Abstract
Shiga toxins are potent cytotoxins that inhibit host cell protein synthesis, leading to cell death. Classically, these toxins are associated with intestinal infections due to Shiga toxin-producing Escherichia coli or Shigella dysenteriae serotype 1 and infections with these strains can lead to hemolytic uremic syndrome. Over the past decade there is increasing recognition that Shiga toxin is produced by additional Shigella species. We recently reported the presence and expression of stx genes in Shigella flexneri 2a clinical isolates. The toxin genes were carried by a new stx-encoding bacteriophage and infection with these strains correlated with recent travel to Haiti or the Dominican Republic. In this study we further explored the epidemiological link to this region by utilizing the French National Reference Center for Escherichia coli, Shigella and Salmonella collection to survey the frequency of Stx-producing Shigella species isolated from French travelers returning from the Caribbean. About 21% of the isolates tested were found to encode and produce Stx. These isolates included strains of S. flexneri 2a, S. flexneri Y, and S. dysenteriae 4. All of the travelers whom were infected with Stx-producing Shigella had recently traveled to Haiti, the Dominican Republic, or French Guiana. Furthermore, whole genome sequencing found that the toxin genes were encoded by a prophage that was highly identical to the phage we identified in our previous study. These findings demonstrate that this new stx-encoding prophage is circulating within that geographical area, has spread to other continents, and is capable of spreading to multiple Shigella serogroups.
Keywords: Shigella, Shiga toxin, Haiti, Dominican Republic
INTRODUCTION
Shiga toxins are cytotoxins that act by inhibiting eukaryotic protein synthesis, eventually leading to host cell death [1]. Shiga toxins are classified as AB5 toxins based on their structure [2]. They consist of an enzymatically active A subunit that exhibits RNA N-glycosidase activity and a B pentamer which is responsible for binding of the toxin to glycolipid receptors on the target cell surface. After binding, Shiga toxin enters a mammalian cell by endocytosis and eventually traffics to the endoplasmic reticulum where the A subunit is proteolytically cleaved to an inactive, A2 subunit and an active A1 subunit which binds to and inactivates the host cell ribosome [3]. Infections with bacteria that produce Shiga toxin can cause hemorrhagic colitis and lead to more serious complications like hemolytic uremic syndrome (HUS), a potentially deadly condition [4].
While Shiga toxins are commonly made by Shigella dysenteriae serotype 1 and Shiga toxin–producing Escherichia coli (STEC), recently Shiga toxin genes have been found in other Shigella species [5–7]. S. dysenteriae 1 produces the prototypical Stx which is encoded on the chromosome within a defective bacteriophage [8]. Stx is secreted by S. dysenteriae 1 via an unknown mechanism. In STEC the Shiga toxin family is comprised of two different branches, Stx1 and Stx2, which contain many subtypes and variants that are antigenically related [9]. Shiga toxins in the Stx1 family are nearly identical to S. dysenteriae 1 toxin whereas subtypes from the Stx2 family share approximately 50% homology to Stx [10, 11]. In contrast to S. dysenteriae 1, the toxin genes in STEC are encoded by lambdoid prophages and toxin release occurs through lytic induction of the prophage [12–14].
In a previous study, we analyzed 26 clinical isolates from United States public health department laboratories of S. flexneri 2a that produce and release Stx [6]. The toxin genes in these isolates are carried by a new stx-converting phage, ϕPOC-J13. These S. flexneri isolates were identified based on their shared pulsed-field gel electrophoresis (PFGE) pattern in the Centers for Disease Control and Prevention (CDC) PulseNet database. Additionally, of the patients whom reported foreign travel, ~60% had recently visited the island of Hispaniola (Haiti and the Dominican Republic), suggesting that the emergence of these strains is associated with that region.
Here we have further investigated this link between infection with Stx-producing Shigella and travel to Hispaniola by surveying the occurrence of stx-encoding Shigella species in French travelers returning from the Caribbean. Approximately 50–60% of all Shigella isolated in France and its overseas “départements” (administrative subdivisions) are reported to the French National Reference Center for Escherichia coli, Shigella and Salmonella (FNRC-ESS), located at the Institut Pasteur, Paris, France. The collection includes all serogroups of Shigella and epidemiological data (date and site of isolation, gender, age and international travel history) are recorded for each case. We utilized the FNRC-ESS collection of Shigella species from French travelers who had reported recent travel to the Caribbean to screen for stx. The findings reported here support our hypothesis that emergence of Stx-producing Shigella is occurring within Hispaniola, shows that the stx-converting phage responsible has spread to other Shigella species, and demonstrates that Stx-producing Shigella has spread globally.
METHODS
Bacterial strains and growth conditions
Shigella strains were grown in Tryptic Soy Broth (BD Difco, Franklin Lakes, NJ, USA) at 37°C with aeration or on Tryptic Soy Broth plates containing 1.5% agar and 0.025% Congo red (Sigma-Aldrich, St. Louis, MO, USA). E. coli K-12 strain MG1655 was grown in Luria-Bertani (LB) broth and on LB agar plates.
Taxonomic identification of isolates
Shigella “species” identification was confirmed using conventional methods and serotyping was done by slide agglutination assays using a complete set of antisera allowing recognition of all described Shigella serotypes [15]. The results of whole genome sequencing confirmed identification of the isolates.
PCR analysis of Shigella clinical isolates
DNA was extracted from the Shigella clinical isolates with the InstaGene matrix kit (Bio-Rad, Hercules, CA, USA) and screened by PCR for stx using the previously described primers Lin 5′ and Lin 3′ which detect stx and its variants [16, 17]. Subsequent PCR with primers Lin 5′ and VT1b allowed detection of most variants of stx1 and a PCR reaction with primers Lin 5′ and stx2-R allowed detection of most variants of stx2. Similarly, DNA extracts were screened for stx2 using primers Lin 5′ and stx2-R [16, 18]. Strains that were positive by PCR for stx were further subtyped according to the consensus international methods described in Scheutz et al. [9]. The PCR reactions were carried out using a PCR Taq DNA polymerase kit (Applied Biosystem/Roche, Foster City, CA).
Cell lysates from the stx-encoding isolates were analyzed by PCR to show that stx was phage encoded using primer pairs Stx1R2/Phage_stxR2 and Phage_stx1F2/Stx1F2 [6]. The insertion site of the phage into locus S1742 or a homologous gene was determined by PCR using primers to the upstream region of S1742 and an early phage gene (primers S1742_up/Stx-_phage_up) and by amplifying a late phage gene and the downstream region of S1742 with primers Stx_phage_dn/S1742_dn [6]. These PCR reactions were carried out using PCR Master Mix 2X according to the manufacturer’s specification (Fermentas, Pittsburgh, PA, USA).
Cytotoxicity assay
Whole cell lysates and supernatants from the stx-encoding strains were tested in a Vero cell cytotoxicity assay as previously described [6].
Determination of plaque forming units
Phage particles were isolated from overnight supernatants and absorbed onto E. coli MG1655 as previously described [6]. Plaque plates were incubated overnight at 37°C before plaque forming units were enumerated. The plaques observed were verified to be due to an stx-encoding phage by spotting 50 μl of phage prepared from overnight supernatants onto a soft agar overlay of MG1655. After overnight incubation at 37°C the zone of clearing from where the phage preparation was spotted was removed and analyzed by PCR with primers stx1-det-F1 and stx1-seq-R1 to detect stx [9]. To confirm that the PCR product from the overlay was not due to bacterial contamination, supernatant from an stx-positive, non-phage producing strain was spotted and shown to be stx-negative.
Whole genome sequencing and analysis
Genomic DNA was extracted from overnight cultures using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA). Sequencing libraries were prepared with either the TruSeq DNA Sample Prep Kit (Illumina, San Diego, CA, USA) or the Nextera DNA Sample Prep Kit (Illumina). DNAs were sequenced on the Illumina MiSeq Platform, generating paired-end 250 bp reads in sufficient quantity to provide over 35X coverage for each genome. Raw reads were trimmed and draft genome sequences were assembled de novo with CLC Genomics Workbench v6.5.1 or v7.0.4 (CLC bio, Boston, MA, USA). In most cases the entire phage harboring stx was contained on one contig; otherwise two contigs were bioinformatically joined to obtain the entire phage sequence and this was then verified by mapping the reads onto the phage sequence.
The stx-encoding prophage sequences were extracted from the genomic assemblies of the strains investigated and aligned to the ΦPOC-J13 phage reference sequence (GenBank accession KJ603229) using the Mauve algorithm within the MegAlign Pro module of the Lasergene software package (DNASTAR Inc., Madison, WI, USA). Phylogenetic analysis of identified single nucleotide polymorphisms (SNPs) was conducted with SplitsTree 4 [19], using the neighbor-net algorithm and untransformed p distances.
Pulsed-field gel electrophoresis
Pulsed-field gel electrophoresis (PFGE) was performed according to the protocol developed by the CDC (http://www.cdc.gov/pulsenet/pathogens/index.html), using Salmonella enterica serotype Braenderup H9812 as the control strain. Agarose-embedded DNA was digested with 50 U of XbaI (Roche Diagnostics Corp, Indianapolis, IN, USA) for at least 2 hours in a water bath at 37°C. The restriction fragments were separated by electrophoresis in 0.5X TBE buffer (Invitrogen, Carlsbad, CA, USA) at 14°C for 18 h using a Chef Mapper electrophoresis system (Bio-Rad) with pulse times of 2.16 – 54.17 s. The gels were stained with GelRed Nucleic Acid Stain (Phenix Research, Candler, North Carolina, USA), and DNA bands were visualized with UV transillumination (Bio-Rad). PFGE results were analyzed using the BioNumerics Software v6.6 (Applied-Maths, Kortrijk, Belgium), and banding pattern similarity was compared using a 1.5% band position tolerance.
Nucleotide sequence accession numbers
The Whole Draft Genome sequences have been deposited at DDBJ/EMBL/GenBank under the accession numbers listed in the Table.
Table.
stx-positive Shigella isolates from French travelers
| Strain | Species | Isolation Year | Reported Travel | Agea | Gender | GenBank Accession no. |
|---|---|---|---|---|---|---|
| BS1021 | S. flexneri 2a | 2003 | Haiti | 1–5 | F | LAHV01000000 |
| BS1022 | S. flexneri 2a | 2004 | Dominican Republic | 15–64 | M | LAHW01000000 |
| BS1023 | S. flexneri 2a | 2005 | Dominican Republic | 8 | M | LAHX01000000 |
| BS1024 | S. flexneri 2a | 2005 | French Guiana | 15–64 | M | LAHY01000000 |
| BS1025 | S. flexneri 2a | 2008 | Haiti | 13 | M | LAHZ01000000 |
| BS1041 | S. flexneri 2a | 1999 | Dominican Republic | 4 | F | LAIA01000000 |
| BS1042 | S. flexneri 2a | 2005 | Dominican Republic | 4 | F | LAIB01000000 |
| BS1043 | S. flexneri Y | 2005 | Haiti | 39 | M | LAIC01000000 |
| BS1044 | S. flexneri 2a | 2005 | Dominican Republic | 1–5 | F | LAID01000000 |
| BS1045 | S. flexneri 2a | 2007 | Dominican Republic | 31 | M | LAIE01000000 |
| BS1046 | S. flexneri 2a | 2008 | Dominican Republic | 4 | F | LAIF01000000 |
| BS1047 | S. dysenteriae 4 | 2008 | Haiti | 50 | M | LAIG01000000 |
For some patients the exact age was not recorded but rather described as a range.
RESULTS
A review of records between 1994 and 2008 found 67 Shigella strains submitted to the FNRC-ESS that were isolated from patients who had reported recent travel to Haiti or the Dominican Republic. Of the 67 strains, 51 isolates were tested for stx by PCR. The remaining 16 strains were either not found in the collection or were not viable. Four randomly selected Shigella isolates from French Guiana, a French overseas “département” in South America, plus one strain from a traveler returning from French Guiana were also included in the analysis. The isolates included all serogroups of Shigella (S. boydii, S. dysenteriae, S. flexneri, and S. sonnei). Of the 51 isolates where travel to either Haiti or the Dominican Republic had been reported, 11 were found to be stx-positive. This included nine strains of S. flexneri 2a, one S. dysenteriae 4, and one S. flexneri Y. An S. flexneri 2a isolate from a traveler returning from French Guiana was also stx-positive, however, the four randomly selected French Guiana isolates were negative for stx by PCR. Additionally, all of the isolates analyzed were found to be stx2-negative. Limited clinical data were available, however, the patients presented with symptoms of an intestinal infection characteristic of shigellosis. Travel, isolation date, and patient information for the 12 stx-positive isolates are listed in the Table.
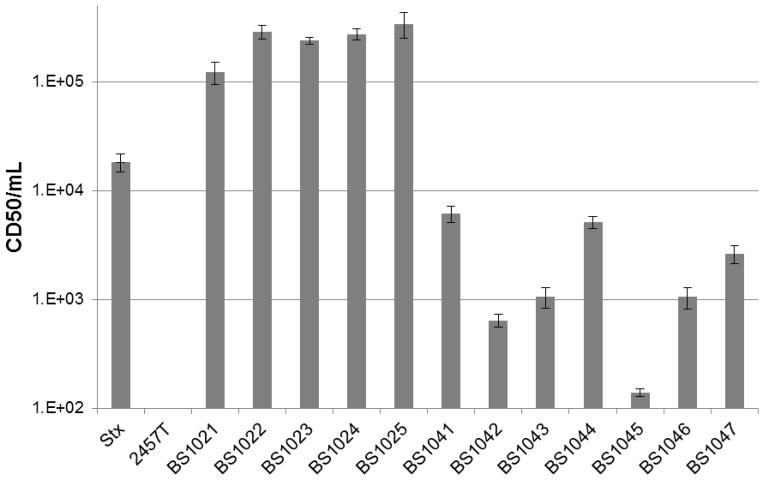
The 12 stx-positive isolates were further characterized by determining if they produced a functional toxin. Supernatants from overnight cultures of the stx-encoding Shigella isolates were cytotoxic to Vero cells compared to a Shiga toxin-negative laboratory strain of S. flexneri, 2457T (Figure 1). The CD50/ml values for the clinical isolates ranged between ~1.5x102 and ~5x105, demonstrating that all of the stx-positive isolates produced and released toxin. Since the toxin genes are typically encoded by lambdoid prophages we also monitored for the presence of viable phage progeny in overnight supernatants by performing a plaque assay using the E. coli indicator strain MG1655. Eight of the isolates formed plaques on MG1655. Viable phage progeny were not detected from three S. flexneri 2a strains (BS1022, BS1045, and BS1046) and the S. flexneri Y isolate (BS1043). Failure of supernatants from these four strains to produce plaques on MG1655 may be due to resistance of MG1655 to the phage or due to mutations that have resulted in a defective phage (see sequencing analysis below).
Figure 1.
stx-encoding Shigella species from French travelers release a functional toxin. Overnight supernatants were serially diluted 10-fold in media and applied to Vero cells to test for toxicity. Stx from S. dysenteriae 1 was included as a positive control. CD50/ml is defined as the reciprocal of the dilution of Stx that kills 50% of Vero cells. Data are an average of three independent experiments.
Although the plaque assay demonstrates that the strains are making viable phage progeny, we wanted to confirm that the plaques observed were due to an stx-encoding phage. In order to harvest enough DNA we used a phage spotting assay on MG1655 and did PCR analysis from the zone of clearing. All of the strains that formed plaques on MG1655 formed a zone of clearing and were stx-positive except for BS1047 (data not shown). Although it is unclear why BS1047 was capable of forming plaques when incubated with MG1655 in liquid culture but did not produce a zone of clearing in the spotting assay, it is likely due to differences between the two assays.
In our previous study, we designed primers based on the ϕPOC-J13 sequence to show that the stx genes in all 26 of the isolates analyzed in that study were flanked by phage sequence. We utilized those primers to analyze the 12 stx-positive isolates from the French travelers and found that their stx locus was also surrounded by phage sequence similar to ϕPOC-J13 (data not shown). Additionally, our previous analysis showed that ϕPOC-J13 is inserted in the S. flexneri chromosome into locus S1742, which encodes a putative oxidoreductase. Based upon our primer sequences designed from ϕPOC-J13, we determined that the phage in each of the 12 stx-positive French isolates had also inserted into locus S1742 or a homologous gene (data not shown).
Our PCR analyses and the travel link to Hispaniola suggested that the phage in the Shigella isolates from the French travelers is also ϕPOC-J13. To investigate the similarity to ϕPOC-J13, whole genome sequencing was performed on all 12 isolates. The DNA sequences of the stx-encoding prophages from the 12 strains were aligned and compared to ϕPOC-J13 (GenBank accession KJ603229) (Figure 2A). The set of 13 prophages were nearly identical in sequence with only 13 single nucleotide polymorphisms (SNPs) identified among them, mostly within hypothetical or putative proteins. Three insertion/deletion (indel) sites were observed in strains BS1024, BS1041, BS1045 and BS1046. In addition to the 13 SNPs and three indels, four IS elements were also observed among the strains investigated (Figure 2A). Strain BS1022 contained an IS1 element in an intergenic region and an IS2 element in a 2022 bp gene encoding a tail fiber protein. Strain BS1046 also contained an IS2 element inserted in a large 8.4 kb hypothetical protein encoding gene. Lastly, strain BS1047 possessed an IS2 element within a 546 bp putative tail fiber adhesin encoding gene. It is uncertain what affect these mutations may have on Shiga toxin production and production of infectious phage particles, however, it is possible that they may account for the varying results that were observed in our assays above.
Figure 2.
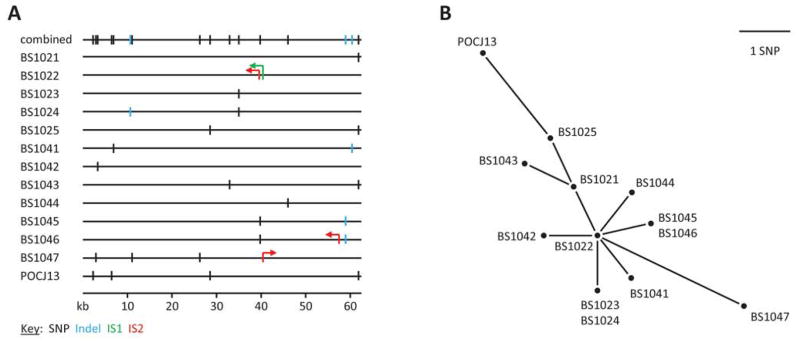
stx-encoding prophage sequence similarity. A) SNPs, indels, and IS elements within the prophage sequences of the strains investigated. SNP locations are indicated by vertical black lines, while vertical blue lines show indels. The locations and orientations of the IS1 and IS2 elements are indicated by the green and red arrows, respectively. B) SNP-based phylogenetic relationships of the prophages.
The 13 SNPs identified were used to construct a phylogenetic tree of the relationships among the stx-encoding prophages from the 12 strains investigated and ϕPOC-J13 (Figure 2B). The resulting unrooted phylogeny places the prophage from strain BS1022 (minus the two IS elements) as the potential founder with eight of the prophages being only one SNP different from the BS1022 prophage sequence. The prophage from strain BS1047 and ϕPOCJ13 are the most divergent with three and four SNP differences from the BS1022 prophage, respectively. These findings indicate that all of the Stx-producing Shigella species isolated have acquired the same phage.
The Stx-producing S. flexneri 2 strains from our previous study were identified in PulseNet based on their shared PFGE pattern, JZXX01.0357. To determine whether these new strains shared the same signature pattern, PFGE was performed on seven of the isolates from the French travelers. None of the patterns matched JZXX01.0357 (BS937). Moreover, each of the seven PFGE patterns was different (Figure 3). Only one of the isolates (S. flexneri 2a, BS1045) had a pattern number already in the PulseNet Database, JZXX01.1361. We used this pattern to search for matches and found two strains that were a 100% match: one isolate from the Massachusetts state laboratory and one isolate from the Maryland state laboratory.
Figure 3.
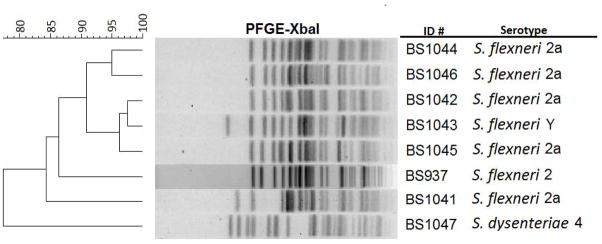
Dendrogram of PFGE analysis (XbaI) of Stx-producing Shigella. The PFGE patterns from seven of the Stx-producing Shigella strains from French travelers (BS1044, BS1046, BS1042, BS1043, BS1045, BS1041, and BS1047) are compared to BS937 (PulseNet PFGE pattern JZXX01.0357).
DISCUSSION
Overall, 12/56 (~21%) Shigella isolates analyzed from the FNRC-ESS collection of French travelers returning from the Caribbean were found to produce Stx. This finding reinforces our hypothesis that the emergence of stx-encoding Shigella species is originating from Haiti and the Dominican Republic. Furthermore, the finding that the isolates from the French travelers did not share the same PFGE pattern amongst each other, or from the previously published Stx-producing S. flexneri 2 strains, highlights the importance of using multiple approaches to identify these new strains of stx-encoding Shigella.
This is the first report of this new stx-encoding phage harbored in different Shigella species and serotypes, indicating that ϕPOC-J13 and homologous stx-encoding phages are capable of spreading to multiple Shigella species. Because we have limited clinical information on the isolates, the health consequences of infection with Stx-producing Shigella remain unclear. However, infections with S. dysenteriae 1 and STEC result in complications of HUS in ~10% of cases [20]. Therefore, these new Stx-producing Shigella isolates have the potential to cause more severe disease than typically associated with non-S. dysenteriae type 1 infections.
Finally, we have now identified Stx-producing Shigella in both French and American travelers who had recently visited the island of Hispaniola. We have also isolated stx-positive Shigella from Haitian children in Haiti (manuscript in preparation). It is still uncertain what environmental factors have contributed to the emergence of these species in that region. However, our findings imply that travelers are capable of spreading these Shigella strains globally. It is impossible to predict the extent of international spread of Stx-producing Shigella. Nonetheless, one could speculate that as the strains spread they may become capable of persisting in the ecosystems of other regions, as well as the stx-encoding phage spreading to other Shigella species in those regions. If either of those possibilities occurs, infections with Stx-producing Shigella may become more prevalent worldwide.
Acknowledgments
We thank all the corresponding laboratories of the FNRC-ESS network. We thank Isabelle Carle, Monique Lejay-Collin, Corinne Ruckly, Stephen Darnell and Reinaldo Fernandez for their excellent technical assistance.
The French National Reference Center for E. coli, Shigella and Salmonella (FNRC-ESS) is co-funded by the Institut de Veille Sanitaire. The Unité des Bactéries Pathogènes Entériques belongs to the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence funded by the French Government’s Investissement d’Avenir” program (grant no. ANR-10-LABX-62-IBEID). This work was also supported by grant R01A124656 from the National Institute of Allergy and Infectious Diseases.
Footnotes
The results included herein were previously presented at the 49th U.S.-Japan Conference on Cholera and Other Enteric Bacterial Infections and the 2015 Mid-Atlantic Microbial Pathogenesis Meeting.
The opinions or assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the Uniformed Services University of the Health Sciences.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tesh VL, O’Brien AD. The pathogenic mechanisms of Shiga toxin and the Shiga-like toxins. Mol Microbiol. 1991;5:1817–1822. doi: 10.1111/j.1365-2958.1991.tb00805.x. [DOI] [PubMed] [Google Scholar]
- 2.Bergan J, Dyve Lingelem AB, Simm R, Skotland T, Sandvig K. Shiga toxins. Toxicon. 2012;60:1085–1107. doi: 10.1016/j.toxicon.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Melton-Celsa AR. Shiga Toxin (Stx) Classification, Structure, and Function. Microbiol Spectr. 2014:2. doi: 10.1128/microbiolspec.EHEC-0024-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler T. Haemolytic uraemic syndrome during shigellosis. Trans R Soc Trop Med Hyg. 2012;106:395–399. doi: 10.1016/j.trstmh.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Beutin L, Strauch E, Fischer I. Isolation of Shigella sonnei lysogenic for a bacteriophage encoding gene for production of Shiga toxin. Lancet. 1999;353:1498. doi: 10.1016/S0140-6736(99)00961-7. [DOI] [PubMed] [Google Scholar]
- 6.Gray MD, Lampel KA, Strockbine NA, et al. Clinical Isolates of Shiga Toxin 1a-Producing Shigella flexneri with an Epidemiological Link to Recent Travel to Hispaniola. Emerg Infect Dis. 2014;20:1669–1677. doi: 10.3201/eid2010.140292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta SK, Strockbine N, Omondi M, et al. Emergence of Shiga toxin 1 genes within Shigella dysenteriae type 4 isolates from travelers returning from the Island of Hispanola. Am J Trop Med Hyg. 2007;76:1163–1165. [PubMed] [Google Scholar]
- 8.McDonough MA, Butterton JR. Spontaneous tandem amplification and deletion of the shiga toxin operon in Shigella dysenteriae 1. Mol Microbiol. 1999;34:1058–1069. doi: 10.1046/j.1365-2958.1999.01669.x. [DOI] [PubMed] [Google Scholar]
- 9.Scheutz F, Teel LD, Beutin L, et al. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J Clin Microbiol. 2012;50:2951–2963. doi: 10.1128/JCM.00860-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calderwood SB, Auclair F, Donohue-Rolfe A, Keusch GT, Mekalanos JJ. Nucleotide sequence of the Shiga-like toxin genes of Escherichia coli. Proc Natl Acad Sci U S A. 1987;84:4364–4368. doi: 10.1073/pnas.84.13.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newland JW, Strockbine NA, Neill RJ. Cloning of genes for production of Escherichia coli Shiga-like toxin type II. Infect Immun. 1987;55:2675–2680. doi: 10.1128/iai.55.11.2675-2680.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neely MN, Friedman DI. Functional and genetic analysis of regulatory regions of coliphage H-19B: location of shiga-like toxin and lysis genes suggest a role for phage functions in toxin release. Mol Microbiol. 1998;28:1255–1267. doi: 10.1046/j.1365-2958.1998.00890.x. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt H. Shiga-toxin-converting bacteriophages. Res Microbiol. 2001;152:687–695. doi: 10.1016/s0923-2508(01)01249-9. [DOI] [PubMed] [Google Scholar]
- 14.Wagner PL, Livny J, Neely MN, et al. Bacteriophage control of Shiga toxin 1 production and release by Escherichia coli. Mol Microbiol. 2002;44:957–970. doi: 10.1046/j.1365-2958.2002.02950.x. [DOI] [PubMed] [Google Scholar]
- 15.Bopp C, Brenner F, Fields P, Wells J, Strockbine N. Escherichia, Shigella, and Salmonella. In: Murray P, Baron E, Jorgensen J, Pfaller M, Yolken R, editors. Manual of Clinical Microbiology. 8. Washington, DC: American Society for Microbiology; 2003. pp. 645–671. [Google Scholar]
- 16.Lin Z, Kurazono H, Yamasaki S, Takeda Y. Detection of various variant verotoxin genes in Escherichia coli by polymerase chain reaction. Microbiol Immunol. 1993;37:543–548. doi: 10.1111/j.1348-0421.1993.tb01675.x. [DOI] [PubMed] [Google Scholar]
- 17.Pollard DR, Johnson WM, Lior H, Tyler SD, Rozee KR. Rapid and specific detection of verotoxin genes in Escherichia coli by the polymerase chain reaction. J Clin Microbiol. 1990;28:540–545. doi: 10.1128/jcm.28.3.540-545.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paton AW, Paton JC. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J Clin Microbiol. 1998;36:598–602. doi: 10.1128/jcm.36.2.598-602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- 20.Walker CL, Applegate JA, Black RE. Haemolytic-uraemic syndrome as a sequela of diarrhoeal disease. J Health Popul Nutr. 2012;30:257–261. doi: 10.3329/jhpn.v30i3.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]