Abstract
Objective
Diet modification may improve body composition and respiratory parameters in children with respiratory insufficiency. Our objective was to examine the effect of an individualized diet intervention on changes in weight, lean body mass (LBM), minute ventilation (MV) and carbon dioxide production (VCO2) in children on home mechanical ventilator support.
Design
Prospective, open-labeled interventional study.
Setting
Study subjects' homes
Patients
Children, ages 1 month to 17 years, dependent on at least 12 hours per day of transtracheal mechanical ventilator support.
Intervention
Twelve weeks of an individualized diet modified to deliver energy at 90-110% of measured energy expenditure (MEE) and protein intake per age-based guidelines.
Measurements & Main Results
During a multidisciplinary home visit we obtained baseline values of height and weight, LBM% by bioelectrical impedance analysis, actual energy and protein intake by food record, and MEE by indirect calorimetry. An individualized diet was then prescribed to optimize energy and protein intake. After 12 weeks on this interventional diet we evaluated changes in weight, height, LBM%, MV and VCO2. Sixteen subjects, mean age 9.3y (SD 4.9), 8 male, completed the study. For the diet intervention, a majority of subjects required a change in energy and protein prescription. The mean percentage of energy delivered as carbohydrate was significantly decreased, 51.7% at baseline vs. 48.2% at follow-up, p=0.009. Mean height and weight increased on the modified diet. Mean LBM% increased from 58.3% to 61.8%. MV was significantly lower (0.18 L/min/kg vs. 0.15 L/min/kg, p=0.04) and we observed a trend towards lower VCO2 (5.4 ml/min*kg vs. 5.3 ml/min*kg, p=0.06) after 12 weeks on the interventional diet.
Conclusions
Individualized diet modification is feasible and associated with a significant decrease in minute ventilation, a trend towards significant reduction in carbon dioxide production, and improved body composition in children on long-term mechanical ventilation. Optimization of respiratory parameters and LBM by diet modification may benefit children with respiratory insufficiency in the intensive care unit.
Keywords: children, long-term mechanical ventilation, diet intervention, body composition, minute ventilation
Introduction
Mechanically ventilated children represent a vulnerable cohort at risk for malnutrition, inadequate protein intake and lower lean body mass (LBM).[1-3] Inadequate macronutrient intake has been associated with loss of muscle mass, whereas overfeeding has been associated with increased carbon dioxide burden and increased fat deposition, all of which can alter respiratory mechanics.[4-9] Low protein intake and LBM depletion have been associated with respiratory muscle weakness, increased respiratory effort, and decreased physical conditioning.[4, 5, 10] The impact of diet intervention on clinical outcomes has been previously explored in adult patients with respiratory insufficiency. Diet modification to optimize energy and protein intake has been associated with weight gain, increased LBM, improved handgrip strength and respiratory mechanics in this cohort.[6, 11, 12] A strategy of individualized diet intervention and its impact on respiratory and nutritional outcomes in children on long-term mechanical ventilation has not been previously evaluated.
We aimed to assess the feasibility of individualized diet intervention in children dependent on long-term mechanical ventilator support using a multidisciplinary model. The model would allow for diet modification based on comprehensive nutrition, metabolic and respiratory assessments performed in the subjects' home. We hypothesized that multidisciplinary assessments and individualized diet modifications would be feasible in the subject's home. In addition, we aimed to examine the effect of individualized diet optimization on nutritional status, body composition, CO2 production (VCO2) and minute ventilation (MV). We hypothesized that optimizing energy and protein intake would be associated with improved nutritional status, particularly increment in LBM, and decrease in VCO2 and MV. We chose the home ventilator dependent cohort to test our hypothesis in this pilot intervention study. If nutritional optimization is associated with positive outcomes in our study cohort, this concept could be generalized to a wider patient population with respiratory insufficiency.
Material & Methods
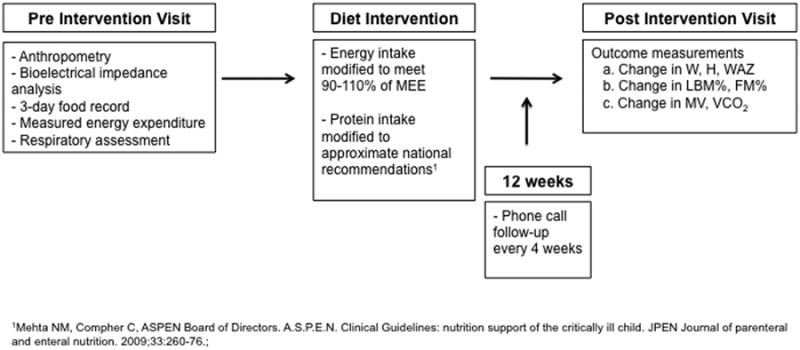
In a prospective interventional cohort study in children on home ventilator support, we used a mobile and multidisciplinary team model to make individualized diet modifications. Figure 1 depicts the study design further described in detail below. The Boston Children's Hospital Institutional Review Board approved this protocol and we obtained written informed consent from all study subjects' parents or guardians and assent from eligible subjects.
FIGURE 1. Schematic of the study protocol.
Study Population
Children aged 1 month to 17 years were elibigle if they were dependent on at least 12 hours of mechanical ventilation per day via a cuffed tracheostomy tube, with a fractional oxygen requirement (FiO2) less than 0.6, and a leak less than 10% around the cuff at the time of data collection. Exclusion criteria were illness such as fever, seizures and/or infectious disease in the 24 hours preceding the study, and/or respiratory decompensation requiring an increase in ventilator settings of >20% from baseline in the 72 hours preceding the study. Children were recruited from the Critical Care, Anesthesia, Perioperative Extension (CAPE) and Home Ventilation Program at our institution. In addition to their vulnerability for nutritional inadequacy, this cohort is characterized by children with static mechanical ventilatory requirements, and deemed suitable for assessing the impact of dietary changes on body composition and respiratory parameters. For this pilot study we determined a priori a sample size of 20 patients, to detect a 15% reduction in MV and 25% reduction in VCO2, with statistical power > 80% and alpha of 0.05. We recruited patients consecutively from the eligible pool in the home ventilation program until the first 20 subjects were enrolled.
Pre-intervention: Baseline Assessment
Baseline nutritional, metabolic and respiratory assessments were completed in the subject's home by the multidisciplinary team. Details of study procedures and results of these baseline assessments have been previously reported.(1) In brief, the procedures during the home visit included the following assessments. (A) Anthropometric measurements, specifically weight and height (or length in recumbent subjects and those under the age of 1 year), mid-upper arm circumference, and skinfold measurements were obtained. Weight for age z-score (WAZ) and BMI z-scores were calculated and used to classify nutritional status based on World Health Organization (WHO) criteria for children under 2 years of age, and Center for Disease Control and Prevention (CDC) criteria for those over 2 years of age.[13, 14] (B) Body composition was assessed by bioelectrical impedance analysis (BIA) using a multi-frequency impedance device (Bodystat Quadscan 4000®, Bodystat, Ltd., Tampa, FL). BIA procedures have been previously described.[1, 15] Using the principle of differential electrical conduction through body tissues, BIA calculates total body water from measured impedance values to 4 current frequencies flowing through the body. Current injector electrodes are placed on upper and lower extremities for this test. Total body water measurement is then used to derive lean body mass (LBM%) and fat mass (FM%) percentages using established published equations.[16] In subjects aged 3 years or older, FM% values by BIA were compared to age, gender and race-matched population norms.[17, 18] (C) Daily average energy intake (AEI, kcal/day), and its macronutrient components, protein (grams/day), carbohydrate (grams/day) and fat (grams/day), were determined using a 3-day food record and the ESHA Food Processor® software (ESHA Research, Salem, OR). Based on the ratio of AEI to MEE by indirect calorimetry, subjects were classified as underfed (AEI:MEE <90%), adequately fed (AEI:MEE 90-110%), overfed (AEI:MEE >110%). Protein intake adequacy was determined by comparing the actual intake (g/kg/d) of each subject to the American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) age-based recommendations; 2 - 3 g/kg/day in children 0-2 years old, 1.5-2 g/kg/day in children 2-13 years old, and 1.5 g/kg/day in children >13 years old.[19] (D) Metabolic and respiratory assessments were obtained by indirect calorimetry. MEE was assessed by indirect calorimetry using the CCM Express® (MedGraphics, St Paul, MN). We have previously reported the accuracy of this device over a range of tidal volumes relevant to the pediatric cohort.[1] The device was calibrated at the subject's home according to manufacturer's specifications. Breath to breath gas exchange measurements of volumetric oxygen consumption (VO2) and VCO2 were recorded for 30 minutes. A proximal flow sensor was affixed between the tracheostomy and the ventilator circuit in subjects who were mechanically ventilated 24 hours a day or directly unto the tracheostomy with no ventilator circuit in subjects who would have not been on mechanical ventilation per their regimen at the time of visit. The level of activity during the IC test was recorded for each subject. VO2 and VCO2 measurements were averaged over the steady state period, defined as a consecutive period ≥ 5 minutes when VO2 and VCO2 variance was less than 10%.[20] MEE, kcal/day, was calculated from gas exchange measurements, using the modified Weir equation.[21] VCO2 and MV were recorded from the indirect calorimetry test during the home visit.
Diet Intervention: Individualized Energy and Protein Intake Modification
In subjects who were underfed (AEI:MEE <90%) or overfed (AEI:MEE>110%), energy prescription was modified to meet 90 to 110% of the MEE. Protein intake was adjusted, as required, to meet the A.S.P.E.N. age-based recommendations in all subjects.[19] During the diet modification, we aimed to maintain macronutrient distribution within recommended ranges.[22] Wherever possible, the patient's baseline formula was used in the modified diet. The diet prescription was reviewed with the patient's primary gastroenterologist and/or dietitian prior to implementation. The modified diet was prescribed for a period of 12 weeks. In a randomized trial in adults with chronic lung disease 12 weeks of diet intervention was shown to be associated with significant changes in body composition, hand grip strength and lung function.[11] We did not change the subjects' usual route of feeding (gastrostomy, jejunostomy or oral) for the study. Follow-up was conducted every 4 weeks via telephone with the parents or guardians to ensure diet adherence, address any questions, and to monitor for intolerance. The research team was available to discuss any questions or concerns throughout the study period. During the 12-week period we recorded any significant intercurrent illness (hospitalization, change in mechanical ventilation support, change in FiO2, and prolonged period of no nutrition). Adherence to the prescribed diet was evaluated by comparing the AEI and protein intake recorded at the post-intervention assessment to the prescribed regimen using a paired t-test.
A post-intervention follow-up home visit was completed for each subject after 12 weeks on the modified diet. All the procedures completed in the baseline pre-intervention visit were repeated. The post-intervention visit was scheduled at the same time of day period as the pre-intervention visit. In subjects who had a significant intercurrent illness the post-intervention visit was scheduled when the subject had returned and maintained their baseline health and respiratory support status for at least 2 weeks.
Data analyses
The primary outcomes for this study were, a) change in nutritional parameters- weight, WAZ, height, LBM% and FM%, and b) change in the respiratory parameters- MV and VCO2, between the pre- and post-intervention assessments.
Statistical analyses were performed using GraphPad Prism version 5.04, (GraphPad Software, La Jolla, CA). Demographic and clinical data was tested for normality using the D'Agostino & Pearson normality test.[23] Normally distributed data are presented as mean (SD) and non-normally distributed data as median (IQR). Categorical data are presented as frequency (%). Statistical significance, where applicable, was set at p< 0.05. Paired t-tests were performed to evaluate for significant changes between pre-intervention and post-intervention weight, WAZ, height, BMI z-score, LBM%, FM%, MV, and VCO2.
Results
Twenty eligible children were enrolled and 16 completed all the study procedures. Two subjects were withdrawn from the study prior to diet intervention for non-clinical reasons and two were withdrawn from the study due to perceived enteral nutrition intolerance by the subjects' parents or guardians. Concern for nutrition intolerance was identified soon after initiating the interventional diet and the subjects were returned to their pre-study nutrition regimen. Follow-up by telephonic conversations was provided and no complications were identified through the end of the study period. There were no significant differences in the baseline characteristics of the 16 patients who completed the study and the complete cohort that was enrolled (Table 1).
TABLE 1. Baseline demographic and clinical characteristics of enrolled subjects on trans-tracheal mechanical ventilation.
| Characteristic | Mean (SD) or n (%) | |
|---|---|---|
|
| ||
| Subjects with Diet Intervention completed N=16 | All enrolled subjects N=20 | |
|
| ||
| Age, y | 9.3 ± 4.9 | 8.4 ± 4.8 |
|
| ||
| Gender, Male | 8 (50%) | 11 (55%) |
|
| ||
| Years since initiation of mechanical ventilation, y | 3.9 ± 5 | 3.9 ± 4.7 |
|
| ||
| Hours of mechanical ventilation per day | ||
| 12h | 9 (56%) | 11 (55%) |
| 24h | 7 (44%) | 9 (45%) |
|
| ||
| Primary Mode of Mechanical Ventilation | ||
| Pressure Control | 10 (62.5%) | 11 (68.8%) |
| Volume Control | 3 (19%) | 5 (25%) |
| Pressure Support | 3 (19%) | 4 (20%) |
|
| ||
| Route of Nutrition (# of patients) | ||
| PO | 2 (12.5%) | 2 (10%) |
| PO + GT/JT | 1 (6.3%) | 2 (10%) |
| GT/JT | 13 (81.3%) | 16 (80%) |
|
| ||
| Energy Adequacy (# of patients) | ||
|
| ||
| Underfed, AEI:MEE <90% | 7 (44%) | 9/19 (47.4%) |
|
| ||
| Overfed, AEI:MEE >110% | 4 (25%) | 4/19 (21%) |
|
| ||
| Mean Protein Intake, g/kg/d | 1.5 ± 0.6 | 1.6 ± 0.6 |
|
| ||
| Measured Energy Expenditure, kcal | 1086 ± 381.1a | 1080 ± 388.8 |
|
| ||
| Estimated Energy Expenditure by Schofield equation, kcal | 1105 ± 279.9 | 1072 ± 278.4 |
|
| ||
| Diagnosesb (# of patients) | ||
|
| ||
| Multifactorial chronic respiratory insufficiency | 12 (75%) | 15 (75%) |
|
| ||
| Neuromuscular Disorder- SMA, muscular dystrophy, NOS | 5 (41.7%) | 7 (35%) |
|
| ||
| Seizure disorder | 5 (31%) | 6 (30%) |
|
| ||
| Congenital Heart Disease | 4 (25%) | 4 (20%) |
|
| ||
| Spastic Quadriplegia | 4 (25%) | 4 (20%) |
|
| ||
| Endocrinopathies | 3 (19%) | 3 (15%) |
|
| ||
| Central Apnea/ Hypoventilation syndromes | 2 (13%) | 3 (15%) |
|
| ||
| Metabolic disorder | 1 (6.3%) | 2 (10%) |
PO- per os; GT- gastrostomy tube; JT- jejunostomy tube; AEI- average energy intake as determined from 3-day food diary; MEE- measured energy expenditure as determined by indirect calorimetry; SMA-spinal muscular atrophy; NOS- not otherwise specified;
N=15 for MEE because one subject did not achieve steady state;
Diagnoses are not mutually exclusive, some subjects had more than one common diagnosis.
Pre-intervention
Table 1 shows baseline characteristics as well as the energy and protein intake. Eleven subjects (69%) were either underfed or overfed based on AEI:MEE. Mean protein intake for the cohort was 1.5 g/kg/d. Protein intake in 9/16 (56%) subjects was below the lower range of age-based recommendations.
Pre- and post-intervention nutritional and body composition parameters are shown in Table 2. Five of 16 (31%) subjects had a WAZ ≤-1 and four of 16 (25%) subjects had a BMI z-score ≥ 1. In subjects 3 years and older, mean FM% was significantly higher than age, gender and race matched norms (41.6% vs. 22.8%, p< .0001).
TABLE 2. Energy and protein intake adequacy, anthropometry and body composition in subjects who completed the diet intervention.
| Variable | Mean (SD) or n (%) | ||
|---|---|---|---|
|
| |||
| Pre-Intervention | Post-Intervention | P-value | |
|
| |||
| ENERGY & PROTEIN INTAKE ADEQUACY (N=16) | |||
|
| |||
| AEI:MEE | |||
|
| |||
| <90% | 7 (44%) | 0 (0%) | N/A |
| 90-110% | 5 (31%) | 13 (81%) | |
| >110% | 4 (25%) | 3 (19%) | |
|
| |||
| Protein intake (g/kg/d) | 1.5 ± 0.62 | 1.9 ± 0.57 | 0.02 |
|
| |||
| ANTHROPOMETRY & BODY COMPOSITION (N=16) | |||
|
| |||
| Weight, kg | 32.5 ± 17.9 | 34.2 ± 17.9 | 0.0005 |
|
| |||
| Weight for age, z-score | -0.37 ± 1.7 | -0.17 ± 1.6 | 0.16 |
|
| |||
| Height, cm | 124.5 ± 24.6 | 127.1 ± 25.2 | 0.0014 |
|
| |||
| BMI, kg/m2 | 19.7 ± 6.1 | 19.9 ± 6.1 | 0.53 |
|
| |||
| BMI z-score | 0.41 ± 1.4 | 0.44 ± 1.2 | 0.84 |
|
| |||
| BIA LBM, % | 58.3 ± 13.2 | 61.8 ± 14.9 | 0.12 |
|
| |||
| BIA FM, % | 39.7 ± 12.2 | 38.2 ± 14.9 | 0.36 |
AEI- average energy intake; MEE-measured energy expenditure; BMI- body mass index; LBM- lean body mass; FM- fat mass; BIA- bioelectric impedance analysis
Table 3 shows the baseline respiratory quotient (RQ), gas exchange values and the respiratory parameters, minute ventilation (L/min) per kg and VCO2 (mL/min) per kg. The RQ for the cohort ranged from 0.79 to 1.12.
TABLE 3.
Metabolic and respiratory outcomes in subjects who completed the diet intervention
| Characteristic | Mean (SD) | ||
|---|---|---|---|
| Pre-Intervention | Post-Intervention | P-value | |
| Minute Ventilation, L/kg | 0.18 ± 0.12 | 0.15 ± 0.09 | 0.041 |
| VO2, ml/min*kg ˆ | 6.43 ± 3.7 | 5.96 ± 3.1 | 0.13 |
| VCO2, ml/min*kg ˆ | 5.45 ± 3.5 | 5.28 ± 2.8 | 0.057 |
| RQ ˆ | 0.92 ± 0.1 | 0.89 ± 0.08 | 0.16 |
kg- kilogram; VO2- volumetric oxygen consumption; VCO2- volumetric carbon dioxide production; RQ- respiratory quotient;
These data are from a sample size of 15. One subject did not reach steady state on indirect calorimetry and therefore was excluded from this analysis.
Diet Intervention
Figure 2 shows the percentage of calories from carbohydrate, protein and fat in the baseline and in the interventional diets. On average for the entire cohort, the percent fraction of energy delivered as carbohydrate and fat was significantly decreased, whereas the percent fraction of energy delivered as protein was increased in the interventional diet. The macronutrient composition remained within acceptable clinical range based on national dietary reference intake standards.[22]
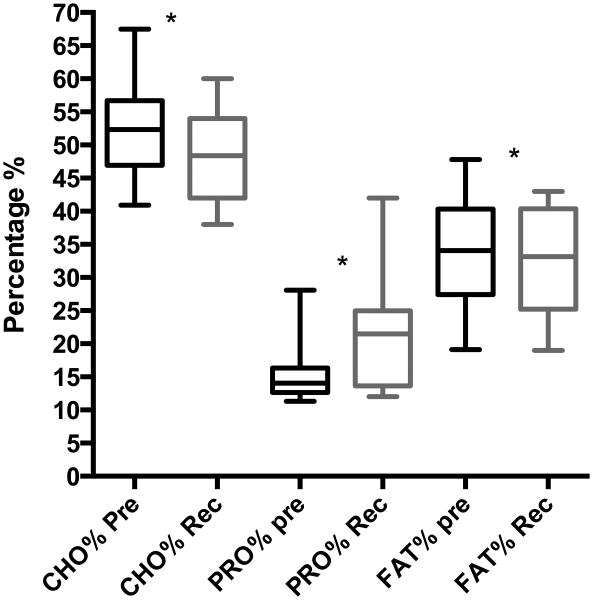
FIGURE 2.
Mean percentages of calories from carbohydrate, protein and fat in subjects' baseline diet and modified diet. This includes all 16 subjects in whom diet modification was recommended and completed. Statistical difference in the mean percentage of calories from carbohydrate, protein and fat between the baseline pre intervention diet and the recommended modified diet was examined by paired t-test and demonstrated a significant difference for (51.7% vs 48.2%, p=0.0087), protein (15.8% vs 21.1%, p=0.0008) and fat (33.8% vs 31.8%, p=0.02). *p-value <0.05. CHO% - percentage of calories from carbohydrate in the subjects' diet; PRO%- percentage of calories from protein in the subjects' diet; FAT%- percentage of calories from fat in the subjects' diet; Pre- baseline pre intervention diet; Rec- recommended modified diet; *p value < 0.05
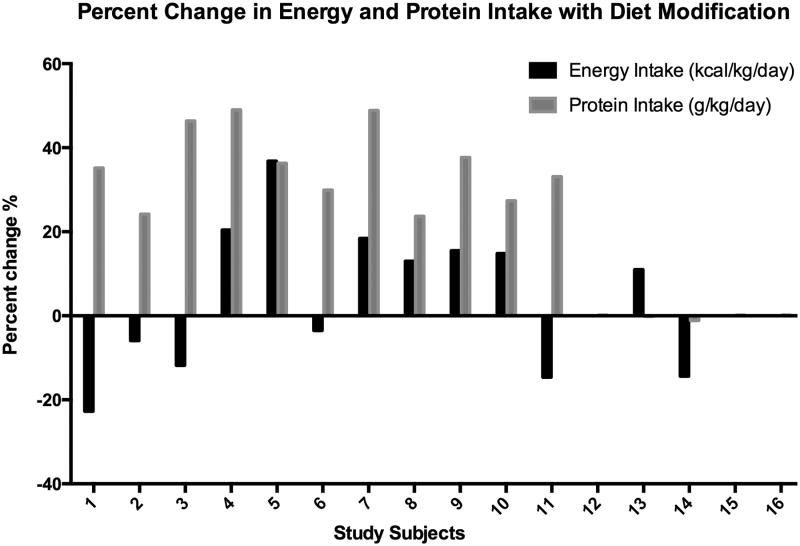
Diet modification resulted in improved energy intake adequacy (AEI:MEE) with only 3 subjects having an AEI:MEE ratio outside of the 90-110% range after the intervention. Energy intake was increased in 7 patients and was decreased in 6 patients. This was accomplished by modifying the volume or quantities of their baseline formula or foods. The mean daily protein intake for the cohort was significantly increased with the diet intervention from 1.5 g/kg/d at baseline to 2.0 g/kg/d post-intervention, p=0.0002. Daily protein intake was increased in 11 patients, including 9 subjects below the lower range of recommended intake and in 2 subjects secondary to energy modification. Increasing protein intake was accomplished by increasing formula or food, or by adding modular protein supplement. Figure 3 depicts individual subjects' energy and protein percent change as a result of the diet intervention.
FIGURE 3.
Individual subject percent change in energy and protein with diet modification. Black bars represent a positive or negative change in energy intake. Gray bars represent a positive or negative change in protein intake. All 16 subjects completed the study. No bars represents no change in energy or protein intake.
As a measure of compliance to the interventional diet, there were no significant differences between the prescribed diet and actual intake of energy (p=0.52) and protein (p=0.11) according to the post-intervention visit report.
Post-Intervention
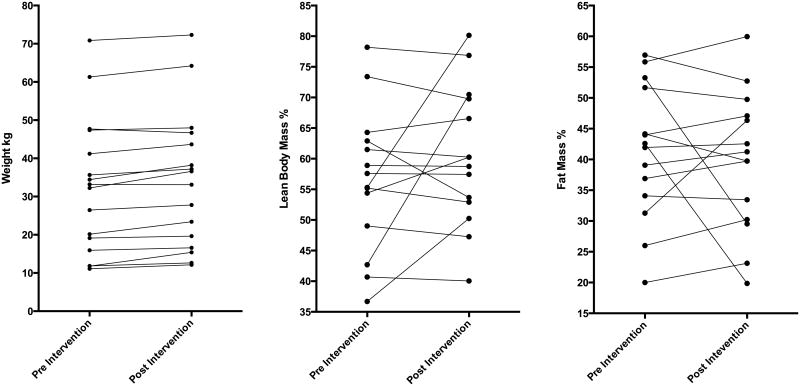
Mean values for weight (kg) and height (cm) were significantly higher at the post-intervention assessments.(Table 2) Mean WAZ for the cohort changed from -0.37 to -0.17. Mean LBM% increased and mean FM% decreased at the post-intervention assessments. For individual subjects, the LBM% and FM% either did not change or a trend towards increasing LBM% and associated decrease in FM% was noted post-intervention. The direction of change in LBM% and FM% were not aligned with corresponding weight change (Figure 4).
FIGURE 4.
Line plots of individual subject pre and post intervention for A. weight (kg), B. lean body mass % and C. fat mass % change.
Mean MV (L/min/kg) was significantly different between pre- and post-intervention assessments, 0.18 (SD 0.12) L/min per kg vs. 0.15 (SD 0.09) L/min per kg, p=0.041. Compared to the pre-intervention value, mean VCO2 (ml/min) per kg was lower at the post-intervention but this difference did not reach statistical significance, 5.4 (SD 3.5) (ml/min) per kg vs. 5.3 (SD 2.8) (ml/min) per kg, p=0.057. (Table 3) Level of activity was similar for all subjects during the metabolic assessment pre- and post-intervention. Two subjects required hospitalization for less than 7 days during the intervention period, one for tracheitis and the other for pneumonia. The second visits for these subjects were completed 4 weeks after the hospitalization, and both were at baseline health and respiratory support status.
Discussion
We have reported the feasibility of individualized optimization of energy and protein intake in children with chronic respiratory failure, using a unique nutritional intervention model. We recorded a significant decline in MV and a trend towards a decline in VCO2 following 12 weeks on the modified diet in this vulnerable cohort. In addition, subjects showed improved body composition with a trend towards decreased FM% and increased or maintained LBM%; while mean weight and height showed increments at the end of the intervention period. The population of children dependent on long-term mechanical ventilation is steadily growing and managed in special care units, long-term care facilities, or the patient's home. These graduates of the intensive care unit are frequently readmitted for intercurrent illness. The results of this study, and the concept of nutritional optimization to improve respiratory and body composition variables, may be applicable to a patients in the subacute and convalescent phase of respiratory insufficiency in the pediatric intensive care unit. To our knowledge, this is the first prospective study examining the impact of a diet intervention on clinical outcomes in children on long-term mechanical ventilation.
In this study, we successfully and safely modified diets of individual subjects to optimize energy intake (to match 90-110% of MEE) in 13 (81%) subjects, and protein intake (to match age-based recommended values) in 11 (69%) subjects. A majority of the cohort (80%) completed the diet intervention, and there were no complications. Our diet intervention was achieved without impeding growth. Our model of home visits by a mobile multidisciplinary team for nutritional intervention is innovative, and our study has shown this approach to be feasible and safe. A similar multidisciplinary, non-mobile approach could be replicated in children with respiratory insufficiency in the intensive care unit or the outpatient clinic setting. The impact of these nutritional and respiratory changes on clinical outcomes and quality of life needs to be examined. The cost-benefit analysis of this approach will need to be studied before adopting it in general practice.
The significant decrease in MV and a trend towards a significant decrease in VCO2 in our cohort after 12 weeks of diet modification is exciting and mimics similar findings in prior studies in adults with respiratory insufficiency.[11, 24] Excessive energy intake, in particular carbohydrates, has been shown to impact respiratory parameters in adults.[6, 7, 9, 25] In our current study, measured energy expenditure by indirect calorimetry facilitated a new paradigm for planning nutrition prescriptions, especially in cohorts that are vulnerable to the consequences of underfeeding and overfeeding due to reliance on inaccurate equations for estimating energy expenditure. Reduction in the carbohydrate fraction of the modified diet was associated with a reduction in MV and VCO2, irrespective of the total caloric intake. Although the number of subjects is small, this observation emphasizes the importance of optimal macronutrient composition of the diet in addition to the total energy provided. Improved MV and VCO2 may lead to improved clinical outcomes such as decreased ventilatory requirements, decreased work of breathing, subjective comfort, growth, and potentially a more favorable response to intercurrent respiratory illness. Although we did not aim to explore these outcomes in our current pilot study, future investigations must examine the effect of nutritional optimization on these outcomes in other cohorts of children with respiratory insufficiency, including those admitted to the intensive care unit.
Low BMI and LBM have been associated with poorer quality of life, worse pulmonary function and higher risk for mortality in adults with chronic respiratory insufficiency.[4, 5, 10, 26] Diet modification with subsequent improvement in weight and LBM is associated with improved respiratory muscle strength, pulmonary function, and quality of life in this cohort.[11, 27, 28] In our current study increasing protein intake and optimizing energy intake in the modified diet was associated with an increase in LBM% and corresponding loss in FM% (Figure 4). BIA allowed noninvasive assessment of body composition. The use of BIA for examination of body composition has been validated in multiple adult and pediatric cohorts, including in children with cerebral palsy and neuromuscular disorders.[15, 29-31] The change in LBM% was variable among subjects and the small sample size did not allow for a detailed examination of patient characteristics and other factors predicting the magnitude of this change. The relationship between nutrition and body composition is likely complex and should be further explored. Our results suggest the importance of optimizing protein intake in mechanically ventilated children in the intensive care unit in whom the protein catabolism is high and preservation of LBM is an important goal of nutritional therapy.
The small sample size and heterogeneity of underlying comorbidities in our study cohort are important study limitations. These do not permit for any subgroup analysis and identification of patient populations that might benefit the most from the studied intervention. The heterogeneity of the cohort, however, supports the need for an individualized approach to nutrition. The duration of our intervention, 12 weeks, is within an adequate time frame previously shown to effectively examine outcomes after a diet intervention.[11] If indeed there is a dose effect of this association, a greater change in measured variables might be expected from an intervention longer than 12 weeks. Indirect calorimteric testing performed over 30-minutes, has been used as a surrogate for 24-hour energy expenditure in mechanically ventilated adults and children.[32, 33] However, metabolic rate may be altered by illness, diurnal pattern, and respiratory support status. To account for these variables influencing energy expenditure, we recorded the level of activity at each IC test, scheduled second visit only after recovery from intercurrent illness, and at a time that was similar to the previous visit.
Conclusion
Individualized diet modification is feasible and is associated with a significant decrease in minute ventilation in children on long-term mechanical ventilatory support. The diet intervention was also associated with trends towards reduction in carbon dioxide production and improved body composition. The role of our innovative, multidisciplinary nutritional intervention to improve nutritional and respiratory outcomes should be explored in other vulnerable populations including children with respiratory failure in the pediatric intensive care unit.
Acknowledgments
We would like to thank Nicole Stenquist, BS, Michelle Hernon, BS and Lauren Perlman, RRT for their assistance. We would like to thank all of the participating families. All authors met full authorship criteria.
Financial Support- This study was partially funded by the Medical Staff Organization Boston Children's Hospital House Officer Development Award. The metabolic measurement device was funded by the BCH Payer-Provider Quality Initiative Grant for the CAPE Program. CD was supported in part by K24HD058795
Non-Standard Abbreviations
- AEI
average energy intake
- BIA
bioelectrical impedance analysis
- BMI
Body Mass Index
- FM
fat mass
- LBM
lean body mass
- MEE
measured energy expenditure
- RQ
respiratory quotient
- VCO2
volumetric carbon dioxide production
- VO2
volumetric oxygen consumption
- WAZ
weight for age z-score
Footnotes
Study Location- The multidisciplinary team was based at Boston Children's Hospital. The study patients were selected from the Boston Children's Hospital Critical Care, Anesthesia, Perioperative Extension and Home Ventilation Program, although the study assessments were performed in subjects' individual homes.
References
- 1.Martinez EE, Smallwood CD, Bechard LJ, Graham RJ, Mehta NM. Metabolic assessment and individualized nutrition in children dependent on mechanical ventilation at home. The Journal of pediatrics. 2015;166(2):350–7. doi: 10.1016/j.jpeds.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 2.Mehta NM, Bechard LJ, Cahill N, Wang M, Day A, Duggan CP, et al. Nutritional practices and their relationship to clinical outcomes in critically ill children--an international multicenter cohort study*. Critical care medicine. 2012;40(7):2204–11. doi: 10.1097/CCM.0b013e31824e18a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hulst JM, van Goudoever JB, Zimmermann LJ, Hop WC, Albers MJ, Tibboel D, et al. The effect of cumulative energy and protein deficiency on anthropometric parameters in a pediatric ICU population. Clinical nutrition. 2004;23(6):1381–9. doi: 10.1016/j.clnu.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Cano NJ, Roth H, Court-Ortune I, Cynober L, Gerard-Boncompain M, Cuvelier A, et al. Nutritional depletion in patients on long-term oxygen therapy and/or home mechanical ventilation. Eur Respir J. 2002;20(1):30–7. doi: 10.1183/09031936.02.01812001. [DOI] [PubMed] [Google Scholar]
- 5.Budweiser S, Jorres RA, Riedl T, Heinemann F, Hitzl AP, Windisch W, et al. Predictors of survival in COPD patients with chronic hypercapnic respiratory failure receiving noninvasive home ventilation. Chest. 2007;131(6):1650–8. doi: 10.1378/chest.06-2124. [DOI] [PubMed] [Google Scholar]
- 6.Lo HC, Lin CH, Tsai LJ. Effects of hypercaloric feeding on nutrition status and carbon dioxide production in patients with long-term mechanical ventilation. JPEN Journal of parenteral and enteral nutrition. 2005;29(5):380–7. doi: 10.1177/0148607105029005380. [DOI] [PubMed] [Google Scholar]
- 7.Liposky JM, Nelson LD. Ventilatory response to high caloric loads in critically ill patients. Critical care medicine. 1994;22(5):796–802. doi: 10.1097/00003246-199405000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Klein CJ, Stanek GS, Wiles CE., 3rd Overfeeding macronutrients to critically ill adults: metabolic complications. J Am Diet Assoc. 1998;98(7):795–806. doi: 10.1016/S0002-8223(98)00179-5. [DOI] [PubMed] [Google Scholar]
- 9.Efthimiou J, Mounsey PJ, Benson DN, Madgwick R, Coles SJ, Benson MK. Effect of carbohydrate rich versus fat rich loads on gas exchange and walking performance in patients with chronic obstructive lung disease. Thorax. 1992;47(6):451–6. doi: 10.1136/thx.47.6.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hitzl AP, Jorres RA, Heinemann F, Pfeifer M, Budweiser S. Nutritional status in patients with chronic respiratory failure receiving home mechanical ventilation: impact on survival. Clinical nutrition. 2010;29(1):65–71. doi: 10.1016/j.clnu.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Planas M, Alvarez J, Garcia-Peris PA, de la Cuerda C, de Lucas P, Castella M, et al. Nutritional support and quality of life in stable chronic obstructive pulmonary disease (COPD) patients. Clinical nutrition. 2005;24(3):433–41. doi: 10.1016/j.clnu.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Broekhuizen R, Creutzberg EC, Weling-Scheepers CA, Wouters EF, Schols AM. Optimizing oral nutritional drink supplementation in patients with chronic obstructive pulmonary disease. Br J Nutr. 2005;93(6):965–71. doi: 10.1079/bjn20051437. [DOI] [PubMed] [Google Scholar]
- 13.WHO. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age:methods and development. WHO; Geneva, Switzerland: pp. 1–312. [Google Scholar]
- 14.CDC. Vital and Health Statistics, 2000 CDC Growth Charts for the United States: Methods and Development. Department of Health and Human Services, National Center for Health Statistics; 2002. [PubMed] [Google Scholar]
- 15.Mehta NM, Raphael B, Guteirrez IM, Quinn N, Mitchell PD, Litman HJ, et al. Comparison of body composition assessment methods in pediatric intestinal failure. J Pediatr Gastroenterol Nutr. 2014;59(1):99–105. doi: 10.1097/MPG.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pace N. Studies on Body Composition-III The Body Water and Chemically Combined Nitrogen Content in Relation to Fat Content. Journal of Biological Chemistry. 1945;158:685–91. [Google Scholar]
- 17.Ellis KJ, Abrams SA, Wong WW. Body composition of a young, multiethnic female population. Am J Clin Nutr. 1997;65(3):724–31. doi: 10.1093/ajcn/65.3.724. [DOI] [PubMed] [Google Scholar]
- 18.Ellis KJ. Body composition of a young, multiethnic, male population. Am J Clin Nutr. 1997;66(6):1323–31. doi: 10.1093/ajcn/66.6.1323. [DOI] [PubMed] [Google Scholar]
- 19.Mehta NM, Compher C ASPEN Board of Directors. A.S.P.E.N. Clinical Guidelines: nutrition support of the critically ill child. JPEN Journal of parenteral and enteral nutrition. 2009;33(3):260–76. doi: 10.1177/0148607109333114. [DOI] [PubMed] [Google Scholar]
- 20.McClave S, Snider H. Use of indirect calorimetry in clinical nutrition. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 1992:207–21. doi: 10.1177/0115426592007005207. [DOI] [PubMed] [Google Scholar]
- 21.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109(1-2):1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panel on Macronutrients' Dietary Reference Intakes for Energy, Carbohydrate Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) Washington DC: National Academies Press; 2005. [Google Scholar]
- 23.D'Agostino RB, B A, D'Agostino RB., Jr A suggestion for using powerful and informative tests of normality. The American Statistician. 1990;44(4):316–21. [Google Scholar]
- 24.Ferreira IM, Brooks D, White J, Goldstein R. Nutritional supplementation for stable chronic obstructive pulmonary disease. The Cochrane database of systematic reviews. 2012;12:CD000998. doi: 10.1002/14651858.CD000998.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talpers SS, Romberger DJ, Bunce SB, Pingleton SK. Nutritionally associated increased carbon dioxide production. Excess total calories vs high proportion of carbohydrate calories. Chest. 1992;102(2):551–5. doi: 10.1378/chest.102.2.551. [DOI] [PubMed] [Google Scholar]
- 26.Kyle UG, Janssens JP, Rochat T, Raguso CA, Pichard C. Body composition in patients with chronic hypercapnic respiratory failure. Respir Med. 2006;100(2):244–52. doi: 10.1016/j.rmed.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 27.Pison CM, Cano NJ, Cherion C, Caron F, Court-Fortune I, Antonini MT, et al. Multimodal nutritional rehabilitation improves clinical outcomes of malnourished patients with chronic respiratory failure: a randomised controlled trial. Thorax. 2011;66(11):953–60. doi: 10.1136/thx.2010.154922. [DOI] [PubMed] [Google Scholar]
- 28.Collins PF, Elia M, Stratton R. Nutritional support and functional capacity in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respirology. 2013;18(4):616–29. doi: 10.1111/resp.12070. [DOI] [PubMed] [Google Scholar]
- 29.Rieken R, van Goudoever JB, Schierbeek H, Willemsen SP, Calis EA, Tibboel D, et al. Measuring body composition and energy expenditure in children with severe neurologic impairment and intellectual disability. Am J Clin Nutr. 2011;94(3):759–66. doi: 10.3945/ajcn.110.003798. [DOI] [PubMed] [Google Scholar]
- 30.Mok E, Letellier G, Cuisset JM, Denjean A, Gottrand F, Hankard R. Assessing change in body composition in children with Duchenne muscular dystrophy: anthropometry and bioelectrical impedance analysis versus dual-energy X-ray absorptiometry. Clinical nutrition. 2010;29(5):633–8. doi: 10.1016/j.clnu.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Mok E, Beghin L, Gachon P, Daubrosse C, Fontan JE, Cuisset JM, et al. Estimating body composition in children with Duchenne muscular dystrophy: comparison of bioelectrical impedance analysis and skinfold-thickness measurement. Am J Clin Nutr. 2006;83(1):65–9. doi: 10.1093/ajcn/83.1.65. [DOI] [PubMed] [Google Scholar]
- 32.McClave SA, Spain DA, Skolnick JL, Lowen CC, Kieber MJ, Wickerham PS, et al. Achievement of steady state optimizes results when performing indirect calorimetry. JPEN Journal of parenteral and enteral nutrition. 2003;27(1):16–20. doi: 10.1177/014860710302700116. [DOI] [PubMed] [Google Scholar]
- 33.Smallwood CD, Mehta NM. Accuracy of abbreviated indirect calorimetry protocols for energy expenditure measurement in critically ill children. JPEN Journal of parenteral and enteral nutrition. 2012;36(6):693–9. doi: 10.1177/0148607112441948. [DOI] [PubMed] [Google Scholar]