Abstract
When synthetic cannabinoid compounds became controlled by state and federal governments, different, non-controlled compounds began to appear as marijuana substitutes. Unlike the scheduled cannabinoids, the newer compounds have not been characterized for potency and efficacy in preclinical studies. The purpose of these experiments was to determine whether some of the more recent synthetic compounds sold as marijuana substitutes have behavioral effects similar to those of Δ9-tetrahydrocannabinol (Δ9-THC), the pharmacologically active compound in marijuana. The compounds UR-144, XLR-11, AKB-48 (APINACA), PB-22 (QUPIC), 5F-PB-22 and AB-FUBINACA were tested for locomotor depressant effects in male Swiss-Webster mice and subsequently for their ability to substitute for Δ9-THC (3 mg/kg, i.p.) in drug discrimination experiments with male Sprague-Dawley rats. UR-144, XLR-11, AKB-48, and AB-FUBINACA each decreased locomotor activity for up to 90 min, whereas PB-22 and 5F-PB-22 produced depressant effects lasting 120-150 min. Each of the compounds fully substituted for the discriminative stimulus effects of Δ9-THC. These findings confirm the suggestion that these compounds have marijuana-like psychoactive effects and abuse liability.
Keywords: cannabinoids, drug discrimination, locomotor activity, abuse liability, mouse, rat
Introduction
Recreational use of synthetic cannabinoids has been increasing despite efforts to control the availability of these compounds (Drug Enforcement Administration, 2014b). New, unregulated compounds appear once older compounds become controlled under state and national laws. Most of these compounds have been described in the scientific literature or patented as potential lead compounds, however; others, e.g., APINACA (AKB-48, N-(1-adamantyl)-1-pentyl-1H-indazole-3-carboxamide) seem to have been synthesized expressly for recreational drug trade and have not appeared in the scientific literature. Based on the rapid appearance of new compounds, the Drug Enforcement Agency (DEA) has been requesting temporary scheduling of recreationally-used compounds when they are discovered instead of waiting for scientific analysis. UR-144 (1-pentylindol-3-yl)(2,2,3,3-tetramethylcyclopropyl)methanone), XLR-11 (5F-UR144, [1-(5-fluoro-pentyl)-1H-indol-3-yl](2,2,3,3-tetramethylcyclopropyl)methanone), and AKB-48 were temporarily scheduled on May 16, 2013 (Drug Enforcement Administration, 2013), and PB-22 (QUPIC, quinolin-8-yl 1-pentyl-1H-indole-3-carboxylate), 5F-PB-22 (quinolin-8-yl 1-(5-fluoropentyl)-1H-indole-3-carboxylate), and AB-FUBINACA (N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(4- fluorobenzyl)-1H-indazole-3-carboxamide) were temporarily scheduled on January 10, 2014 (Drug Enforcement Administration, 2014a).
These compounds were identified as high risk by the DEA, and independent investigators have confirmed their sale and use. Four of the six compounds (UR-144, XLR-11, AKB-48, and AB-FUBINACA) have been identified in samples of synthetic cannabinoids obtained on the street (Kavanagh et al., 2013; Uchiyama et al., 2013; Strano Rossi et al., 2014). All six of the compounds have been found in blood or urine samples (or identified by verbal report) of users reporting adverse effects (Behonick et al., 2014; Gugelmann et al., 2014; Mohr et al., 2014; Strano Rossi et al., 2014), and the use of UR-144 or XLR-11 has been reported in cases of driving under the influence (Lemos, 2014; Musshoff et al., 2014). Of further concern, several of these compounds have been reported to produce significant adverse effects. For example, PB-22 caused convulsions in humans and canines (Gugelmann et al., 2014) and 5F-PB-22 was present in three cases of sudden death (Behonick et al., 2014). Renal toxicity associated with the use of synthetic cannabinoids has also been reported, with XLR-11 being identified in several of the cases (Centers for Disease Control and Prevention, 2013; Buser et al., 2014).
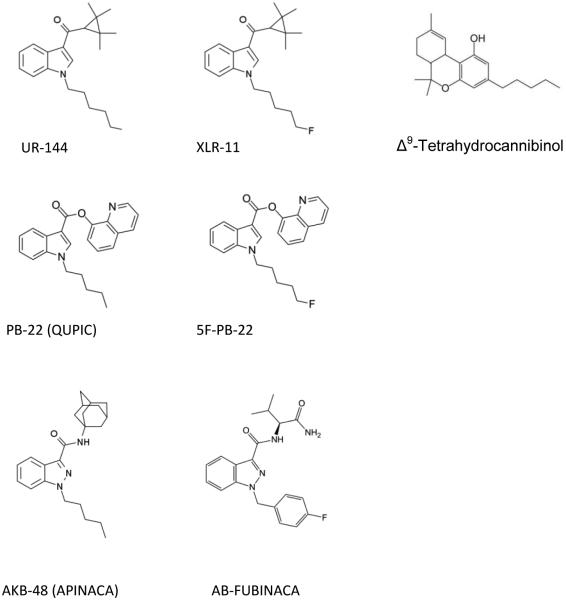
It has been previously noted that synthetic cannabinoids are not merely other forms of Δ9-THC (Fantegrossi et al., 2013). These compounds have chemical structures unrelated to Δ9-THC, different metabolism, and often greater toxicity (Fantegrossi et al., 2014). As shown in Figure 1, UR-144, XLR-11, PB-22, and 5F-PB-22 have central indole rings, whereas AKB-48 and AB-FUBINACA have indazole rings. Although pharmacological information on these compounds is scant, it has been reported that UR-144 binds to both CB1 and CB2 receptors (Frost et al., 2010) and that UR-144 and XLR-11 depress locomotor activity and fully substitute for the discriminative stimulus effects of Δ9-THC (Wiley et al., 2013).
Fig. 1.
Chemical structures of the six cannabinoid test compounds.
The purpose of the present studies was to determine whether the six cannabinoids listed above are psychoactive and may produce subjective effects similar to that of Δ9-THC. The mouse locomotor activity test was used to measure depressant effects common to cannabinoids. Drug discrimination procedures, which have a long history as a useful animal model of the subjective effects of drugs that may predict abuse liability (Balster, 1991; Horton et al., 2013), were used in rats to test for the ability of the six cannabinoid compounds to produce discriminative-stimulus effects similar to those of Δ9-THC.
Methods
Subjects
Male ND4 Swiss–Webster mice were obtained from Harlan Laboratories (Indianapolis, IN) at approximately 8 weeks of age and maintained in the University of North Texas Health Science Center (UNTHSC) animal facility for two weeks prior to testing. Mice were housed 3-4 per cage on a 12:12-h light/dark cycle (lights on at 07:00 h) and were allowed free access to food and water except during test sessions. Male Sprague-Dawley rats were obtained from Harlan Laboratories. All rats were housed individually and were maintained on a 12:12 light/dark cycle (lights on at 07:00 h). Body weights were maintained at 320-350 g by limiting food to 15 g/day, which included the food received during operant sessions. Water was continuously available in the home cage. All housing and procedures were in accordance with Guidelines for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Locomotor Activity
Each study was conducted using 32 Digiscan locomotor activity testing chambers (40.5 × 40.5 × 30.5 cm) (Omnitech Electronics, Columbus OH), each housed within a sound-attenuating chamber that provided dim illumination. A panel of 16 infrared beams and corresponding photodetectors were located in the horizontal direction along the sides of each activity chamber. Separate groups of 8 mice were injected with either vehicle (ethanol/Cremophor EL/0.9% saline 1:1:18) or a cannabinoid: Δ9-THC (1 – 30 mg/kg), UR-144 (1 – 30 mg/kg), XLR-11 (1 – 30 mg/kg), AKB-48 (1 – 30 mg/kg), PB-22 (0.05 – 0.5 mg/kg), 5F-PB-22 (0.1 – 1.0 mg/kg), AB-FUBINACA (0.5 – 5 mg/kg), immediately prior to locomotor activity testing. Only 7 mice were tested following the 1 mg/kg dose of UR-144. Each dose range included doses that were without effect to those producing at least 50% depression below vehicle control. In all studies, horizontal activity (interruption of photocell beams) was measured for 8 h within 10-min periods, beginning at 08.00 h (1 h after lights on). Behavioral observations were recorded on each mouse at 30, 120 and 480 min following the highest dose tested.
Discrimination Procedures
Standard behavior-testing chambers (Coulbourn Instruments, Allentown, PA) were connected to IBM-PC compatible computers via Med Associates interfaces (East Fairfield, VT). The computers were programmed in Med-PC for Windows, version IV (Med Associates, East Fairfield, VT) for the operation of the chambers and collection of data.
A pool of rats were first trained to discriminate Δ9-THC (3 mg/kg) from vehicle (ethanol/Cremophor EL/0.9% saline 1:1:18) using a two-lever choice methodology. Thirty minutes prior to the training sessions, rats received an injection of either saline or Δ9-THC and were subsequently placed in the behavior-testing chambers, where food (45 mg food pellets; Bio-Serve, Frenchtown, NJ) was available as a reinforcer for every ten responses on a designated injection-appropriate lever. Each training session lasted a maximum of 10 min, and the rats could earn up to 20 food pellets. Rats were used in tests of substitution of the experimental compounds once they had achieved 9 of 10 sessions at 85% or greater injection-appropriate responding for both the first reinforcer and total session, which occurred after approximately 60 training sessions. The training sessions occurred on separate days in a double alternating fashion (drug-drug-vehicle-vehicle-drug; etc.) until the training phase was complete, after which substitution tests were introduced into the training schedule such that at least one vehicle and one drug session occurred between each test (drug-vehicle-test-vehicle-drug-test-drug; etc.). The substitution tests occurred only if the rats had achieved 85% injection-appropriate responding on the two prior training sessions.
During test sessions, both levers were active, such that 10 consecutive responses on either lever led to reinforcement. For dose-effect experiments, data were collected until the first reinforcer was obtained, or for a maximum of 20 min. Each compound was tested in a separate group of six rats using a repeated-measures design such that each rat was tested at all doses of a given drug. Vehicle and Δ9-THC (3 mg/kg) controls were tested before the start of each compound evaluation. Intraperitoneal injections (1 ml/kg) of vehicle, UR-144 (0.1 – 5 mg/kg), AKB-48 (0.025 – 2.5 mg/kg), and 5F-PB-22 (0.01 – 0.5 mg/kg) occurred 30 min prior to the start of the test session. Administration of XLR-11 (0.05 – 1 mg/kg), PB-22 (0.1 – 0.5 mg/kg), AB-FUBINACA (0.05 – 1 mg/kg) were administered 15 min prior to the start of the test session. A dose range was tested from no effect (<20% Δ9-THC-appropriate responding) to full effect (≥80% Δ9-THC-appropriate responding or suppression of responding to less than 20% of vehicle control). Pretreatment times were based on the time of peak depression for each compound in the locomotor activity testing.
For the time course experiments, a repeated-measures design was used, such that each rat was tested at several time points following a single administration of the test compound. The lowest dose that fully substituted without significant rate effects in the dose-effect studies was selected. The rats were injected with the test compound and placed in the test chambers 5 min after administration. Data were collected until the first reinforcer was obtained, or for a maximum of 5 min, and the rats were immediately removed from the chambers. Testing was repeated at 15, 30, 60, and 120 min after administration. If necessary, testing was continued at 4, 8, 24, and 48 h after administration until Δ9-THC-appropriate responding had decreased to below 30-40%.
Drugs
Δ9-Tetrahydrocannabinol, UR-144 ((1-pentylindol-3-yl)(2,2,3,3-tetramethylcyclopropyl)methanone), XLR-11 ([1-(5-fluoro-pentyl)-1H-indol-3-yl](2,2,3,3-tetramethylcyclopropyl)methanone), AKB-48 (N-(1-adamantyl)-1-pentyl-1H-indazole-3-carboxamide), PB-22 (quinolin-8-yl 1-pentyl-1H-indole-3-carboxylate), 5F-PB-22 (quinolin-8-yl 1-(5-fluoropentyl)-1H-indole-3-carboxylate), and AB-FUBINACA (N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(4- fluorobenzyl)-1H-indazole-3-carboxamide) were provided by the National Institute on Drug Abuse Drug Supply Program. All drugs were dissolved in ethanol/Cremophor EL/0.9% saline (1:1:18) and were administered i.p. in a volume of 1 ml/kg.
Data Analysis
Locomotor activity data were expressed as the mean number of photocell counts in the horizontal plane (ambulation counts) during each 10-min period of testing. A 30-min period, beginning when maximal depression of locomotor activity first appeared as a function of dose, was used for analysis of dose-response data and calculation of ED50 values. OriginGraph (OriginLab Corporation, Northampton, MA) was used to estimate the maximal depression induced by each cannabinoid. The ED50 values were calculated by estimating the dose producing ½ of maximal depression (0 photocell counts) from the descending linear portion of the dose response curve. A two-way analysis of variance, with dose as a between-groups factor and time as a within-subjects factor, was conducted on horizontal activity counts/10 min interval. Subsequently, a one-way analysis of variance was conducted on horizontal activity counts for the 30-min period of maximal effect, and planned comparisons were conducted for each dose against the vehicle control using single degree-of-freedom F tests.
Drug discrimination data are expressed as the mean percentage of drug-appropriate responses occurring in each test period. Rates of responding were expressed as a function of the number of responses made divided by the time to the first reinforcer. Graphs for percent drug-appropriate responding and response rate were plotted as a function of dose of test compound (log scale). Percent drug-appropriate responding was shown only if at least 3 rats completed the first fixed ratio, whereas all rats are shown for the response rate data. Full substitution was defined as ≥80% drug-appropriate responding and not statistically different from the training drug. The potencies of UR-144, XLR-11, AKB-48, PB-22, 5F-PB-22, and AB-FUBINACA were calculated by fitting straight lines to the dose-response data for each compound by means of OriginGraph (OriginLab Corporation, Northampton, MA). Straight lines were fitted to the linear portion of dose-effect curves, including not more than one dose producing <20% of the maximal effect and not more than one dose producing >80% of the maximal effect. Other doses were excluded from the analyses. Response-rate data were analyzed by one-way repeated measures analysis of variance. Effects of individual doses were compared to the vehicle control value using a priori contrasts. The criterion for significance was set a priori at p<0.05.
Results
Locomotor Activity
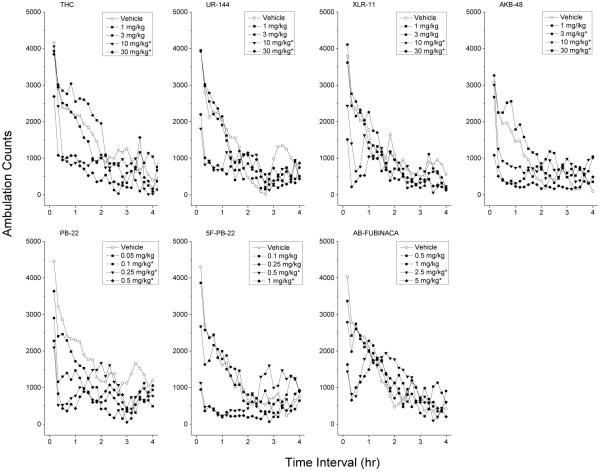
Figure 2 shows average horizontal activity counts in 10-min bins as a function of time and dose of each test compound. Each compound decreased locomotor activity as dose increased (ED50 values are shown in Table 1) and the time-course of these effects was complete within 4 h. No unusual behavioral observations were recorded for any of the test compounds.
Fig. 2.
Time course of locomotor activity. Mean horizontal activity (Ambulation counts) as a function of time (10 min bins) and dose for each test compound (left to right). Only data from the first four hours are shown. Data are from independent groups of 8 mice per dose, except the 1 mg/kg dose of UR-144 (n=7). * indicates depressant effects (p < 0.05) relative to vehicle control.
Table 1.
ED50 values (mg/kg) for discriminative stimulus effects of cannabinoids in THC-trained rats and locomotor depressant effects in mice. Data are mean ± standard error.
| Drug | Drug Discrimination |
Locomotor Activity |
|---|---|---|
| THC | 0.85±0.12 | 11.14±0.10 |
| UR-144 | 0.45±0.12 | 7.68±0.09 |
| XLR-11 | 0.18±0.09 | 10.29±0.08 |
| AKB-48 (APINACA) | 0.21±0.17 | 2.18±0.02 |
| PB-22 | 0.20±0.04 | 0.12±0.05 |
| 5F-PB-22 | 0.039±0.21 | 0.25±0.05 |
| AB-FUBINACA | 0.18±0.10 | 1.71±0.05 |
Depressant effects of Δ9-THC occurred within 10 to 50 min following injection and lasted 90-140 min. A two-way analysis of variance conducted on horizontal activity counts/10 min indicated significant effects of Treatment F(4,35)=3.47, p<.02, 10-Minute Periods F(47,1645)=29.80, p<.001, and the Periods × Treatment interaction F(188,1645)=2.38, p<.001. Maximal depressant effects were observed 30 to 60 minutes following 10 and 30 mg/kg F(4,35)=10.77, p<.001. The 10 and 30 mg/kg doses of UR-144 produced a depression of locomotor activity within 10 minutes following injection that lasted 60-90 minutes. The two-way analysis of variance for this compound indicated significant effects of Treatment F(4,34)=3.78, p=.012, 10-Minute Periods F(47,1598)=34.12, p<.001, and the Periods × Treatment interaction F(188,1598)=2.89, p<.001. Maximal depressant effects were observed 10 to 40 minutes following 10 and 30 mg/kg F(4,34)=18.24, p<0.001.
Depressant effects of XLR-11 occurred within 10 minutes following administration and lasted 40-60 minutes. A two-way analysis of variance conducted on horizontal activity counts/10 min indicated significant effects of Treatment F(4,35)=8.43, p<.001, 10-Minute Periods F(47,1645)=30.05, p<.001, and the Periods × Treatment interaction F(188,1645)=2.15, p<.001. Maximal depressant effects of 10 and 30 mg/kg occurred 10 to 40 minutes following injection F(4,35)=17.15, p<.001. AKB-48 produced depressant effects lasting 70 to 100 minutes which began within 10 to 20 minutes following injection. The two-way analysis of variance for this test compound indicated significant effectx of Treatment F(4,35)=5.69, p<.001, 10-Minute Periods F(47,1645)=14.52, p<.001, and the Periods × Treatment interaction F(188,1645)=2.86, p<.001. Maximal depressant effects occurred 10 to 40 minutes following injection of 3 to 30 mg/kg [F(4,35)=21.01, p<0.001].
Depressant effects of PB-22 occurred within 10 minutes following injection and lasted 90 to 150 minutes. A two-way analysis of variance conducted on horizontal activity counts/10 min failed to indicate a significant effect of Treatment F(4,35)=1.03, p=.408, although significant effects were observed for the 10-Minute Periods F(47,1645)=12.46, p<.001, and the Periods × Treatment interaction F(188,1645)=1.98, p<.001. Maximal depressant effects of 0.1 to 1 mg/kg occurred 0 to 30 minutes following injection [F(4,35)=17.15, p<0.001]. 5F-PB-22 produced depressant effects at 0.5 and 1 mg/kg within 10 minutes following injection; these effects lasted 110 minutes. A two-way analysis of variance conducted on horizontal activity counts/10 min failed to indicate a significant effect of Treatment F(4,35)=1.79, p=.153, although significant effects were observed for the 10-Minute Periods F(47,1645)=16.10, p<.001, and the Periods × Treatment interaction F(188,1645)=4.45, p<.001. Maximal depressant effects occurred 0 to 30 minutes following injection [F(4,35)=45.27, p<0.001].
Depressant effects of 2.5 and 5 mg/kg AB-FUBINACA occurred within 10 minutes following injection and lasted 50-60 minutes. A two-way analysis of variance conducted on horizontal activity counts/10 min failed to indicate a significant effect of Treatment F(4,35)=0.38, p=.821, although significant effects were observed for the 10-Minute Periods F(47,1645)=28.28, p<.001, and the Periods × Treatment interaction F(188,1645)=2.21, p<.001. Peak effects of 2.5 and 5 mg/kg AB-FUBINACA were observed between 0 and 30 minutes following injection [F(4,35)=17.36, p<.001].
Drug Discrimination
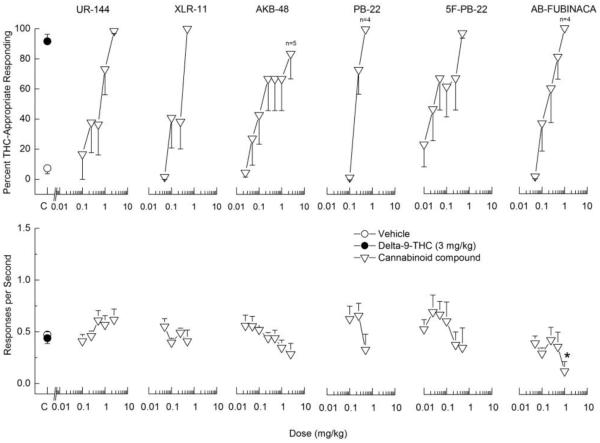
All of the test compounds (UR-144, XLR-11, AKB-48, PB-22, 5F-PB-22, and AB-FUBINACA) fully substituted for the discriminative stimulus effects of 3 mg/kg Δ9-THC (Figure 3). The ED50 values for each compound are shown in Table 1. UR-144, XLR-11, and 5F-PB-22 produced no effects on response rate. AKB-48 did not produce statistically significant decreases in response rate, but responding was suppressed in one of six rats at the dose that fully substituted (2.5 mg/kg). PB-22 decreased response rate F(3,15)=5.20, p<0.02 and two of six rats failed to respond at the dose that fully substituted (0.5 mg/kg). AB-FUBINACA also suppressed response rate F(5,25)=2.88, p<.05, and two of six rats failed to respond at the dose that fully substituted (1 mg/kg).
Fig. 3.
Substitution for the discriminative stimulus effects of Δ9-THC: Dose-Effect. Upper panels show percentage of total responses made on the drug-appropriate lever. Lower panels show rate of responding in responses per second (r/s). All of the cannabinoids fully substituted for the discriminative stimulus effects of Δ9-THC (>80% drug-appropriate responding). n=6 except where shown. C indicates vehicle and Δ9-THC control values. * indicates response rate different from vehicle control (p < 0.05).
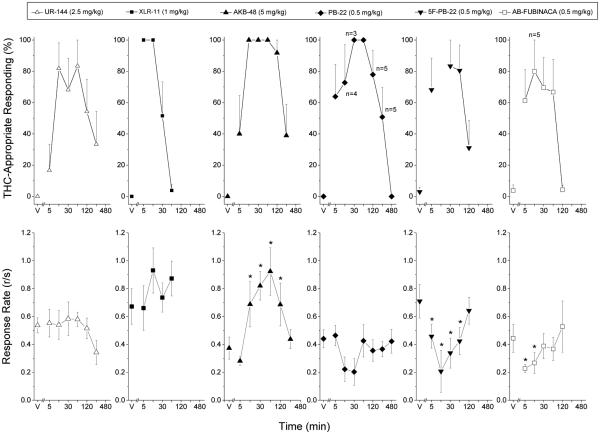
The results of the time course studies are shown in Figure 4. UR-144 (2.5 mg/kg) fully substituted for the discriminative stimulus effects of 3 mg/kg Δ9-THC at 15 and 60 min after administration and drug-appropriate responding was diminished to less than 40% after 4 h. No effect of UR-144 on response rate was observed. XLR-11 (1 mg/kg) fully substituted from 5 to 15 min following administration and drug-appropriate responding was nearly absent by 60 min. No effect on response rate was observed for this dose of XLR-11. AKB-48 (5 mg/kg) produced full substitution for the discriminative stimulus effects of Δ9-THC from 15 to 120 min after administration and drug- appropriate responding had diminished to less than 50% at 4 h following administration. Response rate was increased at 15, 30, 60 and 120 min after administration of AKB-48 F(6,30)=4.85 p<0.001.
Fig. 4.
Time course of the discriminative stimulus effects of synthetic cannabinoids. Percentage of total responses made on the drug-appropriate lever as a function of time (shown in log increments). Each panel shows the effects of the peak dose of each compound. n=6 except where shown. V indicates vehicle values. * indicates rate different (p < 0.05) from vehicle control.
PB-22 (0.5 mg/kg) fully substituted from 30 to 60 min following administration and drug-appropriate responding had decreased to vehicle levels after 8 h. Response rates were suppressed such that 2 of 6 rats failed to earn at least one reinforcer at 15 min, and 3 of 6 rats failed to earn at least one pellet at 30 min after administration. However, no statistically significant effect on response rate was observed, perhaps because response rates stayed the same or increased for the remaining rats, leading to increased variability. 5F-PB-22 (0.5 mg/kg) produced full substitution for the discriminative stimulus effects of Δ9-THC from 30 to 60 min after administration. Drug-appropriate responding was attenuated by 120 min following administration. Rates of responding were decreased at 5, 15, 30, and 60 min after 5F-PB-22 F(5,25)=3.87 p<0.01, with marked suppression at 15 min after administration, such that 4 of 6 rats did not earn a food pellet. For AB-FUBINACA (0.5 mg/kg), substitution for Δ9-THC was present at 15 min and absent by 2 h following administration. Greater than 60% drug- appropriate responding persisted up to 1 h following administration. Depression of response rate was observed at 5 and 15 min following administration F(5,25)=2.68 p<0.05.
Discussion
Six compounds temporarily assigned to schedule 1 by the DEA (UR-144, XLR-11, AKB-48, PB-22, 5F-PB-22, and AB-FUBINACA) were found to be active in rodent behavior assays. Each compound produced dose-dependent depression of spontaneous locomotor activity similar to that produced by Δ9-THC. The synthetic cannabinoids were all at least as potent as Δ9-THC, and some were as much as 100-fold more potent than Δ9-THC. Rank order of potency for depression of locomotor activity was PB-22 = 5F-PB-22 > AB-FUBINACA = AKB-48 > UR-144 = XLR-11 = Δ9-THC. These findings confirm and extend previous findings that UR-144 and XLR-11 decrease locomotor activity in mice (Wiley et al., 2013), as well as those of earlier studies indicating that a range of synthetic cannabinoids depress locomotor activity (Wiley et al., 1998; Gatch and Forster, 2014).
All six of the test compounds also fully substituted for the discriminative stimulus effects of Δ9-THC in rats. These results confirm and extend previous findings that UR-144 and XLR-11 fully substitute in rats trained to discriminate Δ9-THC (Wiley et al., 2013). Likewise, a number of other synthetic cannabinoids also fully substitute for the discriminative stimulus effects of Δ9-THC in mice (Brents et al., 2012), rats (Järbe et al., 2011; Wiley et al., 2014; Gatch and Forster, 2014), and monkeys (Ginsburg et al., 2012). The test compounds appeared to be more potent at producing stimulus effects than Δ9-THC, but none of the calculated ED50 values differed significantly from Δ9-THC.
Unlike Δ9-THC, some of the test compounds substituted only at doses that also decreased rates of responding (PB-22, 5F-PB-22, and AB-FUBINACA). PB-22 produced full substitution at 0.5 mg/kg when tested in the dose-effect study using a 15 min pretreatment time. However, in the time course study, only 73% drug-appropriate responding was observed at 15 min. The variability between these results may be attributable to the rate-decreasing effects of PB-22. Only 4 of 6 rats responded following 0.5 mg/kg at 15 min after administration in both the time-course and dose-effect studies. However, 100% THC-appropriate responding was observed at 30 and 60 min following 0.5 mg/kg PB-22, with little rate suppression at 60 min.
AKB-48 (2.5 mg/kg) appeared to barely reach the 80% criterion for full substitution, and indeed less than 50% THC-appropriate responding was observed in a time course study of the 2.5 mg/kg dose (data not shown). However, 100% THC-appropriate responding was observed at 15 to 60 min following 5 mg/kg AKB-48, which indicates that AKB-48 can produce robust Δ9-THC discriminative stimulus effects depending on the parameters of the study. Similarly, UR-144 and AB-FUBINACA produced around 80% THC-appropriate responding in the time-course studies, but produced close to 100% THC-appropriate responding in the dose-effect studies. These differences between dose-effect and time-course studies are likely due to the variability in potency of these compounds across groups of animals. It is important to note that all of the test compounds produced substantial THC-like discriminative stimulus effects at a particular dose and time point, indicating their potential for abuse liability similar to that of cannabis.
Similar to a previous study of synthetic cannabinoids (Gatch and Forster, 2014), duration of the locomotor depressant effects of the test compounds did not predict the duration of their discriminative stimulus effects, nor did potency for the LMA depressant effects predict potency for substitution. For most of the test compounds, the ED50 values for drug discrimination were smaller than those for locomotor activity: the differences ranged from 3-fold to 60-fold. This difference in potency is in contrast to our work with psychostimulants, in which the locomotor stimulant effects of the test compounds in mice are highly predictive of the potency for the discriminative stimulus effects in rats, despite the physiological differences between the species (Gatch et al., 2013, 2014). The apparent disparity in the current studies may suggest that sedative or anxiolytic actions of cannabinoids are not a salient component of their discriminative stimulus effects.
Before advancing to behavioral testing, all six of the test compounds were tested in assays developed and conducted by NovaScreen (PerkinElmer, Waltham, MA) under contract with the National Institute on Drug Abuse Addiction Treatment Discovery Program (NIDA ATDP) and the data were provided by the Program. All of the compounds bind with nanomolar affinities to human recombinant CB1 receptors expressed in HEK-293 cells, and act as full agonists—again, at nanomolar affinities--in CHO cells that expressed the human CB1 receptor (Table 2). It is noteworthy that functional activity at CB1 among the six test compounds was correlated with potency for Δ9-THC substitution (r2=0.72, p=0.02), but not locomotor depression (r2=0.21, p=0.20).
Table 2.
Binding affinity and functional activity of test compounds at human CB1 cannabinoid receptors. Dose effect curves were tested in triplicate and the values shown are means of the three determinations.
| Drug | hCB1 Binding IC50/Ki (nM) |
Functional Activity EC50 (nM) |
Maximum Effect (%) |
|---|---|---|---|
| UR-144 | 578.5 | 1295 | 95.28 |
| XLR-11 | 7.92 | 359 | 104.95 |
| AKB-48 (APINACA) | 304.5 | 585 | 107.88 |
| PB-22 | 0.73 | 2.47 | 101.54 |
| 5F-PB-22 | 0.27 | 0.95 | 98.31 |
| AB-FUBINACA | 2.38 | 3 | 99.52 |
In summary, UR-144, XLR-11, AKB-48, PB-22, 5F-PB-22, and AB-FUBINACA produced locomotor depressant and discriminative stimulus effects similar to those produced by Δ9-THC. These findings, in combination with confirmation that they act as CB1 receptor agonists, suggest that these six compounds may have abuse liability similar to Δ9-THC, and to other illicit, controlled cannabinoids. Some of the test compounds produced substantial depressant effects on response rate, but no other adverse effects were observed at the doses and time points tested. Evidence that these compounds will produce reinforcing effects and maintain compulsive drug-seeking is necessary to establish that they have substantial abuse liability and need to be controlled to the same degree as Δ9-THC.
Acknowledgements
Funding was provided by the Addiction Treatment Discovery Program of the National Institute on Drug Abuse (NIH N01DA-2-8822 and N01DA-13-8908). Program staff were involved in selection of compounds and test parameters. The ATDP had no further role in study design; the collection, analysis and interpretation of data; or the writing of the report. They have granted permission for the submission of this data for publication.
References
- Balster RL. Drug abuse potential evaluation in animals. Brit J Addiction. 1991;86:1549–1558. doi: 10.1111/j.1360-0443.1991.tb01747.x. [DOI] [PubMed] [Google Scholar]
- Behonick G, Shanks KG, Firchau DJ, Mathur G, Lynch CF, Nashelsky M, Jaskierny DJ, Meroueh C. Four Postmortem Case Reports with Quantitative Detection of the Synthetic Cannabinoid, 5F-PB-22. J. Anal. Toxicol. 2014;38:559–562. doi: 10.1093/jat/bku048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Gallus-Zawada A, Radominska-Pandya A, Vasiljevik T, Prisinzano TE, Fantegrossi WE, Moran JH, Prather PL. Monohydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain intermediate to high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity. Biochem Pharmacol. 2012;83:952–961. doi: 10.1016/j.bcp.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser GL, Gerona RR, Horowitz BZ, Vian KP, Troxell ML, Hendrickson RG, Houghton DC, Rozansky D, Su SW, Leman RF. Acute kidney injury associated with smoking synthetic cannabinoid. Clin. Toxicol. (Phila) 2014;52:664–673. doi: 10.3109/15563650.2014.932365. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Acute kidney injury associated with synthetic cannabinoid use--multiple states, 2012. Morb. Mortal. Wkly. Rep. 2013;62:93–98. [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration Schedules of controlled substances: temporary placement of three synthetic cannabinoids into Schedule I. Final order. Fed Regist. 2013;78:28735–28739. [PubMed] [Google Scholar]
- Drug Enforcement Administration Schedules of controlled substances: temporary placement of four synthetic cannabinoids into Schedule I. Final order. Fed Regist. 2014a;79:7577–7582. [PubMed] [Google Scholar]
- Drug Enforcement Administration . National Forensic Laboratory Information System Special Report: Synthetic Cannabinoids and Synthetic Cathinones Reported in NFLIS, 2010–2013. U.S. Drug Enforcement Administration; Springfield, VA: 2014b. [Google Scholar]
- Fantegrossi WE, McCain KR, Moran JH, Hoffman RS. Not simply synthetic tetrahydrocannabinol. J. Pediatr. 2013;163:1797–1798. doi: 10.1016/j.jpeds.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Moran JH, Radominska-Pandya A, Prather PL. Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Δ(9)-THC: mechanism underlying greater toxicity? Life Sci. 2014;97:45–54. doi: 10.1016/j.lfs.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, El-Kouhen O, Yao BB, Hsieh GC, Pai M, Zhu CZ, Chandran P, Meyer MD. Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity. J. Med. Chem. 2010;53:295–315. doi: 10.1021/jm901214q. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ. Δ9-Tetrahydrocannabinol-like discriminative stimulus effects of compounds commonly found in K2/Spice. Behav. Pharmacol. 2014;25:750–757. doi: 10.1097/FBP.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of 'bath salt' cathinones. Behav Pharmacol. 2013;24:437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Rutledge M, Forster MJ. Discriminative and locomotor effects of five synthetic cathinones in rats and mice. Psychopharmacology (Berl) 2015;232:1197–1205. doi: 10.1007/s00213-014-3755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg BC, Schulze DR, Hruba L, McMahon LR. JWH-018 and JWH-073: Δ9-tetrahydrocannabinol-like discriminative stimulus effects in monkeys. J Pharmacol Exp Ther. 2012;340:37–45. doi: 10.1124/jpet.111.187757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugelmann H, Gerona R, Li C, Tsutaoka B, Olson KR, Lung D. 'Crazy Monkey' Poisons Man and Dog: Human and canine seizures due to PB-22, a novel synthetic cannabinoid. Clin. Toxicol. (Phila) 2014;52:635–638. doi: 10.3109/15563650.2014.925562. [DOI] [PubMed] [Google Scholar]
- Horton DB, Potter DM, Mead AN. A translational pharmacology approach to understanding the predictive value of abuse potential assessments. Behav Pharmacol. 2013;24:10–36. doi: 10.1097/FBP.0b013e3283644d2e. [DOI] [PubMed] [Google Scholar]
- Järbe TU. Cannabinergic aminoalkylindoles, including AM678=JWH018 found in 'Spice', examined using drug (Δ(9)-tetrahydrocannabinol) discrimination for rats. Behav Pharmacol. 2011;22:498–507. doi: 10.1097/FBP.0b013e328349fbd5. H. D, K. VS and A. M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh P, Grigoryev A, Savchuk S, Mikhura I, Formanovsky A. UR-144 in products sold via the Internet: identification of related compounds and characterization of pyrolysis products. Drug Test Anal. 2013;5:683–692. doi: 10.1002/dta.1456. [DOI] [PubMed] [Google Scholar]
- Lemos NP. Driving Under the Influence of Synthetic Cannabinoid Receptor Agonist XLR-11. J. Forensic Sci. 2014;59:1679–1683. doi: 10.1111/1556-4029.12550. [DOI] [PubMed] [Google Scholar]
- Mohr AL, Ofsa B, Keil AM, Simon JR, McMullin M, Logan BK. Enzyme-Linked Immunosorbent Assay (ELISA) for the Detection of Use of the Synthetic Cannabinoid Agonists UR-144 and XLR-11 in Human Urine. J Anal Toxicol. 2014;38:427–431. doi: 10.1093/jat/bku049. [DOI] [PubMed] [Google Scholar]
- Musshoff F, Madea B, Kernbach-Wighton G, Bicker W, Kneisel S, Hutter M, Auwärter V. Driving under the influence of synthetic cannabinoids ("Spice"): a case series. Int. J. Legal Med. 2014;128:56–64. doi: 10.1007/s00414-013-0864-1. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the Care and Use of Laboratory Animals. The National Academies Press; Washington, D.C.: 2011. [PubMed] [Google Scholar]
- Strano Rossi S, Odoardi S, Gregori A, Peluso G, Ripani L, Ortar G, Serpelloni G, Romolo FS. An analytical approach to the forensic identification of different classes of new psychoactive substances (NPSs) in seized materials. Rapid Commun. Mass. Spectrom. 2014;28:1904–1916. doi: 10.1002/rcm.6969. [DOI] [PubMed] [Google Scholar]
- Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y. URB-754: a new class of designer drug and 12 synthetic cannabinoids detected in illegal products. Forensic Sci. Int. 2013;227:21–32. doi: 10.1016/j.forsciint.2012.08.047. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, Martin BR. Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther. 1998;285:995–1004. [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Lefever TW, Grabenauer M, Moore KN, Thomas BF. Cannabinoids in disguise: Δ9-tetrahydrocannabinol-like effects of tetramethylcyclopropyl ketone indoles. Neuropharmacology. 2013;75:145–154. doi: 10.1016/j.neuropharm.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Lefever TW, Cortes RA, Marusich JA. Cross-substitution of Δ(9)-tetrahydrocannabinol and JWH-018 in drug discrimination in rats. Pharmacol. Biochem. Behav. 2014;124:123–128. doi: 10.1016/j.pbb.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]