Abstract
Negative urgency is a facet of impulsivity that reflects mood-based rash action and is associated with various maladaptive behaviors in humans. However, the underlying neural mechanisms of negative urgency are not fully understood. Several brain regions within the mesocorticolimbic pathway, as well as the neurotransmitters dopamine (DA) and serotonin (5-HT), have been implicated in impulsivity. Extracellular DA and 5-HT concentrations are regulated by DA transporters (DAT) and 5-HT transporters (SERT); thus, these transporters may be important molecular mechanisms underlying individual differences in negative urgency. The current study employed a reward omission task to model negative urgency in rats. During reward trials, a cue light signaled the non-contingent delivery of one sucrose pellet; immediately following the non-contingent reward, rats responded on a lever to earn sucrose pellets (operant phase). Omission trials were similar to reward trials, except that non-contingent sucrose was omitted following the cue light prior to the operant phase. As expected, contingent responding was higher following omission of expected reward than following delivery of expected reward, thus reflecting negative urgency. Upon completion of behavioral training, Vmax and Km were obtained from kinetic analysis of [3H]DA and [3H]5-HT uptake using synaptosomes prepared from nucleus accumbens (NAc), dorsal striatum (Str), medial prefrontal cortex (mPFC), and orbitofrontal cortex (OFC) isolated from individual rats. Vmax for DAT in NAc and for SERT in OFC were positively correlated with negative urgency scores. The current findings suggest that mood-based impulsivity (negative urgency) is associated with enhanced DAT function in NAc and SERT function in OFC.
Keywords: Negative urgency, dopamine transporter, serotonin transporter, nucleus accumbens, orbitofrontal cortex, rat
1. Introduction
Impulsivity is often fractioned into two broad categories, impulsive choice (i.e., inability to delay gratification) and impulsive action (i.e., inability to inhibit a prepotent response) [1]. In addition to these facets of impulsivity, negative urgency has received considerable consideration in clinical research. Negative urgency is the tendency to act rashly during a negative mood state and is one of four measures of impulsivity included in the UPPS personality questionnaire [2]. Similar to other facets of impulsivity, negative urgency is a predictor of several maladaptive behaviors, including drug abuse, binge eating, pathological gambling, risky sex, and problematic alcohol use [3–5; see 6 for a full review].
Currently, animal models of negative urgency are lacking, thus limiting exploration of the neurobiological mechanisms involved in this facet of impulsivity. In one study, a reward omission test was used to model negative urgency in rats [7]. In this paradigm, rats learned to associate a stimulus light with delivery of one sucrose pellet and then responded on a lever to earn sucrose pellets. When expected food delivery was omitted, rats showed increased lever responding on the lever associated with food delivery. This finding translated to human participants who showed a similar increase in response rate following omission of expected monetary reward, with the increase in response rate being associated with negative urgency scores on the UPPS [7]. These findings suggest that a reward omission procedure may be useful model of negative urgency in both humans and laboratory animals.
One advantage of using animal models is the ability to elucidate the underlying neural mechanisms of negative urgency. Understanding the neurobiological mechanisms involved in negative urgency may help explain why these individuals are more likely to engage in maladaptive behaviors. Several brain regions within the mesocorticolimbic pathway, including nucleus accumbens (NAc), dorsal striatum (Str), medial prefrontal cortex (mPFC), and orbitofrontal cortex (OFC), have been implicated as being involved in various facets of impulsivity [8–9]. Furthermore, dopamine (DA) and serotonin (5-HT) systems are important mediators of impulsive behavior [8–10]. Extracellular DA and 5-HT concentrations are regulated by DA transporters (DAT) and 5-HT transporters (SERT). Polymorphisms in genes encoding DAT and SERT are associated with impulsivity, as well as neuropsychiatric conditions associated with increased impulsivity, such as attention deficit hyperactivity disorder (ADHD) and substance use disorders [11–16]. The role of DAT and SERT in impulsivity is further supported by pharmacological evidence showing that DAT and SERT inhibitors alter impulsive behavior in humans [17–19] and rats [20–23]. However, it is unclear if DAT and SERT mediate negative urgency behavior. Thus, the goal of the present study was to determine the role of mesocorticolimbic DAT and SERT function in negative urgency behavior in rats using a reward omission task as previously described [7].
2. Materials and Methods
2.1. Materials
[3H]5-HT (5-[1,2-3H(N)-hydroxytryptamine creatinine sulfate; specific activity, 27.1 Ci/mmol) and [3H]DA (3,4-ethyl-2 [N-3H] dihydroxyphenylethylamine; specific activity, 31 Ci/mmol) were purchased from Perkin Elmer Life Sciences (Boston, MA). 5-Hydroxytryptamine creatinine sulfate (5-HT), dopamine HCl, desipramine HCl, nomifensine maleate,1-(2-bis(4-fluorphenyl)-methoxy)-ethyl-4-(3-phenyl-propyl) piperazine HCl (GBR 12909), fluoxetine HCl, pargyline HCl, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), catechol, L-ascorbic acid and D-glucose were purchased from Sigma-Aldrich (St. Louis, MO). Paroxetine HCl was provided generously by Beecham Pharmaceuticals (Surrey, UK). All other chemicals were purchased from Fisher Scientific Co. (Pittsburgh, PA).
2.2. Animals
Forty-seven male, experimentally-naïve Sprague Dawley rats (250–275 g at the beginning of operant training) were obtained from Harlan Laboratories (Indianapolis, IN) and were housed individually upon arrival. Rats were acclimated in a colony room held at constant temperature and handled for 7 days before operant training. Light and dark phases were on a 12:12 h cycle, and all procedures occurred during the light phase. Rats were food restricted (approximately 85% of free feed body weight) during behavioral studies. All procedures were in accordance with the “Guide for the Care and Use of Laboratory Animals” [24] and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
2.3. Behavioral apparatus
Operant conditioning chambers (28 × 21 × 21 cm; ENV-008; MED Associates, St. Albans, VT) located inside sound-attenuating chambers (ENV-018M; MED Associates) were used. The front and back walls of the experimental chambers were made of aluminum, while the side walls were made of Plexiglas. A recessed food tray (5 x 4.2 cm) was located 2 cm above the floor in the bottom-center of the front wall. A 28-V white cue light was located 6 cm above each response lever. A white house light was mounted in the center of the back wall of the chamber. All responses and scheduled consequences were recorded and controlled by a computer interface using Med-IV software.
2.4. Procedure
2.4.1. Reward Omission Task
The reward omission task employed has been previously described [7]. Rats were given 10 sessions consisting of 32 light-sucrose Pavlovian associations/session. During these sessions, either the left or right stimulus light (counterbalanced across rats) was illuminated for 5 sec, followed by delivery of one sucrose pellet (F0021 dustless precision pellet, Bio-Serve, Frenchtown, NJ). Following a 2-sec delay in the dark, the houselight was illuminated for 10 sec (intertrial interval; ITI).
Rats then were given 8 sessions of operant conditioning training consisting of 32 2-min trials/session. Each trial was separated by a 10-sec ITI. During these sessions, both levers were extended into the chamber. One lever was designated as active, in which responses resulted in delivery of one sucrose pellet. The other lever was designated as inactive, in which responses were recorded, but had no programmed consequence. The response requirement increased every 2 sessions (FR-1, 3, 5, and 10). Levers designated as active and inactive were counterbalanced across rats.
Following operant training, rats were given 30 baseline training sessions for the reward omission task. Each baseline session consisted of 32 trials separated into 2 components. Each trial began with a light-sucrose Pavlovian association component. Following delivery of the sucrose pellet, a 2-sec delay in the dark was imposed, followed immediately by the extension of both levers. Each lever was presented for 2 min. Rats completed a FR-10 schedule of reinforcement on the active lever to receive one sucrose pellet. No time-out period was imposed following reinforcement. After 2 min, a 10-sec ITI occurred, signaled by illumination of the houselight.
Following the baseline training sessions, rats received an alternating schedule of training and test sessions, such that 4 training sessions separated each of 3 test sessions. During a test session, rats were given 24 reward trials and 8 omission trials, randomly intermixed. Reward trials were identical to those presented during baseline training sessions. Omission trials were similar to reward trials, except that rats did not receive a sucrose pellet following presentation of the stimulus light during the Pavlovian component of the trial. Negative urgency scores were calculated using the equation U = [(O-R)/R] * 100%, where U is the negative urgency score, O is the average number of responses during omission trials, and R is the average number of responses during reward trials. Increasing positive values indicate greater levels of negative urgency behavior. Immediately following the final test session, DAT and SERT function were assessed as described below.
2.4.2. [3H]DA and [3H]5-HT uptake
Immediately following the final test session, DAT and SERT function were determined in NAc, Str, mPFC and OFC from individual rats using saturation analysis of [3H]DA and [3H]5-HT uptake, respectively. Twenty-four rats were used in [3H]DA uptake assays, and 23 rats were used for [3H]5-HT uptake assays. Kinetic parameters of transporter function, Vmax (pmol/min/mg) and Km (μM), for [3H]DA and [3H]5-HT uptake were determined using a previously published method [25]. Each brain region obtained from each rat was homogenized in 20 ml of ice-cold sucrose solution (0.32 M sucrose and 5 mM sodium bicarbonate, pH 7.4) with 16 passes of a Teflon pestle homogenizer (clearance, ~0.003”). Homogenates were centrifuged at 2000g for 10 min at 4°C, and resulting supernatants were centrifuged at 20,000g for 17 min at 4°C. Resulting pellets were resuspended in 2.2 ml of ice-cold assay buffer (125 mM NaCl, 5 mM KCl, 1.5 mM MgSO4, 1.25 mM CaCl2, 1.5 mM KH2PO4, 10 mM glucose, 25 mM HEPES, 0.1 mM EDTA, 0.1 mM pargyline, and 0.1 mM L-ascorbic acid, saturated with 95% O2/5% CO2, pH 7.4) to obtain synaptosomal suspensions.
Synaptosomes prepared from NAc (~50 μg protein/30 μl for DA uptake and ~80 μg protein/50 μl for 5-HT uptake), Str (~50 μg protein/30 μl for DA uptake and ~80 μg protein/50 μl for 5-HT uptake), mPFC (~40 μg protein/100 μl for both DA and 5-HT uptake), and OFC (~50 μg protein/100 μl for both DA and 5-HT uptake) were incubated in a metabolic shaker for 5 min at 34°C. [3H]DA uptake in mPFC and OFC synaptosomes was assessed in the presence of desipramine (5 nM; 25 μl) and paroxetine (5 nM; μl) to inhibit norepinephrine transporter (NET) and SERT function, respectively, isolating uptake by DAT [22]. [3H]5-HT uptake in mPFC and OFC synaptosomes was determined using buffer containing desipramine (1 μM; 25 μl) and GBR 12909 (50 nM; 25 μl) to inhibit NET and DAT, respectively, isolating uptake by SERT [26–28]. [3H]5-HT uptake into NAc and Str synaptosomes was determined using buffer containing GBR12909 (50 nM; 25 μl) to inhibit DAT, isolating uptake via SERT [24]. To evaluate DA uptake, 7 [3H]DA concentrations (0.01–1 μM; 25 μl for mPFC and OFC, 50 μl for NAc and Str) were included during incubation. To evaluate 5-HT uptake in the 4 brain regions, [3H]5-HT (1–300 nM, 25 μl) was incubated. For the DA and 5-HT uptake assays, a total incubation volume of 250 μl was used for both mPFC and OFC. For both NAc and Str, total incubation volume was 250 μl for 5-HT and 500 μl for DA. To determine nonspecific [3H]DA uptake, nomifensine (10 μM) was included during incubation (25 μl for mPFC and OFC; 50 μl for NAc and Str). To determine nonspecific [3H]5-HT uptake, fluoxetine (10 μM) was included during incubation (25 μl for mPFC and OFC; 25 μl for NAc and Str). Incubations continued for 5 min at 34°C and were terminated by addition of 3 ml of ice-cold assay buffer. Samples were filtered immediately through Whatman GF/B glass fiber filters (presoaked with 1 mM pyrocatechol for 3 hr) using a Brandel cell harvester (model MP-43RS; Brandel Inc., Gaithersburg, MD). Filters were washed 3 times with 3 ml of ice-cold buffer containing 1 mM pyrocatechol. Radioactivity was determined by liquid scintillation spectrometry (model B1600TR; PerkinElmer Life and Analytical Sciences). Protein concentrations were determined using bovine serum albumin as the standard [29]. Vmax and Km values were determined using the commercially available Graph-Pad Prism 4.0 program (Graph-Pad Software Inc., San Diego, CA).
2.5. Data and statistical analysis
If negative urgency scores (averaged across each test session) were greater than 1.5 times the interquartile range, rats were considered statistical outliers and excluded from behavioral and neurochemical analyses. Of the 47 rats evaluated in the behavioral assay, behavioral data from 3 rats were determined to be outliers, leaving 22 rats for each of the [3H]DA and [3H]5-HT uptake assays. Data were analyzed using a one-sample t test to compare average negative urgency scores to 0. To determine if negative urgency scores differed across sessions, a repeated-measures ANOVA was conducted, with session as a within-subjects factor. For [3H]DA and [3H]5-HT uptake assays, kinetic parameters for maximal transport velocity (Vmax, pmol/min/mg) and affinity (Km, μM) were estimated by fitting the data to the Michaelis-Menten equation. Log-transformed Km values were used for statistical analyses. Pearson r correlations were used to correlate negative urgency scores with either Vmax or Km (log-transformed) for [3H]DA and [3H]5-HT uptake in NAc, Str, mPFC, and OFC. For the DA uptake assay, brain region dissection isolating NAc and Str was not successful in 2 rats. For the [3H]DA uptake assay, mPFC and OFC were excluded for 5 and 4 rats, respectively, from the data analysis due to lack of saturation of uptake, which did not allow for determination of Vmax. Statistical tests were considered significant at p < 0.05.
3. Results
3.1. Reward omission task
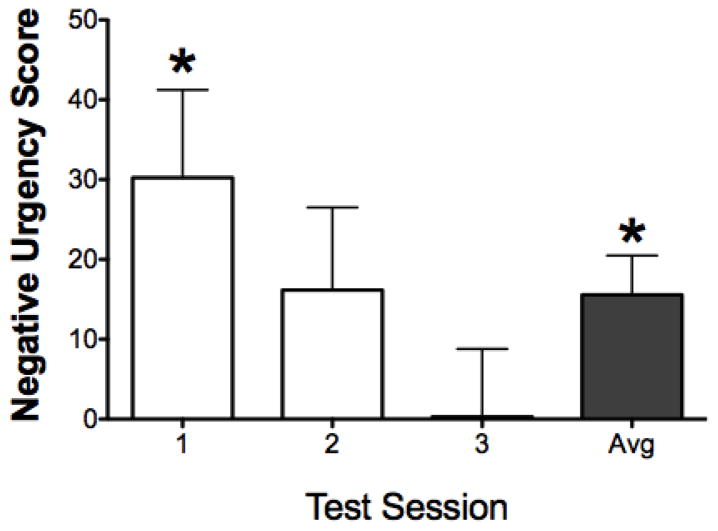
Figure 1 shows negative urgency scores across the 3 test sessions. One-sample t tests showed that responses on the active lever increased 30.2% following omission of an expected food pellet relative to delivery of an expected food pellet on test session 1 (t(43) = 2.75, p < .05). Averaged across all 3 test sessions, rats showed a 15.6% increase in lever responding following omission of expected reward relative to delivery of expected reward (t(43) = 3.16, p < .05). While negative urgency scores tended to decrease across test sessions, repeated-measures ANOVA did not reveal a significant difference across sessions.
Figure 1.
Negative urgency scores are plotted as a function of test session (n = 44). Note, a negative urgency score of 0 indicates no difference in responding between reward delivery and omission trials, positive values indicate greater responding on reward omission trials, and negative values indicate greater responding on reward delivery trials. Error bars represent SEM. *p <0.05, compared to a negative urgency score of 0.
3.2. [3H]DA and [3H]5-HT uptake in NAc, Str, mPFC, and OFC
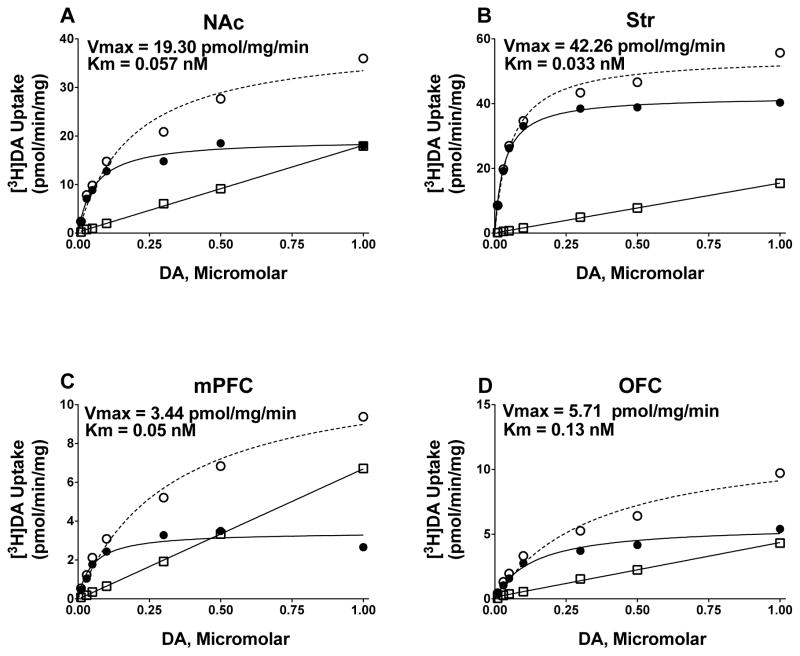
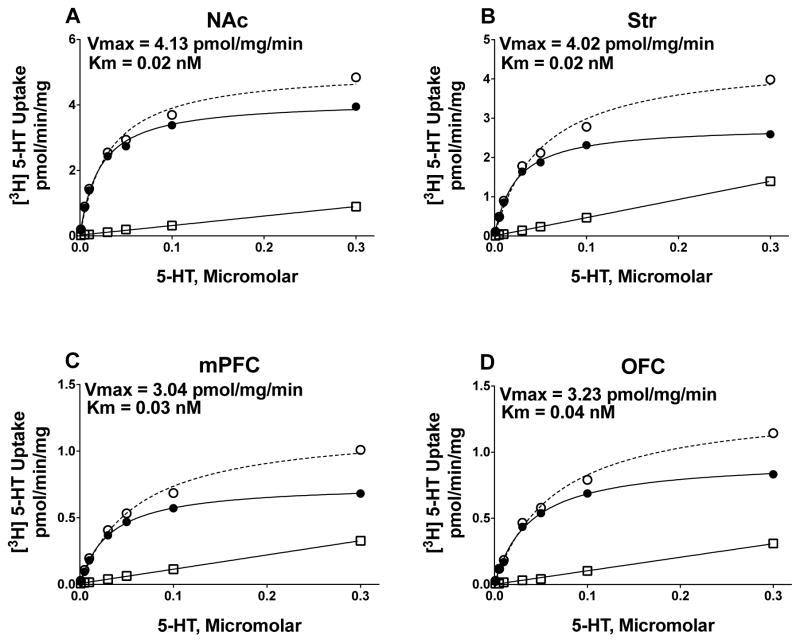
DAT and SERT function were determined using saturation analyses of [3H]DA and [3H]5-HT uptake in NAc, Str, mPFC and OFC. As illustrated in Figure 2 (DAT uptake) and Figure 3 (SERT uptake), specific uptake was saturable for both [3H]DA and [3H]5-HT in NAc, Str, mPFC, and OFC obtained from individual rats.
Figure 2.
Saturation curves for [3H]DA uptake at DAT in NAc, Str, mPFC, and OFC synaptosomal preparations from a representative individual rat. Nonspecific [3H]DA uptake was determined in the presence of 10 μM nomifensine. Specific uptake (closed circles) was obtained by subtracting nonspecific uptake (open squares) from total uptake (open circles). Vmax represents the maximal velocity of [3H]DA uptake, and Km represents affinity of DA for DAT.
Figure 3.
Saturation curves for [3H]5-HT uptake at SERT in NAc, Str, mPFC, and OFC synaptosomal preparations from a representative individual rat. Nonspecific [3H]5-HT uptake was determined in the presence of 10 μM fluoxetine. Specific uptake (closed circles) was obtained by subtracting nonspecific uptake (open squares) from total uptake (open cirlces). Vmax represents the maximal velocity of [3H]5-HT uptake, and Km represents affinity of 5-HT for SERT.
3.3. Correlation between negative urgency scores and DAT or SERT kinetic parameters
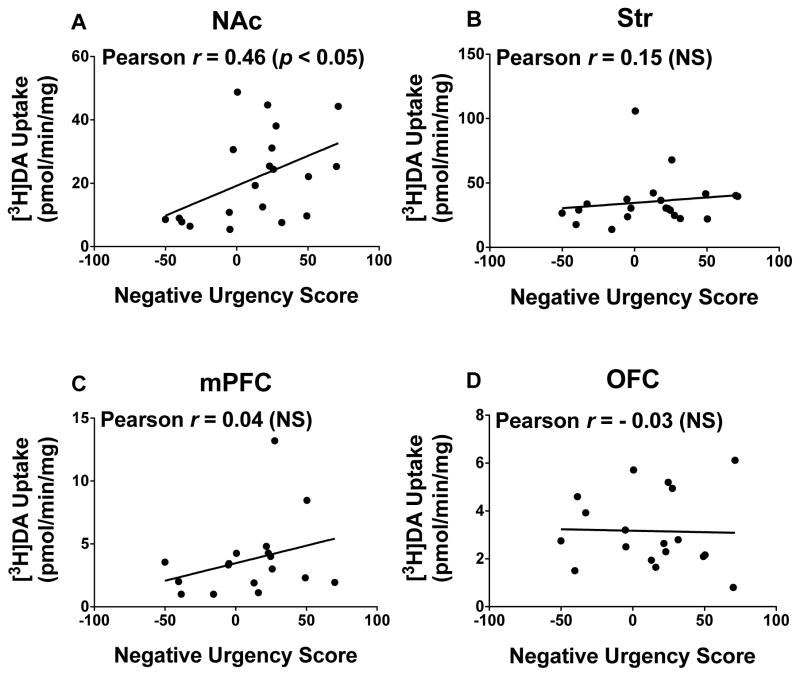
Pearson r correlation coefficients were obtained to determine whether individual differences in DAT or SERT kinetic parameters (Vmax and Km) for [3H]DA and [3H]5-HT uptake were associated with negative urgency scores in the reward omission task. With respect to DAT function, Vmax for [3H]DA uptake in NAc from individual rats was positively correlated with negative urgency scores (r = 0.46, p < 0.05; Figure 4a). However, no correlations were found between Vmax for [3H]DA uptake in Str, mPFC or OFC and negative urgency scores (Figures 4b-d). No correlations were found between Km of [3H]DA uptake in any brain region and negative urgency scores (data not shown).
Figure 4.
Pearson r correlation coefficients of Vmax for [3H]DA uptake in (a) NAc (n = 20), (b) Str (n = 21), (c) mPFC (n = 17), and (d) OFC (n = 18) with negative urgency scores. Data points represent individual rats.
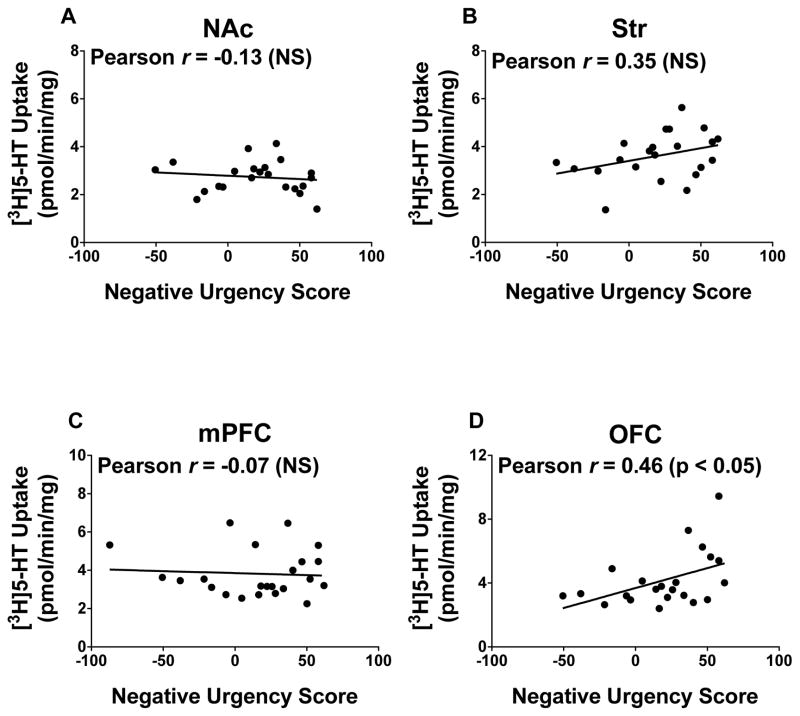
With respect to SERT function, no correlations were found between Vmax for [3H]5-HT uptake in NAc, Str, and mPFC and negative urgency scores (Figures 5a-c). Vmax for [3H]5-HT uptake in OFC was positively correlated with negative urgency scores (r = 0.46, p < 0.05; Figure 5d). No correlations were obtained between Km of [3H]5-HT uptake in any brain region and negative urgency scores (data not shown).
Figure 5.
Pearson r correlation coefficients of Vmax for [3H]5-HT uptake in (a) NAc (n = 22), (b) Str (n = 22), (c) mPFC (n = 22), and (d) OFC (n = 22) with negative urgency scores. Data points represent individual rats.
4. Discussion
The goal of the current study was to determine if individual differences in DAT or SERT function within mesocorticolimbic brain regions is associated with performance in a reward omission task. The reward omission task used here models negative urgency behavior in humans and rats [7]. Overall, rats responded significantly more (30.2% increase) following reward omission relative to reward delivery on test session 1. More importantly, the current results show that rats exhibiting increased responding following reward omission had greater [3H]DA uptake in NAc and greater [3H]5-HT uptake in OFC. The current results suggest that NAc DAT and OFC SERT may be important mechanisms underlying increased levels of negative urgency.
Rats showed increased responding following reward omission, which is consistent with a previous study [7]. Gipson et al. [7] administered a single test session to measure the effect of reward omission on food responding, whereas the present study examined the effect of reward omission across several sessions. The current results showed that the increase in responding for food following reward omission dissipated after three test sessions. Of note, Gipson et al. [7] showed that rats self-administering d-amphetamine displayed negative urgency-like behavior across multiple sessions, which suggests that reinforcer type is an important mediator of the effect of reward omission. Overall, the current results support the use of the reward omission task to model negative urgency-like behavior.
[3H]DA and [3H]5-HT uptake kinetics were analyzed in several brain regions that have been reported to be critical mediators of various facets of impulsivity [8–9]. We observed greater DAT function within NAc in animals exhibiting greater negative urgency scores. While we did not measure extracellular dopamine, the greater DAT function observed with high negative urgency suggests decreased extracellular dopamine concentrations and decreased dopamine signaling in NAc. Importantly, NAc responds to anticipated rewards [30] and plays a critical role in pathological gambling [31], aggression [32], and addiction [33]. NAc has also been implicated in impulsivity, as lesions to NAc core increase impulsive choice in rats [34–37], and inactivation of NAc shell increases impulsivity in the five choice serial reaction time (5CSRT) task [38]. Furthermore, decreased NAc activation is observed following reward omission in humans [39]. Drugs effective in treating impulse-control disorders (e.g., ADHD) increase DA levels primarily by reversing and/or inhibiting DAT function [40–41]. Differential NAc DAT expression has been linked to impulsivity. For example, overexpression of DAT within NAc increases impulsive and risky decision making in rats [42].
The current results extend these findings by showing that increased negative urgency is associated with increased [3H]DA uptake in NAc. Thus, in addition to playing a role in impulsive choice and negative mood [43], decreased NAc DA levels may an important mediator of mood-based rash action. The current results also show that SERT uptake in OFC is positively correlated with negative urgency scores. OFC is implicated in various forms of decision making, most importantly in updating the value of an expected reward on the basis of past experience [44]. OFC also is implicated in drug abuse, as hypoactivity within this region is observed in individuals with cocaine addiction [45]. Evidence also shows that OFC is involved in impulsive behavior, although some inconsistencies exist in the literature. For example, several studies have reported an increase in impulsive choice following lesions to OFC [46–49]; however, other studies have observed either a decrease in impulsive choice [50–51] or no change in impulsive choice following temporary inactivation via GABA agonists or following excitotoxic lesions [52–56]. Despite these discrepancies regarding impulsive choice, damage to OFC increases impulsive action [57–58]. Further, clinical evidence indicates that low levels of 5-HT are linked to several impulsive behaviors, such as risk taking, aggression, violence, and suicide [59–61]. Preclinical work also shows that 5-HT activity within OFC is involved in impulsivity, with forebrain 5-HT depletion increasing impulsive choice in a delay discounting procedure [62–65] and impulsive action in the 5CSRT task [66–67]. The finding that negative urgency was positively correlated with OFC SERT function is consistent with our observations that increased impulsive choice is associated with increased OFC SERT function [Darna et al., submitted]. Overall, these results show that increased SERT function in OFC, likely leading to decreased extracellular 5-HT levels in OFC, is associated with increased negative urgency behavior.
Finally, although Str and mPFC are involved in impulsive behavior [8–9] and ventral PFC activation is associated with reward omission in humans [68], DAT and SERT function in these regions was not associated with negative urgency scores in the current study. These results provide support that the underlying neural mechanisms mediating negative urgency are distinct from other facets of impulsivity. Although the current results are promising, more work will be required to elucidate the underlying neural mechanisms of negative urgency. Specifically, future studies are needed to determine the potential role of the amygdala in the reward omission task. Amygdala is known to be involved in negative affect [69] and has been suggested to be an important mediator of negative urgency [70]. Considering the basolateral nucleus of the amygdala shares a reciprocal connection with OFC [71–72] and projects glutamatergic neurons to NAc [73], this brain region may be a critical mediator of negative urgency as modeled by the reward omission task.
Highlights.
Rats increased lever responding for food following omission of expected reward.
DAT function in NAc positively correlated with negative urgency scores.
SERT function in OFC positively correlated with negative urgency scores.
Acknowledgments
This work was supported by National Institutes of Health grants P50 DA05312, R01 DA12964, UL1 TR000117, and T32 DA016176. The authors would like to thank Emily Denehy and Travis McCuddy for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34:1306–18. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whiteside S, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Pers Individ Dif. 2001;30:669–89. [Google Scholar]
- 3.Fischer S, Settles RE, Collins B, Gunn R, Smith GT. The role of negative urgency and expectancies in problem drinking and disordered eating testing a model of comorbidity in pathological and at-risk samples. Psychol Addict Behav. 2012;26:112–23. doi: 10.1037/a0023460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Settles RE, Fischer S, Cyders MA, Combs JL, Gunn RL, Smith GT. Negative urgency: a personality predictor of externalizing behavior characterized by neuroticism, low conscientiousness, and disagreeableness. J Abnorm Psychol. 2012;121:160–72. doi: 10.1037/a0024948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verdejo-García A, Bechara A, Recknor EC, Pérez-García M. Negative emotion-driven impulsivity predicts substance dependence problems. Drug Alcohol Depend. 2007;91:213–9. doi: 10.1016/j.drugalcdep.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 6.Cyders MA, Smith GT. Emotion-based dispositions to rash action: positive and negative urgency. Psychol Bull. 2008;134:807–28. doi: 10.1037/a0013341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gipson CD, Beckmann JS, Adams ZW, Marusich JA, Nesland TO, Yates JR, et al. A translational behavioral model of mood-based impulsivity: implications for substance abuse. Drug Alcohol Depend. 2012;122:93–9. doi: 10.1016/j.drugalcdep.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardinal RN. Neural systems implicated in delayed and probabilistic reinforcement. Neural Networks. 2006;19:1277–1301. doi: 10.1016/j.neunet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–60. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Winstanley CA. The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br J Pharmacol. 2011;164:1301–21. doi: 10.1111/j.1476-5381.2011.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carver CS, Johnson SL, Joormann J, Kim Y, Nam JY. Serotonin transporter polymorphism interacts with childhood adversity to predict aspects of impulsivity. Psychol Sci. 2011;22:589–95. doi: 10.1177/0956797611404085. [DOI] [PubMed] [Google Scholar]
- 12.Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–57. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- 13.Paloyelis Y, Asherson P, Mehta MA, Faraone SV, Kuntsi J. DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention deficit/hyperactivity disorder and healthy controls. Neuropsychopharmacology. 2010;35:2414–26. doi: 10.1038/npp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonuga-Barke EJ, Kumsta R, Schlotz W, Lasky-Su J, Marco R, Miranda A, et al. A functional variant of the serotonin transporter gene (SLC6A4) moderates impulsive choice in attention-deficit/hyperactivity disorder boys and siblings. Biol Psychiatry. 2011;70:230–6. doi: 10.1016/j.biopsych.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waldman ID, Rowe DC, Abramowitz A, Kozel ST, Mohr JH, Sherman SL, et al. Association and linkage of the dopamine transporter gene and attention-deficit hyperactivity disorder in children: heterogeneity owing to diagnostic subtype and severity. Am J Hum Genet. 1998;63:1767–76. doi: 10.1086/302132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walderhaug DL, Herman AI, Magnusson A, Morgan MJ, Landrø NI. The short (S) allele of the serotonin transporter polymorphism and acute tryptophan depletion both increase impulsivity in men. Neurosci Lett. 2010;473:208–11. doi: 10.1016/j.neulet.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crunelle CL, van den Brink W, Dom G, Booij J. Dopamine transporter occupancy by methylphenidate and impulsivity in adult ADHD. Br J Psychiatry. 2014;204:486–7. doi: 10.1192/bjp.bp.113.132977. [DOI] [PubMed] [Google Scholar]
- 18.de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–25. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 19.Pietras CJ, Cherek DR, Lane SD, Tcheremissine OV, Steinberg JL. Effects of methylphenidate on impulsive choice in adult humans. Psychopharmacology. 2003;170:390–8. doi: 10.1007/s00213-003-1547-2. [DOI] [PubMed] [Google Scholar]
- 20.Bizot JC, Thiébot MH, Le Bihan C, Soubrié P, Simon P. Effects of imipramine-like drugs and serotonin uptake blockers on delay of reward in rats. Possible implication in the behavioral mechanism of action of antidepressants. J Pharmacol Exp Ther. 1988;246:1144–51. [PubMed] [Google Scholar]
- 21.Bizot JC, Le Bihan C, Puech AJ, Hamon M, Thiébot M. Serotonin and tolerance to delay of reward in rats. Psychopharmacology. 1999;146:400–12. doi: 10.1007/pl00005485. [DOI] [PubMed] [Google Scholar]
- 22.van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 23.van Gaalen MM, Brueggeman RJ, Bronius PF, Schoffelmeer AN, Vanderschuren LJ. Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology. 2006;187:73–85. doi: 10.1007/s00213-006-0396-1. [DOI] [PubMed] [Google Scholar]
- 24.National Research Council. Guide for the care and use of laboratory animals. 8. Washington: National Academy Press; 2010. [Google Scholar]
- 25.Marusich JA, Darna M, Charnigo RJ, Dwoskin LP, Bardo MT. A multivariate assessment of individual differences in sensation seeking and impulsivity as predictors of amphetamine self-administration and prefrontal dopamine function in rats. Exp Clin Psychopharm. 2011;19:275–84. doi: 10.1037/a0023897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Helligenstein JH, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 27.Norrholm SD, Horton DB, Dwoskin LP. The promiscuity of the dopamine transporter: implications for the kinetic analysis of [3H]serotonin uptake in rat hippocampal and striatal synaptosomes. Neuropharmacology. 2007;53:982–9. doi: 10.1016/j.neuropharm.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou FC, Lesch KP, Murphy DL. Serotonin uptake into dopamine neurons via dopamine transporters: a compensatory alternative. Brain Res. 2002;942:109–19. doi: 10.1016/s0006-8993(02)02709-9. [DOI] [PubMed] [Google Scholar]
- 29.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 30.Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci. 1992;12:4595–610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adriani W, Boyer F, Leo D, Canese R, Podo F, Perrone-Capano C, et al. Social withdrawal and gambling-like profile after lentiviral manipulation of DAT expression in the rat accumbens. Int JNeuropsychopharmacol. 2010;13:1329–42. doi: 10.1017/S1461145709991210. [DOI] [PubMed] [Google Scholar]
- 32.Albert DJ, Petrovic DM, Walsh ML, Jonik RH. Medial accumbens lesions attenuate testosterone-dependent aggression in male rats. Physiol Behav. 1989;46:625–31. doi: 10.1016/0031-9384(89)90342-9. [DOI] [PubMed] [Google Scholar]
- 33.Belin D, Belin-Rauscent A, Murray JE, Everitt BJ. Addiction: failure of control over maladaptive incentive habits. Curr Opin Neurobiol. 23:564–72. doi: 10.1016/j.conb.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 34.Bezzina G, Cheung TH, Asgari K, Hampson CL, Body S, Bradshaw CM, et al. Effects of quinolinic acid-induced lesions of the nucleus accumbens core on intertemporal choice: a quantitative analysis. Psychopharmacology. 2007;195:71–84. doi: 10.1007/s00213-007-0882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats be lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- 36.da Costa Araújo S, Body S, Hampson CL, Langley RW, Deakin JF, Anderson IM, et al. Effects of lesions of the nucleus accumbens core on inter-temporal choice: further observations with an adjusting-delay procedure. Behav Brain Res. 2009;202:272–7. doi: 10.1016/j.bbr.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Pothuzien HH, Jongen-Relo AL, Feldon J, Yee BK. Double dissociation of the effects of selective nucleus accumbens core and shell lesions on impulsive-choice behaviour and salience learning in rats. Eur J Neurosci. 2005;22:2605–16. doi: 10.1111/j.1460-9568.2005.04388.x. [DOI] [PubMed] [Google Scholar]
- 38.Feja M, Hayn L, Koch M. Nucleus accumbens core and shell inactivation differentially affects impulsive behaviours in rats. Prog NeuroPsychopharmacol Biol Psychiatry. 2014;54:31–42. doi: 10.1016/j.pnpbp.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Pedroni A, Koeneke S, Velickaite A, Jäncke L. Differential magnitude coding of gains and omitted rewards in the ventral striatum. Brain Res. 2011;1411:76–86. doi: 10.1016/j.brainres.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 40.Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison to amphetamine. J Neurochem. 1997;68:2032–37. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- 41.Volkow ND, Fowler JS, Wang GJ, Ding YS, Gatley SJ. Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. Eur Neuropsychopharmacol. 2002;12:557–66. doi: 10.1016/s0924-977x(02)00104-9. [DOI] [PubMed] [Google Scholar]
- 42.Adriani W, Boyer F, Giolosa L, Macri S, Dreyer JL, Laviola G. Increased impulsive behavior and risk proneness following lentivirus-mediated dopamine transporter over-expression in rats’ nucleus accumbens. Neuroscience. 2009;159:47–58. doi: 10.1016/j.neuroscience.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 43.Volman SF, Lammel S, Margolis EB, Kim Y, Richard JM, Roitman MF, Lobo MK. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. J Neurosci. 2013;33:17569–76. doi: 10.1523/JNEUROSCI.3250-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Ann Rev Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- 45.Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey SL, Bendriem B, et al. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148:621–6. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- 46.Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, et al. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2002;160:290–8. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- 47.Kheramin S, Body S, Mobini S, Ho MY, Velázquez-Martinez DN, Bradshaw CM, et al. Effects of quinolinic acid-induced lesions of the orbital prefrontal cortex on inter-temporal choice: a quantitative analysis. Psychopharmacology. 2002;165:9–17. doi: 10.1007/s00213-002-1228-6. [DOI] [PubMed] [Google Scholar]
- 48.Kheramin S, Body S, Mobini S, Ho MY, Velázquez-Martinez DN, Bradshaw CM, et al. Effects of orbital prefrontal cortex dopamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology. 2004;175:206–14. doi: 10.1007/s00213-004-1813-y. [DOI] [PubMed] [Google Scholar]
- 49.Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MF. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9:1161–8. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- 50.Mar AC, Walker AL, Theobald DE, Eagle DM, Robbins TW. Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J Neurosci. 2011;31:6398–404. doi: 10.1523/JNEUROSCI.6620-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24:4718–22. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abela AR, Chudasama Y. Dissociable contributions of the ventral hippocampus and orbitofrontal cortex to decision-making with a delayed or uncertain outcome. Eur J Neurosci. 2013;37:640–7. doi: 10.1111/ejn.12071. [DOI] [PubMed] [Google Scholar]
- 53.Churchwell JC, Morris AM, Heurtelou NM, Kesner RP. Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci. 2009;123:1185–96. doi: 10.1037/a0017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jo S, Kim KU, Lee D, Jung MW. Effect of orbitofrontal cortex lesions on temporal discounting in rats. Behav Brain Res. 2013;245:22–8. doi: 10.1016/j.bbr.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mariano TY, Bannerman DM, McHugh SB, Preston TJ, Rudebeck PH, Rudebeck SR, et al. Impulsive choice in hippocampal but not orbitofrontal cortex-lesioned rats on a nonspatial decision-making maze task. Eur J Neurosci. 2009;30:472–84. doi: 10.1111/j.1460-9568.2009.06837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stopper CM, Green EB, Floresco SB. Selective involvement by the medial orbitofrontal cortex in biasing risky, but not impulsive, choice. Cereb Cortex. 2014;24:154–62. doi: 10.1093/cercor/bhs297. [DOI] [PubMed] [Google Scholar]
- 57.Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–19. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 58.Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, Robbins TW. Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb Cortex. 2008;18:178–88. doi: 10.1093/cercor/bhm044. [DOI] [PubMed] [Google Scholar]
- 59.Åsberg M, Träskman L, Thorén P. 5-HIAA in the cerebrospinal fluid: a biochemical suicide predictor. Arch Gen Psychiatry. 1976;33:1193–97. doi: 10.1001/archpsyc.1976.01770100055005. [DOI] [PubMed] [Google Scholar]
- 60.Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci. 1983;33:2609–14. doi: 10.1016/0024-3205(83)90344-2. [DOI] [PubMed] [Google Scholar]
- 61.Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, et al. Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am J Psychiatry. 1994;151:1485–91. doi: 10.1176/ajp.151.10.1485. [DOI] [PubMed] [Google Scholar]
- 62.Bizot J, Le Bihan C, Puech AJ, Hamon M, Thiébot M. Serotonin and tolerance to delay of reward in rats. Psychopharmacology. 1999;146:400–12. doi: 10.1007/pl00005485. [DOI] [PubMed] [Google Scholar]
- 63.Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Effects of central 5-hydroxytryptamine depletion on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2000;152:390–7. doi: 10.1007/s002130000542. [DOI] [PubMed] [Google Scholar]
- 64.Schweighofer N, Bertin M, Shishida K, Okamoto Y, Tanaka SC, Yamawaki S, et al. Low-serotonin levels increase delayed reward discounting in humans. J Neurosci. 2008;28:4528–32. doi: 10.1523/JNEUROSCI.4982-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wogar MA, Bradshaw CM, Szabadi E. Effect of lesions of the ascending 5-hydroxytryptaminergic pathways on choice between delayed reinforcers. Psychopharmacology. 1993;111:239–43. doi: 10.1007/BF02245530. [DOI] [PubMed] [Google Scholar]
- 66.Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology. 1997;133:329–42. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- 67.Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Fractioning impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 2004;29:1331–43. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- 68.Adler B, Walter H, Erk S. Neural correlates of frustration. Neuroreport. 2005;16:669–72. doi: 10.1097/00001756-200505120-00003. [DOI] [PubMed] [Google Scholar]
- 69.Barrett LF, Bliss-Moreau E, Duncan SL, Rauch SL, Wright CI. The amygdala and the experience of affect. Soc Cog Affect Neurosci. 2007;2:73–83. doi: 10.1093/scan/nsl042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cyders MA, Smith GT. Emotion-based dispositions to rash action: positive and negative urgency. Psychol Bull. 2008;134:807–28. doi: 10.1037/a0013341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol. 1977;172:687–722. doi: 10.1002/cne.901720408. [DOI] [PubMed] [Google Scholar]
- 72.McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- 73.Christie MJ, Summers RJ, Stephenson JA, Cook CJ, Beart PM. Excitatory amino acid projections to the nucleus accumbens septi in the rat: a retrograde transport study utilizing D[3H]aspartate and [3H]GABA. Neuroscience. 1987;22:425–439. doi: 10.1016/0306-4522(87)90345-9. [DOI] [PubMed] [Google Scholar]