Abstract
Long-range synchrony between distant brain regions accompanies multiple forms of behavior. This review compares and contrasts the methods by which long-range synchrony is evaluated in both humans and model animals. Three examples of behaviorally-relevant long-range synchrony are discussed in detail: gamma-frequency synchrony during visual perception; hippocampal-prefrontal synchrony during working memory; and prefrontal-amygdala synchrony during anxiety. Implications for circuit mechanism, translation, and clinical relevance are discussed.
Keywords: Oscillations, Coherence, Hippocampus, Prefrontal Cortex, Gamma, Theta
Introduction
The rich tapestry formed by the trillions of connections between far-flung brain regions demonstrates the complexity of the brain. New imaging techniques artfully display these connections (Figure 1A), which facilitate cooperation amongst distributed elements of neural systems. Yet the connectivity of the brain does not statically derive from these anatomical pathways; it dynamically fluctuates with mood and cognitive states, influenced by stimuli and influencing behavior. Detecting and quantifying connectivity provides a key to understanding this dynamism.
Figure 1.
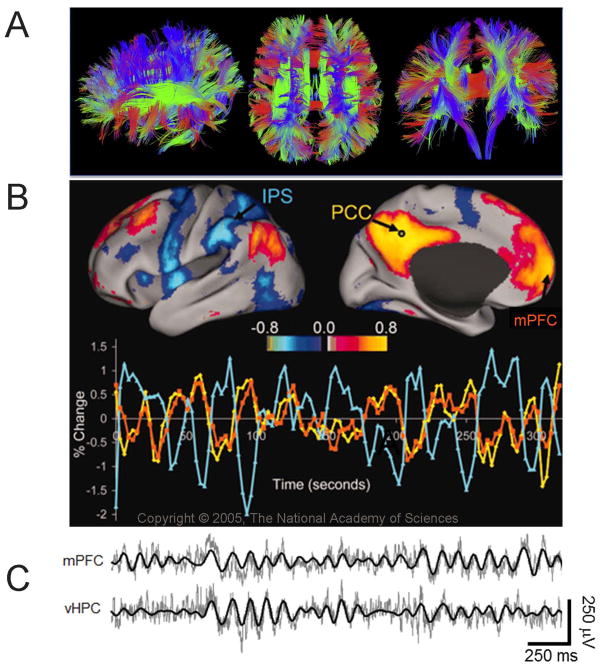
Structural and dynamic connectivity in the brain. A. Diffusion tensor imaging tractography in the human brain. Reproduced with permission from (Setsompop et al 2013). B. Top, Resting state fMRI image illustrating the correlations observed for a single resting subject between a seed region in the posterior cingulate/precuneus (PCC) and all other voxels in the brain. Warm colors represent positive correlations while cool colors reflect negative correlations. Bottom, An example time course of the PCC (yellow) signal, along with a positively correlated region, the medial prefrontal cortex (mPFC, orange), and a negatively correlated region, the intraparietal sulcus (IPS, blue). Reproduced with permission from (Fox et al 2005). C. Simultaneously recorded local field potentials from depth electrode in the mPFC (top) and ventral hippocampus (vHPC; bottom) in a mouse during active exploration. Raw traces are plotted in gray and theta filtered traces are overlaid in black (adapted with permission from (Adhikari et al 2010)).
Studying long-range neural synchrony has proven invaluable for this purpose. This research assays the degree to which brain regions are functionally connected by measuring the degree to which their neural activity patterns are synchronized. Neural synchrony can be quantified using a wide range of tools, including magnetoencephalography (MEG), electroencephalography (EEG) and functional neuroimaging (Figure 1B), as well as direct neurophysiological recordings (Figure 1C).
Numerous studies have established compelling if correlative links between synchrony and behavioral states, identifying several common themes. The brain shows high synchrony even at rest; such baseline or “resting state” synchrony tends to generally follow from anatomical connectivity. During tasks, synchrony typically changes within the activated regions, often in specific task phases or with specific perceptual or cognitive demands. Disease states may have altered synchrony, often correlated with associated alterations in behavior. These themes suggest that long-range synchrony, while supported by anatomical connectivity, changes on behavioral timescales. This review focuses on three sets of studies that illustrate these themes. In citing key examples of the relationship between long-range synchrony and behavior, this review builds on the growing momentum in the literature. Both animal and human studies identify functional connectivity correlates of behavior. Increasingly, researchers have applied such methods to large clinical samples and sophisticated animal models to examine the role of dysconnectivity in neuropsychiatric disease. Moreover, the advent of technologies to manipulate specific circuits and cell types allows direct testing of causal hypotheses generated from these data. These simultaneous developments promise to move the field beyond beautiful pictures and elegant correlations, towards establishing the causal relationship between long-range neural synchrony and behavior.
Methods: Measuring long-range synchrony
The advent of technologies that measure neural activity over time make examining long-range synchrony possible. These technologies include functional neuroimaging (primarily functional magnetic resonance imaging [fMRI]), magnetoencephalography (MEG), and electroencephalography (EEG) in humans. In model animals, studies of long-range synchrony mostly use intracranial electrophysiological techniques that permit simultaneous, fast measurements of neural activity in multiple brain regions during behavior. Rare human intracranial recordings of neural activity supplement the data acquired through non-invasive methods. The temporal and anatomical precision of these technologies differ considerably, both because of the origins of the biological signals they rely on, and the techniques used to capture these signals.
A few pertinent details of non-invasive approaches to measuring neural activity in humans will aid the discussion of neural synchrony (for full review see (Bandettini 2009, Ioannides 2007, Pan et al 2011, Sakkalis 2011)). fMRI relies on anatomically localized changes in blood flow induced by neural activity. Increases in metabolic demand (driven by neural activity) boost blood flow, which in turn raises the blood oxygen level. The resulting signal, blood-oxygen-level dependent (BOLD) contrast, changes slowly relative to neural activity. By contrast, EEG, MEG and intracranial recordings measure electrical activity directly, or via the magnetic fields that activity induces. The extracranial signals picked up by EEG and MEG result from the coordinated (and often rhythmic) activity of large numbers of neurons; only the current generated by the activation of a substantial number of neurons can induce currents or magnetic fields large enough to be detected outside the skull. Electrical measures have sub-millisecond time resolutions, compared to the seconds-long time course of the BOLD signal. However, locating the origin of these electrical signals depends on modeling how intracranial sources give rise to extracranial signals, a challenging endeavor (Pascual-Marqui et al 2002).
Intracranial recordings can measure neural activity simultaneously from multiple brain regions with a high degree of anatomical and temporal precision. These electrodes yield two types of neural activity: spikes and local field potentials (LFPs). Spikes represent the extracellular manifestation of action potentials, while slow, large voltage fluctuations caused by synchronous synaptic activity of many neurons produce LFPs. Depth electrodes can capture both spikes and LFPs in targeted structures, while electrocorticography (ECOG) captures LFPs from the surface of the brain using flexible, multi-channel arrays.
The principle behind long-range synchrony is to measure activity simultaneously from multiple sites, and ask if activity at these distributed sites tends to change in a coordinated manner. For fMRI, one tracks activity in individual voxels or regions of interest (ROIs) and measures correlations in these time series. Correlations can be positive (meaning activity in the two areas tends to go up and down together) or negative (meaning activity in one area correlates with less activity in the second area, and vice-versa). Given the slow timescale of the BOLD signal, fluctuations in fMRI activity occur slowly (0.01–0.1 Hz).
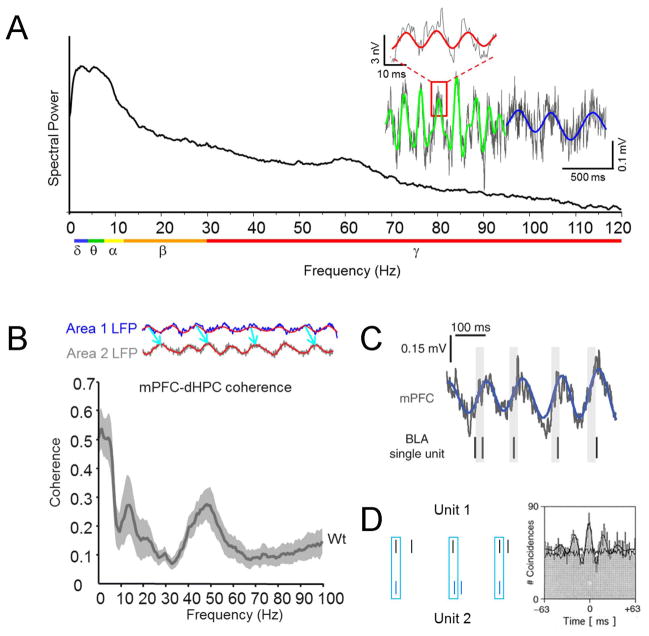
LFPs and EEGs recorded from the behaving brain consist of oscillatory activity in characteristic frequency ranges, such as delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz) and gamma (30–120 Hz) (Figure 2A). We call LFPs recorded simultaneously from two regions synchronous if their peaks and valleys align (which we will call phase coherence) or if their amplitudes correlate (power correlation). The mathematical term, coherence - typically calculated as a function of frequency - encompasses both of these aspects of LFP-LFP synchrony. The coherence spectrum between, for example, the hippocampus and prefrontal cortex, demonstrates peaks in coherence at delta, theta and gamma frequencies ((Adhikari et al 2010, Jones & Wilson 2005a, O’Neill et al 2013, Sigurdsson et al 2010); Figure 2B), indicating that these two regions strongly synchronize in these frequency ranges.
Figure 2.
Oscillations and synchrony in local field potentials. A. The power spectrum of a local field potential (LFP) recorded from the nucleus accumbens of an actively exploring mouse. The frequency range conventions are color coded below the x-axis (blue: delta, green: theta, yellow: alpha, orange: beta, red: gamma). Right, The raw local field potential is plotted in gray and a band-filtered trace is overlaid to highlight segments with prominent theta (green), delta (blue) and gamma (red) oscillations. B. Top, Two cartoon LFP traces displaying a consistent phase relationship. Bottom, The mPFC and dHPC show peaks in coherence in the delta, theta and gamma frequency range (adapted with permission from Sigurdsson et al 2010). C. Raw (gray) and theta-filtered (blue) mouse mPFC LFP traces, along with simultaneously recorded basolateral amygdala (BLA) single-unit activity illustrating phase locking. Gray bars are aligned on zero phase (reproduced with permission from Stujenske et al 2014). D. Left, schematic of synchronously firing spike trains. Right, cross-correlations of two neurons recorded in middle temporal area of a monkey watching a visual stimulus. The black line outlining the cross-correlogram represents the fitted function used to quantify correlation strength, and the thin black line corresponds to that coherence expected by chance (adapted with permission from (Kreiter & Singer 1996)).
Spikes can be used to measure synchrony in two ways. Phase-locking refers to the temporal relationship between spikes and LFP oscillations, quantified by the degree to which the spikes align with particular phases of the oscillation (Jones & Wilson 2005a, O’Keefe & Recce 1993); Figure 2C). Alternatively, cross-correlations of spikes recorded simultaneously in both regions measure the degree to which neurons in the two regions synchronize their action potentials (Brown et al 2004); Figure 2D).
Simultaneous fMRI and electrophysiological synchrony studies have related these two measures. Task-evoked fMRI synchrony seems to arise from common fluctuations in gamma oscillations (Goense & Logothetis 2008, Nir et al 2007, Shmuel & Leopold 2008). But this description can be misleading. Electrophysiological measures rely on fast synchrony – precise, rapid alignment of neuronal activity across brain regions on the order of milliseconds. By contrast, synchrony measured with BOLD seems to respond to correlations in power: BOLD signals reflect slow fluctuations in the strength of these faster oscillations. Theoretically, for example, the strength of gamma oscillations might rise and fall together in two brain regions, while the oscillations themselves need not by synchronized. Such methodological differences may be crucial to understanding how circuits perform computations, and how such computations go awry in disease. Given the emphasis in the animal literature on long-range coherence in behavior and the utility of fMRI for assaying synchrony in disease states, clarifying the relationship between slow and fast synchrony has important translational implications.
Synchrony in the visual system
Binding by synchrony: evidence from animal models
The framework for studying long-range neural synchrony emerged from visual system research. Over 25 years ago, Singer and colleagues recorded synchronous neural activity in neurons with non-overlapping receptive fields located in cortical columns up to 7 mm apart in the cat primary visual cortex (Gray et al 1989). Visual stimuli moving in the same direction across distant receptive fields induced weak synchrony, while the same stimuli moving in opposite directions failed to do so. A single, long stimulus that simultaneously activated both receptive fields resulted in robust synchrony.
These physiological findings evoke a behavioral phenomenon: that of binding the disparate features of visual stimuli into a unified perception of an object. The possibility that gamma synchrony could solve the “binding problem” is grounded in previous theoretical models (Grossberg 1976, Malsburg 1981, Milner 1974). Subsequent work found stimulus-induced synchrony in the middle temporal area of awake, behaving monkeys (Kreiter & Singer 1996), and inter-regional synchrony between activity in primary visual cortex and a visual association area in the cat (Engel et al 1991a, Engel et al 1991b). Together, these studies suggest that synchronous neural firing emerges under the Gestalt psychophysical principles that define the perception of a single object, such as spatial continuity and coherent motion (Wagemans et al 2012).
If synchrony underlies the perception of bound objects, rather than mere receptive field stimulation, one would expect synchrony only from stimuli that are perceived as bound. In strabismic cats, each eye forms a separate representation of a stimulus; the discrepancy, known as “binocular rivalry”, is resolved by suppressing the image from the non-dominant eye. Fries and colleagues (Fries et al 1997) predicted that synchrony should only exist for objects perceived through the dominant eye. Indeed, neural activity shows enhanced gamma-range synchrony only in the receptive fields of the dominant eye (Fries et al 1997).
If synchrony underlies the perception of bound objects, attending to an object should enhance perceptual binding and thus neural synchrony. Indeed, when monkeys attend to one visual stimulus while ignoring a distractor, gamma power in the spike-triggered average of LFPs recorded in area V4 increases only for attended stimuli (Fries et al 2001). The strength of this increase in local synchrony correlates with the monkeys’ performance on the task (Womelsdorf et al 2006). Moreover, the LFP activity recorded in V4 is coherent in the gamma range with LFPs recorded in V1, again only for attended stimuli (Bosman et al 2012). Granger causality analysis, a method to assess the directionality of information flow between brain regions, suggested that V1 activity drove V4 activity. Collectively, these data suggest that neural synchrony between brain regions reflects behaviorally relevant circuit communication, although they do not distinguish neural synchrony driven by task demands from synchrony specifically devoted to binding visual objects.
Synchrony in human studies of perception
Studies conducted in animals share the limitation that the subjects cannot directly report their perceptual experience. However, long-range synchrony studies in humans rely on techniques with less temporal and spatial resolution than those used in animals. As noted above, structured EEG signals result from large groups of simultaneously active neurons; non-synchronous firing would result in little net activity. Thus, the power of oscillatory signals serves a proxy for local neural synchrony. Gamma power increases in the central lead with the perception of a coherent shape, even if that shape is generated with illusory contours (Tallon-Baudry et al 1996). Similarly, beta and gamma power in both occipital and frontal leads increases as subjects find a hidden image (Tallon-Baudry et al 1997). Synchronous activity across EEG leads provides additional evidence of long-range synchrony. For instance, both gamma power within, and phase synchrony between, frontal and posterior electrodes increases during facial perception (Rodriguez et al 1999). Similarly, attending to a unilaterally presented visual stimulus induces widespread increases in gamma phase synchronization of EEG electrodes located over the posterior visual cortical areas contralateral to the stimulus. These data imply that perception of a coherent image results in widespread increases in gamma power and synchrony.
Not all studies find widespread gamma synchrony with visual perception. Von Stein and Sarnthein (von Stein & Sarnthein 2000) observed increases in EEG gamma and alpha power that remained localized to the occipital leads. Long-range coherence in the theta and low beta ranges only developed with tasks designed to involve multiple brain regions, such as visuo-spatial working memory or cross-sensory object recognition (von Stein & Sarnthein 2000). One of the few human studies to use intracortical electrodes to measure stimulus-evoked synchrony in the visual cortex found decreases in local gamma power followed by increases in beta synchrony during a visual working memory task (Tallon-Baudry et al 2001). Thus, while most studies agree that inter-area synchrony marks long-range circuit communication for visual tasks, the precise frequency range of this synchrony may vary.
As discussed earlier, animal studies show gamma range synchrony as binocular rivalry resolves. To study binocular rivalry in humans, Tononi and colleagues “frequency tagged” visual stimuli monocularly presented to each eye by flickering sets of images at slightly different frequencies (Tononi et al 1998). Doing so generates a sharp increase in MEG signal recorded at the presented frequencies. By measuring the topographic distribution and coherence of these tagged signals, two different groups found evidence for widespread increased synchrony as binocular rivalry resolved (Cosmelli et al 2004, Srinivasan et al 1999). Similarly, transient gamma and long lasting theta phase synchrony between frontal and posterior leads emerges prior to the resolution of binocular rivalry (Doesburg et al 2005). Broadly, these experiments support the conclusion that long-range synchrony develops as subjects perceive bound objects.
This observation of transient gamma followed by long-lasting theta synchrony implies that gamma synchrony might signal shifts in perception, while lower frequencies may maintain a given percept. Supporting this hypothesis, parietal and frontal EEG electrodes develop transient gamma phase synchrony, followed by sustained occipital lead alpha power increases just as subjects report a perceptual switch of a Necker cube - an image that spontaneously shifts three-dimensional perspective (Nakatani & van Leeuwen 2006). Similar transient increases in gamma power in both frontal and occipital EEG leads also occurred as subjects viewed another image with changing perspectives (Keil et al 1999).
Perhaps because of the transient nature of neural activity and resultant changes in power and synchrony, few studies of visually-induced synchrony have linked electrical activity with fMRI. Although evoked fMRI BOLD signal correlates with LFP activity, especially in the gamma range (Goense & Logothetis 2008, Ossandon et al 2011, Scheeringa et al 2011), fMRI connectivity correlates best with fluctuations in the power of low frequency (0.1 – 4 Hz) electrical activity (He et al 2008, Nir et al 2008, Scholvinck et al 2010). One study that did simultaneously record MEG and fMRI compared brain activity as subjects either fixated on a target of an otherwise blank screen or watched a short segment of a popular movie (Betti et al 2013). MEG synchrony was measured by calculating power in different frequency ranges and examining how this power fluctuates on slower timescales (<0.3 Hz). Broadly, the authors found that watching the movie caused a decrease in connectivity in the resting state network when assayed both with fMRI and with slow fluctuations in MEG alpha/beta power. Conversely, there were parallel increases in specific node-to-node MEG power correlations in the theta, beta and gamma regions, which often, but not always, corresponded to fMRI connectivity. This study serves as a proof of principle that one can tailor methods to find agreement across MRI and MEG synchrony measures.
Does synchrony amount to binding?
The idea that synchronous activity encodes binding remains controversial, with critics raising objections on both theoretical and experimental grounds. In an influential paper, Shadlen and Movshon argue that the primary visual cortex lacks the requisite information to determine if elements belong to a single object and therefore synchronous firing in V1 cannot represent binding (Shadlen & Movshon 1999). They pointedly note that the perception of an object often requires binding receptive fields with opposite motion, such as the two ends of a spinning propeller (Merker 2013), yet such stimuli reportedly decrease synchrony. Moreover, they argue that in the active brain, synchronous spike activity often occurs by chance, rendering a system that uses synchrony to code information implausible. Instead, they suggest that observed synchrony reflects shared connectivity (Shadlen & Movshon 1999).
Several studies do not support the binding-by-synchrony hypothesis (Dong et al 2008, Lamme & Spekreijse 1998, Palanca & DeAngelis 2005, Roelfsema et al 2004, Thiele & Stoner 2003). For instance, Palanca and DeAngelis (2005) recorded multiunit activity in the medial temporal area as monkeys watched a single object moving versus unconnected objects with similar receptive field properties. Although they found a modest increase in coherence in the single object condition, binding-associated synchrony accounted for only 0.1% of the variance in a generalized linear model, while basic visual stimulus properties, such as overlapping receptive fields and preferred directions, accounted for up to 56%. Moreover, in a clever experiment designed to address whether binding per se was associated with enhanced neural synchrony, the authors showed monkeys single and unconnected objects, allowing the animals to form perceptual binding before the objects disappeared behind a mask with apertures that made equivalent features visible. This experiment varies only whether the monkeys formed perceptual binding on the object, keeping the visual features constant. Under these conditions, bound single objects are not associated with enhanced synchrony (Palanca & DeAngelis 2005).
Moving beyond binding: function and mechanism
The data presented so far suggest that while synchrony may not fully encode binding (see (Uhlhaas et al 2009)), visual tasks are indeed associated with local and long-range synchrony. How can we interpret the observed synchrony? A conservative explanation is that LFP oscillations simply represent the “hum” of “wheels turning” during local neural activity (Merker 2013). Synchrony reflects the simultaneous participation, and likely communication, between distant regions. Synchrony is nonetheless still important; to turn Merker’s analogy around: just as one could infer a vehicle’s direction and speed by analyzing changes in wheel hum, one could determine temporal and spatial characteristics of functional neural circuit connectivity by studying long range synchrony.
Yet the ubiquitous nature of neural oscillations as well as the propensity of particular neural subtypes to resonate at specific frequencies (reviewed in (Wang 2010)), suggests that oscillatory synchrony may not only reflect neural communication, but also facilitate it. Fries (Fries 2005) proposed that long-range coherence of oscillations ensures that a given region provides input in a temporal window when the downstream target is appropriately receptive. Along similar lines, Lisman and Jensen (Lisman & Jensen 2013) suggested that fast oscillations provide a temporal window within which the most excited cells fire synchronously, punctuated by pauses; this scheme avoids mixing messages from multiple inputs. Nesting fast (i.e., gamma) oscillations within slower (i.e., theta) oscillations provides a framework for encoding and faithfully transmitting sequenced information.
Long-range synchrony and working memory
Hippocampal-prefrontal synchrony in rodents
Another canonical example of long-range synchrony occurs between the hippocampus and prefrontal cortex during spatial working memory in the rodent. Spatial working memory involves the short-term storage of spatial information in order to solve a task. In the rodent, spatial working memory requires both the hippocampus and medial prefrontal cortex (mPFC) (Aggleton et al 1986, Izaki et al 2001, Lee & Kesner 2003, Yoon et al 2008). Disconnection experiments show spatial working memory requires cooperation between the two structures. The hippocampus and mPFC have predominantly unilateral connections (Thierry et al 2000, Verwer et al 1997). Bilaterally disrupting either the hippocampus or the mPFC significant impairs performance on spatial working memory, while unilateral disruption on the same side has no effect, suggesting that the surviving pair of structures suffices to support spatial working memory. Disrupting the hippocampus on one side, and the mPFC on the other, impairs task performance, suggesting that connectivity between the two structures is required (Floresco et al 1997, Wang & Cai 2006).
Inspired by these findings, Matt Wilson and colleagues set out to measure connectivity between the hippocampus and mPFC during working memory. They demonstrated that mPFC neurons synchronize with theta-frequency oscillations in the mPFC (Jones & Wilson 2005b, Siapas et al 2005), and that the strength of this synchrony increases during the choice phase of a task, in which rats must execute a rule-based decision using their memory of the previous reward location (Jones & Wilson 2005a). Several other groups have confirmed this increase in synchrony, adding refinements that speak to the role it might play in the behavior (Gordon 2011, Hyman et al 2005, Hyman et al 2010). Theta-frequency synchrony increases gradually throughout the choice phase, peaking as the animal makes its decision (Benchenane et al 2010); gamma synchrony between the entorhinal cortex and hippocampus peaks at the same time point (Yamamoto et al 2014). Greater synchrony in both frequency ranges appears during correct trials compared to error trials (Jones & Wilson 2005c, Korotkova et al 2010, Yamamoto et al 2014). And deficits in synchrony accompany deficits in working memory performance in various models (Belforte et al 2010, Korotkova et al 2010, Sigurdsson et al 2010, Yamamoto et al 2014).
These experiments provide evidence for synchrony of these two regions. But what does this synchrony actually mean? The prevailing thought is that it reflects information flow. Consistent with this notion, synchrony in the hippocampal-prefrontal system exists not only in working memory, but also in other behavioral states, including sleep (Siapas & Wilson 1998), when consolidation of long-term memory is thought to take place. The evidence from these studies suggests that synchrony reflects effective connectivity, and perhaps effective information transfer between the two structures. The specific frequency (theta, ripple, etc.) involved may simply reflect the dominant mode of activity within the hippocampus at the time. Since theta-frequency oscillations dominate the hippocampus during navigation, spatial working memory during navigation results in theta-frequency synchrony.
Evidence that synchrony reflects information transfer from the hippocampus to the prefrontal cortex comes from attempts to determine the directionality of these interactions by examining the temporal relationship between activity in the two regions. Activity in the hippocampus tends to lead activity in the prefrontal cortex (Jones & Wilson 2005a, Siapas et al 2005, Sigurdsson et al 2010). Moreover, hippocampal lesions disrupt some forms of task-relevant coding in the mPFC (Burton et al 2009). Among other task-relevant information, mPFC neurons encode information about spatial location (Jung et al 1998); our lab, among several others, is currently investigating whether such place information requires hippocampal input.
Inferences from human electrophysiological studies
Given the plethora of studies linking hippocampal-prefrontal synchrony to working memory in rodents, multiple studies have examined this system in humans. The results have been mixed. Typically, working memory studies in people utilize either visuospatial or linguistic tasks. The former relies primarily on visual decoding of object location on a 2-dimensional screen. The latter relies on remembering short strings of digits, letters or words. Neither of these tasks utilize the kind of place-based coding seen in the rodent hippocampus. Perhaps accordingly, they do not typically implicate the hippocampal-prefrontal circuit.
Working memory tasks in humans do induce changes in oscillatory strength and synchrony in EEGs. The strength of theta-, alpha-, and gamma-frequency activity increases with working memory demand across multiple leads (Raghavachari et al 2001, Roux & Uhlhaas 2014, Watrous et al 2014). Coherence between pairs of leads also increases, though the details vary from study to study. Payne and Kounios (Payne & Kounios 2009), using a visual letter recognition task, found that theta coherence between frontal and parietal leads increased with memory load. Using a similar visuospatial task, Sauseng et al. (Sauseng et al 2005) also saw increases in theta coherence between frontal and parietal sites, along with decreases in alpha coherence frontally. By contrast, alpha coherence increases in a working memory task requiring interpretation of semantic meaning (Haarmann & Cameron 2005).
The interpretation of these working memory-related coherence changes between distant EEG leads is unclear. Since the resistance of the skull creates significant problems with volume conduction, strong oscillations emerging from a single site could be recorded from multiple distant leads, causing an artifactual increase in coherence. Ideally, convincing evidence of active synchronization would show pockets of highly coherent leads in two distinct regions with asynchronous activity in between. Unfortunately, where studies do examine the distribution of coherence, they typically show large, contiguous swaths of cortex oscillating synchronously (c.f., Fig. 2 in (Sauseng et al 2005)). Indeed, in one careful examination of this issue (Raghavachari et al 2006), intracranial recordings from cortical surface arrays found that coherence decreases monotonically as a function of distance between electrode pairs, suggesting that volume conduction accounts for any synchrony. These caveats make EEG studies difficult to interpret.
MEG offers distinct advantages over EEG, as magnetic fields more easily cross the skull, reducing issues of volume conduction and permitting better source localization. Findings from MEG studies have confirmed and extended EEG findings, recapitulating increases in oscillatory power at various frequencies in frontal areas (Jensen et al 2002, Jensen & Tesche 2002, Kaiser et al 2003, Roux & Uhlhaas 2014), as well as other regions (Bonnefond & Jensen 2012, Haegens et al 2010, Roux et al 2012). Using source reconstruction techniques, Guitart-Masip et al.(Guitart-Masip et al 2013) mapped the increase in theta power to discrete sources in the anterior hippocampus and anterior cingulate cortex; they then showed that the hippocampal theta source synchronizes with several prefrontal regions, particularly during a reversal-learning task that requires maintenance of shape-location associations. Few studies of synchrony in other frequency bands exist, though one network-based analysis suggested that lower (<25 Hz) frequency bands comprise the main effects of working memory (Palva et al 2010).
Intracranial recordings and imaging
Intracranial recordings offer perhaps the most definitive method for characterizing long-range synchrony between defined brain regions, and have begun to corroborate the extracranial studies. In a series of recordings from patients with epilepsy, Axmacher, Fell and colleagues reported increased synchrony between LFPs in the hippocampus and other temporal lobe structures, including the rhinal cortices and the inferior temporal cortex (Axmacher et al 2008, Fell et al 2008). In a few subjects also implanted with ECOG arrays over the prefrontal cortex, they also found evidence of gamma-frequency synchrony between the hippocampus and prefrontal sites (Axmacher et al 2008). By contrast, Rissman et al. (Rissman et al 2008) found that as working memory load increases in a face recognition working memory task, so does connectivity between the hippocampus and the fusiform face area. These findings suggest that the hippocampus synchronizes with specific cortical targets of relevance to the particular task used.
Similar to electrophysiological studies, imaging studies fail to consistently identify synchrony between the hippocampus and prefrontal cortex. Some fMRI studies have demonstrated increases in hippocampal-prefrontal synchrony with working memory demand, for example, during face or letter recognition tasks (Finn et al 2010, Rissman et al 2008). Others have reported decreases in synchrony between the prefrontal cortex and hippocampus, during a face recognition task with fMRI (Axmacher et al 2008) or a numerical recognition task with PET (Meyer-Lindenberg et al 2001, Meyer-Lindenberg et al 2005).
One possibility, given the findings above, is that hippocampal-prefrontal synchrony is specifically involved in spatial forms of working memory. To test this, Meyer-Lindenberg and colleagues recently devised a virtual reality version of the classic rodent spatial working memory test (Bähner et al 2015). Using this task, they found increased synchrony between the hippocampus and dorsolateral prefrontal cortex, as well as an extended prefrontal-parietal network.
Clinical relevance: deficits in synchrony in schizophrenia
The rough correspondence between findings in humans and rodents raises the possibility of translational relevance. Working memory disruptions accompany multiple neuropsychiatric illnesses, including schizophrenia (Krieger et al 2005, Piskulic et al 2007). It is possible that deficits in long-range synchrony may underlie such behavioral deficits; indeed, studies have suggested alterations in hippocampal-prefrontal synchrony (Ford et al 2002, Lawrie et al 2002, Meyer-Lindenberg et al 2001, Meyer-Lindenberg et al 2005) as well as deficits in global connectivity (Argyelan et al 2014, Bassett et al 2012, Venkataraman et al 2012) in schizophrenia patients. Understanding the neurobiological mechanisms underlying these deficits may reveal novel therapeutic targets.
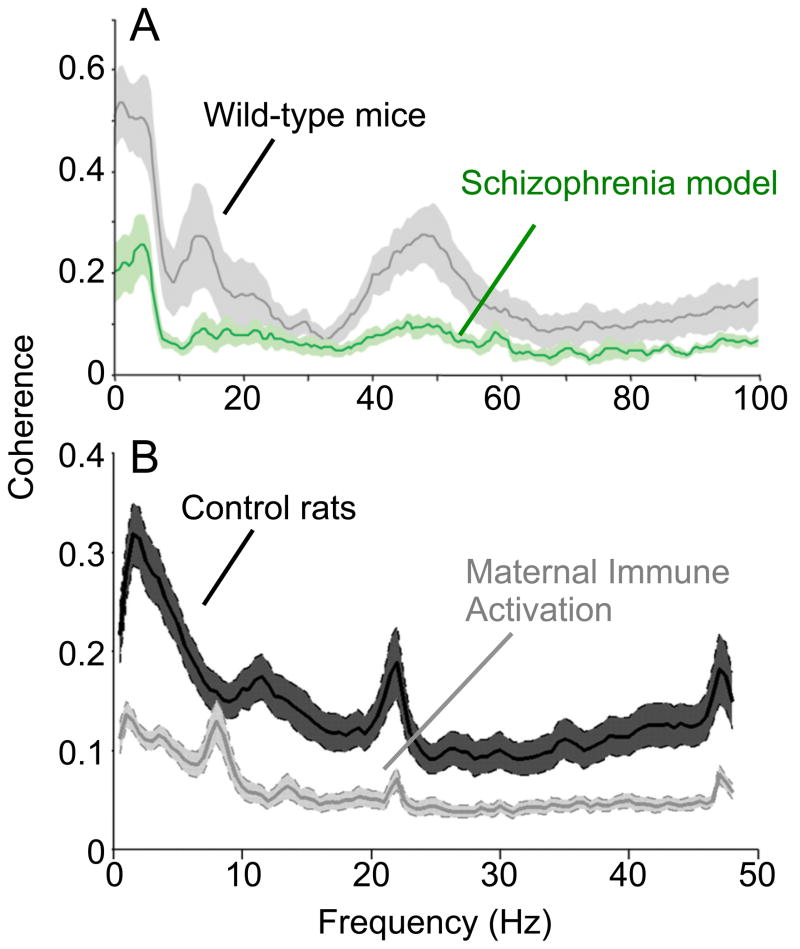
Deficits in hippocampal-prefrontal synchrony have been identified in both genetic and environmental animal models of schizophrenia predisposition. Mice modeling a microdeletion on chromosome 22 that increases the risk of schizophrenia about 30-fold (Karayiorgou et al 1995) have working memory deficits (Stark et al 2008), as do patients with the microdeletion (Lajiness-O’Neill et al 2005, Lewandowski et al 2007, Sobin et al 2005, van Amelsvoort et al 2004). Hippocampal-prefrontal synchrony is reduced in these mice (Figure 3A, (Sigurdsson et al 2010)), and the reduction correlates with deficits in working memory.
Figure 3.
Synchrony deficits in rodent models of schizophrenia predisposition. A. Coherence between LFPs recorded from the hippocampus and medial prefrontal cortex of a mouse model of a microdeletion that raises the risk of schizophrenia by 30-fold (green) and wild-type littermates (gray). Shaded areas are +/− s.e.m. Adapted from Sigurdsson et al., 2010. B. Coherence between hippocampal and medial prefrontal LFPs in a rat model of prenatal infection, a risk factor that raises the risk of schizophrenia by 2–3 fold (gray) and wild-type controls (black). Conventions as in A. Adapted from Dickerson et al., 2010, with permission.
Infection during gestation is a significant environmental risk factor for schizophrenia, increasing the risk for contracting the disorder by 2–3 fold (Canetta & Brown 2012). The risk seems similar regardless of the infectious agent, suggesting that activation of the maternal immune system, rather than infection itself, is deleterious. Accordingly, offspring of female rats exposed to immune activation during pregnancy develop a panoply of behavioral abnormalities reminiscent of schizophrenia, including working memory deficits (Canetta & Brown 2012). These offspring also have deficits in hippocampal-prefrontal synchrony remarkably similar to those seen in the genetic model (Figure 3B; (Dickerson et al 2010). Long range synchrony thus appears to be a shared pathophysiological consequence of at least two risk models, suggesting that synchrony could be an intermediate phenotype of relevance to schizophrenia in general.
Fear and anxiety
Fear conditioning as a means to probe anxiety-related circuitry
Pavlovian fear conditioning, a well-characterized model applicable across many species, provides a rich avenue of insight into the neural mechanisms of fear (reviewed in (Maren 2001)). In this paradigm, a neutral cue such as a tone (the conditioned stimulus, or CS) is paired with an aversive experience, such as a mild shock (the unconditioned stimulus, or UCS) that elicits behavioral manifestations of fear, such as freezing or escape. Over time, the subject associates the CS with the aversive stimulus and exhibits anxious behavior when presented with the CS, even in the absence of the UCS. Extinction, a second form of learning pertinent to anxiety disorders, occurs after fear conditioning, when the CS is repeatedly experienced without a paired UCS. The subject learns that the CS no longer predicts the UCS and stops exhibiting anxious behaviors.
Long-range neural synchrony has been observed in the circuits involved in fear conditioning in both humans and model animals. Fear conditioning induces synchronous EEG activity in healthy human subjects (Keil et al 2001, Keil et al 2007, Miltner et al 1999, Mueller et al 2014). For example, coherence increases between EEG leads overlying the visual and somatosensory cortices during the establishment of light (CS) – shock (UCS) associations (Miltner et al 1999). Moreover, this synchrony disappears with extinction; it seems specific to the expression of fear.
The limited spatial resolution of EEG precludes probing subcortical structures with known involvement in fear circuitry, such as the amygdala (Mueller et al 2014). However, fMRI can reveal the connectivity of the amygdala with cortical structures during fear conditioning. One recent study examined large-scale network connectivity during predictable (CS paired with UCS) and unpredictable (UCS alone) threat (Wheelock et al 2014); unpredictable threat produced more intense anxiety. The authors analyzed the network activity of 15 brain regions activated by both kinds of threats. For predictable threats, the dorsolateral prefrontal cortex formed an outward hub, communicating with the greatest number of structures, including the insular cortex, which served as a secondary hub. By contrast, for unpredictable threat, the dorsomedial prefrontal cortex (dmPFC) formed the primary hub, while the amygdala comprised a secondary hub (Wheelock et al 2014). These results suggest that the dorsolateral prefrontal cortex and insular cortex provide emotional regulation during predictable stress. For unpredictable stress, however, the amygdala and dmPFC orchestrate a reactive stress response.
While the slow time course of fMRI signals prevents a real-time parsing of amygdala-prefrontal interactions, recent work in model animals provides insight into their dynamics. Paz and colleagues (Klavir et al 2013) recorded neural activity in the amygdala and dorsal anterior cingulate cortex in monkeys that had been trained that a particular CS predicted the UCS, while a different CS predicted its absence. They then switched the contingencies and observed the firing responses of neurons in both regions to the surprising mismatches of CS and unexpected outcome. Some neurons in the amygdala fired to any type of mismatch between CS and outcome (representing “unsigned prediction errors”, which merely indicate surprise). Others fired only for specific types of mismatch (representing “signed prediction errors,” whether positive or negative). Amygdala neurons that represent unsigned prediction errors fired slightly before neurons in the anterior cingulate, while amygdala neurons that represent signed prediction errors fired slightly after anterior cingulate neurons (Klavir et al 2013). These data suggest that the amygdala provides information to the dorsal anterior cingulate cortex about surprising threat contingencies while the cingulate cortex instructs the amygdala about valance (positive or negative value). Interestingly, anterior cingulate-to-amygdala directionality is associated with the resistance to extinction (Livneh & Paz 2012), raising the intriguing possibility that pathologically persistent fear represents a failure of the anterior cingulate to properly connect with the amygdala.
Recent work from our laboratory also investigated synchronous neural activity between the mPFC and the amygdala during discrimination between safe and threatening cures (Likhtik et al 2014, Stujenske et al 2014). Mice were trained with a CS+ paired with a mild shock, and an explicitly unpaired CS−. Mice that distinguished the CS+ and CS− had elevated theta (4–5 Hz) coherence between the mPFC and the amygdala. The directionality of this synchrony is modulated dynamically. During the CS+, the two structures equally influence each other; during the CS−, a predominant mPFC-to-amygdala directionality emerges (Likhtik et al 2014). This mPFC lead inversely correlates with freezing, suggesting that it represents safety. During the CS−, mPFC input synchronizes a subset of amygdala neurons, generating a local gamma-frequency oscillation that is coupled to theta in the mPFC (Stujenske et al 2014). Extinction is also associated with an mPFC lead (Lesting et al 2013, Narayanan et al 2011, Stujenske et al 2014). Collectively, these data suggest that synchrony in the mPFC-to-BLA circuit reflects a dynamic rivalry of signals conveying fear and safety.
Synchrony also arises with fear memory consolidation. Studies by Pape and colleagues showed that fear conditioned stimuli induce synchrony between low theta-frequency (4–5 Hz) oscillations in LFPs recorded from the prefrontal cortex, hippocampus, and amygdala (Lesting et al 2013, Narayanan et al 2007a, Seidenbecher et al 2003). Elevated hippocampal-amygdala synchrony appears 24 hours after training, not earlier or later (Narayanan et al 2007a), matching the timecourse of memory consolidation. Synchrony also increases 24 hours after the memory reactivation (Narayanan et al 2007b), implicating hippocampal-amygdala circuitsin reconsolidation, the fascinating phenomenon which renders memories labile (Nader et al 2000). Consistent with a role for long-range synchrony in memory consolidation, Paré and colleagues (Popa et al 2010) found that synchrony across the hippocampal-prefrontal-amygdala circuit emerges during REM sleep after training. The extent and directionality of synchrony within the circuit predicts the strength of the resultant fear memory.
Innate and stress-induced anxiety
Animals and people both have innate defensive reactions to stimuli that do not require learning. Such stimuli include predator smells or dark, confined spaces (for people; rodents find bright, open spaces aversive). While both forms of anxiety appear to rely on the amygdala and prefrontal cortex (Deacon et al 2002, Gonzalez et al 2000, Lacroix et al 2000, Shah et al 2004, Shah & Treit 2003), studies in rodents suggest that innate forms of anxiety also require an extended network of additional brain regions, including the hippocampus and bed nucleus of the stria terminals (Bannerman et al 2003, Deacon et al 2002, File & Gonzalez 1996, Kim et al 2013, Kjelstrup et al 2002). Consistent with these findings, we have found increased LFP coherence and spike-LFP phase locking between the mPFC and the hippocampus in mice exploring anxiety-provoking environments, such as the open field and the elevated plus maze, which both rely on open spaces to induce anxiety (Adhikari et al 2010). Similar increases in synchrony are also seen between the mPFC and the amygdala (Likhtik et al 2014, Stujenske et al 2014), where, just as in learned fear, an mPFC-to-amygdala directionality predominates during relative safety.
In humans, studies of anxiety find differences in baseline connectivity between individuals with high and low anxiety. High anxiety individuals show positive amygdala -ventromedial prefrontal cortex fMRI correlations and negative amygdala-dorsomedial cortex correlations, while low anxiety individuals show roughly the opposite results (Kim et al 2011). Amygdala-anterior insular cortex synchrony correlates with state (moment-to-moment) anxiety, while increased anatomical connectivity, measured with diffusion tensor imaging (DTI), correlates with trait (lifetime tendency) anxiety (Baur et al 2013). Similarly, adolescents with high cortisol reactivity have higher resting state fMRI connectivity between the salience network (including the anterior cingulate cortex and the insula) and the subgenual cingulate cortex (Thomason et al 2011). These results suggest that individuals with anxiety have both structural and functional differences in connectivity involving the insular cortex, prefrontal cortex and amygdala, even in the absence of a specific anxiogenic task.
These studies lead to the conclusion that in highly anxious individuals, “resting state” functional connectivity patterns may reflect free-floating anxiety, rather than a neutral state (Baur et al 2013, Kim et al 2011). The finding that functional connectivity (measured by synchrony) correlates with anxiety state while anatomical connectivity (as measured by DTI) correlates with anxiety trait emphasizes the distinction between dynamics and structure. In anxiety disorders, stress might evoke anxiety states by altering dynamic connectivity, overlaid on a baseline of disturbed structural connectivity in susceptible individuals.
Indeed, stress evokes changes in synchrony within an extended network that includes the amygdala, insula and prefrontal cortex. In healthy volunteers, watching a stressful video increases cortisol, noradrenergic activity, and fMRI connectivity in a “salience network” that includes the insula, cingulate, amygdala, midbrain and thalamus. The strength of network connectivity correlates with cortisol and negative affect (Hermans et al 2011). Blocking noradrenergic activity, but not cortisol, reduces this salience network (Hermans et al 2011). These data imply that the salience network reflects attention to stressful stimuli, rather than the stress response itself.
Further insight into the relationship between networks mediating attentiveness and aversiveness comes from studies of network activity during anxious anticipation. One study, utilizing factor analysis of fMRI data, suggested three successive phases that unfold over time: first, the salience network increases in activity and connectivity; next the anterior insula and bed nucleus synchronize; finally, nucleus accumbens activity (typically associated with reward-related activity) decreases (McMenamin et al 2014). In this study, amygdala activity did not increase, but its connectivity with other brain regions widened (McMenamin et al 2014). A similar study did find increases in amygdala activity, as well as increased synchrony between the amygdala and parts of dorsal prefrontal cortex; the strength of this connectivity correlated both with reaction time speed for identifying emotional stimuli as well trait anxiety scores (Robinson et al 2012).
Functional connectivity in generalized anxiety disorder
Data from both rodents and humans suggest that long-range synchronous activity conveys neural communication in circuits active during anxiety. The networks identified in both learned fear and innate anxiety appear similar. However, differing anxiety levels within healthy subjects can manifest as different functional connectivity patterns. These findings raise the possibility that anxiety disorders result from disruption in long-range synchrony within these circuits.
Several studies have compared the functional connectivity patterns of healthy controls and patients with generalized anxiety disorder (GAD). The findings are in broad agreement, though again, the specifics vary. In a combined fMRI and DTI study, healthy controls had the typical negative correlation between activity in the dorsal prefrontal cortex and amygdala (Tromp et al 2012), suggesting that one of these regions tends to suppress activity in the other. In patients with GAD, this negative correlation was weaker (Tromp et al 2012). A similar negative correlation between activity in the amygdala and ventrolateral prefrontal cortex was induced in healthy adolescents exposed to emotional pictures; this correlation was also weaker in adolescents with GAD (Monk et al 2008). These deficits in connectivity appear to correspond with decreased cognitive control over emotional responses (Etkin et al 2010).
These studies of circuit dysfunction in GAD also tie in to an emerging literature examining the molecular and circuit basis of anxiety in genetic mouse models. Mice lacking either of two components of the serotonin system, the serotonin 1A receptor or the serotonin transporter, have phenotypes of increased innate anxiety. Recordings from the mPFC of serotonin 1A receptor knockout mouse suggest a failure of the mPFC to form representations of anxiety-provoking environments (Adhikari et al 2011), while multi-site recordings from serotonin transporter knockout mice show altered amygdala-mPFC synchrony (Narayanan et al 2011). Further studies investigating the mechanisms by which long-range synchrony is altered in these or other animal models may well provide greater insight into the neurobiology of GAD, and identify novel approaches toward improved treatment of anxiety disorders.
Open questions and future directions
The three examples discussed above demonstrate a substantial amount of concordance across paradigms and species regarding the relationship between synchrony and behavior. The take home message is that long-range synchrony – between visual cortical areas and parietal areas during perception; between the hippocampus and prefrontal cortex during working memory; and between the amygdala and prefrontal cortex during anxiety – correlates with behavior. What this means, in terms of mechanism, causality, translatability, and clinical relevance, remains to be determined.
First, we need a better mechanistic understanding of how cross-regional synchrony emerges. A better circuit level understanding of long-range synchrony would help guide experiments aimed at testing whether such synchrony is necessary for behavior. After identifying specific circuit elements – cell types, synapses, pathways, etc. – one could disrupt and augment synchrony with the basic neuroscientists’ arsenal of tools. For example, optogenetic inhibition of a specific connection could disrupt synchrony between directly connected brain regions. Conversely, stimulating the same connection (at specific frequencies) could enhance synchrony. The resulting effects of these manipulations on behavior could then be assayed. Already, this approach has begun to clarify the circuit mechanisms underlying gamma synchrony and its relevance to behavior (Cardin et al 2009, Colgin et al 2009, Lasztoczi & Klausberger 2014, Sohal et al 2009, Yamamoto et al 2014). A similar approach for lower frequency oscillations would help resolve the meaning of lower frequency power changes, such as alpha and theta (reviewed in (Lisman & Jensen 2013, Merker 2013, Palva & Palva 2007).
While understanding mechanisms will require animal models, differences in how synchrony is measured raise issues of how to properly translate findings between animals and humans. Of particular concern, findings obtained through fMRI and those obtained from electrophysiological methods have vastly different time courses. fMRI measures activity changes that occur over a few seconds; electrophysiological synchrony is measured in milliseconds. How do they agree at all? As noted above, activity within an area measured by fMRI seems to correlate with the strength of gamma oscillations (Goense & Logothetis 2008, Nir et al 2007, Shmuel & Leopold 2008). Thus, correlations in fMRI-measured activity may represent common fluctuations in gamma strength. This explanation provides a framework with which to interpret some of the findings in the literature. For example, while we and others have found increases in theta-frequency synchrony between the hippocampus and prefrontal cortex during the retrieval phase of spatial working memory tasks in rodents (Benchenane et al 2010, Hyman et al 2005, Hyman et al 2010, Jones & Wilson 2005b, Sigurdsson et al 2010), the Meyer-Lindenberg group found increases in synchrony during the encoding portion of their task (Bähner et al 2015). The discrepancy may reflect species or task differences. However, it is also possible that the fMRI captures gamma, rather than theta synchrony. Consistent with this notion, we recently found enhanced gamma-frequency synchrony between the hippocampus and mPFC during the encoding phase of a spatial working memory task in mice (Spellman et al, submitted).
These details have direct clinical relevance, since deficits in synchrony at different frequencies may have far different circuit-level mechanisms, and developing new treatments will require first understanding which specific circuit elements to target. The findings presented here make it clear that long-range synchrony accompanies behavior. The next steps, establishing mechanism, evaluating causality, translating findings across species and determining clinical relevance, are already underway, and promise to have considerable impact on our understanding of how the networked brain functions, and how that function goes awry in disease.
Acknowledgments
Supported by NIMH R01 MH081968; R01 MH096274; P50 MH096891; and T32 MH015144; and the Hope for Depression Research Foundation.
Contributor Information
Alexander Z. Harris, Email: ah2835@columbia.edu.
Joshua A. Gordon, Email: jg343@columbia.edu.
Bibliography
- Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257–69. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari A, Topiwala MA, Gordon JA. Single Units in the Medial Prefrontal Cortex with Anxiety-Related Firing Patterns Are Preferentially Influenced by Ventral Hippocampal Activity. Neuron. 2011;71:898–910. doi: 10.1016/j.neuron.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Hunt PR, Rawlins JN. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behav Brain Res. 1986;19:133–46. doi: 10.1016/0166-4328(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Argyelan M, Ikuta T, DeRosse P, Braga RJ, Burdick KE, et al. Resting-state fMRI connectivity impairment in schizophrenia and bipolar disorder. Schizophr Bull. 2014;40:100–10. doi: 10.1093/schbul/sbt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Schmitz DP, Wagner T, Elger CE, Fell J. Interactions between medial temporal lobe, prefrontal cortex, and inferior temporal regions during visual working memory: a combined intracranial EEG and functional magnetic resonance imaging study. J Neurosci. 2008;28:7304–12. doi: 10.1523/JNEUROSCI.1778-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähner F, Plichta MM, Demanuele C, Schweiger J, Gerchen MF, et al. Hippocampal-dorsolateral prefrontal coupling as a species-conserved cognitive mechanism: A human translational imaging study. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.13. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini PA. What’s new in neuroimaging methods? Ann N Y Acad Sci. 2009;1156:260–93. doi: 10.1111/j.1749-6632.2009.04420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Nelson BG, Mueller BA, Camchong J, Lim KO. Altered resting state complexity in schizophrenia. Neuroimage. 2012;59:2196–207. doi: 10.1016/j.neuroimage.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur V, Hanggi J, Langer N, Jancke L. Resting-state functional and structural connectivity within an insula-amygdala route specifically index state and trait anxiety. Biol Psychiatry. 2013;73:85–92. doi: 10.1016/j.biopsych.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, et al. Postnatal ablation of NMDA receptors in corticolimbic interneurons leads to schizophrenia-related phenotypes. Nat Neurosci. 2010 doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, et al. Coherent Theta Oscillations and Reorganization of Spike Timing in the Hippocampal-Prefrontal Network upon Learning. Neuron. 2010;66:921–36. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Betti V, Della Penna S, de Pasquale F, Mantini D, Marzetti L, et al. Natural scenes viewing alters the dynamics of functional connectivity in the human brain. Neuron. 2013;79:782–97. doi: 10.1016/j.neuron.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefond M, Jensen O. Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Curr Biol. 2012;22:1969–74. doi: 10.1016/j.cub.2012.08.029. [DOI] [PubMed] [Google Scholar]
- Bosman CA, Schoffelen JM, Brunet N, Oostenveld R, Bastos AM, et al. Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron. 2012;75:875–88. doi: 10.1016/j.neuron.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN, Kass RE, Mitra PP. Multiple neural spike train data analysis: state-of-the-art and future challenges. Nat Neurosci. 2004;7:456–61. doi: 10.1038/nn1228. [DOI] [PubMed] [Google Scholar]
- Burton BG, Hok V, Save E, Poucet B. Lesion of the ventral and intermediate hippocampus abolishes anticipatory activity in the medial prefrontal cortex of the rat. Behav Brain Res. 2009;199:222–34. doi: 10.1016/j.bbr.2008.11.045. [DOI] [PubMed] [Google Scholar]
- Canetta SE, Brown AS. Prenatal Infection, Maternal Immune Activation, and Risk for Schizophrenia. Transl Neurosci. 2012;3:320–27. doi: 10.2478/s13380-012-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–7. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–7. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- Cosmelli D, David O, Lachaux JP, Martinerie J, Garnero L, et al. Waves of consciousness: ongoing cortical patterns during binocular rivalry. Neuroimage. 2004;23:128–40. doi: 10.1016/j.neuroimage.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Bannerman DM, Rawlins JN. Anxiolytic effects of cytotoxic hippocampal lesions in rats. Behav Neurosci. 2002;116:494–7. doi: 10.1037//0735-7044.116.3.494. [DOI] [PubMed] [Google Scholar]
- Dickerson DD, Wolff AR, Bilkey DK. Abnormal long-range neural synchrony in a maternal immune activation animal model of schizophrenia. J Neurosci. 2010;30:12424–31. doi: 10.1523/JNEUROSCI.3046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doesburg SM, Kitajo K, Ward LM. Increased gamma-band synchrony precedes switching of conscious perceptual objects in binocular rivalry. Neuroreport. 2005;16:1139–42. doi: 10.1097/00001756-200508010-00001. [DOI] [PubMed] [Google Scholar]
- Dong Y, Mihalas S, Qiu F, von der Heydt R, Niebur E. Synchrony and the binding problem in macaque visual cortex. J Vis. 2008;8:30, 1–16. doi: 10.1167/8.7.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Konig P, Kreiter AK, Singer W. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science. 1991a;252:1177–9. doi: 10.1126/science.252.5009.1177. [DOI] [PubMed] [Google Scholar]
- Engel AK, Kreiter AK, Konig P, Singer W. Synchronization of oscillatory neuronal responses between striate and extrastriate visual cortical areas of the cat. Proc Natl Acad Sci U S A. 1991b;88:6048–52. doi: 10.1073/pnas.88.14.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167:545–54. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J, Ludowig E, Rosburg T, Axmacher N, Elger CE. Phase-locking within human mediotemporal lobe predicts memory formation. Neuroimage. 2008;43:410–9. doi: 10.1016/j.neuroimage.2008.07.021. [DOI] [PubMed] [Google Scholar]
- File SE, Gonzalez LE. Anxiolytic effects in the plus-maze of 5-HT1A-receptor ligands in dorsal raphe and ventral hippocampus. Pharmacol Biochem Behav. 1996;54:123–8. doi: 10.1016/0091-3057(95)02108-6. [DOI] [PubMed] [Google Scholar]
- Finn AS, Sheridan MA, Kam CL, Hinshaw S, D’Esposito M. Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. J Neurosci. 2010;30:11062–7. doi: 10.1523/JNEUROSCI.6266-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–90. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51:485–92. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–80. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–3. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Fries P, Roelfsema PR, Engel AK, Konig P, Singer W. Synchronization of oscillatory responses in visual cortex correlates with perception in interocular rivalry. Proc Natl Acad Sci U S A. 1997;94:12699–704. doi: 10.1073/pnas.94.23.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goense JB, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol. 2008;18:631–40. doi: 10.1016/j.cub.2008.03.054. [DOI] [PubMed] [Google Scholar]
- Gonzalez LE, Rujano M, Tucci S, Paredes D, Silva E, et al. Medial prefrontal transection enhances social interaction. I: behavioral studies. Brain Res. 2000;887:7–15. doi: 10.1016/s0006-8993(00)02931-0. [DOI] [PubMed] [Google Scholar]
- Gordon JA. Oscillations and hippocampal-prefrontal synchrony. Curr Opin Neurobiol. 2011;21:486–91. doi: 10.1016/j.conb.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–7. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Grossberg S. Adaptive pattern classification and universal recoding: II. Feedback, expectation, olfaction, illusions. Biol Cybern. 1976;23:187–202. doi: 10.1007/BF00340335. [DOI] [PubMed] [Google Scholar]
- Guitart-Masip M, Barnes GR, Horner A, Bauer M, Dolan RJ, Duzel E. Synchronization of medial temporal lobe and prefrontal rhythms in human decision making. J Neurosci. 2013;33:442–51. doi: 10.1523/JNEUROSCI.2573-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarmann HJ, Cameron KA. Active maintenance of sentence meaning in working memory: evidence from EEG coherences. Int J Psychophysiol. 2005;57:115–28. doi: 10.1016/j.ijpsycho.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Haegens S, Osipova D, Oostenveld R, Jensen O. Somatosensory working memory performance in humans depends on both engagement and disengagement of regions in a distributed network. Hum Brain Mapp. 2010;31:26–35. doi: 10.1002/hbm.20842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc Natl Acad Sci U S A. 2008;105:16039–44. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, et al. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334:1151–3. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–49. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Working memory performance correlates with prefrontal-hippocampal theta interactions but not with prefrontal neuron firing rates. Frontiers in Integrative Neuroscience. 2010;4 doi: 10.3389/neuro.07.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannides AA. Dynamic functional connectivity. Curr Opin Neurobiol. 2007;17:161–70. doi: 10.1016/j.conb.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Izaki Y, Maruki K, Hori K, Nomura M. Effects of rat medial prefrontal cortex temporal inactivation on a delayed alternation task. Neurosci Lett. 2001;315:129–32. doi: 10.1016/s0304-3940(01)02366-7. [DOI] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex. 2002;12:877–82. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15:1395–9. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Jones M, Wilson M. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial working memory task. PLoS Biol. 2005a;2:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Phase precession of medial prefrontal cortical activity relative to the hippocampal theta rhythm. Hippocampus. 2005b;15:867–73. doi: 10.1002/hipo.20119. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005c;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, Qin Y, McNaughton BL, Barnes CA. Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cereb Cortex. 1998;8:437–50. doi: 10.1093/cercor/8.5.437. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Ripper B, Birbaumer N, Lutzenberger W. Dynamics of gamma-band activity in human magnetoencephalogram during auditory pattern working memory. Neuroimage. 2003;20:816–27. doi: 10.1016/S1053-8119(03)00350-1. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, et al. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci U S A. 1995;92:7612–6. doi: 10.1073/pnas.92.17.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Muller MM, Gruber T, Wienbruch C, Stolarova M, Elbert T. Effects of emotional arousal in the cerebral hemispheres: a study of oscillatory brain activity and event-related potentials. Clin Neurophysiol. 2001;112:2057–68. doi: 10.1016/s1388-2457(01)00654-x. [DOI] [PubMed] [Google Scholar]
- Keil A, Muller MM, Ray WJ, Gruber T, Elbert T. Human gamma band activity and perception of a gestalt. J Neurosci. 1999;19:7152–61. doi: 10.1523/JNEUROSCI.19-16-07152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Stolarova M, Moratti S, Ray WJ. Adaptation in human visual cortex as a mechanism for rapid discrimination of aversive stimuli. Neuroimage. 2007;36:472–9. doi: 10.1016/j.neuroimage.2007.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011;21:1667–73. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496:219–23. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99:10825–30. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavir O, Genud-Gabai R, Paz R. Functional connectivity between amygdala and cingulate cortex for adaptive aversive learning. Neuron. 2013;80:1290–300. doi: 10.1016/j.neuron.2013.09.035. [DOI] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–69. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Kreiter AK, Singer W. Stimulus-dependent synchronization of neuronal responses in the visual cortex of the awake macaque monkey. J Neurosci. 1996;16:2381–96. doi: 10.1523/JNEUROSCI.16-07-02381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger S, Lis S, Janik H, Cetin T, Gallhofer B, Meyer-Lindenberg A. Executive function and cognitive subprocesses in first-episode, drug-naive schizophrenia: an analysis of N-back performance. Am J Psychiatry. 2005;162:1206–8. doi: 10.1176/appi.ajp.162.6.1206. [DOI] [PubMed] [Google Scholar]
- Lacroix L, Spinelli S, Heidbreder CA, Feldon J. Differential role of the medial and lateral prefrontal cortices in fear and anxiety. Behav Neurosci. 2000;114:1119–30. doi: 10.1037//0735-7044.114.6.1119. [DOI] [PubMed] [Google Scholar]
- Lajiness-O’Neill RR, Beaulieu I, Titus JB, Asamoah A, Bigler ED, et al. Memory and learning in children with 22q11.2 deletion syndrome: evidence for ventral and dorsal stream disruption? Child Neuropsychol. 2005;11:55–71. doi: 10.1080/09297040590911202. [DOI] [PubMed] [Google Scholar]
- Lamme VA, Spekreijse H. Neuronal synchrony does not represent texture segregation. Nature. 1998;396:362–6. doi: 10.1038/24608. [DOI] [PubMed] [Google Scholar]
- Lasztoczi B, Klausberger T. Layer-specific GABAergic control of distinct gamma oscillations in the CA1 hippocampus. Neuron. 2014;81:1126–39. doi: 10.1016/j.neuron.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51:1008–11. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. J Neurosci. 2003;23:1517–23. doi: 10.1523/JNEUROSCI.23-04-01517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesting J, Daldrup T, Narayanan V, Himpe C, Seidenbecher T, Pape HC. Directional theta coherence in prefrontal cortical to amygdalo-hippocampal pathways signals fear extinction. PLoS One. 2013;8:e77707. doi: 10.1371/journal.pone.0077707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski KE, Shashi V, Berry PM, Kwapil TR. Schizophrenic-like neurocognitive deficits in children and adolescents with 22q11 deletion syndrome. Am J Med Genet B Neuropsychiatr Genet. 2007;144:27–36. doi: 10.1002/ajmg.b.30379. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 2014;17:106–13. doi: 10.1038/nn.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Jensen O. The theta-gamma neural code. Neuron. 2013;77:1002–16. doi: 10.1016/j.neuron.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh U, Paz R. Amygdala-prefrontal synchronization underlies resistance to extinction of aversive memories. Neuron. 2012;75:133–42. doi: 10.1016/j.neuron.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Malsburg C. The correlation theory of brain function. Göttingen, West Germany: Max Planck Institute for Biophysical Chemistry; 1981. [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- McMenamin BW, Langeslag SJ, Sirbu M, Padmala S, Pessoa L. Network organization unfolds over time during periods of anxious anticipation. J Neurosci. 2014;34:11261–73. doi: 10.1523/JNEUROSCI.1579-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merker B. Cortical gamma oscillations: the functional key is activation, not cognition. Neurosci Biobehav Rev. 2013;37:401–17. doi: 10.1016/j.neubiorev.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, et al. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158:1809–17. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–86. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- Milner PM. A model for visual shape recognition. Psychol Rev. 1974;81:521–35. doi: 10.1037/h0037149. [DOI] [PubMed] [Google Scholar]
- Miltner WH, Braun C, Arnold M, Witte H, Taub E. Coherence of gamma-band EEG activity as a basis for associative learning. Nature. 1999;397:434–6. doi: 10.1038/17126. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–76. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller EM, Panitz C, Hermann C, Pizzagalli DA. Prefrontal oscillations during recall of conditioned and extinguished fear in humans. J Neurosci. 2014;34:7059–66. doi: 10.1523/JNEUROSCI.3427-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–6. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nakatani H, van Leeuwen C. Transient synchrony of distant brain areas and perceptual switching in ambiguous figures. Biol Cybern. 2006;94:445–57. doi: 10.1007/s00422-006-0057-9. [DOI] [PubMed] [Google Scholar]
- Narayanan RT, Seidenbecher T, Kluge C, Bergado J, Stork O, Pape HC. Dissociated theta phase synchronization in amygdalo-hippocampal circuits during various stages of fear memory. Eur J Neurosci. 2007a;25:1823–31. doi: 10.1111/j.1460-9568.2007.05437.x. [DOI] [PubMed] [Google Scholar]
- Narayanan RT, Seidenbecher T, Sangha S, Stork O, Pape HC. Theta resynchronization during reconsolidation of remote contextual fear memory. Neuroreport. 2007b;18:1107–11. doi: 10.1097/WNR.0b013e3282004992. [DOI] [PubMed] [Google Scholar]
- Narayanan V, Heiming RS, Jansen F, Lesting J, Sachser N, et al. Social defeat: impact on fear extinction and amygdala-prefrontal cortical theta synchrony in 5-HTT deficient mice. PLoS One. 2011;6:e22600. doi: 10.1371/journal.pone.0022600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Fisch L, Mukamel R, Gelbard-Sagiv H, Arieli A, et al. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr Biol. 2007;17:1275–85. doi: 10.1016/j.cub.2007.06.066. [DOI] [PubMed] [Google Scholar]
- Nir Y, Mukamel R, Dinstein I, Privman E, Harel M, et al. Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat Neurosci. 2008;11:1100–8. doi: 10.1038/nn.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Recce M. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–30. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- O’Neill PK, Gordon JA, Sigurdsson T. Theta oscillations in the medial prefrontal cortex are modulated by spatial working memory and synchronize with the hippocampus through its ventral subregion. J Neurosci. 2013;33:14211–24. doi: 10.1523/JNEUROSCI.2378-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossandon T, Jerbi K, Vidal JR, Bayle DJ, Henaff MA, et al. Transient suppression of broadband gamma power in the default-mode network is correlated with task complexity and subject performance. J Neurosci. 2011;31:14521–30. doi: 10.1523/JNEUROSCI.2483-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanca BJ, DeAngelis GC. Does neuronal synchrony underlie visual feature grouping? Neuron. 2005;46:333–46. doi: 10.1016/j.neuron.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Palva S, Monto S, Palva JM. Graph properties of synchronized cortical networks during visual working memory maintenance. Neuroimage. 2010;49:3257–68. doi: 10.1016/j.neuroimage.2009.11.031. [DOI] [PubMed] [Google Scholar]
- Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007;30:150–8. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Pan H, Epstein J, Silbersweig DA, Stern E. New and emerging imaging techniques for mapping brain circuitry. Brain Res Rev. 2011;67:226–51. doi: 10.1016/j.brainresrev.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Esslen M, Kochi K, Lehmann D. Functional imaging with low-resolution brain electromagnetic tomography (LORETA): a review. Methods Find Exp Clin Pharmacol. 2002;24(Suppl C):91–5. [PubMed] [Google Scholar]
- Payne L, Kounios J. Coherent oscillatory networks supporting short-term memory retention. Brain Res. 2009;1247:126–32. doi: 10.1016/j.brainres.2008.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskulic D, Olver JS, Norman TR, Maruff P. Behavioural studies of spatial working memory dysfunction in schizophrenia: a quantitative literature review. Psychiatry Res. 2007;150:111–21. doi: 10.1016/j.psychres.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Popa D, Duvarci S, Popescu AT, Lena C, Pare D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc Natl Acad Sci U S A. 2010;107:6516–9. doi: 10.1073/pnas.0913016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, et al. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;21:3175–83. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE, Tully M, Madsen JR, Bromfield EB, Kahana MJ. Theta oscillations in human cortex during a working-memory task: evidence for local generators. J Neurophysiol. 2006;95:1630–8. doi: 10.1152/jn.00409.2005. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Dynamic adjustments in prefrontal, hippocampal, and inferior temporal interactions with increasing visual working memory load. Cereb Cortex. 2008;18:1618–29. doi: 10.1093/cercor/bhm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Charney DR, Overstreet C, Vytal K, Grillon C. The adaptive threat bias in anxiety: amygdala-dorsomedial prefrontal cortex coupling and aversive amplification. Neuroimage. 2012;60:523–9. doi: 10.1016/j.neuroimage.2011.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ. Perception’s shadow: long-distance synchronization of human brain activity. Nature. 1999;397:430–3. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, Lamme VA, Spekreijse H. Synchrony and covariation of firing rates in the primary visual cortex during contour grouping. Nat Neurosci. 2004;7:982–91. doi: 10.1038/nn1304. [DOI] [PubMed] [Google Scholar]
- Roux F, Uhlhaas PJ. Working memory and neural oscillations: alpha-gamma versus theta-gamma codes for distinct WM information? Trends Cogn Sci. 2014;18:16–25. doi: 10.1016/j.tics.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Roux F, Wibral M, Mohr HM, Singer W, Uhlhaas PJ. Gamma-band activity in human prefrontal cortex codes for the number of relevant items maintained in working memory. J Neurosci. 2012;32:12411–20. doi: 10.1523/JNEUROSCI.0421-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakkalis V. Review of advanced techniques for the estimation of brain connectivity measured with EEG/MEG. Comput Biol Med. 2011;41:1110–7. doi: 10.1016/j.compbiomed.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Schabus M, Doppelmayr M. Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int J Psychophysiol. 2005;57:97–103. doi: 10.1016/j.ijpsycho.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Fries P, Petersson KM, Oostenveld R, Grothe I, et al. Neuronal dynamics underlying high- and low-frequency EEG oscillations contribute independently to the human BOLD signal. Neuron. 2011;69:572–83. doi: 10.1016/j.neuron.2010.11.044. [DOI] [PubMed] [Google Scholar]
- Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci U S A. 2010;107:10238–43. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–50. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- Setsompop K, Kimmlingen R, Eberlein E, Witzel T, Cohen-Adad J, et al. Pushing the limits of in vivo diffusion MRI for the Human Connectome Project. Neuroimage. 2013;80:220–33. doi: 10.1016/j.neuroimage.2013.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Movshon JA. Synchrony unbound: a critical evaluation of the temporal binding hypothesis. Neuron. 1999;24:67–77. 111–25. doi: 10.1016/s0896-6273(00)80822-3. [DOI] [PubMed] [Google Scholar]
- Shah AA, Sjovold T, Treit D. Inactivation of the medial prefrontal cortex with the GABAA receptor agonist muscimol increases open-arm activity in the elevated plus-maze and attenuates shock-probe burying in rats. Brain Res. 2004;1028:112–5. doi: 10.1016/j.brainres.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Shah AA, Treit D. Excitotoxic lesions of the medial prefrontal cortex attenuate fear responses in the elevated-plus maze, social interaction and shock probe burying tests. Brain Res. 2003;969:183–94. doi: 10.1016/s0006-8993(03)02299-6. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Hum Brain Mapp. 2008;29:751–61. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–51. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–8. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal – prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–67. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Daniels S, Khuri J, Taylor L, et al. Neuropsychological characteristics of children with the 22q11 Deletion Syndrome: a descriptive analysis. Child Neuropsychol. 2005;11:39–53. doi: 10.1080/09297040590911167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Russell DP, Edelman GM, Tononi G. Increased synchronization of neuromagnetic responses during conscious perception. J Neurosci. 1999;19:5435–48. doi: 10.1523/JNEUROSCI.19-13-05435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark K, Xu B, Bagchi A, Lai W, Liu H, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40:751–60. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- Stujenske JM, Likhtik E, Topiwala MA, Gordon JA. Fear and safety engage competing patterns of theta-gamma coupling in the basolateral amygdala. Neuron. 2014;83:919–33. doi: 10.1016/j.neuron.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Permier J. Oscillatory gamma-band (30–70 Hz) activity induced by a visual search task in humans. J Neurosci. 1997;17:722–34. doi: 10.1523/JNEUROSCI.17-02-00722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J Neurosci. 1996;16:4240–9. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Fischer C. Oscillatory synchrony between human extrastriate areas during visual short-term memory maintenance. J Neurosci. 2001;21:RC177. doi: 10.1523/JNEUROSCI.21-20-j0008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele A, Stoner G. Neuronal synchrony does not correlate with motion coherence in cortical area MT. Nature. 2003;421:366–70. doi: 10.1038/nature01285. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Degenetais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;10:411–9. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Hamilton JP, Gotlib IH. Stress-induced activation of the HPA axis predicts connectivity between subgenual cingulate and salience network during rest in adolescents. J Child Psychol Psychiatry. 2011;52:1026–34. doi: 10.1111/j.1469-7610.2011.02422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Srinivasan R, Russell DP, Edelman GM. Investigating neural correlates of conscious perception by frequency-tagged neuromagnetic responses. Proc Natl Acad Sci U S A. 1998;95:3198–203. doi: 10.1073/pnas.95.6.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromp DP, Grupe DW, Oathes DJ, McFarlin DR, Hernandez PJ, et al. Reduced structural connectivity of a major frontolimbic pathway in generalized anxiety disorder. Arch Gen Psychiatry. 2012;69:925–34. doi: 10.1001/archgenpsychiatry.2011.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Lima B, Melloni L, Neuenschwander S, et al. Neural synchrony in cortical networks: history, concept and current status. Front Integr Neurosci. 2009;3:17. doi: 10.3389/neuro.07.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amelsvoort T, Henry J, Morris R, Owen M, Linszen D, et al. Cognitive deficits associated with schizophrenia in velo-cardio-facial syndrome. Schizophr Res. 2004;70:223–32. doi: 10.1016/j.schres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Venkataraman A, Whitford TJ, Westin CF, Golland P, Kubicki M. Whole brain resting state functional connectivity abnormalities in schizophrenia. Schizophr Res. 2012;139:7–12. doi: 10.1016/j.schres.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwer RW, Meijer RJ, Van Uum HF, Witter MP. Collateral projections from the rat hippocampal formation to the lateral and medial prefrontal cortex. Hippocampus. 1997;7:397–402. doi: 10.1002/(SICI)1098-1063(1997)7:4<397::AID-HIPO5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38:301–13. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Wagemans J, Elder JH, Kubovy M, Palmer SE, Peterson MA, et al. A century of Gestalt psychology in visual perception: I. Perceptual grouping and figure-ground organization. Psychol Bull. 2012;138:1172–217. doi: 10.1037/a0029333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GW, Cai JX. Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behav Brain Res. 2006;175:329–36. doi: 10.1016/j.bbr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90:1195–268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watrous AJ, Fell J, Ekstrom AD, Axmacher N. More than spikes: common oscillatory mechanisms for content specific neural representations during perception and memory. Curr Opin Neurobiol. 2014;31C:33–39. doi: 10.1016/j.conb.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MD, Sreenivasan KR, Wood KH, Ver Hoef LW, Deshpande G, Knight DC. Threat-related learning relies on distinct dorsal prefrontal cortex network connectivity. Neuroimage. 2014;102(Pt 2):904–12. doi: 10.1016/j.neuroimage.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P, Mitra PP, Desimone R. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature. 2006;439:733–6. doi: 10.1038/nature04258. [DOI] [PubMed] [Google Scholar]
- Yamamoto J, Suh J, Takeuchi D, Tonegawa S. Successful execution of working memory linked to synchronized high-frequency gamma oscillations. Cell. 2014;157:845–57. doi: 10.1016/j.cell.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Yoon T, Okada J, Jung MW, Kim JJ. Prefrontal cortex and hippocampus subserve different components of working memory in rats. Learn Mem. 2008;15:97–105. doi: 10.1101/lm.850808. [DOI] [PMC free article] [PubMed] [Google Scholar]