Abstract
Practice variation in transplant physician management of immune suppression (IS) after allogeneic hematopoietic cell transplantation (HCT) is anticipated to have important consequences, but has not been characterized to date. We conducted a national survey of transplant physician members of the American Society for Blood and Marrow Transplantation to discern variation in IS management, characterize the burden of graft-versus-host disease (GVHD) emerging in the setting of IS taper, and describe the proportion of HCT recipients who successfully discontinue IS by 2 and 5 years post-HCT. There was marked heterogeneity in IS discontinuation practice, with variation in initiation of taper, sequence of agents tapered, frequency of changes, and strategy utilized. Twenty-five percent reported no consistent strategy in their usual practice. Confidence in therapeutic decision making was limited. The majority indicated that they could not predict who would develop GVHD on taper of IS, and reported a resultant burden of both acute and chronic GVHD (aGVHD, cGVHD) emerging or recurring in the setting of IS taper. HCT physicians projected rates of IS discontinuation that increased from 2 to 5 years post-HCT, and differed significantly according to donor relation and stem cell source utilized. The marked variation in practice, burden of GVHD emerging in the setting of IS taper, and limited confidence in therapeutic decision making all highlight shortcomings in an essential component of HCT physicians' scope of practice. These data argue for more rigorous study of IS management post-HCT so that evidence-based practice guidelines can be developed.
Keywords: Immune suppression, Practice variation, Physician survey, Graft-versus-host disease
Introduction
Although significant advances have been made in the prevention and therapy of acute and chronic graft-versus-host disease (aGVHD, cGVHD) [1-6], less systematic attention has been directed to management of immune suppression (IS) after allogeneic hematopoietic cell transplantation (HCT) and the problem of recurrent GVHD in this setting. In contradistinction to solid organ transplantation, most recipients of allogeneic HCT will eventually discontinue IS, thereafter maintaining a state of immune tolerance. However, there is little published data on the rates of successful rates of IS discontinuation after HCT [7]. As well, current practice in management of immunosuppressive agents after HCT is empiric and circumscribed by the inability of physicians to discern which patients can safely taper or discontinue immunosuppressive agents. Drug-suppressed immune response cannot be distinguished from the development of immune tolerance based on clinical assessment, and therefore attempts at IS discontinuation are often met with emergence or recurrence of aGVHD and cGVHD.
The optimal duration of IS administration is not known. Investigators have studied the impact of prolonged cyclosporine (CSA) administration after HCT on the incidence of aGVHD and cGVHD, but with mixed conclusions. Although some reports indicated decreased risk with prolonged administration of CSA [8,9], prospective trials have not consistently demonstrated a benefit. In a randomized trial of allogeneic related or unrelated marrow transplant recipients, the hazard for development of cGVHD did not significantly differ between 6 and 24 months of CSA administration post-HCT [10]. Others have demonstrated in peripheral blood stem cell (PBSC) grafts from matched related donors significantly reduced incidence of cGVHD in those treated with 12 versus 6 months of CSA post-HCT [11]. In the setting of nonmyeloablative HCT, retrospective analysis did not demonstrate significant differences in rates of extensive cGVHD among 3 tapering schedules [12]. These published data and unpublished observations indicate variation in center-based and individual transplant physician practice in the management of IS after HCT. This practice variation has not been formally characterized to date.
Heterogeneity in physician practice has been demonstrated in a number of important studies in the field of HCT, demonstrating variation in the following: aGVHD prophylaxis and therapy [13]; antimicrobial prophylaxis [14]; diagnosis, grading, and management of cGVHD [15]; management of steroid-refractory aGVHD [16]; recommendation for transplantation therapy, timing of this therapy, and transplantation conditions [17]; and pretransplantation evaluation and supportive care practices [18].
To examine the variation in transplant physician management of IS, characterize the burden of GVHD incurred in this setting, and to describe the proportion of patients who are able to successfully discontinue IS after transplantation, we conducted a vignette-based national survey of HCT physicians through the American Society for Blood and Marrow Transplantation (ASBMT).
Methods
Development of Survey
The survey was developed to describe vignette-based clinical scenarios that require decisions on IS management after allogeneic HCT. Vignettes were utilized so that standardized information could be presented to respondents to facilitate decision making. Following a base case, each additional vignette was constructed to differ by 1 variable to examine how the practices differed according to each variable. These were organized to reflect nuances in HCT conditions that may impact IS management decisions, including hematopoietic stem cell source (peripheral blood mobilized versus bone marrow [BM] harvested), donor relation (matched related versus matched unrelated), and conditioning regimen intensity (myeloablative versus reduced intensity). Because there is potential variation in practice according to conditions treated with HCT, all vignettes were standardized to describe only acute myelogenous leukemia (AML). AML was chosen, because it is currently the most common indication for allogeneic HCT. Additional vignettes examined decision making with AML recurrence after HCT, and after successful treatment of aGVHD and cGVHD.
The survey was developed by the authors, and then tested with transplant physicians at the Moffitt Cancer Center and Fred Hutchinson Cancer Research Center. Survey items were modified based on feedback obtained on the following elements: content validity, face validity, presentation of information, ability of survey respondent to interpret the essential information and understand each item, as well as ability to complete the survey within the anticipated time. The final survey instrument consisted of 7 clinical vignettes, 34 associated questions examining practice in response to these vignettes, and an additional 17 questions on the respondent's perception of sociodemographic and transplant practice characteristics. These included characteristics of physician practice (transplant center, pediatric versus adult transplantation practice, years of experience as a transplant physician, academic versus clinical practice setting, percent of practice comprised of clinical versus research or administrative responsibilities, and number of faculty in the transplant group), characteristics of allogeneic transplant patients treated at the respondent's center (number of HCT performed per year, proportion performed with myeloablative conditioning, proportion of related versus unrelated donors, proportion with matched donors, proportion of unrelated donors utilizing umbilical cord blood, median age of transplant recipients, proportion utilizing peripheral blood versus BM stem cells, and proportion in remission at time of transplantation), and self-reported proportion of HCT patients with aGVHD and cGVHD. The survey was anticipated to take 10 to 15 minutes to complete.
Survey Distribution and Data Collection
This study was approved by the University of South Florida institutional review board as a national survey of transplant physician practice. The requirement for signed informed consent for participation was waived. The electronic, Internet-based survey was maintained by the Survey Methods Core Program at the Moffitt Cancer Center. Invitation to ASBMT members to participate was facilitated by e-mail notification by the ASBMT. Potential respondents were provided a link to the electronic survey, which described the nature of the study, potential risks and benefits involved, lack of financial or other reimbursement for participation, and the right to decline participation.
Statistical Methods
The intent of this study was exploratory in nature without a priori hypotheses. Descriptive statistics were utilized to summarize responses for items related to each clinical vignette and the subsequent sociodemographic and transplantation items. Association between item responses and survey respondent sociodemographic and transplantation practice variables were explored with the chi-square, Gamma test, or Cochran-Armitage trend test as appropriate per the nature of the variables examined.
Results
Characteristics of Survey Respondents
The initial notification was distributed by the ASBMT on December 14, 2009. Two reminder e-mails followed on January 12, 2010, and February 8, 2010. Two additional targeted e-mail invitations were sent to nonresponders on May 4 and 11, 2010. The total number of recipients was 1457. From this sample, a total of 1100 were transplant physicians. Undeliverable or returned messages were not tracked by the ASBMT. Of the 1457 recipients, 2 actively declined to participate. A total of 225 respondents completed the survey, resulting in an overall response rate of 15% and a physician response rate of 21%. This was lower than the anticipated response [19-21]. A total of 74 responded after the first notification, 57 after the second, and 48 after the third notification. An additional 22 and 24 responded after 2 targeted invitations to nonresponders, respectively. The self-reported HCT physician demographic, HCT center and patient, and baseline GVHD characteristics are summarized in Table 1.
Table 1. Survey Respondent Self-Report of HCT Physician, HCT Center, and Patient, and Baseline GVHD and GVHD Prophylaxis Characteristics.
| HCT Physician Characteristics | Number (%) |
|---|---|
| Practice setting | |
| Academic | 192 (87%) |
| Community | 28 (13%) |
| % effort in clinical duties | |
| <20% | 8 (4%) |
| 20% to 39% | 31 (14%) |
| 40% to 59% | 62 (28%) |
| 60% to 79% | 74 (33%) |
| 80% to 100% | 46 (21%) |
| Duration of practice as HCT physician | |
| Fellow/training | 8 (4%) |
| <5 years | 32 (15%) |
| 5 to 9 years | 78 (35%) |
| 10 to 14 years | 34 (15%) |
| 15 to 19 years | 68 (31%) |
| ≥20 years | 0 (0%) |
| Number of HCT faculty in group | |
| <5 | 81 (37%) |
| 5 to 9 | 94 (43%) |
| 10 to 14 | 24 (11%) |
| 15 to 19 | 12 (5%) |
| ≥20 | 10 (5%) |
| Adult | 178 (81%) |
| Pediatric | 43 (19%) |
|
| |
| HCT Center and Patient Characteristics | Number (%) |
|
| |
| # HCT/year | |
| <25 | 39 (18%) |
| 25 to 49 | 56 (25%) |
| 50 to 99 | 61 (28%) |
| 100 to 149 | 29 (13%) |
| 150 to 199 | 9 (4%) |
| ≥200 | 27 (12%) |
| Median age HCT patients | |
| <20 | 36 (17%) |
| 20 to 39 | 20 (9%) |
| 40 to 59 | 156 (72%) |
| ≥60 | 6 (3%) |
| % in remission at HCT | |
| <25% | 3 (1%) |
| 25% to 49% | 27 (12%) |
| 50% to 74% | 72 (33%) |
| 75% to 100% | 117 (53%) |
| % PBSCs | |
| <25% | 35 (16%) |
| 25% to 49% | 19 (9%) |
| 50% to 74% | 55 (25%) |
| 75% to 100% | 109 (50%) |
| % related donors | |
| <25% | 31 (14%) |
| 25% to 49% | 123 (56%) |
| 50% to 74% | 47 (21%) |
| 75% to 100% | 19 (9%) |
| % HLA matched | |
| <25% | 8 (4%) |
| 25% to 49% | 36 (16%) |
| 50% to 74% | 64 (29%) |
| 75% to 100% | 111 (51%) |
| % RIC or NMA | |
| <25% | 23 (10%) |
| 25% to 49% | 84 (38%) |
| 50% to 74% | 87 (40%) |
| 75% to 100% | 26 (12%) |
| % unrelated donor utilizing cord blood | |
| <25% | 169 (78%) |
| 25% to 49% | 24 (11%) |
|
| |
| HCT Center and Patient Characteristics | Number (%) |
|
| |
| 50% to 74% | 16 (7%) |
| 75% to 100% | 9 (4%) |
|
| |
| GVHD Characteristics | Number (%) |
|
| |
| GVHD prophylaxis | |
| CSA/MTX | |
| <20% | 45 (28%) |
| 20% to 29% | 34 (22%) |
| 40% to 59% | 25 (16%) |
| 60% to 79% | 31 (20%) |
| 80% to 100% | 23 (15%) |
| CSA/MMF | |
| <20% | 51 (40%) |
| 20% to 29% | 39 (31%) |
| 40% to 59% | 17 (13%) |
| 60% to 79% | 13 (10%) |
| 80% to 100% | 7 (6%) |
| TAC/MTX | |
| <20% | 29 (21%) |
| 20% to 29% | 31 (23%) |
| 40% to 59% | 27 (20%) |
| 60% to 79% | 29 (21%) |
| 80% to 100% | 21 (15%) |
| TAC/MMF | |
| <20% | 53 (48%) |
| 20% to 29% | 27 (24%) |
| 40% to 59% | 18 (16%) |
| 60% to 79% | 6 (5%) |
| 80% to 100% | 7 (6%) |
| TAC/SIR | |
| <20% | 53 (66%) |
| 20% to 29% | 15 (19%) |
| 40% to 59% | 5 (6%) |
| 60% to 79% | 5 (6%) |
| 80% to 100% | 2 (3%) |
| Proportion with II-IV acute GVHD | |
| <25% | 29 (13%) |
| 25% to 49% | 129 (59%) |
| 50% to 74% | 58 (26%) |
| 75% to 100% | 4 (2%) |
| Proportion with moderate/severe chronic GVHD | |
| <25% | 51 (23%) |
| 25% to 49% | 115 (52%) |
| 50% to 74% | 52 (24%) |
| 75% to 100% | 2 (1%) |
HCT indicates allogeneic hematopoietic cell transplantation; GVHD, graft-versus-host disease; PBSCs, peripheral blood stem cells; HLA, human leukocyte antigen; RIC, reduced-intensity conditioning; NMA, nonmyeloablative; CSA, cyclosporine; MTX, methotrexate; MMF, myco-phenolate mofetil; TAC, tacrolimus; SIR, sirolimus.
IS Management Post-HCT
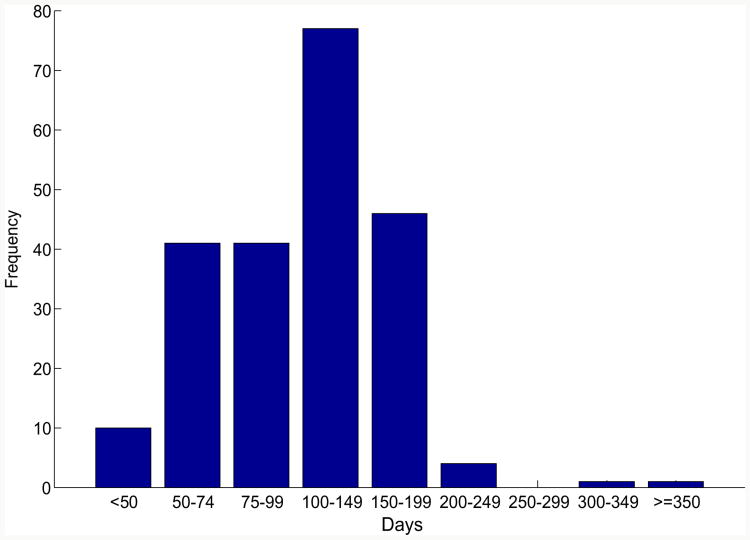
The base case of the survey described a 35-year-old man with AML with normal cytogenetics in his first complete remission, who was treated with a myeloablative regimen (cyclophosphamide and total-body irradiation) followed by matched sibling donor peripheral blood HCT. Survey respondents were asked to describe their usual practice of IS management in this setting. Figure 1 depicts the frequency distribution of time of initiation of taper of any IS agents used in GVHD prophylaxis in the absence of any clinical GVHD.
Figure 1.
Frequency histogram of reported day post-HCT for initiation of immune suppression taper.
Respondents most frequently indicated an IS taper initiation between days 100 and 149 post-HCT. IS taper start date was significantly associated with the following factors: Physicians treating pediatric patients who underwent HCT started the IS taper earlier than physicians who treated adults (P = .004). Earlier IS taper start date was also associated with a greater proportion of myeloablative regimens utilized (P =.036) and a greater proportion of HCT patients in remission at the time of HCT (P < .001). Of those who indicated use of cyclosporine as GVHD prophylaxis, 53% indicated they would taper this agent first. Of those indicating the use of tacrolimus for GVHD prophylaxis, 45% indicated they would taper this first. From those using mycophenolate mofetil in GVHD prophylaxis, 71% indicated that they would first taper this agent. Finally, of those indicating use of sirolimus in GVHD prophylaxis, 20% indicated that they would taper this agent first.
There was considerable variation in the strategy utilized for IS taper: 56 (25%) indicated that they followed no consistent strategy; 121 (55%) tapered by percent dose reduction (eg, 10% reduction); and 44 (20%) tapered according to fixed dose reduction (eg, by 0.5 mg). Heterogeneity was also observed in the frequency of IS taper: days, or less than weekly 8 (4%); weekly 100 (45%); biweekly 88 (40%); monthly 19 (9%); every 6 weeks 4 (2%); bimonthly 2 (1%); none indicated taper on a greater than every 2 month basis. Significant associations were detected between the frequency of IS taper and the following factors: those with an institutional practice guideline or protocol for IS management tapered more frequently (P = .002); those indicating less comfort in decision making tapered less frequently (P = .016); adult HCT physicians tapered less frequently than pediatric (P = .009); a greater proportion of peripheral blood HCT utilized was associated with less frequent IS taper (P = .008); and greater utilization of cord blood HCT was associated with more frequent IS taper (P = .007).
A second clinical vignette mirrored the first, with the exception of utilization of BM harvested stem cells rather than PBSCs. The majority of respondents (82%) indicated that stem cell source does not influence their practice of IS management after HCT. Of those who did indicate differential practice according to stem cell source, the majority (83%) indicated earlier start of IS taper with BM compared with peripheral blood stem cell transplantation (PBSCT). Use of similar IS management strategies for PBSC and BM was significantly associated with self-report of the ability to distinguish those who will develop GVHD on IS taper (P < .009), adult versus pediatric HCT physician status (P < .006), and older age of the respondent's center HCT patients (P < .005).
A third vignette mirrored the base case, with the exception that it depicted the use of a matched unrelated donor peripheral blood HCT. Forty-three percent of respondents indicated no difference in their management of IS agents after HCT in the setting of matched unrelated donor versus matched sibling donor HCT. Of those who did indicate differential management, the majority (87%) started IS taper later after unrelated donor HCT. Sixteen percent reported tapering less frequently, and 18% indicated tapering by lesser amounts. Similar approaches to IS management after unrelated and related donor HCT was associated with a larger number of faculty in the BMT group (P = .012), more HCT performed/year (P < .0001), and greater degree of agreement with the statement, “my current strategy for IS taper is adequate” (P = .045).
A fourth clinical vignette mirrored the base case, with the exception of the use of a reduced-intensity conditioning (RIC) regimen. Forty percent of respondents indicated no difference in their management of IS following RIC compared with myeloablative conditioning therapy. Of the remainder, 60% reported tapering earlier, and 34% indicated that their IS management in the setting of RIC is determined by donor chimerism post-HCT. No difference in management by physicians was significantly associated with adult versus pediatric HCT (P = .01), a larger number of HCT/year (P = .004), and a higher median age of HCT recipients (P = .005).
Management of IS after Relapse
In a fifth vignette, respondents described their management of IS in the case of a 35-year-old man with relapsed AML (peripheral blood cytopenias, 26% myeloblasts on BM) by day 60 after a prior myeloablative HCT utilizing matched sibling donor PBSCs. Twenty-four percent tapered IS, and 71% endorsed completely discontinuing all IS agents. Fifty percent delivered donor-lymphocyte infusion (DLI), and 48% treated with chemotherapy. Discontinuation of all IS was associated with a feeling of adequacy of IS strategy (P = .003), decreased number of faculty in the group (P = .0003), decreased number of HCT/year performed (P < .0001), and a lesser median age of HCT patients treated (P = .03). Delivery of chemotherapy was associated with a larger number of HCT/year performed (P = .02). When presented with aGVHD following DLI, 2% reported no change in management, 38% resumed prior IS agents stopped after relapse developed, 72% added systemic glucocorticoid therapy, and 10% started additional nonsteroid IS agents. No change to IS therapy in the setting of aGVHD following DLI was associated with a shorter duration of practice as an HCT physician (P = .027). DLI administration, resumption of prior IS agents, and addition of steroids all differed according to adult versus pediatric HCT physician status (all P < .05). Interestingly, 58% of respondents indicated that they either agreed or strongly agreed with the statement, “I am willing to accept grade II-IV aGVHD that develops in the setting of stopping IS agents and DLI if there is primary disease relapse after HCT.” Agreement with this statement was associated with a longer duration of practice as an HCT physician (P < .0001).
Management of IS after Resolution of GVHD
In a sixth vignette, respondents were asked to describe their management of IS after the resolution of an overall grade III biopsy confirmed aGVHD of the skin and gastrointestinal (GI) tract on day 30 in the case of the patient originally described in vignette 1. He was described as having achieved a complete remission of aGVHD manifestations after 1 mg/kg of steroid therapy. Seventeen percent indicated that they would taper steroids immediately upon resolution of the aGVHD manifestations. Others reported tapering steroids after a postresolution period of days (11%), 1 week (25%), 2 weeks (29%), 1 month (11%), 2 months (1%), or >2 months (1%). Allowing a longer period following resolution of aGVHD before tapering glucocorticoid therapy was associated with community practice setting (P = .03), smaller number of HCT/year performed (P = .006), and younger median age of HCT patients (P = .02). Nonsteroid IS agents were tapered at the following time points: with active aGVHD (1%), immediately upon resolution (2%), within days (1%), 1 week (3%), 2 weeks (7%), 1 month (27%), 2 months (19%), and >2 months (40%).
In the final vignette, the original base case patient was depicted as having developed at day 120 post-HCT cGVHD including lichen planus-like changes in the mouth without limitation in oral intake, sicca syndrome requiring eye drops twice daily, arthralgias, and new increase in liver function tests at 2× the upper limit of normal; these manifestations were then described as completely resolved after 1 mg/kg of glucocorticoid therapy. There was marked variation in timing of glucocorticoid taper: taper with active cGVHD (6%), immediately on resolution (10%), days (4%), 1 week (11%), 2 weeks (24%), 1 month (28%), 2 months (9%), and >2 months (7%). Nonsteroid agents were tapered at the following: with active cGVHD (2%), at resolution (1%), days (1%), 1 week (1%), 2 weeks (4%), 1 month (19%), 2 months (19%), and >2 months (53%).
GVHD following IS Taper
Following each previously described clinical vignette, survey respondents were asked to estimate the frequency at which they observe recurrent aGVHD following the taper of IS agents. The frequency distribution across these clinical vignettes is depicted in Figure 2. With the exception of the comparison of myeloablative conditioning with PBSCT and RIC PBSCT, the distributions between groups significantly differed.
Figure 2.
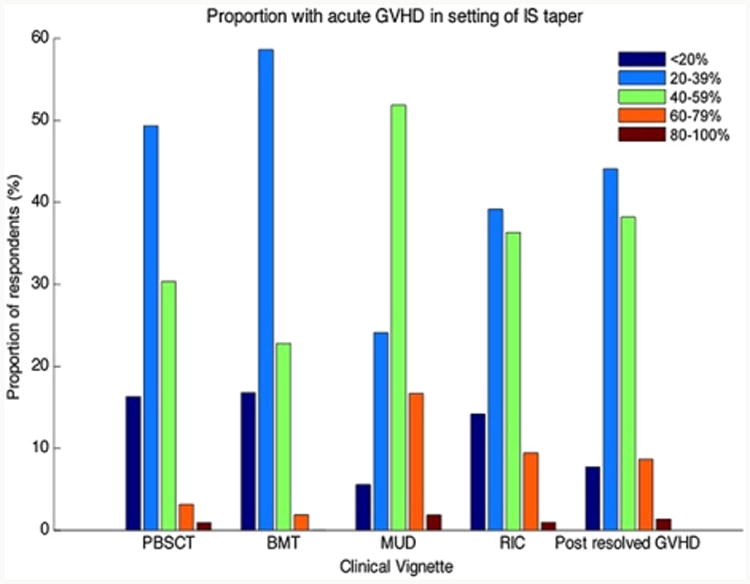
Frequency distribution of estimated rates of recurrent aGVHD following IS taper. PBSCT = ablative conditioning, peripheral blood stem cells; BMT = ablative conditioning, bone marrow; MUD = ablative conditioning, unrelated donor peripheral blood stem cells; RIC = reduced-intensity conditioning, peripheral blood stem cells; post-resolved GVHD = recurrent aGVHD in setting of IS taper following prior successfully treated episode of aGVHD (P <.05 for comparisons between each group, with exception of the comparison of myeloablative PBSCT and RIC PBSCT).
When presented with the occurrence of recurrent aGVHD following IS taper, respondents varied in their management strategy: 44% resumed the original dose of IS agents before taper; 73% started 1 to 2 mg/kg of glucocorticoid therapy; and 16% started both 1 to 2 mg/kg glucocorticoids and an additional systemic IS agent not part of the original pretaper regimen. Respondents indicated the proportion of these cases of recurrent aGVHD in the setting of IS taper, which can be successfully treated to complete resolution: <20% of cases (1%); 20% to 39% of cases (15%); 40% to 59% of cases (52%); 60% to 79% of cases (31%), and 80% to 100% of cases (2%). The self-reported burden of cases of recurrent aGVHD realized in the setting of IS taper that cannot be treated to resolution thus highlights the gravity of this problem.
Following the final vignette depicting a case of cGVHD successfully treated with systemic glucocorticoids, respondents indicated the proportion of cases in which recurrent cGVHD develops upon IS taper (Figure 3). These data support an overall large burden of recurrent cGVHD in the setting of IS taper. A greater proportion of reported recurrent cGVHD was associated with greater use of PBSCs over BM (P = .036).
Figure 3.
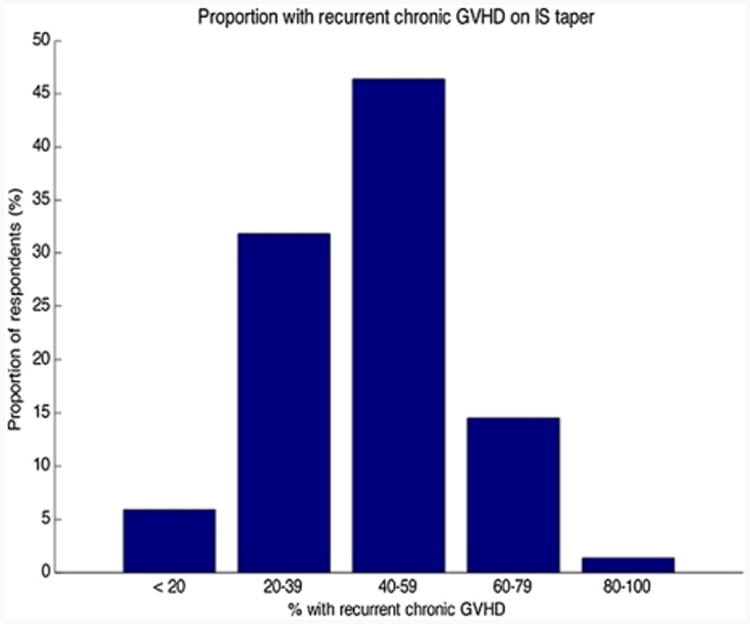
Estimated recurrent cGVHD in the setting of IS taper following successful control of a prior episode of cGVHD.
Discontinuation of IS
Following each clinical vignette, survey respondents estimated the proportion of their own HCT patients, consistent with each of the described clinical scenarios, who are able to completely discontinue IS by 2 and 5 years. The frequency distribution for IS discontinuation across these clinical vignettes is depicted in Figures 4 and 5. Reported IS discontinuation rates at 2 and 5 years significantly differed according to clinical vignette in all comparisons, with the exception of myeloablative conditioning with PBSCT versus RIC PBSCT.
Figure 4.
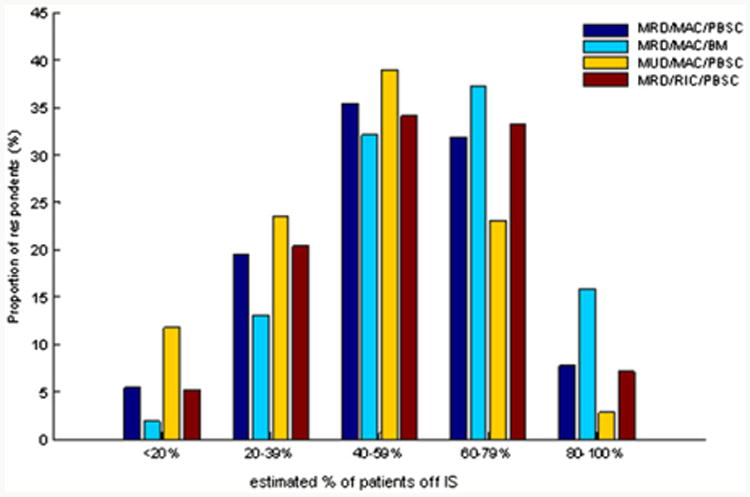
Frequency distribution of estimated rate of IS discontinuation at 2 years after HCT according to clinical vignette. MRD = matched related donor; MUD = matched unrelated donor; MAC = myeloablative conditioning; RIC = reduced-intensity conditioning; PBSC = peripheral blood stem cells; BM = bone marrow stem cells. The proportions off IS are compared between each of these clinical vignettes by Bowker's test with P <.05 for each, except the comparison of MRD/MAC/PBSC and MRD/RIC/PBSC vignettes, where P = NS.
Figure 5.
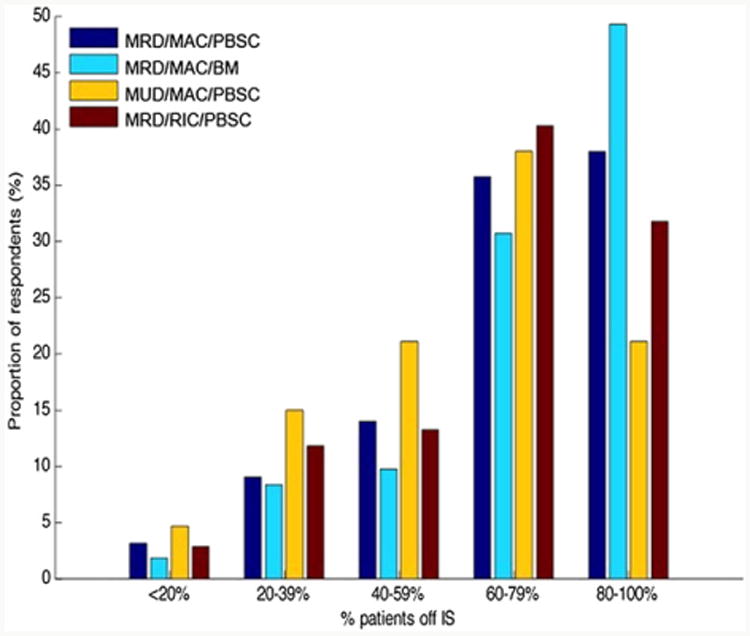
Frequency distribution of estimated rate of IS discontinuation at 5 years after HCT according to clinical vignette. MRD = matched related donor; MUD = matched unrelated donor; MAC = myeloablative conditioning; RIC = reduced-intensity conditioning; PBSC = peripheral blood stem cells; BM = bone marrow stem cells. The proportions off IS are compared between each of these clinical vignettes by Bowker's test with P <.05 for each, except the comparison of MRD/MAC/PBSC and MRD/RIC/PBSC vignettes, where P = NS.
Decision-Making Confidence
The majority (51%) indicated that they had no institutional practice guideline or protocol for IS taper. Forty-one percent indicated that their current strategy for IS taper after HCT is not adequate; perceived inadequacy was associated with the absence of guidelines (P = .0007). Twenty-six percent indicated that they were either uncomfortable or very uncomfortable with IS taper and management decisions. Increasing level of comfort in decision making was associated with adult HCT physician status (P = .049), academic practice setting (P = .03), greater proportion of cord blood HCT among unrelated donor sources (P = .016), and smaller proportion of PBSCT compared with BMT (P = .02). A total of 62% either disagreed or strongly disagreed with the statement, “I am able to distinguish those patients who will have recurrent GVHD after IS taper versus those who will not”; 26% neither disagreed nor agreed; and 12% agreed. Increasing level of agreement with this statement was associated with the absence of institutional guidelines or protocol (P = .01), and shorter duration of practice as an HCT physician (P = .005).
Qualitative Results
Survey respondents were afforded an opportunity to offer comments on their perceptions of the survey itself. These instructive comments highlighted both limitations of this instrument, as well as colorfully depicted the variation in HCT physician practice. First, some (18 respondents total) reported that the vignettes did not capture all subtle nuances relevant to decision making. In summary, these included the following: inadequate details on combination of patient age, comorbidity, disease risk, and performance status; inadequate information for estimation of anticipated tolerance of GVHD; no mention of the use of antithymocyte globulin (ATG) or alemtuzumab in GVHD prophylaxis; and no allowance for staged response of multiple therapeutic options in sequence. Other comments presented extremes of practice variation, ranging from all decisions uniformly being driven by protocol to, alternatively, a total absence of guidelines or a standardized approach. One respondent stated, “We need standard guidelines for IS taper and treatment of aGVHD and cGVHD. Failure is due in part to intra- and interinstitutional variation in the absence of these guidelines.”
Discussion
Variation in management of IS following HCT is anticipated, given the lack of evidence- or consensus-based practice guidelines. However, this practice variation has not been systematically studied to date. We have conducted a national survey of transplant physician management of IS following HCT, with the principal aims of examining variation in practice of IS management, describing the perceived burden of recurrent GVHD incurred in this setting, as well as the estimated rates of successful discontinuation of IS post-HCT. The resulting data demonstrate marked variation in the management of IS, with heterogeneity in timing of taper onset, IS agents utilized and sequence of tapering, the frequency of taper, and the strategy employed in patients with and without GVHD. Strikingly, 25% of respondents reported that they have no strategy that they consistently follow.
The data demonstrate that practice varies according to a number of transplant variables: In the setting of unrelated donor HCT, the majority of transplant physicians indicated caution, starting IS taper later than matched sibling HCT; a smaller proportion also endorsed tapering less frequently and by lesser amounts. The majority endorsed differential management of IS following RIC, both tapering earlier after HCT, and with particular attention to donor chimerism. In the setting of relapse, a robust majority stopped all IS, and a lesser proportion favored the use of DLI. Not surprisingly, of those who utilized DLI for the treatment of relapse, the majority indicated that they were willing to accept the risk of aGVHD inherent with this procedure.
Several other relationships emerged in the examination of response according to physician and transplant center characteristics: Pediatric HCT physicians tapered IS earlier and more frequently. Adult HCT physicians were more likely to indicate no difference in management according to stem cell source and conditioning regimen intensity and to report more comfort in their decision making surrounding IS management compared with pediatricians. Duration of practice as an HCT physician also emerged as an important determinant of practice: Those with shorter duration of experience were more likely to endorse no escalation in therapy when confronted with aGVHD after DLI. Conversely, those with longer duration of practice were more likely to accept the risk of GVHD inherent in the use of DLI for post-HCT relapse. These data may reflect differences in maturity in decision making, as well as differential focus on the poor outcome associated with relapsed disease post-HCT. Interestingly, those with shorter practice duration were more likely to endorse that they could distinguish those who will develop GVHD versus not in the setting of IS taper. This likely represents limited experience because it is not clear that recent insights have emerged in the clinical prediction of GVHD in the setting of IS taper that could explain this disparity. Although there was limited representation of self-identified community HCT physicians in this sample, the data indicate that community practice setting was associated with both allowing more time after resolution of GVHD before IS taper, as well as a lesser degree of confidence in decision making. Finally, the utilization of PBSC emerged as a consistent transplant center variable important for physician decision making and their reported outcomes: Greater utilization of PBSCs was associated with less frequent taper of IS, less confidence in decision making, greater projected burden of recurrent cGVHD on IS taper, greater perceived proportion of recurrent aGVHD, and a smaller estimated proportion of successful discontinuation of IS at 2 and 5 years post-HCT.
Physicians consistently reported a large burden of both recurrent aGVHD and cGVHD in the setting of IS taper, but differed in their resultant therapeutic approach: Many utilized glucocorticoid therapy in this setting, whereas smaller proportions endorsed resuming prior doses of IS agents or both starting glucocorticoids and adding new IS agents. The consequences of GVHD emerging or recurring in the setting of IS taper is clearly a grave problem, as supported by HCT physicians' self-reported proportion of these cases that cannot be treated to resolution.
The data also provide insight into transplant physicians' perceptions of rates of successful IS discontinuation following HCT. These data indicate that HCT physicians largely expect the majority of HCT recipients to have discontinued IS by 5 years post-HCT, and that their expectations differ according to stem cell source and donor relation. Differences in estimated IS discontinuation rates were not detected according to conditioning regimen intensity.
Our measurement of decision-making confidence provides additional insight into the shortcomings of current practice. The majority of respondents indicated that they have no institutional guidelines or protocols that they follow in their practice of IS discontinuation post-HCT. As well, 41% reported that their current strategy for IS management post-HCT is not adequate. Alarmingly, 26% admitted that they are either uncomfortable or very uncomfortable with making decisions in the management of IS post-HCT. Although this was a confidential survey, this is likely a low estimate, given the conflict involved in acknowledging and reporting uncertainty and discomfort in the HCT physician's scope of practice. As anticipated, the majority indicated that they are not able to distinguish those who will or will not develop GVHD upon taper of IS. This level of uncertainty and discomfort involved in the decision making of HCT physicians has potentially serious complications, given the attendant risk and consequences of GVHD emerging in the setting of IS taper.
Although these results are intriguing, we acknowledge the limitation of low response rate, which threatens the validity of these data. The response rate appears lower than anticipated in physician surveys; however, the true denominator of respondents is not certain. Undeliverable or returned messages were not tracked by the ASBMT. As well, reasons for nonparticipation are largely unknown, given the limited number of those who actively declined to participate. Methods employed to encourage response included both serial invitations, as well as the later targeted requests to nonresponders. Given the observed declining trend in proportion responding per invitation, it is unlikely that further invitations would have dramatically increased the total response rate within a reasonable time frame. Provision of both paper and electronic survey, as well as monetary incentive, may have increased the response rate, but was not possible.
In summary, these data support a lack of consistent practice and argue for more rigorous study of IS management post-HCT. Moving forward from these findings, we suggest several potential approaches to determine best practice in IS taper and discontinuation following HCT. Retrospective studies examining successful IS discontinuation rates according to tapering schedule may provide an initial understanding. Prospective observational studies could capture physician practice in IS taper and the associated outcomes. Lessons learned from these studies would be important to develop a prospective randomized trial comparing best approaches for tapering and discontinuation of IS. Finally, if validated biomarkers of post-HCT immune tolerance can be developed, these could facilitate a personalized and informed strategy for IS discontinuation after HCT.
Acknowledgments
Financial disclosure: The authors have nothing to disclose.
Footnotes
Financial disclosure: See Acknowledgments on page 1535.
References
- 1.Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–2314. [PubMed] [Google Scholar]
- 2.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 3.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 4.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 5.Cutler C, Li S, Ho VT, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109:3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao NJ, Chen BJ. Prophylaxis and treatment of acute graft-versus-host disease. Semin Hematol. 2006;43:32–41. doi: 10.1053/j.seminhematol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Stewart BL, Storer B, Storek J, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104:3501–3506. doi: 10.1182/blood-2004-01-0200. [DOI] [PubMed] [Google Scholar]
- 8.Ruutu T, Volin L, Elonen E. Low incidence of severe acute and chronic graft-versus-host disease as a result of prolonged cyclosporine prophylaxis and early aggressive treatment with corticosteroids. Transplant Proc. 1988;20:491–493. [PubMed] [Google Scholar]
- 9.Lonnqvist B, Aschan J, Ljungman P, Ringden O. Long-term cyclosporin therapy may decrease the risk of chronic graft-versus-host disease. Br J Haematol. 1990;74:547–548. doi: 10.1111/j.1365-2141.1990.tb06356.x. [DOI] [PubMed] [Google Scholar]
- 10.Kansu E, Gooley T, Flowers ME, et al. Administration of cyclosporine for 24 months compared with 6 months for prevention of chronic graft-versus-host disease: a prospective randomized clinical trial. Blood. 2001;98:3868–3870. doi: 10.1182/blood.v98.13.3868. [DOI] [PubMed] [Google Scholar]
- 11.Mengarelli A, Iori AP, Romano A, et al. One-year cyclosporine prophylaxis reduces the risk of developing extensive chronic graft-versus-host disease after allogeneic peripheral blood stem cell transplantation. Haematologica. 2003;88:315–323. [PubMed] [Google Scholar]
- 12.Burroughs L, Mielcarek M, Leisenring W, et al. Extending postgrafting cyclosporine decreases the risk of severe graft-versus-host disease after nonmyeloablative hematopoietic cell transplantation. Transplantation. 2006;81:818–825. doi: 10.1097/01.tp.0000203556.06145.5b. [DOI] [PubMed] [Google Scholar]
- 13.Ruutu T, Niederwieser D, Gratwohl A, Apperley JF. A survey of the prophylaxis and treatment of acute GVHD in Europe: a report of the European Group for Blood and Marrow, Transplantation (EBMT). Chronic Leukaemia Working Party of the EBMT. Bone Marrow Transplant. 1997;19:759–764. doi: 10.1038/sj.bmt.1700745. [DOI] [PubMed] [Google Scholar]
- 14.Trifilio S, Verma A, Mehta J. Antimicrobial prophylaxis in hematopoietic stem cell transplant recipients: heterogeneity of current clinical practice. Bone Marrow Transplant. 2004;33:735–739. doi: 10.1038/sj.bmt.1704423. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Vogelsang G, Gilman A, et al. A survey of diagnosis, management, and grading of chronic GVHD. Biol Blood Marrow Transplant. 2002;8:32–39. doi: 10.1053/bbmt.2002.v8.pm11846354. [DOI] [PubMed] [Google Scholar]
- 16.Hsu B, May R, Carrum G, Krance R, Przepiorka D. Use of antithymocyte globulin for treatment of steroid-refractory acute graft-versus-host disease: an international practice survey. Bone Marrow Transplant. 2001;28:945–950. doi: 10.1038/sj.bmt.1703269. [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Joffe S, Artz AS, et al. Individual physician practice variation in hematopoietic cell transplantation. J Clin Oncol. 2008;26:2162–2170. doi: 10.1200/JCO.2007.15.0169. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ, Astigarraga CC, Eapen M, et al. Variation in supportive care practices in hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:1231–1238. doi: 10.1016/j.bbmt.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorpe C, Ryan B, McLean SL, et al. How to obtain excellent response rates when surveying physicians. Fam Pract. 2009;26:65–68. doi: 10.1093/fampra/cmn097. [DOI] [PubMed] [Google Scholar]
- 20.McCloskey SA, Tao ML, Rose CM, Fink A, Amadeo AM. National survey of perspectives of palliative radiation therapy: role, barriers, and needs. Cancer J. 2007;13:130–137. doi: 10.1097/PPO.0b013e31804675d4. [DOI] [PubMed] [Google Scholar]
- 21.Asch DA, Jedrziewski MK, Christakis NA. Response rates to mail surveys published in medical journals. J Clin Epidemiol. 1997;50:1129–1136. doi: 10.1016/s0895-4356(97)00126-1. [DOI] [PubMed] [Google Scholar]