | 3.26 | 8.97 | 134 | 44.7 | 49.8 | 15 | 301 | 1235 | 6.92 | 621.4 |
| Mean | 3.89 | 9.1 | 138 | 46.1 | 50.7 | 15.2 | 301 | 1027 | 7.7 | 698.9 | |
| SD | 1.16 | 0.22 | 4 | 1.2 | 1.3 | 0.3 | 1 | 315 | 2.1 | 179.7 | |
| Change (fold) | 1.1 | 0.9 | 0.9 | 0.9 | 1.1 | 1 | 1 | 1.2 | 1.3 | 1.2 | |
| Inauhzin-C | 24001 | 2.15 | 8.84 | 133 | 42.9 | 48.5 | 15 | 309 | 776 | 6.57 | 580.8 |
| High/♀ | 24002 | 6.28 | 8.69 | 131 | 43.5 | 50 | 15.1 | 302 | 1164 | 7.41 | 649.5 |
| 120 mg/kg | 24003 | 2.32 | 9.61 | 146 | 48.6 | 50.5 | 15.2 | 302 | 1062 | 7.54 | 724.1 |
| Mean | 3.58 | 9.05 | 137 | 45 | 49.7 | 15.1 | 304 | 1001 | 7.17 | 651.5 | |
| SD | 2.34 | 0.49 | 8 | 3.1 | 1 | 0.1 | 4 | 201 | 0.53 | 71.7 | |
| Change (fold) | 1 | 0.9 | 0.9 | 0.9 | 1 | 1 | 1 | 1.1 | 1.3 | 1.1 | |
| Group no. | Animal no. | Abs_neuts (×109 cells/L) | %NEUT (%) | Abs_lymphs (×109 cells/L) | %LYM (%) | Abs_monos (×109 cells/L) | %MONO (%) | Abs_eos (×109 cells/L) | %EOS (%) | Abs_basos (×109 cells/L) | %BASO (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control/♀ | 21001 | 1.01 | 24.2 | 2.93 | 69.9 | 0.13 | 3.1 | 0.08 | 1.9 | 0.01 | 0.2 |
| 0 mg/kg | 21002 | 1.12 | 31.4 | 2.27 | 63.8 | 0.07 | 1.9 | 0.07 | 2 | 0.01 | 0.4 |
| 21003 | 0.86 | 26.9 | 2.06 | 64.9 | 0.07 | 2.1 | 0.17 | 5.3 | 0 | 0.1 | |
| Mean | 1 | 27.5 | 2.42 | 66.2 | 0.09 | 2.37 | 0.11 | 3.07 | 0.01 | 0.23 | |
| SD | 0.13 | 3.6 | 0.45 | 3.3 | 0.03 | 0.64 | 0.06 | 1.93 | 0.01 | 0.15 | |
| Change (fold) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Inauhzin-C | 22001 | 1.19 | 29.6 | 2.6 | 64.9 | 0.12 | 2.9 | 0.07 | 1.8 | 0.01 | 0.1 |
| Low/♀ | 22002 | 1.06 | 25 | 2.85 | 67.3 | 0.18 | 4.1 | 0.12 | 2.7 | 0.01 | 0.2 |
| 30 mg/kg | 22003 | 1.48 | 40.5 | 1.86 | 51 | 0.14 | 3.9 | 0.13 | 3.5 | 0.01 | 0.4 |
| Mean | 1.24 | 31.7 | 2.44 | 61.1 | 0.15 | 3.63 | 0.11 | 2.67 | 0.01 | 0.23 | |
| SD | 0.22 | 8 | 0.51 | 8.8 | 0.03 | 0.64 | 0.03 | 0.85 | 0 | 0.15 | |
| Change (fold) | 1.2 | 1.2 | 1 | 0.9 | 1.6 | 1.5 | 1 | 0.9 | 1.5 | 1 | |
| Inauhzin-C | 23001 | 1.01 | 31.6 | 1.99 | 62.4 | 0.09 | 2.7 | 0.06 | 1.9 | 0.01 | 0.3 |
| Mid/♀ | 23002 | 2.36 | 45.2 | 2.51 | 48 | 0.17 | 3.3 | 0.1 | 1.9 | 0.02 | 0.4 |
| 60 mg/kg | 23003 | 1.15 | 35.3 | 1.87 | 57.4 | 0.13 | 4.1 | 0.09 | 2.6 | 0.01 | 0.3 |
| Mean | 1.51 | 37.4 | 2.12 | 55.9 | 0.13 | 3.37 | 0.08 | 2.13 | 0.01 | 0.33 | |
| SD | 0.74 | 7 | 0.34 | 7.3 | 0.04 | 0.7 | 0.02 | 0.4 | 0.01 | 0.06 | |
| Change (fold) | 1.5 | 1.4 | 0.9 | 0.8 | 1.4 | 1.4 | 0.8 | 0.7 | 2 | 1.4 | |
| Inauhzin-C | 24001 | 1.15 | 53.4 | 0.81 | 37.7 | 0.05 | 2.1 | 0.11 | 5.2 | 0.01 | 0.4 |
| High/♀ | 24002 | 2.51 | 39.9 | 3.55 | 56.5 | 0.06 | 1 | 0.08 | 1.2 | 0.03 | 0.4 |
| 120 mg/kg | 24003 | 1.04 | 44.7 | 1.15 | 49.3 | 0.04 | 1.5 | 0.06 | 2.7 | 0.01 | 0.5 |
| Mean | 1.57 | 46 | 1.84 | 47.8 | 0.05 | 1.53 | 0.08 | 3.03 | 0.02 | 0.43 | |
| SD | 0.82 | 6.8 | 1.49 | 9.5 | 0.01 | 0.55 | 0.03 | 2.02 | 0.01 | 0.06 | |
| Change (fold) | 1.6 | 1.7 | 0.8 | 0.7 | 0.6 | 0.6 | 0.8 | 1 | 2.5 | 1.9 | |
Biochemical analysis of animal blood samples showed the increase in aspartate aminotransferase (AST) level in 120 mg/kg group only. However, it could not be concluded as dose-related due to absence of dose-dependency in all other dose levels (Table 3). The elevation of alanine transarninase (ALT) in 120 mg/kg group was due to an abnormal ALT level in a single animal (24004). Therefore, it was considered as an isolated incidence. DBIL and ALP were reduced in a dose dependent and statistically significant manner (P < 0.05 and P < 0.01, respectively), suggesting that INZ might cause liver toxicity that is associated with the elevation of DBIL and ALP levels.
Table 3.
Serum chemistry, female, Phase B.
| Group no. | Animal no. | ALT (U/L) | AST (U/L) | TBIL (μmol/L) | DBILa (μmol/L) | ALP (U/L)a | Urea (mmol/L) | CREA (μmol/L) |
|---|---|---|---|---|---|---|---|---|
| Control/♀ | 21004 | 35.1 | 81.8 | 1.9 | 1.1 | 169 | 5.2 | 12.5 |
| 0 mg/kg | 21005 | 35.5 | 108 | 1.3 | 0.7 | 141.9 | 5.9 | 12.6 |
| 21006 | 35.2 | 78.3 | 1.9 | 1.2 | 172.6 | 5.9 | 13.5 | |
| Mean | 35.3 | 89.4 | 1.7 | 1 | 161.2 | 5.7 | 12.9 | |
| SD | 0.2 | 16.2 | 0.3 | 0.3 | 16.8 | 0.4 | 0.6 | |
| Change (fold) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Inauhzin-C | 22004 | 24.9 | 75.9 | 1.2 | 0.8 | 133.9 | 5 | 13.2 |
| Low/♀ | 22005 | 50.1 | 143.1 | 1 | 0.7 | 151.1 | 6 | 13.3 |
| 30 mg/kg | 22006 | 24.1 | 69.5 | 0.9 | 0.6 | 140.1 | 6.9 | 14 |
| Mean | 33 | 96.2 | 1 | 0.7 | 141.7 | 6 | 13.5 | |
| SD | 14.8 | 40.8 | 0.2 | 0.1 | 8.7 | 1 | 0.4 | |
| Change (fold) | 0.9 | 1.1 | 0.6 | 0.7 | 0.9 | 1.1 | 1 | |
| Inauhzin-C | 23004 | 28 | 66.2 | 1.4 | 0.7 | 97.1 | 6.7 | 10.9 |
| Mid/♀ | 23005 | 32.4 | 98.3 | 0.8 | 0.5 | 115.9 | 5.9 | 12.8 |
| 60 mg/kg | 23006 | 24.1 | 67.9 | 0.9 | 0.5 | 153.2 | 6.7 | 16 |
| Mean | 28.2 | 77.5 | 1 | 0.6 | 122.1 | 6.4 | 13.2 | |
| SD | 4.2 | 18.1 | 0.3 | 0.1 | 28.6 | 0.5 | 2.6 | |
| Change (fold) | 0.8 | 0.9 | 0.6 | 0.6 | 0.8 | 1.1 | 1 | |
| Inauhzin-C | 24004 | 101 | 119.8 | 0.9 | 0.5 | 76 | 3.8 | 9.8 |
| High/♀ | 24005 | 37.2 | 110 | 1 | 0.3 | 58.2 | 5 | 14.8 |
| 120 mg/kg | 24006 | 37.1 | 116.7 | 1.3 | 0.6 | 73.4 | 5.1 | 13.5 |
| Mean | 58.4 | 115.5 | 1.1 | 0.5 | 69.2 | 4.6 | 12.7 | |
| SD | 36.9 | 5 | 0.2 | 0.2 | 9.6 | 0.7 | 2.6 | |
| Change (fold) | 1.7 | 1.3 | 0.6 | 0.5 | 0.4 | 0.8 | 1 | |
Dose dependent, and statistically significant P < 0.05.
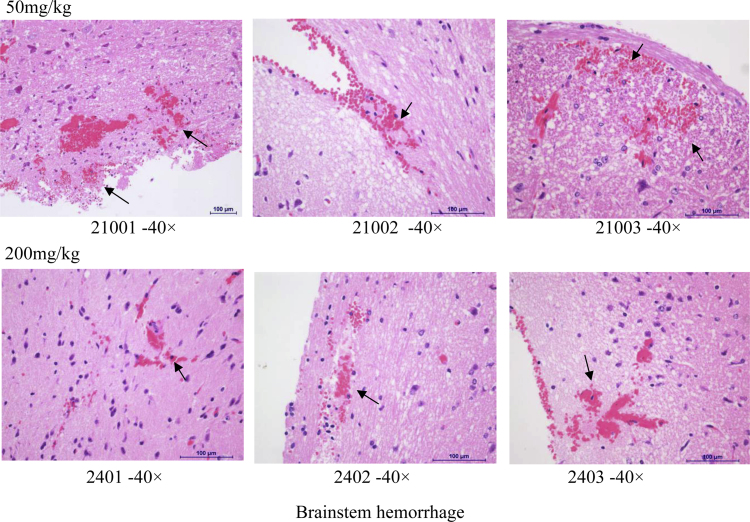
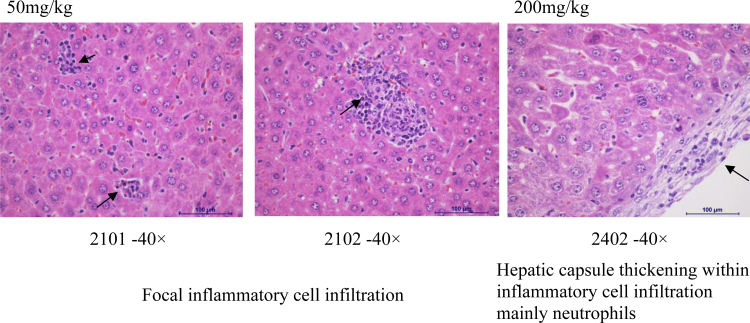
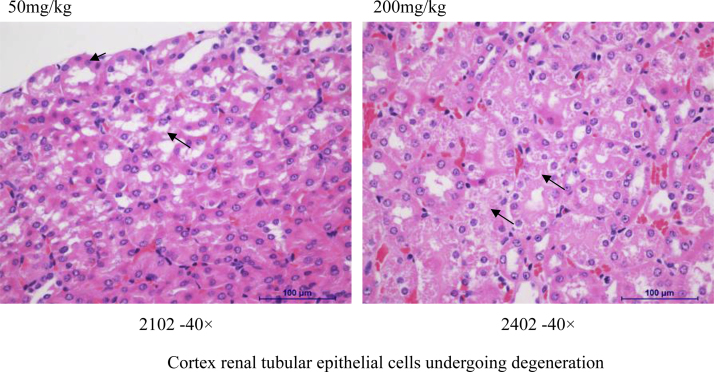
Histopathology assessment was conducted in control and high dose groups only (Suppl. Table 5, Fig. 2, Fig. 3, Fig. 4). Abnormal morphological changes were observed in all the brains (hemorrhage) and kidneys (cortex renal tubular epithelial cells undergoing degeneration), and sporadically in livers (infiltration of focal inflammatory cells mainly macrophage) of both control and high dose groups. Therefore, they were not considered as drug-related, probably were artifacts. We observed the hepatic capsule thickening within inflammatory cell infiltration mainly in neutrophils at doses of INZ higher than 50 mg/kg, which might be related to the increase of DBIL and ALP levels (Table 3, Fig. 3 and Suppl. Table 5).
Fig. 2.
Some representative images of morphologic changes in brain.
Fig. 3.
Some representative images of morphologic changes in mouse liver.
Fig. 4.
Some representative images of morphologic changes in mouse kidney.
In summary of the Phase B study, based on the absence of clinical findings in all animals, minimal body weight and food consumption changes, it was concluded that administration of INZ (C) via 5-day consecutive intraperitoneal injection is tolerated by female CD-1 mice under current study condition of Phase B at all dose levels tested, although there were some dose-dependent changes in hematology parameters (absolute/relative neutrophil and basophil counts), which were considered tolerable. Gross findings observed in the terminal necropsy were considered the irritation of INZ (C) formulations with the multiple i.p. injection. Findings observed at microscopic examination of tissues from control and high dose groups were not considered as drug-related due to presenting in both groups. Hence, our compound INZ (C) is quite safe to this strain of mice within the 120 mg/kg dose range via i.p. administration.
4. Discussion
In this study, a dose range-finding (ORF) study has been performed to assess the toxicological profile of INZ analog, INZ (C) (Compound 8 [4]), following a single dose administration in CD-1 mice in Phase A study. The established MTD of 200 mg/kg for female and 250 mg/kg for male, respectively, offers the rationale for selecting the doses for a 5-day toxicity study with female mice in Phase B study. The Phase B study has been performed at 4 dose levels (0 = control, 30, 60 and 120 mg/kg) of INZ (C) administered daily by i.p. for 5 days, and different parameters, such as mortality, body weight, food consumption, clinical pathology and gross pathology, were examined during and/or at termination of the study. Major organs, such as brain, lung, liver, aderanal gland, kidney, spleen, heart, ovary and uterus/prostate and testis were collected for possible histopathology evaluation. The biochemical assays were performed to measure the liver and kidney toxicity of INZ (C). For the Phase A study, clinical findings and lethality were observed in females at 250 mg/kg. For the Phase B study, it is concluded that administration of INZ (C) could be tolerated by 5-day consecutive dosing via intraperitoneal injection in female CD-1 mice under current study condition as no changes were observed in mean body weight, body weight gain and food consumption, although findings were noticed in gross/microscopic examination and part of the clinical pathology parameters. These toxicity studies demonstrate that INZ (C) is a quite safe compound within one week of treatment at the dose of less than 120 mg/kg via i.p.
Translating a promising SIRT1 and IMPDH2 dual inhibitor with excellent in vitro properties into an anticancer drug requires that the inhibitor has excellent pharmacological properties and is nontoxic or less to toxic to normal tissues. We have proved the efficacy of INZ in several preclinical models of lung and colon cancer [1], [2]. The first example is the xenograft tumors derived from aggressive H460 cells in severe combined immunodeficiency (SCID) mice. At the dose of 30 mg/kg via i.p. injection once every other day (Q.O.D.) for 3 weeks, INZ significantly reduced the average tumor weight at the end of the experiment by nearly 40%. Moreover, INZ-treated H460 tumors displayed elevated p53 compared to the vehicle-treated tumors by immunoblotting. In another xenograft tumor model, INZ was very effective in inhibition of HCT116 tumor growth as it significantly reduced tumor growth and weight by ∼70% after 30 mg/kg via i.p. once per day. In addition, we demonstrated that INZ and Nutlin-3 synergistically induces p53 and suppresses HCT116 xenograft tumor growth [8]. At the dose of 15 mg/kg of INZ and 150 mg/kg of Nutlin-3, each of the compounds could individually reduce the average tumor volume by less than 20% after treatment for 21 days. However, when combining the two treatments at the same doses, the final average volume of tumors was significantly decreased by 60%. Recently, we have further tested the combined anti-tumor effects of INZ and current first-line chemotherapeutic drugs, such as cisplatin, in the H460 xenograft tumor model. As a result, we found the enhanced growth suppression of xenografted lung cancer with combination of INZ and cisplatin at lower doses [3]. All these results demonstrate that INZ cooperates with other drugs that have different in vivo mechanisms of action, such as the MDM2 inhibitor Nutlin-3 or the DNA-damaging chemotherapeutic drug cisplatin, efficiently activate p53 and suppress xenograft tumor growth in animals. In line with the results in this report here, the effective dose of INZ has been shown to display little toxicity to the animals used in those previous studies [1]. Taken together, all of these studies including this report demonstrate that our previously reported effective doses of 30–60 mg/kg via i.p. are in the safe range to the small rodent animals.
Our biochemical toxicity studies, such as BUN (blood urea nitrogen), ALT (alanine aminotransferase), AST (aspartate aminotransferase), ALP (alkaline phosphatase), and total bilirubin (TBIL) biochemical assays, and histopathology assessments showed that INZ (C) does not appear to cause apparent hepatotoxic and kidney toxic in mice at all the dose levels tested in Phase B (Table 2, Table 3, Fig. 2, Fig. 3, Fig. 4 and Suppl. Table 5). In addition, through the whole Phase B toxicity experiment, both groups of animals, control and INZ (C)-treated groups, were healthy without apparent changes in their behavior, food appetite and body weight. These results, therefore, are in line with expectations that INZ (C) is a potent and non-toxic anti-cancer agent, and provide solid evidence for the further evaluation of INZ as a potential promising therapy in cancers.
Significant difference in the toxicity of INZ at 250 mg/kg was found between different sexes after 2 days (Suppl. Table 1). The INZ(C)-treated female mice showed many abnormalities, including decreased motor activity, piloerection, discharge both eyes, eye(s) partly closed, cold to touch and deep respiration. In contrast, no such abnormalities were observed in male mice and they became tolerated to the treatment. Since no toxicities were found besides the liver toxicity associated with the dose dependence of DBIL and ALT levels in Phase B studies (Table 2, Table 3 and Fig. 2, Fig. 3, Fig. 4), the sex-related metabolism in liver microsomes may result in the sex differences in INZ(C) induced liver toxicity. We will investigate the metabolisms from both sexes in the liver microsomes by HPLC/MS in our future studies, and determine which cytochrome P450s (CYPs) responsible for INZ(C) metabolism is male-specific or predominant.
For animal studies, the i.p. route is a practical and alternative route compared to other conventional drug delivery routes. It is most useful for substances that are degraded in gastrointestinal (GI) tract and insufficiently absorbed. Bioavailability of i.p. is almost similar to intravenous (i.v.), and greater than oral routes. In addition, substances are administered during clinical trials, by several routes, depending on the tumor type. Most recently, the i.p. route has been used for chemotherapy in patients with intra abdominal malignancies, i.e. gynecological and gastrointestinal cancers, and has shown very promising results. In patients with peritoneal surface malignancies, such as ovary cancers, chemotherapy via i.p. has resulted in satisfactory results [9].
INZ (C) has a short half-life in liver microsomes (date not shown) and does not have desired oral bioavailability. Thus, the oral route is not suitable for INZ (C) in the toxicity studies. Because of these caveats, we have been trying to improve the solubility and the metabolic stability of INZ (C) by optimizing the structure and developing oral formulations. Moreover, we are testing INZ (C) in a variety of tumors in orthotopic cancer murine models, including colon cancers and ovary cancers. The eventual therapeutic dosing route for INZ or its optimized derivative will depend on its physiochemical property and which tumors it affects mostly.
Tests and abbreviation.
| Hematology | |
| Red blood cell (erythrocyte) count (RBC) | Platelet count (PLT) |
| Hemoglobin | White blood cell (leukocyte) count (WBC) |
| Hematocrit | |
| Mean corpuscular volume (MCV) | |
| Mean corpuscular hemoglobin (MCH) | Differential blood cell count |
| Mean corpuscular hemoglobin concentration (MCHC) | Reticulocyte count (RETIC) |
| Clinical chemistry | |
| Blood urea nitrogen (BUN) | Alanine aminotransferase (ALT) |
| Creatinine (CREA) | Alkaline phosphatase (ALP) |
| Bilirubin (BIL) | Aspartate aminotransferase (AST) |
Conflict of interest
We declare there is no conflict of interest in our study.
Transparency document
Acknowledgements
We thank the Shanghai ChemPartner Ltd. for their assistance in this study. H.L. was supported in part by Ladies Leukemia League, Inc. in LA, and by NIH-NCI Grants CA095441 and CA172468.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:10.1016/j.toxrep.2015.02.011.
Appendix A. Supplementary data
The following is supplementary data to this article:
References
- 1.Zhang Q., Zeng S.X., Zhang Y., Ding D., Ye Q., Meroueh S.O., Lu H. A small molecule Inauhzin inhibits SIRT1 activity and suppresses tumour growth through activation of p53. EMBO Mol. Med. 2012;4(4):298–312. doi: 10.1002/emmm.201100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y., Zhang Q., Zeng S.X., Mayo L.D., Lu H. Inauhzin and Nutlin3 synergistically activate p53 and suppress tumor growth. Cancer Biol. Ther. 2012;13(10):915–924. doi: 10.4161/cbt.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y., Zhang Q., Zeng S.X., Hao Q., Lu H. Inauhzin sensitizes p53-dependent cytotoxicity and tumor suppression of chemotherapeutic agents. Neoplasia. 2013;15(5):523–534. doi: 10.1593/neo.13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Q., Ding D., Zeng S.X., Ye Q.Z., Lu H. Structure and activity analysis of Inauhzin analogs as novel antitumor compounds that induce p53 and inhibit cell growth. PLoS One. 2012;7(10):e46294. doi: 10.1371/journal.pone.0046294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao J.M., Zeng S.X., Zhou X., Lu H. Global effect of Inauhzin on human p53-responsive transcriptome. PLoS One. 2012;7(12):e52172. doi: 10.1371/journal.pone.0052172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vousden K.H., Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137(3):413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q., Zhou X., Wu R., Mosley A., Zeng S.X., Xing Z., Lu H. The role of IMP dehydrogenase 2 in Inauhzin-induced ribosomal stress. eLife. 2014:3. doi: 10.7554/eLife.03077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lain S., Hollick J.J., Campbell J., Staples O.D., Higgins M., Aoubala M., McCarthy A., Appleyard V., Murray K.E., Baker L., Thompson A., Mathers J., Holland S.J., Stark M.J., Pass G., Woods J., Lane D.P., Westwood N.J. Discovery, in vivo activity, and mechanism of action of a small-molecule p53 activator. Cancer Cell. 2008;13(5):454–463. doi: 10.1016/j.ccr.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferlay J., Soerjomataram I., Ervik M., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. International Agency for Research on Cancer; Lyon, France: 2014. GLOBOCAN 2012 v1. 1. [Internet] Available from: http://globocan.iarc.fr, accessed on 16/01/2015. [Google Scholar]
- 10.Prabhu V.V., Allen J.E., Hong B., Zhang S., Cheng H., El-Deiry W.S. Therapeutic targeting of the p53 pathway in cancer stem cells. Expert Opin. Ther. Targets. 2012;16(12):1161–1174. doi: 10.1517/14728222.2012.726985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pflaum J., Schlosser S., Müller M. p53 Family and Cellular Stress Responses in Cancer. Front. Oncol. 2014;4:285. doi: 10.3389/fonc.2014.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson S., Delongeas J.L., Donald E., Dreher D., Festag M., Kervyn S. A European pharmaceutical company initiative challenging the regulatory requirement for acute toxicity studies in pharmaceutical drug development. Regul. Toxicol. Pharmacol. 2008;50:345–352. doi: 10.1016/j.yrtph.2007.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.