SUMMARY
In the heart, augmented Ca2+ fluxing drives contractility and ATP generation through mitochondrial Ca2+ loading. Pathologic mitochondrial Ca2+ overload with ischemic injury triggers mitochondrial permeability transition pore (MPTP) opening and cardiomyocyte death. Mitochondrial Ca2+ uptake is primarily mediated by the mitochondrial Ca2+ uniporter (MCU). Here we generated mice with adult and cardiomyocyte-specific deletion of Mcu, which produced mitochondria refractory to acute Ca2+ uptake, augmented ATP production and MPTP opening upon acute Ca2+ challenge. Mice lacking Mcu in the adult heart were also protected from acute ischemia-reperfusion injury. However, resting/basal mitochondrial Ca2+ levels were normal in hearts of Mcu-deleted mice and mitochondria lacking MCU eventually loaded with Ca2+ after stress stimulation. Indeed, Mcu-deleted mice were unable to immediately sprint on a treadmill unless warmed-up for 30 minutes. Hence, MCU is a dedicated regulator of short-term mitochondrial Ca2+ loading underlying a “fight-or-flight” response that acutely matches cardiac workload with ATP production.
INTRODUCTION
Under physiological conditions, mitochondrial Ca2+ loading serves as a signal to enhance mitochondrial energetic output, either by directly binding and activating key dehydrogenases of the tricarboxylic acid cycle or by activation of the ATP synthase, thereby linking momentary cardiac contractile Ca2+ cycling with metabolic output (Glancy and Balaban, 2012). However, prolonged elevations of intracellular Ca2+ can trigger mitochondrial permeability transition pore (MPTP) opening, mitochondrial dysfunction and cardiomyocyte death (Kwong and Molkentin, 2015).
A major pathway for mitochondrial Ca2+ entry is through the mitochondrial Ca2+ uniporter (MCU) complex, a selective Ca2+ channel that facilitates the voltage dependent transport of Ca2+ across the mitochondrial inner membrane (Kamer et al., 2014). The core complex is comprised of the Mcu gene product itself that forms the pore and the regulatory subunits MICU1, MICU2, EMRE and MCUb (Kamer et al., 2014). Specific inhibition of MCU with pharmacological agents such as ruthenium red and Ru360 (Matlib et al., 1998; Zazueta et al., 1999), as well as genetic ablation of MCU complex components blocks acute mitochondrial Ca2+ influx (Baughman et al., 2011; De Stefani et al., 2011; Pan et al., 2013; Sancak et al., 2013). MCU inhibition via drugs or RNA-interference also abrogates cell death in numerous in vitro models, presumably due to less Ca2+ influx and reduced MPTP opening (Dessi et al., 1995; Groskreutz et al., 1992; Qiu et al., 2013).
Recently, viable mice were generated with global deletion of the Mcu gene (Pan et al., 2013). Although mitochondria isolated from these animals had impaired acute Ca2+ uptake, cardiac structure and function were unaffected. Moreover, while Ca2+-induced MPTP opening was abrogated in purified mitochondria lacking Mcu (Pan et al., 2013), cardiac ischemic injury was not reduced as would be predicted from past results with Ru360 or ruthenium red (Garcia-Rivas Gde et al., 2006; Zhang et al., 2006). More recently, Wu and colleagues used a cardiac-specific transgenic approach to overexpress a dominant-negative MCU protein in the heart and found that MCU function was required for cardiac pacemaker cell activity to increase heart rate following catecholamine stimulation (Wu et al., 2015).
RESULTS
Deletion of MCU in the heart blocks acute mitochondrial Ca2+ uptake
To examine the immediate functional effects of the MCU in the heart the Mcu locus was targeted with loxP sites (fl) flanking exons 5 and 6 to generate a conditional loss-of-function allele (Mcufl/fl, Figure 1A). Mcufl/fl mice were then crossed with mice expressing a tamoxifen inducible Cre recombinase (MerCreMer, MCM) driven by the cardiomyocyte specific [.alpha]-myosin heavy chain promoter (Figure 1A). Mcu deletion was induced in 8 week old Mcufl/fl-MCM adult mice by administration of tamoxifen food for 4 weeks followed by an additional 6-week period to allow for MCU protein turnover (Figure 1B). Following this dosing regimen, western blot analyses showed that MCU protein expression was reduced by >80% in the hearts of 18 week old Mcufl/fl-MCM animals when compared with Mcufl/fl and MerCreMer age-matched controls (Figure 1C).
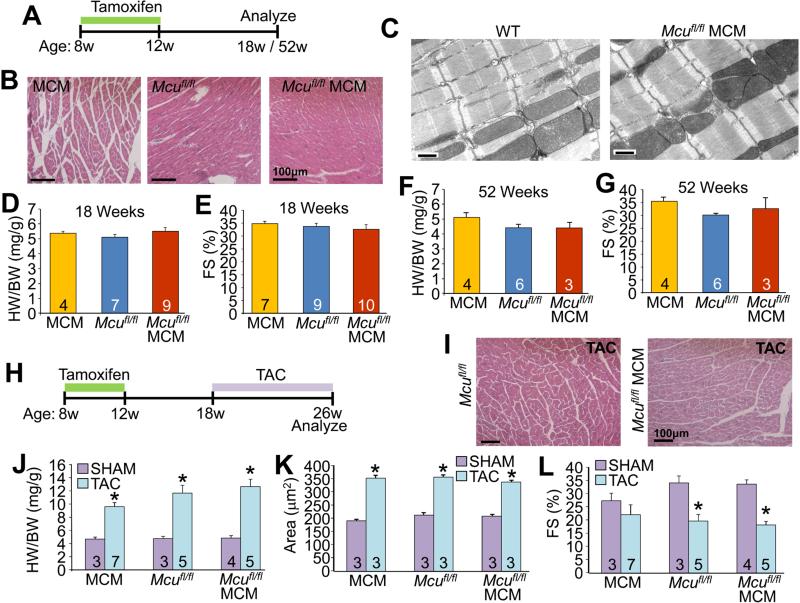
Figure 1. Cardiomyocyte-specific deletion of Mcu impairs mitochondrial Ca2+uptake.
(A) Targeting strategy for the Mcu locus to generate the Mcufl/fl mice where exons 5 and 6 were flanked with LoxP sites (triangles). Mcufl/fl mice were crossed to α-MHC MerCreMer (MCM) mice to generate the Mcufl/fl-MCM animals.
(B) Tamoxifen dosing to induce MerCreMer activity was given to 8 week-old animals for 4 weeks, followed by examination at 18 and 52 weeks of age.
(C) Western blots of MCU and mNCX expression in cardiac mitochondria. The COXI subunit of mitochondrial Complex IV was used as a protein loading control.
(D) Quantification of Ca2+ content from isolated cardiac mitochondria from the indicated genotypes of mice.
(E) Quantification of baseline mitochondrial Ca2+ content in permeabilized myocytes from the indicated genotypes of mice.
(F) The effect of Ru360 (1 μM) on mitochondrial Ca2+ uptake as measured by calcium-green 5N fluorescence in the solution. Mitochondria were challenged with 100 μM CaCl2 additions (arrows).
(G) Mitochondrial Ca2+ uptake in mitochondria from hearts of Mcufl/fl vs. Mcufll/fl-MCM mice. Mitochondria were challenged with 200 μM CaCl2 additions (arrows).
(H) Measurement of mitochondrial Ca2+ uptake in permeabilized myocytes as assessed by Rhod-2 fluorescence in the indicated groups of mice, with or without Ru360.
(I) Quantification of Rhod-2 signal 14 min after Ca2+ addition as shown in H. *P<0.05 vs Mcufl/fl. All values reported as mean ± SEM.
(J) Measurement of mitochondrial Ca2+ efflux as mediated by mNCX and leak, assessed by Rhod-2 fluorescence in adult cardiomyocytes.
(K) Quantification of rates of mNCX Ca2+ efflux as shown in J. All values reported as mean ± SEM. *P<0.05 versus Mcufl/fl
See also Figure S1
Direct measurement of mitochondrial Ca2+ levels with 2 different assays showed no difference in baseline mitochondrial Ca2+ from control hearts versus Mcufl/fl-MCM deleted hearts (Figure 1D and 1E). However, acute cardiac mitochondrial Ca2+ uptake, as assessed with the Ca2+ sensitive dye calcium green-5N, was dramatically inhibited (Figure 1F and 1G). Mcufl/fl control cardiac mitochondria displayed typical mitochondrial Ca2+ uptake at repeated Ca2+ additions, reflected as the rapid decrease in fluorescence signal in the test solution after each Ca2+ pulse, which was inhibited with Ru360 (Figure 1F). Similar to the Ru360 treatment, cardiac mitochondria from Mcufl/f-MCM mice also displayed inhibited mitochondrial Ca2+ uptake (Figure 1G).
Mitochondrial Ca2+ handling was also measured in permeabilized adult cardiac myocytes isolated from 18 week-old Mcufl/fl and Mcufl/fl-MCM mice loaded with Rhod-2, a Ca2+ sensitive dye that accumulates in mitochondria. In this assay, permeabilized Mcufl/fl control myocytes challenged with 2 μM Ca2+ displayed a robust increase in mitochondrial Ca2+ levels that was severely blunted in Mcu deficient cardiomyocytes (Figure 1H and 1I). Importantly, Ru360 treatment of Mcufl/fl-MCM cardiomyocytes did not confer additional inhibition of mitochondrial Ca2+ uptake (Figure 1H and 1I).
To understand how basal mitochondrial Ca2+ content can remain unchanged in the face of impaired MCU activity, we examined the mitochondrial Na+/Ca2+ exchanger (mNCX), which is the major pathway of mitochondrial Ca2+ efflux. Adult cardiac myocytes were isolated from Mcufl/fl-MCM and Mcufl/fl control animals and mitochondria were loaded with Rhod2 and Ca2+ by ionophore permeabilization in conjunction with Ru360 treatment and exposure to buffer containing 2 μM Ca2+. To assess the rate of basal mitochondrial Ca2+ efflux and leak, myocytes were exposed to buffer devoid of both Ca2+ and Na+ then switched to a buffer containing 10 mM Na+ (Figure 1J and 1K). The data show that while leak rates in solution lacking Na+ and Ca2+ were similar, the Na+-induced Ca2+ efflux rate (mediated via mNCX) was much lower in Mcu-deleted myocytes (Figures 1J and 1K). Interestingly, deletion of Mcu resulted in reduced mNCX protein expression, which likely serves as the basis for the observed compensatory decrease in mNCX activity in the absence of MCU (Figure 1C).
MCU does not regulate cardiac adaptation to long-term stress
Despite the defect in acute mitochondrial Ca2+ uptake observed in the hearts of 18 week-old Mcufl/fl-MCM animals, we observed no pathologic effects such as changes in cellular morphology, myofilament or mitochondrial ultrastructure, hypertrophic growth or cardiac ventricular performance at 18 or even 52 weeks of aging (Figure 2A-2G). Furthermore, total cardiomyocyte Ca2+ transient kinetics and amplitude were unaltered, and sarcoplasmic reticulum Ca2+ load was unchanged in Mcufl/fl-MCM myocytes as compared to Mcufl/fl controls (Figure S1). Taken together, these results demonstrate that MCU-mediated mitochondrial Ca2+ import neither contributes to overall cardiac Ca2+ cycling in the heart, nor is it required to support normal cardiac function and adaptation with aging.
Figure 2. Loss of MCU from the adult heart does not lead to pathology at baseline or with pathologic stress stimulation.
(A) Time-course for the analyses of cardiac function in response to aging following Mcu deletion.
(B) Transverse H&E heart sections at 200X magnification.
(C) Representative electron micrographs from heart sections. Scale bar is 500 nm.
(D and F) Heart-weight normalized to body-weight ratios (HW/BW) at 18 and 52 weeks of age.
(E and G) Echocardiographic measurement of fractional shortening (FS%) at 18 and 52 weeks of age.
(H) Time course for generation of mice and analyses of cardiac function following TAC surgery.
(I) H&E-stained transverse heart sections 8 weeks after TAC surgery, at 200X magnification.
(J-L) HW/BW, (K) cardiomyocyte cross-sectional area and (L) FS% in the indicated groups of mice 8 weeks following TAC.
All values reported as mean ± SEM. *P<0.05 versus Mcufl/fl sham
Mouse models with subtle perturbations in baseline cardiac metabolic function or metabolic reserve often display increased pathology under stress stimulation, such as with transverse aortic constriction (TAC) (Abel and Doenst, 2011; Arany et al., 2006; Smeets et al., 2008; Wu et al., 2012). Hence, we subjected Mcufl/fl-MCM, Mcufl/fl and MerCreMer control mice to 8 weeks of cardiac pressure overload with TAC surgery (Figure 2H). All three groups of mice all showed identical pathologic profiles in response to TAC (Figure 2I-L). These results suggest that cardiac adaptation to chronic pressure overload is not dependent on MCU-mediated mitochondrial Ca2+ import.
MCU mediates acute mitochondrial Ca2+ overload in vivo
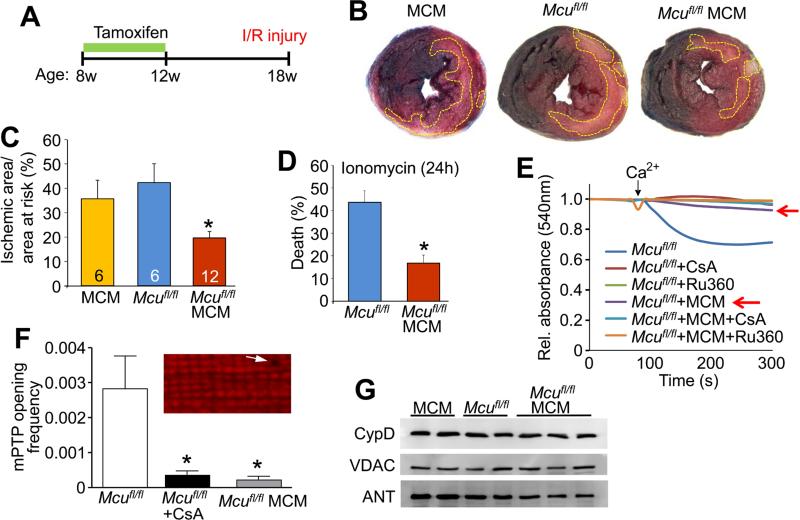
We next sought to determine if MCU played a more selective role in regulating acute Ca2+ responses in the heart, such as after immediate ischemia-reperfusion injury. Here, 18-week old Mcufl/fl-MCM mice and age-matched Mcufl/fl and MerCreMer control animals were subjected to 1 hour of cardiac ischemia followed by 24 hours of reperfusion (Figure 3A). Consistent with past data using Ru360 or ruthenium red, mice with adult and cardiac-specific deletion of Mcu displayed a ~50% reduction in infarct size compared to Mcufl/fl and MerCreMer controls (Figures 3B and 3C). Correspondingly, Mcu deletion in adult cardiomyocytes also conferred protection against acute ionomycin-induced Ca2+ overload cell death (Figure 3D). Mechanistically, acute Ca2+-induced MPTP opening, as measured in isolated cardiac mitochondria, was greatly desensitized in Mcufl/fl-MCM mitochondria as compared to Mcufl/fl controls (Figure 3E). Analyses of MPTP opening in permeabilized adult cardiomyocytes, as reflected by loss of mitochondrial membrane potential, showed inhibition with Mcu deletion similar to cyclosporine A (CsA) treatment of WT control myocytes (Figure 3F). Importantly, expression of MPTP components such as CypD (cyclophilin D), VDAC (voltage-dependent anion channel), and ANT (adenine nucleotide translocator) were unaltered with Mcu deletion (Figure 3G), These results suggest that loss of MCU in the heart inhibits acute Ca2+ induced MPTP opening, resulting in decreased cardiomyocyte damage in response to ischemia-reperfusion injury.
Figure 3. MCU is required for acute mitochondrial Ca2+ stress signaling.
(A) Time-course for the cardiac ischemia-reperfusion experiment.
(B) Representative images of transverse heart sections stained with 2,3,5-triphenyltetrazolium chloride following ischemia-reperfusion injury from the indicated groups. Ischemic area is outlined in yellow.
(C) Quantification of the ischemic area versus area at risk. *P< 0.05 versus all other groups. All values presented as mean ± SEM.
(D) Cardiomyocyte viability in response to ionomycin treatment (625 nM, 24h). *P<0.05 versus control. All values presented as mean ± SEM.
(E) Mitochondrial swelling in response to Ca2+ challenge (200 μM CaCl2). Controls were 5 μM cyclosporine A (CsA) and 2 μM Ru360. The red arrows show the critical experimental group where swelling is inhibited with Mcu deletion alone.
(F) Quantification of MPTP opening frequency in permeabilized cardiomyocytes. MPTP opening is measured by loss of mitochondrial membrane potential (TMRM signal; inset).
Myocytes were challenged with 100 nM free Ca2+ and 1 μM CsA was used as a control. *P< 0.005 versus control. All values presented as mean ± SEM.
(G) Western blots of CypD, VDAC, and ANT protein expression in purified cardiac mitochondria from the indicated genotypes of mice.
MCU is required for acute mitochondrial metabolic-contraction coupling
Oxygen consumption and ATP generation were examined in isolated mitochondria in response to acute Ca2+ loading. At baseline, Mcufl/fl-MCM and Mcufl/fl control mitochondria displayed similar rates of mitochondrial oxygen consumption and ATP synthesis, consistent with unchanged basal Ca2+ levels in the absence of MCU protein (Figure 4A and 4B). Furthermore, potential alternative pathways of oxidation such as reactive oxygen species (ROS) production, NADPH oxidation, superoxide dismutase 2 (SOD2) levels and catalase activity were also unaltered with Mcu deletion (Figure S2). However, while addition of exogenous Ca2+ to Mcufl/fl control mitochondria resulted in an approximate 2-fold increase in respiration and ATP production, this acute effect was blocked by both Mcu deletion and addition of Ru360 to WT control mitochondria (Figure 4A and 4B).
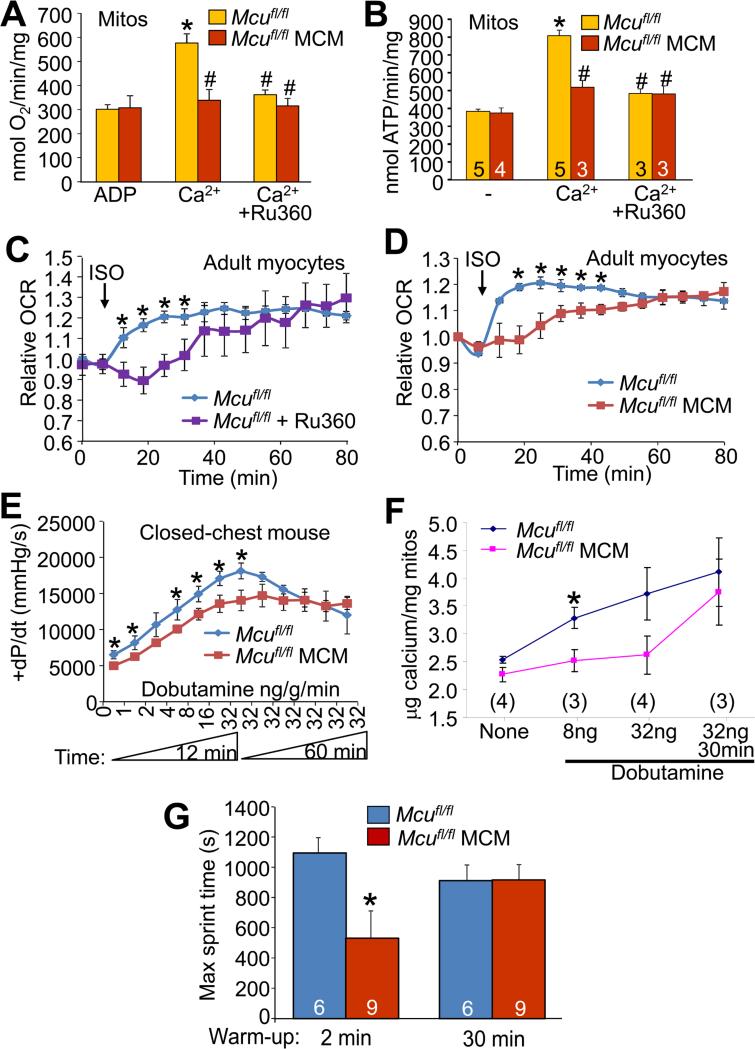
Figure 4. Acute versus chronic regulation of mitochondrial Ca2+ and metabolism due to MCU activity.
(A) State 3 mitochondrial oxygen consumption in purified cardiac mitochondria from the indicated mice stimulated with 100 μM Ca2+ with or without 2 μM Ru360. *P<0.05 versus ADP baseline; #P<0.05 versus Mcufl/fl Ca2+. All values presented as mean ± SEM.
(B) Mitochondrial ATP synthesis in isolated mitochondria at baseline and stimulated with 400 μM CaCl2. 5 μM Ru360 was used as a control. *P<0.05 versus ADP baseline; #P<0.05 versus Mcufl/fl Ca2+. All values presented as mean ± SEM.
(C) Relative oxygen consumption rates (OCR) of Mcufl/fl control adult cardiomyocytes with or without Ru360 (5 μM) in response to 3.125 nM isoproterenol.
(D) OCR in Mcufl/fl-MCM vs. Mcufl/fl adult cardiomyocytes in response to 3.125 nM isoproterenol. *P<0.05 versus control. All values presented as mean ± SEM.
(E) Maximal rates of cardiac contraction as measured in a closed chest mouse in response to increasing doses of dobutamine. Dobutamine was increased from 0 to 32 ng/g/min and then maintained at 32 ng/g/min for an additional hour. *P<0.05 versus control. All values presented as mean ± SEM.
(F) Total mitochondrial Ca2+ content measured from hearts taken at the indicated time-points from indicated groups of mice following dobutamine administration of the experiment shown in E. *P<0.05 versus control. All values presented as mean ± SEM.
(G) Treadmill performance as quantified by maximum sprint time in the Mcufl/fl-MCM vs. Mcufl/fl controls. Animals were subjected to two different protocols where they were allowed either a 2 minute warm-up or a 30 minute warm-up before reaching maximum sprint speed. *P<0.05 versus control. Number of mice used is shown in the bars. All values presented as mean ± SEM.
See also Figures S2 and S3.
To more definitively investigate the hypothesis that MCU is mostly dedicated to short-term mitochondrial Ca2+ regulation, we had to employ an intact system that could sustain extended measurements, such as isolated adult cardiac myocytes treated with the β-adrenergic receptor agonist isoproterenol. Similar to the Ca2+-induced increase in mitochondrial respiration observed in isolated mitochondria (Figure 4A); isoproterenol treatment elicited an immediate increase in oxygen consumption in WT but not Mcu-deleted cardiomyocytes or with Ru360 pretreatment (Figure 4C and 4D). However, over the course of 80 minutes, the isoproterenol stimulated oxygen consumption of the Ru360-inhibited and Mcu-deleted myocytes slowly caught up to the rates observed in control myocytes (Figures 4C and 4D). These results suggest that MCU is specialized for acute matching of mitochondrial energy output with cardiac metabolic demands, but that long-term Ca2+ homeostasis can be achieved through other influx pathways.
We also performed in vivo hemodynamic measurements on Mcufl/fl-MCM and Mcufl/fl control mice challenged with increasing concentrations of the β-adrenergic receptor agonist dobutamine. Mcufl/fl control mice showed a greater short-term increase in the maximal rates of cardiac pressure developed (+dP/dt) compared to Mcufl/fl-MCM animals (Figure 4E). However, with sustained administration of dobutamine at 32 ng/g/min the Mcufl/fl-MCM animals were eventually able to achieve similar maximal rates of ventricular pressure developed as compared to Mcufl/fl controls (Figure 4E). Correspondingly, measurement of total cardiac mitochondrial Ca2+ content in Mcufl/fl-MCM and Mcufl/fl mice showed that while levels were not different at baseline, short-term administration of dobutamine resulted in significantly elevated Ca2+ in only the Mcufl/fl control mitochondria (Figure 4F). However, following 30 minutes of sustained dobutamine, the Ca2+ levels in the Mcufl/fl-MCM mitochondria were substantially higher and had caught up to those observed in Mcufl/fl controls (Figure 4F).
Finally, we challenged Mcufl/fl-MCM and Mcufl/fl control animals to two contrasting treadmill-running regimens to probe further into the acute versus chronic physiologic mechanisms of MCU function (Figure S3). In the first protocol, animals were subjected to high intensity sprinting for 20 minutes at a speed of 20 m/min, but with only a 2 minute warm-up period. In the second protocol, animals were allowed a 30 minute warm-up period prior to the 20 minute sprinting phase at 20 m/min (Figure S3). Under the short warm-up/high-intensity sprinting regimen, Mcufl/fl-MCM animals displayed significantly reduced running capacity as compared to Mcufl/fl controls, but this difference was lost if the mice were allowed a long warm-up period (Figure 4G).
DISCUSSION
In this study we demonstrate that the role of MCU in the heart is to acutely match augmented cardiac work with mitochondrial energy output through mitochondrial Ca2+ loading. Indeed, loss of MCU from the heart resulted in a selective inability to acutely respond to β-adrenergic receptor stimulation or maximal forced exercise by augmenting cardiac metabolic capacity as cardiomyocyte work is enhanced. However, MCU deficient cardiomyocytes or deficient mice eventually catch up to controls in their contractile or metabolic performance, as does total Ca2+ load in the mitochondria.
The proposed model that MCU controls acute mitochondrial Ca2+ entry is also supported by results observed under pathologic conditions. After cardiac ischemia-reperfusion injury, Mcu deletion in the adult heart was protective, a finding that recapitulates the cardioprotection observed with acute Ru360-mediated MCU inhibition in adult animals (Garcia-Rivas Gde et al., 2006; Zhang et al., 2006). This result is also consistent with an acute regulatory role for MCU in loading mitochondria with excessive Ca2+ leading to catastrophic MPTP opening and myocyte necrosis. While cardioprotection was not observed with the constitutive Mcu deletion described earlier (Pan et al., 2013), it should be noted that our targeting approach allowed us to examine uncompensated effects of MCU deletion in the adult heart, circumventing the potential confounding metabolic alterations accompanying long-term and total body MCU loss. Indeed, total somatic Mcu deleted mice displayed growth defects, lactic acidosis, and constitutive phosphorylation of the E1α subunit of the pyruvate dehydrogenase complex (Pan et al., 2013). Such effects were not observed in our adult and cardiac specific Mcu-deleted mice (Figure S2).
Our proposed model also suggests that baseline mitochondrial Ca2+ levels could be regulated by one or more additional influx pathways, either with or independent of MCU. For example, Graier and colleagues showed that as many as 5 different Ca2+ influx currents can be identified in mitoplasts (Jean-Quartier et al., 2012), and a new current was identified in mitoplasts from cells with knock-down of MCU (Bondarenko et al., 2013). In addition, Letm1 is an H+/Ca2+ antiporter that can mediate energy-dependent Ca2+ uptake into mitochondria at low cytosolic Ca2+ concentration (Jiang et al., 2013). Finally, both the ryanodine receptor 1 (RyR1) and transient receptor potential canonical 3 (TRPC3) have been shown to mediate Ca2+ influx into mitochondria independent of the MCU (Beutner et al., 2001; Feng et al., 2013). One known compensatory alteration that we did observe was a significant downregulation in mNCX protein expression and activity, which should partially offset the reduction in acute Ca2+ influx, hence better maintaining overall homeostasis. In summary, we conclude that MCU is the acute fight-or-flight mediator of Ca2+ influx that facilitates immediate increases in mitochondrial metabolism and ATP production associated with augmented cardiac contractile performance.
EXPERIMENTAL PROCEDURES
Animals
The Mcu gene targeting strategy and generation of the Mcu chimeric mice was through the Howard Hughes Medical Institute Gene Targeting Core (Figure S4). Homozygous Mcufl/fl mice were bred to transgenic mice expressing the tamoxifen inducible Cre recombinase under the control of the α-MHC promoter (Sohal et al., 2001). All animal experiments were approved and performed in accordance by Children's Hospital Medical Center's Institutional Animal Care and Use Committee.
Cardiac functional analyses and surgical procedures and treadmill running
Echocardiography was performed using a Hewlett Packard SONOS 5500 imaging system as previously described (Nakayama et al., 2009). The TAC model of cardiac pressure overload was performed as described previously (Kaiser et al., 2004). Ischemia-reperfusion injury was performed as previously described (Wilkins et al., 2004). Adult cardiac myocytes were isolated as previously described (Goonasekera et al., 2012). The enforced sprinting protocols were performed using an Omni-Pacer LC4/M treadmill (Columbus Instruments International), and diagrammed in Figure S3.
Respiration in adult cardiomyocytes
Respiration in adult cardiomyocytes was measured using a Seahorse XF24 Flux Analyzer (Seahorse Biosciences) as described in (Readnower et al., 2012).
Mitochondrial Isolation and analyses
Cardiac mitochondria were isolated as previously described (Kwong et al., 2014). Mitochondrial ATP synthesis rates were measured as described in (Vives-Bauza et al., 2007). Mitochondrial swelling assays were conducted as previously described (Kwong et al., 2014).
Ca2+ sparks and transients measurements
Intact ventricular myocytes were loaded with Fluo-4 AM dye (5 μM Molecular Probes), and transients and sparks were recorded as previously described (Erickson et al., 2013; van Oort et al., 2010).
Statistics
All results are presented as mean ± SEM. Statistical significance was determined by Student's t-test and p<0.05 were considered significant.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health (J.D.M., J.Z., and D.M.B.), and the Howard Hughes Medical Institute (J.D.M.). J.Q.K was supported by an American Heart Association Local Affiliate Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
J.D.M., and J.Q.K., wrote the manuscript. J.Q.K., X.L., R.N.C., R.J.V., M.A.S., J.A.S., and A.J.Y. performed experimentation. J.D.M., J.Z., and D.M.B provided experimental oversight and helped design the study.
Conflict of interest: None (no competing financial interests)
Supplementary information:
Extended methods and Figures S1-S4
REFERENCES
- Abel ED, Doenst T. Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovasc Res. 2011;90:234–242. doi: 10.1093/cvr/cvr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci U S A. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner G, Sharma VK, Giovannucci DR, Yule DI, Sheu SS. Identification of a ryanodine receptor in rat heart mitochondria. J Biol Chem. 2001;276:21482–21488. doi: 10.1074/jbc.M101486200. [DOI] [PubMed] [Google Scholar]
- Bondarenko AI, Jean-Quartier C, Malli R, Graier WF. Characterization of distinct single-channel properties of Ca(2)(+) inward currents in mitochondria. Pflugers Arch. 2013;465:997–1010. doi: 10.1007/s00424-013-1224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessi F, Ben-Ari Y, Charriaut-Marlangue C. Ruthenium red protects against glutamate-induced neuronal death in cerebellar culture. Neurosci Lett. 1995;201:53–56. doi: 10.1016/0304-3940(95)12128-q. [DOI] [PubMed] [Google Scholar]
- Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, et al. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–376. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Li H, Tai Y, Huang J, Su Y, Abramowitz J, Zhu MX, Birnbaumer L, Wang Y. Canonical transient receptor potential 3 channels regulate mitochondrial calcium uptake. Proc Natl Acad Sci U S A. 2013;110:11011–11016. doi: 10.1073/pnas.1309531110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rivas Gde J, Carvajal K, Correa F, Zazueta C. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. Br J Pharmacol. 2006;149:829–837. doi: 10.1038/sj.bjp.0706932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glancy B, Balaban RS. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry. 2012;51:2959–2973. doi: 10.1021/bi2018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonasekera SA, Hammer K, Auger-Messier M, Bodi I, Chen X, Zhang H, Reiken S, Elrod JW, Correll RN, York AJ, et al. Decreased cardiac L-type Ca(2)(+) channel activity induces hypertrophy and heart failure in mice. J Clin Invest. 2012;122:280–290. doi: 10.1172/JCI58227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groskreutz JL, Bronk SF, Gores GJ. Ruthenium red delays the onset of cell death during oxidative stress of rat hepatocytes. Gastroenterology. 1992;102:1030–1038. doi: 10.1016/0016-5085(92)90193-3. [DOI] [PubMed] [Google Scholar]
- Jean-Quartier C, Bondarenko AI, Alam MR, Trenker M, Waldeck-Weiermair M, Malli R, Graier WF. Studying mitochondrial Ca(2+) uptake - a revisit. Mol Cell Endocrinol. 2012;353:114–127. doi: 10.1016/j.mce.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Zhao L, Clish CB, Clapham DE. Letm1, the mitochondrial Ca2+/H+ antiporter, is essential for normal glucose metabolism and alters brain function in Wolf-Hirschhorn syndrome. Proc Natl Acad Sci U S A. 2013;110:E2249–2254. doi: 10.1073/pnas.1308558110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RA, Bueno OF, Lips DJ, Doevendans PA, Jones F, Kimball TF, Molkentin JD. Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia-reperfusion in vivo. J Biol Chem. 2004;279:15524–15530. doi: 10.1074/jbc.M313717200. [DOI] [PubMed] [Google Scholar]
- Kamer KJ, Sancak Y, Mootha VK. The uniporter: from newly identified parts to function. Biochem Biophys Res Commun. 2014;449:370–372. doi: 10.1016/j.bbrc.2014.04.143. [DOI] [PubMed] [Google Scholar]
- Kwong JQ, Davis J, Baines CP, Sargent MA, Karch J, Wang X, Huang T, Molkentin JD. Genetic deletion of the mitochondrial phosphate carrier desensitizes the mitochondrial permeability transition pore and causes cardiomyopathy. Cell Death Differ. 2014;21:1209–1217. doi: 10.1038/cdd.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong JQ, Molkentin JD. Physiological and Pathological Roles of the Mitochondrial Permeability Transition Pore in the Heart. Cell Metab. 2015;21:206–214. doi: 10.1016/j.cmet.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlib MA, Zhou Z, Knight S, Ahmed S, Choi KM, Krause-Bauer J, Phillips R, Altschuld R, Katsube Y, Sperelakis N, et al. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. J Biol Chem. 1998;273:10223–10231. doi: 10.1074/jbc.273.17.10223. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Bodi I, Correll RN, Chen X, Lorenz J, Houser SR, Robbins J, Schwartz A, Molkentin JD. alpha1G-dependent T-type Ca2+ current antagonizes cardiac hypertrophy through a NOS3-dependent mechanism in mice. J Clin Invest. 2009;119:3787–3796. doi: 10.1172/JCI39724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013;15:1464–1472. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Tan YW, Hagenston AM, Martel MA, Kneisel N, Skehel PA, Wyllie DJ, Bading H, Hardingham GE. Mitochondrial calcium uniporter Mcu controls excitotoxicity and is transcriptionally repressed by neuroprotective nuclear calcium signals. Nat Commun. 2013;4:2034. doi: 10.1038/ncomms3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readnower RD, Brainard RE, Hill BG, Jones SP. Standardized bioenergetic profiling of adult mouse cardiomyocytes. Physiol Genomics. 2012;44:1208–1213. doi: 10.1152/physiolgenomics.00129.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Markhard AL, Kitami T, Kovacs-Bogdan E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets PJ, Teunissen BE, Willemsen PH, van Nieuwenhoven FA, Brouns AE, Janssen BJ, Cleutjens JP, Staels B, van der Vusse GJ, van Bilsen M. Cardiac hypertrophy is enhanced in PPAR alpha−/− mice in response to chronic pressure overload. Cardiovasc Res. 2008;78:79–89. doi: 10.1093/cvr/cvn001. [DOI] [PubMed] [Google Scholar]
- Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- van Oort RJ, Respress JL, Li N, Reynolds C, De Almeida AC, Skapura DG, De Windt LJ, Wehrens XH. Accelerated development of pressure overload-induced cardiac hypertrophy and dysfunction in an RyR2-R176Q knockin mouse model. Hypertension. 2010;55:932–938. doi: 10.1161/HYPERTENSIONAHA.109.146449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Bauza C, Yang L, Manfredi G. Assay of mitochondrial ATP synthesis in animal cells and tissues. Methods Cell Biol. 2007;80:155–171. doi: 10.1016/S0091-679X(06)80007-5. [DOI] [PubMed] [Google Scholar]
- Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- Wu R, Wyatt E, Chawla K, Tran M, Ghanefar M, Laakso M, Epting CL, Ardehali H. Hexokinase II knockdown results in exaggerated cardiac hypertrophy via increased ROS production. EMBO Mol Med. 2012;4:633–646. doi: 10.1002/emmm.201200240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Rasmussen TP, Koval OM, Joiner ML, Hall DD, Chen B, Luczak ED, Wang Q, Rokita AG, Wehrens XH, et al. The mitochondrial uniporter controls fight or flight heart rate increases. Nat Commun. 2015;6:6081. doi: 10.1038/ncomms7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazueta C, Sosa-Torres ME, Correa F, Garza-Ortiz A. Inhibitory properties of ruthenium amine complexes on mitochondrial calcium uptake. J Bioenerg Biomembr. 1999;31:551–557. doi: 10.1023/a:1005464927366. [DOI] [PubMed] [Google Scholar]
- Zhang SZ, Gao Q, Cao CM, Bruce IC, Xia Q. Involvement of the mitochondrial calcium uniporter in cardioprotection by ischemic preconditioning. Life Sci. 2006;78:738–745. doi: 10.1016/j.lfs.2005.05.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.