Abstract
The rapid proliferation of myeloid leukemia cells is highly dependent on increased glucose metabolism. Through an unbiased metabolomics analysis of leukemia cells, we found that the glycogenic precursor UDP-D-glucose is pervasively upregulated, despite low glycogen levels. Targeting the rate-limiting glycogen synthase 1 (GYS1) not only decreased glycolytic flux but also increased activation of the glycogen-responsive AMPK (AMP kinase), leading to significant growth suppression. Further, genetic and pharmacological hyper-activation of AMPK was sufficient to induce the changes observed with GYS1 targeting. Cancer genomics data also indicate that elevated levels of the glycogenic enzymes GYS1/2 or GBE1 (glycogen branching enzyme 1) are associated with poor survival in AML. These results suggest a novel mechanism whereby leukemic cells sustain aberrant proliferation by suppressing excess AMPK activity through elevated glycogenic flux and provide a therapeutic entry point for targeting leukemia cell metabolism.
Keywords: CML, AML, metabolic reprogramming, signal transduction, glycogen
INTRODUCTION
Acute myeloid leukemia (AML) and chronic myeloid leukemia (CML) are devastating diseases often associated with activated tyrosine kinases. Targeted therapies have been developed for many of these oncogenic kinases but resistance and disease progression can occur. Initial response rates to treatment are high in AML and very high in CML, but relapse is common in AML and most patients with CML remain PCR positive indefinitely.1,2 Targeting reprogramming of glucose metabolism has emerged as an attractive and novel approach to developing new therapeutics in cancer treatment.3,4 The aberrant use of glucose and other carbon sources is part of a metabolic switch that further leads to fermentation and lactate production, even under aerobic conditions (Warburg effect).5 The molecular changes that lead to this process in leukemic cells are poorly understood, even though the pathways involved in glycolytic activities have been fairly well described in normal cells. We previously found that hyper-active glucose metabolism and mitochondrial electron transport chain activity are essential for increased ROS (reactive oxygen species) production in myeloid malignancies.6,7 Elevated oxidative stress not only contributes to genomic instability, but it is also required for the functioning of redox-sensitive enzymes and virtually all aspects of transformation.8 Also, our data have shown that inhibition of PFKFB3 (phosphofructokinasefructose-2,6-bisphosphatase 3) in leukemic cells reduced metabolic reprogramming, as well as cell growth in vitro and in vivo.9 Targeting metabolic abnormalities with relative sparing of normal cells is therefore predicted to result in significant clinical benefit.
In addition to glucose metabolism, glucose storage in form of glycogen is increased in most cancers as well. Glycogen provides a convenient glucose reservoir during energy stress, glucose deprivation or senescence.10,11 However, glycogen levels are significantly depleted in myeloid malignancies, due to concomitant glycogenolysis,12,13,14 seemingly presenting a disadvantage for transformed cells. The reason for this increased glycogenic flux or its biological consequences for myeloid leukemias are unknown. Glycogen synthesis is controlled in part by the rate-limiting glycogen synthase (GYS). Whereas the so-called muscle GYS1 is more ubiquitously expressed at lower levels, the GYS2 isoform carries a major responsibility for glycogen synthesis in the liver.15-17 Mice with disruption of GYS1 show reduced levels of PFKFB3 as well as reduced glycolysis in muscle cells, hinting at a role of GYS1 beyond its primary function of glycogen synthesis.18 Increased glucose uptake and glycolysis in myeloid leukemia cells9 would result in elevate glucose-6-phosphate levels, as a product of the first glycolysis reaction. Glucose-6-phosphate is already known to be a strong allosteric activator of GYS1 that can also overcome its inhibitory phosphorylation19,20 and it likely plays a much larger role in controlling glycogenic flux than previously thought.21 Inhibitory phosphorylation of GYS1 at S641 is mediated by GSK3 (glycogen synthase kinase 3), downstream of the AKT kinase.22-24 Even though GSK3 proteins were discovered as kinases for GYS, they have since been found to have broader functions that may not necessarily lie within this pathway.25,26 AMPK (AMP-activated protein kinase) has been described as another regulator of GYS1 through inhibitory phosphorylation at S8.27,28 AMPK is also a central and pleiotropic regulator of bioenergetic fuel consumption through targeted phosphorylation of proteins within multiple pathways.29 In general, AMPK activity favors catabolism to the expense of ATP consumption, thus effectively leading to energy conservation and increased ATP production.30 The heterotrimeric protein phosphorylates its substrates through the catalytic α subunit, which is also sensitive to changes in phosphorylation at T 172, predominantly by LKB1 (liver kinase B1) during energy stress 31-34,35. Binding of AMP to the γ subunit increases its activity and the β subunit can interact with glycogen.35
Here, we define the seemingly energy-inefficient glycogenesis pathway as a sensitive regulator of metabolic reprogramming and transformation in myeloid leukemia cells. Knockdown of the rate-limiting GYS1 not only significantly lowered flux towards glycogen, but also reduced cell growth and metabolic reprogramming. Hyper-activation of AMPK mimicked GYS1 targeting and provided a central mechanism for regulating glucose metabolism and cell growth. Importantly, activity of the glycogen-sensitive AMPK inversely correlated with changes in glycogen levels. Thus, analogous to the mechanism associated with loss of the AMPK activating tumor suppressor LKB1 in some solid tumors, such as in lung cancer,36 detrimental hyper-activation of AMPK can be avoided through increased glycogen production by cancer cells. Inhibiting this protective mechanism in myeloid leukemias may aid traditional therapy and hints at additional targets for drug development.
MATERIALS AND METHODS
Metabolomics analysis
The levels of 292 cellular carbon metabolites in extracts of 2.5×106 cultured cells or 4×106 leukemic cells or controls were determined in triplicates. Cell lines were treated for 18h with their respective tyrosine kinase inhibitors at twice the ED50. Extraction of metabolites and quantitative mass spectrometry analysis was done as previously described.37 MetaboAnalyst (http://www.metaboanalyst.ca) was used for statistical analysis of the data.
Expression of active AMPK
A truncated constitutively active HA-tagged form of AMPK-α2 (amino acids 1-312) was generated by PCR and cloned into either pMSCV-IRES-GFP or pMSCV-IRES-DsRed-Express2-puromycin. Retroviruses were generated by co-transfecting HEK293T cells with either pMSCV vector, pMD2.G and pMD-MLV-gag-pol using the TransIT-293 transfection reagent (Mirus, Madison, WI). KU812 cells were sorted for either GFP positive or for DsRed-Express2 positive cells. Cells infected with DsRed-Express2 constructs were selected for three days with puromycin (1μg/ml), prior to sorting.
Semi-quantitative real-time PCR
To measure changes in the expression of genes involved in glucose metabolism in KU812 cells treated overnight with 1μM imatinib or DMSO, RT2 Profiler Glucose Metabolism PCR Arrays (PAHS-006ZA; SABiosciences, Valencia, CA) were used.
Targeted knockdown using lentiviral approaches
Knockdown of GYS1 was done using three different lentiviral constructs (A, B and E; RNAi Screening Facility, Dana-Farber Cancer Institute) containing shRNA against GYS1 and compared to a scrambled control. Lentiviruses were generated by co-transfecting HEK293T cells with viral packaging vectors pMD2.G and pCMVΔ8.91 as well as shRNAs using the TransIT (Mirus, Madison, WI) reagent. KU812 cells were infected in the presence of polybrene (5μg/mL; Millipore, Temecula, CA) and selected for one week in medium containing puromycin (1μg/mL; Sigma).
In vivo mouse studies
KU812 cells with targeted knockdown of GYS1 (construct B) were used and compared to cells containing scrambled shRNA. Animal studies were performed at the Lurie Family Imaging Center (Dana-Farber Cancer Institute) on protocols approved by the Dana-Farber Cancer Institute Animal Care and Use Committee as previously described.9
Statistical analysis
For statistical comparison between test and control groups, the Student's t-test was used. Error bars represent standard deviation of at least three independent experiments. PCR array experiments were analyzed using one-way analysis of variance (ANOVA)/Tukey's multiple comparison test. Mixed models were used to assess the differences in rate of increase in tumor volume and in weight over time in murine models. Models included time as a variable, as well as treatment, and an interaction between treatment and time. P-values below 0.05 were considered statistically significant.
Miscellaneous
Further experimental procedures can be found in the Supplemental Experimental Procedures.
RESULTS
Leukemic cells in myeloid malignancies are associated with increased glycogenesis
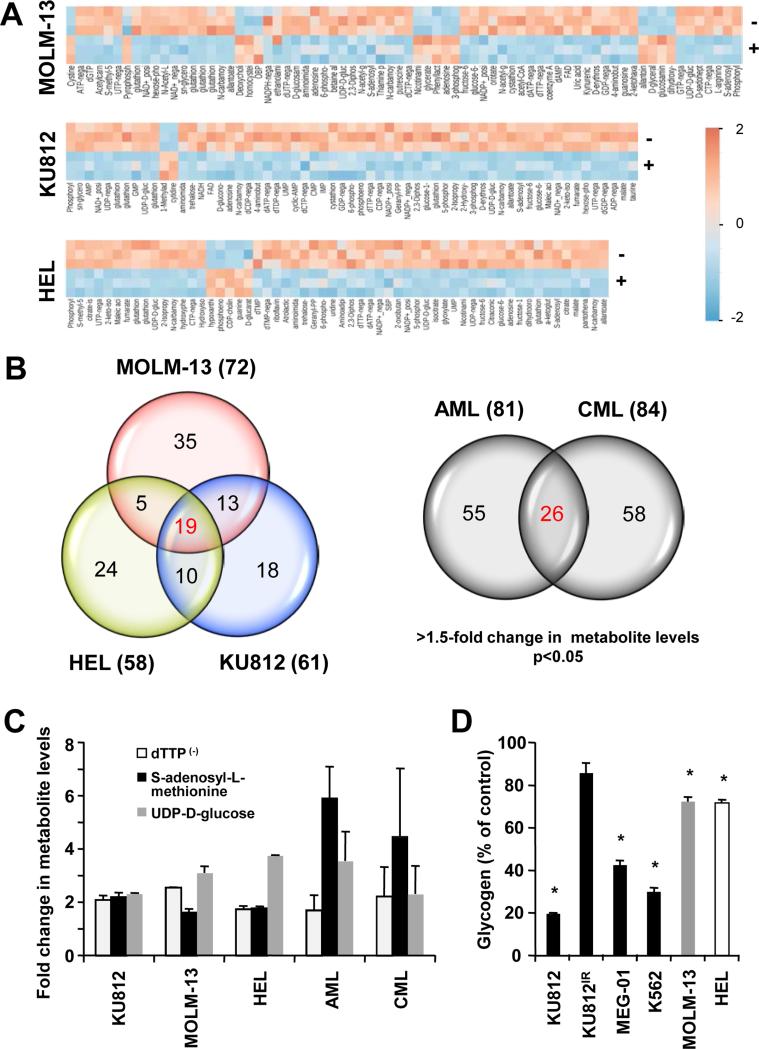
We used a mass spectrometry approach that can reliably detect changes in 292 metabolites associated with carbohydrate metabolism,37 in order to identify abnormalities in metabolic pathways associated with oncogenic transformation, Specifically, the metabolic profiles of patient derived cell lines transformed by different tyrosine kinase oncogenes, including FLT3-ITD (MOLM-13), BCR-ABL (KU812) and JAK2V617F (HEL), were compared. As reduced oncogenic kinase activity also lowers cellular metabolism, most of the metabolites were generally reduced in all inhibitor-treated cell lines. With a threshold of a change in metabolite levels >1.5-fold, we identified a set of metabolites in MOLM-13, KU812 and HEL that was significantly changed (p<0.05) upon tyrosine kinase inhibitor treatment (Figure 1A). In addition, we analyzed and compared the metabolite levels of a limited number of AML and CML patient specimens versus controls (n=3) (Figure S1A). Similar to patient-derived cell lines, we observed inter-patient variability with distinct changes in AML and CML metabolite levels. Further analysis of the cell line metabolite profiles demonstrated that the majority of changes for each of the cell lines were shared with one or the other cell line tested. Distinct signatures of 19 metabolites were found to be reduced in cell lines in response to oncogenic tyrosine kinase inhibitors (Figure 1B, left) and 26 metabolites were found to be elevated in AML and CML patient specimens compared to normal controls (Figure 1B, right).
Figure 1. Metabolomics analysis of human myeloid leukemia cells.
(a) Unsupervised hierarchical clustering of KU812, Molm-13 and HEL metabolite profiles, comparing control treated cells (−) to tyrosine kinase inhibitor treated cells (+; 3.5 μM imatinib, 0.8 nM quizartinib and 400nM ruxolitinib, respectively) (p<0.05; change in metabolite levels >1.5-fold). Reduced metabolite levels are indicated by negative numbers (blue) in the heatmap. (b) Venn diagram obtained from comparison of metabolite profiles of KU812, Molm-13 and HEL in response to inhibitors of their respective oncogenic tyrosine kinases (left) or metabolite profiles of AML and CML patient specimens, compared to normal controls (n=3) (right). (c) Changes in metabolite levels consistently observed in myeloid leukemia cells, as indicated (p<0.05). (d) Changes in glycogen levels were measured in cellular extracts of KU812, MEG-01, K562, MOLM-13 and HEL cells in response to inhibitors of their respective oncogenic tyrosine kinases. *Significant differences (p<0.05) were observed between control and treated cells.
Commonly upregulated metabolites were identified by comparison of both sets, including deoxythymidine triphosphate (dTTP), S-adenosyl-L-methionine and UDP-D-glucose (Figure 1C). Altered levels in these metabolites do not suggest a representation of the overall metabolic changes in these cells but rather hint at robust alterations observed in our sample pool and may represent distinct changes of individual metabolites. Indeed, a pathway analysis involving the metabolite profiles from these results suggests that multiple metabolic or anabolic pathways are affected by the observed changes (Figure S1B). Of particular interest here are increased levels in UDP-D-glucose (reduced levels in response to tyrosine kinase inhibitors in transformed cell lines), a precursor of glycogen synthesis. Our pathway analysis also identified oligo- and polysaccharide synthesis among the pathways with the highest impact score, which corresponds to the extent of changes in metabolite levels that occur within this pathway (Figure S1B). The limited number of metabolites analyzed did not allow for a reliable statistical analysis. An over-active glycogen synthesis pathway is further reflected in our cell line models wherein treatment with inhibitors of oncogenic tyrosine kinases significantly reduced cellular glycogen levels (Figure 1D). It should be noted that these results only demonstrate oncogene-dependent glycogenic flux and do not suggest quantitative changes relative to normal cells. The largest reduction in glycogen was observed in KU812 cells in response to imatinib. This change was blocked when BCR-ABL containing the T315I gatekeeper mutation was introduced into these cells. Further, KU812 cells showed a strong dependency for glycogen production on glucose as a carbon source and not glutamine. Glycogen levels were significantly reduced by 74.2% (p<0.005) in the absence of glucose but not in the absence of glutamine (24.2% reduction; p=0.31) (Figure S1C). Thus, glycogenesis is one of the key metabolic pathways that is specifically altered in leukemic cells.
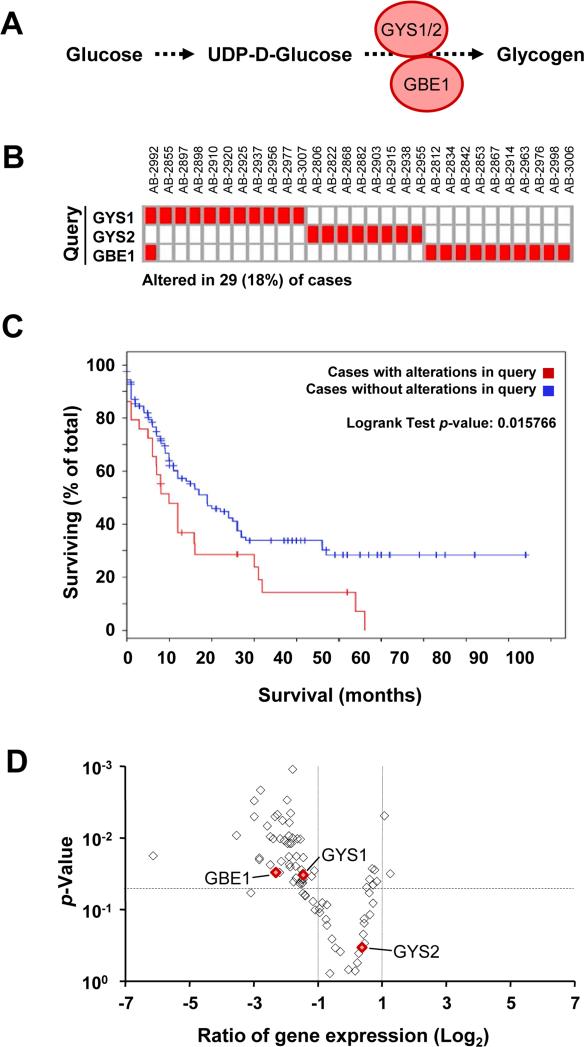
Elevated expression of glycogen synthesis regulating enzymes is associated with poor prognosis
Glycogen synthase (GYS) is a major rate-limiting enzyme involved in glycogen synthesis and works in concert with GBE1 (1,4-alpha-glucan-branching enzyme 1) (Figure 2A).38 We queried the cBioPortal database 39,40 and looked for abnormal expression of GYS1, GYS2 or GBE1 in a set of 163 AML patients. The database also allows for the retrieval of individual patient information and provides the clinical and biological description of each specimen. The results show that there is increased expression of any of the three enzymes in 29 cases, representing 18% of the total patient population (Figure 2B). The changes appeared to be mutually exclusive with the exception of one specimen (AB-2992), in which GYS1 and GBE1 were upregulated at the same time. Of note, changes within this pathway were not unique to AML and a query of the cBioPortal database also revealed high expression of enzymes within this pathway in 13.2% of lung adenocarcinoma and 15.9% of kidney clear cell carcinoma. Increased expression of these glycogenesis enzymes was associated with poor outcome in AML (p= 0.02), lung adenocarcinoma (p=0.003) and clear cell renal cell carcinoma (p=0.04) (Figure 2C and Figure S2A) and showed a decrease in median survival by 47%, 30% and 66%, respectively (Figure S2B). Patient-derived KU812 cells provided a useful model system that reflect some of these core changes, yet still strongly depending on BCR-ABL and allowing for oncogene-targeted intervention. In KU812 cells treated with the ABL inhibitor imatinib, 48 genes coding for enzymes involved in metabolism were changed at least 2-fold in their RNA expression (p<0.05), including GYS1 and GBE1 (Figure 2D). We did not observe significant expression of GYS2 (Figure 2D and not shown). In additional experiments, we found this pathway to be regulated to a somewhat lesser extent in MOLM-13 and HEL cells, which may explain the smaller reduction in glycogen levels in response to tyrosine kinase inhibitors (Figure 1D and not shown).
Figure 2. Dysregulation of the glycogen synthesis pathway in myeloid leukemia cells.
(a) Schematic representation of GYS1, GYS2 (glycogen synthase 1 or 2) and GBE1 (1,4-alpha-glucan-branching enzyme 1) involved in the biochemical pathway for glycogen synthesis from UDP-D-glucose. (b) Elevated levels of GYS1, GYS2, or GBE1 were observed in AML patient specimens, as indicated (changes in expression >1.6-fold). (c) Survival distribution for AML patients with upregulation of GYS1, GYS2 or GBE1. The p-value denotes significant differences in survival between the query group and patients without overexpression of the genes within the query. (d) Changes in expression of genes involved in metabolism in response to imatinib (1μM; ◇) in KU812 cells were determined by real-time PCR relative to their respective p-values (volcano plot). Red symbols indicate changes in expression of GYS1, GYS2 and GBE1. The horizontal dashed line indicates the p=0.005 significance level and the vertical dotted lines indicate 2-fold changes.
Glycogen synthesis through glycogen synthase 1 and its relation to AMPK
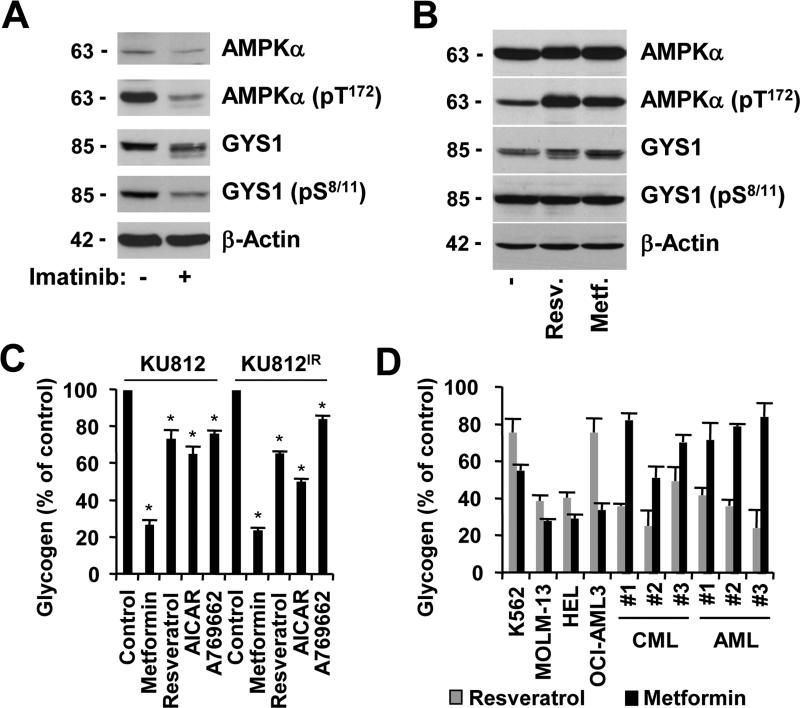
In addition to changes in glucose-6-phosphate levels and GYS1 expression levels, the enzyme activity of GYS1 can also be modulated through inhibitory phosphorylation. The GYS1 kinase AMPK was found to be phosphorylated at its activation site T172 in KU812 cells, and inhibition of the oncogenic BCR-ABL kinase with imatinib reduced AMPK phosphorylation (Figure 3A). Consistent with our gene expression data (Figure 2D), GYS1 protein expression was also reduced upon imatinib treatment, which contributed to the reduction in the pool of S8/11-phosphorylated GYS1. In order to demonstrate that AMPK can induce phosphorylation of GYS1 at S8/11, we treated KU812 cells with the AMPK pathway activators and anti-diabetic small molecule drugs resveratrol and metformin29 (Figure 3B). Interestingly, the amount of GYS1 increased but not the level of phosphorylated protein. Similarly, we detected both GSK3α and GSKβ to be phosphorylated at their respective inhibitory sites S21 and S9,41,42 in KU812 cells and the phosphorylation was diminished after imatinib treatment (Figure S3A). Nevertheless, in contrast to imatinib treatment, inhibition of AKT by MK-2206 upstream of GSK3 was sufficient to reduce inhibitory phosphorylation of GSKβ and increased inhibitory phosphorylation of GYS1 in KU812 cells (Figure S3B). Thus, BCR-ABL-dependent GYS1 expression and phosphorylation are unlikely to be regulated down-stream of AKT in this model. GSK3α may be phosphorylated by other kinases, such as PIM kinases (Figure S3C), which have been previously implicated in energy metabolism.43 Overall, phosphorylation-dependent regulation of GYS may work in concert with altered gene regulation in this pathway, but this process can likely be superseded by allosteric regulation of GYS1 activity.
Figure 3. Glycogen production is regulated at multiple levels.
Expression of AMPKα, AMPKα (pT172), GYS1, GYS1 (pS8/11), GYS1 (pS641) and β-actin was determined by immunoblotting in KU812 cells that were left untreated or treated for 18h with (a) imatinib (1 μM) or (b) resveratrol (10 μM) and metformin (1 mM). Changes in glycogen levels were measured in cellular extracts of (c) KU812 or KU812IR cells in response to treatment (72h) with either metformin (1 mM), resveratrol (10 μM), AICAR (1mM) and A769662 (100 μM) (n=3) or (d) in cellular extracts of cell lines (K562, MOLM-13, HEL and OCI-AML3) as well as primary patient specimens in response to metformin (1 mM) and resveratrol (10 μM). *Significant differences (p<0.05) were observed between control and treated cells.
The role of AMPK was further evaluated using the AMPK pathway stimulators metformin and resveratrol as well as the AMPK activators 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) and A769662.44,45 Treatment was sufficient to reduce glycogen production in myeloid leukemia cells and we did not observe a difference in sensitivity to these drugs in imatinib-resistant KU812 (KU812IR) compared to parental KU812 cells. (Figure 3C). Similar significant reduction in glycogen levels in response to metformin and resveratrol were found in other myeloid leukemia cell lines that are transformed by various oncogenes, including K562 (BCR-ABL), MOLM-13 (FLT3-ITD), HEL (JAK2.V617F) and OCI-AML3 (NRAS.G12D) as well as in primary patient specimens from patients with CML and AML (Figure 3D). Consistent with these results and a previously recognized role of AMPK activators metformin and resveratrol in the inhibition of growth of transformed myeloid cells 46-49, treatment led to a reduction in cell growth (Figure S4A-S4B) as well as reduced glucose uptake (metformin: 64.6 ± 6.5 % of control, p<0.05; resveratrol: 69.6 ± 10.7 % of control, p<0.05) (Figure S4C). Our data thus suggest a role of the AMPK pathway in controlling glycogenesis and metabolic reprogramming, independent of AMPK-induced GYS1 phosphorylation.
Metabolic effects associated with abnormal glycogenesis inversely correlates with AMPK activity
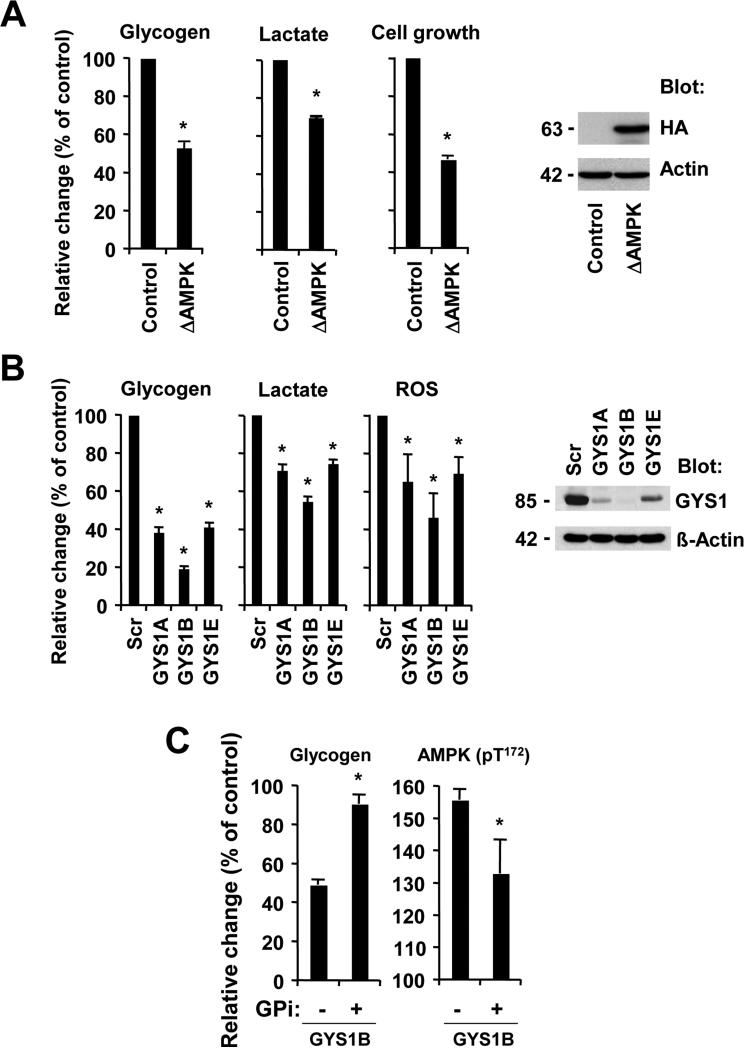
It would be predicted that a constitutively active form of AMPK induces effects that are comparable to AMPK activators in KU812 cells. To test this hypothesis, we expressed constitutively active truncated AMPK 50 (ΔAMPK; amino acids 1-312 of the α2 catalytic subunit) in KU812 cells. Further activation of AMPK by ΔAMPK led to a decrease in glycogen production (−47.5±2.9%, p<0.0005), cell growth (−53.7±2.0%, p<0.0005) as well as lactate production (−30.7±0.5%, p<0.0005) (Figure 4A, left). HA-tagged active ΔAMPK was readily detected in these cells (Figure 4A, right). We also observed reduced ROS levels (72.8 ± 5.6 % of control, p<0.005) and glucose uptake (77.5 ± 3.1% of control, p<0.005) (Figure S5A). Thus, chronic activation of AMPK elicits a response that is quite different from transient effects that are normally associated with signaling through AMPK.
Figure 4. Glycogenesis is required for metabolic reprogramming and associated with decreased AMPK activation.
(a) KU812 cells containing an active form of AMPKα2 (ΔAMPK) were compare to parental KU812 and relative changes in glycogen, cell growth and lactate were measured (left). The expression of HA-tagged ΔAMPK was determined by immunoblotting (right). (b) KU812 cells containing scrambled shRNA (Scr) or GYS1-targeting constructs (A, B or E) were used. Changes in glycogen, lactate, or ROS levels (left) and GYS1 protein expression (right) were measured. (c) KU812 cells expressing GYS1-targeting shRNA construct B were treated with glycogen phosphorylase inhibitor (1 μM) or left untreated. Glycogen levels and AMPK phosphorylation (ELISA) were measured, as indicated. *Significant differences (p<0.05) were observed between control and treated cells.
We next used a lentiviral-based shRNA approach to knockdown GYS1 and evaluate its role in glucose metabolism. GYS1 knockdown reduced glycogen production (19.3%-41.2% of control) as well as lactate production (54.4%-74.2% of control) and intracellular ROS (46.0%-69.3% of control) in KU812 cells (Figure 4B, left). GYS1 knockdown was also associated with reduced glucose uptake (52.3 ± 19.3 % of control, p<0.05) (Figure S5B). Thus, the results did not only show an important role for GYS1 in the maintenance of glycogen levels, but demonstrated an important function for this molecule in glucose and energy metabolism. Out of the six shRNA constructs tested, three were found to significantly knockdown GYS1 in KU812 cells (Figure 4B, right). In order to validate our hypothesis that increased glycogenic flux supports cell growth in myeloid malignancies, we used KU812 cells containing the GYS1B knockdown construct (highest knockdown efficiency) and treated them with glycogen phosphorylase inhibitor (GPi). Inhibitor treatment would be predicted to decrease glycogen degradation and increase its cellular levels. GPi treatment led to a significant increase in intracellular glycogen levels in GYS1 knockdown cells as compared to untreated control cells (Figure 4C, left). The AMPK β subunit contains a regulatory site that is sensitive to changes in glycogen and our experiments show a significant increase in AMPK phosphorylation at its activation site in GYS1 knockdown cells that was diminished upon treatment with GPi (Figure 4C, right). The results demonstrate an inverse relationship between the activation of AMPK and intracellular glycogen levels. In control experiments, the regulation of the AMPK axis by targeted approaches was confirmed by measuring changes in S6 phosphorylation and HIF1 expression, previously found to be downstream of AMPK (Figure S6).51 In myeloid leukemia cells, HIF1 is expressed at low levels, even under normoxic conditions.52
Genetic targeting of GYS1 reduces tumor cell growth
Since altered metabolism is crucial for transformation in leukemic cells, we sought to define the role of GYS1 in cell growth by specifically targeting GYS1. The GYS1 knockdown construct B was used and found to be sufficient to reduce growth of KU812 cells. The effect could further be enhanced in a dose-dependent manner by imatinib treatment (Figure 5A). To test our hypothesis that glycogen levels significantly affect transformation, we treated GYS1 and control knockdown cells with GPi. We observed a dose-dependent increase in cell growth in response to GPi in GYS1B knockdown cells, whereas there was no change in growth in cells containing scrambled shRNA (Figure 5B). The specificity of GYS1-dependent cell growth was confirmed using different GYS1-targeting constructs. Also, low intracellular glucose would be predicted to reduce the amount of substrate available for glycogenic turnover but not the glycogenic reactions themselves. Thus, limiting glucose availability to 0.4 g/L glucose reduced cell growth in all cells tested. In addition, GYS1 knockdown reduced cell growth to a similar extent compared to cells in 4.5 g/L glucose (38.7±1.5% and 38.8±9.8% average reduction, respectively), when compared to their respective control cells (Figure 5C). Consistent with a role of AMPK in this process, we found that knockdown of GYS1 resulted in increased phosphorylation at its activation site with low glucose, compared to control cells (Figure S7A). Neither knockdown of GYS1 nor expression of active AMPK led to a significant loss in cell viability and the reduced cell growth is consistent with reduced metabolic activity due to limited glucose uptake. Further, KU812 GYS1 knockdown cells were injected subcutaneously into SCID/beige mice and tumor growth compared with cells containing scrambled shRNA constructs. The tumor volume increased over time (p<0.0001) and treatment with GYS1 shRNA compared to scrambled shRNA significantly delayed tumor growth (p=0.004) (Figure 5D). Also, the body weights of the GYS1 knockdown mice increased more rapidly, as an indirect reflection of decreased tumor burden, compared to those of the scrambled mice, p=0.002; rates of further increase following day 14 did not differ significantly between the groups, p=0.27. (Figure S7B). Overall, these data implicate GYS1 as an important regulator of cellular transformation in myeloid leukemia cells.
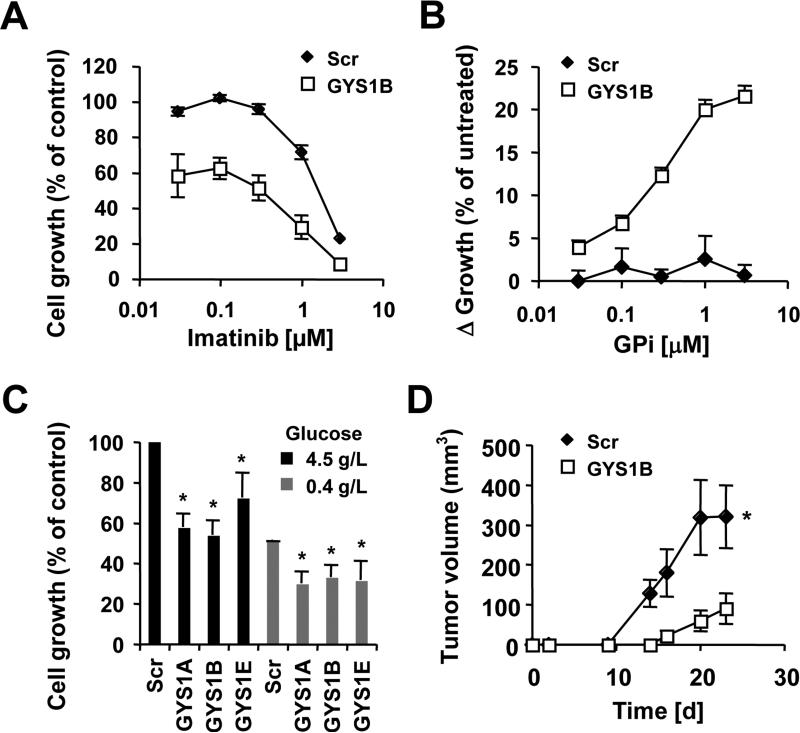
Figure 5. GYS1 is required for increased growth.
Cell growth was measured in KU812 cells expressing Scr shRNA or GYS1-targeting shRNA construct B treated with different concentrations of (a) imatinib or (b) glycogen phosphorylase inhibitor. (c) KU812 cells containing scrambled shRNA (Scr) or GYS1-targeting constructs (A, B or E) were used to measure growth in the presence of 4.5g/L or 0.4 g/L glucose. (d) In vivo tumor formation of KU812 cells with targeted knockdown of GYS1 construct B (◇) or scrambled shRNA (□) was determined in SCID/Beige mice (n=8). The volumes of subcutaneous tumors were measured and compared for 23 days after initial injection, as indicted. *Significant differences (p<0.05) were observed between control and treated cells.
DISCUSSION
Metabolic changes in cancer cells are thought to be broad, but specific regulatory mechanisms or biochemical pathway requirements may lead to distinct differences.3,4 Our metabolomics analysis indicated that the majority of changes driven by tyrosine kinase oncogenes were increases in metabolite levels. Stringent exclusion criteria led to the identification of only three metabolites in commonly dysregulated pathways that were consistently increased in myeloid leukemia cells, involving DNA synthesis (deoxythymidine triphosphate), transmethylation (S-adenosyl-L-methionine) or glycosphingolipid synthesis and glycogenesis (UDP-D-glucose). Of special interest here is the presumably paradoxical and energy inefficient glucose consumption for elevated glycogenesis 12 in cells that have a high demand for monosaccharides. Abnormal glycogenic flux is not unique to myeloid leukemias and appears to be associated with certain proliferating cells, for example in hepatic cancer 53 or rapidly growing Xenopus oocytes.54 However, many solid tumors tend to accumulate glycogen with limited glycogenolysis, suggesting that this pathway may have a different functional role in these diseases.55
The results show that either of the three enzymes (GYS1, GYS2, GBE1) that control the glycogen synthesis reaction are upregulated in 18% of AML patients and that the patient population associated with increased enzyme levels showed significantly shorter survival. Similar data were also found in lung adenocarcinoma as well as kidney cancer, suggesting a broader dependency on this pathway in different cancers. The results did not show an invariable correlation between mutations in commonly known oncogenes and expression of these enzymes (not shown). Thus, even though glycogen turnover is commonly higher in myeloid malignancies, the dysregulation of the enzymes involved may provide a unique growth advantage. Our GYS1 knockdown data in KU812 cells under glucose limiting conditions would argue against a simple growth advantage for stored glycogen as a source of glucose, since we did not observe resistance towards low glucose supplies.
In vivo, there may be a stronger dependency on GYS1 for transformation pathways since our in vitro approach selected for positive growth of cells with only partial GYS1 knockdown. Reduced expression of GYS1 did not only directly affect glycogen levels but had a broader effect on glucose metabolism pathways, ultimately resulting in reduced leukemia cell growth in vitro and in vivo. One major function of these pathways is to cover the energy needs of proliferating cells. In some cancer models, excess energy production in the form of ATP is reduced through active hydrolysis cycles to prevent negative feedback mechanisms and to allow continuing glycolysis.56 Energy-consuming metabolic reactions, such as glycogen synthesis from UDP-D-glucose, have the potential to compensate for this effect, thus acting as part of a floodgate that can temporarily reduce negative pressure from high energy and/or glucose levels on metabolic pathways. We observed increased (AMP+ADP)/ATP ratios in response to GYS1 knockdown (not shown), which may suggest that other mechanisms regulated through GYS1 have a greater impact on cell growth than simple modulation of energy-consumption.
Importantly, inhibition of glycogen synthesis could be either achieved by inhibition of GYS1 activity or by activation of AMPK. This is of interest since binding of glycogen to the glycogen-binding domain within the β-subunit of AMPK is thought to inhibit its kinase activity in cell-free extracts.57 The significance and the biological consequences of this interaction are not known. Our data show that reduced phosphorylation of AMPK at its activation site correlated with high glycogen levels and vice versa. Specifically the branching sites of glycogen elicit inhibitory activity 57 and these sites are not exposed in mature glycogen molecules with tightly packed outer chains.58 Consistent with this, treatment with glycogen phosphorylase inhibitor showed increased glycogen levels in cells with GYS1 knockdown, allowing for exposure of AMPK to a larger amount of glycogen. Since glycogen cannot diffuse within the cell, the regulation of AMPK pathways through AMPK/glycogen complexes is likely to occur within proximity of the glycogen molecules.
Suppression of GYS1 or hyperactivation of AMPK may be a viable strategy to supplement traditional therapy or precision medicine in myeloid malignancies and to take advantage of metabolic changes that occur in transformed cells. Results from mice with GYS1 gene disruption would suggest that targeting this protein with small molecule drugs would have little to no detrimental effects in adults.59,60 On the other hand, loss of liver GYS2 in mice, which is marginally expressed in myeloid leukemia cells, leads to a phenotype that is similar to glycogen storage disease type 0 61 and few studies have been done with GBE1 knockout mice due to the occurrence of hydrops fetalis as a result of glycogen storage disease type 4.62 Inhibiting glycogenesis may also provide a venue to reduce growth of leukemic cells if initial therapy fails or it may work in combination with traditional therapy, as we have shown in BCR-ABL transformed cells. AMPK is of particular interest as a drug target since genomic data in solid tumors, such as lung cancer, have already shown that loss of function mutations in the tumor suppressor LKB1 prevent AMPK activation.32,36,63 However, in neither model AMPK appears to be entirely inactive, leaving the open question whether some basal activity may be required for transformation. It will now be important to develop specific drugs and target glycogenic flux in cells that depend on this pathway for glucose metabolism and cell growth.
Supplementary Material
Acknowledgments
Financial support: This work is supported in part by National Institutes of Health grant CA134660 (M.S.), CA6696 (J.D.G.) and by the Gloria Spivak Faculty Advancement Fund (M.S.).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary information is available at Leukemia's website.
REFERENCES
- 1.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 2.Hehlmann R, Muller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. 2014;32:415–423. doi: 10.1200/JCO.2013.49.9020. [DOI] [PubMed] [Google Scholar]
- 3.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 4.Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364–373. doi: 10.1038/nature11706. [DOI] [PubMed] [Google Scholar]
- 5.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 6.Kim JH, Chu SC, Gramlich JL, Pride YB, Babendreier E, Chauhan D, et al. Activation of the PI3K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood. 2005;105:1717–1723. doi: 10.1182/blood-2004-03-0849. [DOI] [PubMed] [Google Scholar]
- 7.Reddy MM, Fernandes MS, Salgia R, Levine RL, Griffin JD, Sattler M. NADPH oxidases regulate cell growth and migration in myeloid cells transformed by oncogenic tyrosine kinases. Leukemia. 2011;25:281–289. doi: 10.1038/leu.2010.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues MS, Reddy MM, Sattler M. Cell cycle regulation by oncogenic tyrosine kinases in myeloid neoplasias: from molecular redox mechanisms to health implications. Antioxid Redox Signal. 2008;10:1813–1848. doi: 10.1089/ars.2008.2071. [DOI] [PubMed] [Google Scholar]
- 9.Reddy MM, Fernandes MS, Deshpande A, Weisberg E, Inguilizian HV, Abdel-Wahab O, et al. The JAK2V617F oncogene requires expression of inducible phosphofructokinase/fructose-bisphosphatase 3 for cell growth and increased metabolic activity. Leukemia. 2012;26:481–489. doi: 10.1038/leu.2011.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Favaro E, Bensaad K, Chong MG, Tennant DA, Ferguson DJ, Snell C, et al. Glucose utilization via glycogen phosphorylase sustains proliferation and prevents premature senescence in cancer cells. Cell Metab. 2012;16:751–764. doi: 10.1016/j.cmet.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Pelletier J, Bellot G, Gounon P, Lacas-Gervais S, Pouyssegur J, Mazure NM. Glycogen Synthesis is Induced in Hypoxia by the Hypoxia-Inducible Factor and Promotes Cancer Cell Survival. Front Oncol. 2012;2:18. doi: 10.3389/fonc.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seitz JF, Luganova IS. The biochemical identification of blood and bone marrow cells of patients with acute leukemia. Cancer Res. 1968;28:2548–2555. [PubMed] [Google Scholar]
- 13.Valentine WN, Follette JH, Lawrence JS. The glycogen content of human leukocytes in health and in various disease states. J Clin Invest. 1953;32:251–257. doi: 10.1172/JCI102734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner R. Studies on the physiology of the white blood cell; the glycogen content of leukocytes in leukemia and polycythemia. Blood. 1947;2:235–243. [PubMed] [Google Scholar]
- 15.Browner MF, Nakano K, Bang AG, Fletterick RJ. Human muscle glycogen synthase cDNA sequence: a negatively charged protein with an asymmetric charge distribution. Proc Natl Acad Sci U S A. 1989;86:1443–1447. doi: 10.1073/pnas.86.5.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camici M, Ahmad Z, DePaoli-Roach AA, Roach PJ. Phosphorylation of rabbit liver glycogen synthase by multiple protein kinases. J Biol Chem. 1984;259:2466–2473. [PubMed] [Google Scholar]
- 17.Nuttall FQ, Gannon MC, Bai G, Lee EY. Primary structure of human liver glycogen synthase deduced by cDNA cloning. Arch Biochem Biophys. 1994;311:443–449. doi: 10.1006/abbi.1994.1260. [DOI] [PubMed] [Google Scholar]
- 18.Parker GE, Pederson BA, Obayashi M, Schroeder JM, Harris RA, Roach PJ. Gene expression profiling of mice with genetically modified muscle glycogen content. Biochem J. 2006;395:137–145. doi: 10.1042/BJ20051456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouskila M, Hunter RW, Ibrahim AF, Delattre L, Peggie M, van Diepen JA, et al. Allosteric regulation of glycogen synthase controls glycogen synthesis in muscle. Cell Metab. 2010;12:456–466. doi: 10.1016/j.cmet.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Pederson BA, Wilson WA, Roach PJ. Glycogen synthase sensitivity to glucose-6-P is important for controlling glycogen accumulation in Saccharomyces cerevisiae. J Biol Chem. 2004;279:13764–13768. doi: 10.1074/jbc.M312335200. [DOI] [PubMed] [Google Scholar]
- 21.Preller A, Wilson CA, Quiroga-Roger D, Ureta T. Hexokinase and not glycogen synthase controls the flux through the glycogen synthesis pathway in frog oocytes. FEBS Lett. 2013;587:2825–2831. doi: 10.1016/j.febslet.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 22.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 23.Rylatt DB, Aitken A, Bilham T, Condon GD, Embi N, Cohen P. Glycogen synthase from rabbit skeletal muscle. Amino acid sequence at the sites phosphorylated by glycogen synthase kinase-3, and extension of the N-terminal sequence containing the site phosphorylated by phosphorylase kinase. Eur J Biochem. 1980;107:529–537. [PubMed] [Google Scholar]
- 24.Wang Y, Roach PJ. Inactivation of rabbit muscle glycogen synthase by glycogen synthase kinase-3 Dominant role of the phosphorylation of Ser-640 (site-3a). J Biol Chem. 1993;268:23876–23880. [PubMed] [Google Scholar]
- 25.Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107:519–527. [PubMed] [Google Scholar]
- 26.Woodgett JR. Judging a protein by more than its name: GSK-3. Sci STKE. 2001;2001:re12. doi: 10.1126/stke.2001.100.re12. [DOI] [PubMed] [Google Scholar]
- 27.Carling D, Hardie DG. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim Biophys Acta. 1989;1012:81–86. doi: 10.1016/0167-4889(89)90014-1. [DOI] [PubMed] [Google Scholar]
- 28.Jorgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, et al. The alpha2-5′AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes. 2004;53:3074–3081. doi: 10.2337/diabetes.53.12.3074. [DOI] [PubMed] [Google Scholar]
- 29.Burkewitz K, Zhang Y, Mair WB. AMPK at the Nexus of Energetics and Aging. Cell Metab. 2014 doi: 10.1016/j.cmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 31.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP- activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, et al. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 33.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 35.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez-Cespedes M, Parrella P, Esteller M, Nomoto S, Trink B, Engles JM, et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–3662. [PubMed] [Google Scholar]
- 37.Yuan M, Breitkopf SB, Yang X, Asara JM. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc. 2012;7:872–881. doi: 10.1038/nprot.2012.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrer JC, Favre C, Gomis RR, Fernandez-Novell JM, Garcia-Rocha M, de la Iglesia N, et al. Control of glycogen deposition. FEBS Lett. 2003;546:127–132. doi: 10.1016/s0014-5793(03)00565-9. [DOI] [PubMed] [Google Scholar]
- 39.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutherland C, Cohen P. The alpha-isoform of glycogen synthase kinase-3 from rabbit skeletal muscle is inactivated by p70 S6 kinase or MAP kinase-activated protein kinase-1 in vitro. FEBS Lett. 1994;338:37–42. doi: 10.1016/0014-5793(94)80112-6. [DOI] [PubMed] [Google Scholar]
- 42.Sutherland C, Leighton IA, Cohen P. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J. 1993;296(Pt 1):15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beharry Z, Mahajan S, Zemskova M, Lin YW, Tholanikunnel BG, Xia Z, et al. The Pim protein kinases regulate energy metabolism and cell growth. Proc Natl Acad Sci U S A. 2011;108:528–533. doi: 10.1073/pnas.1013214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 46.Estrov Z, Shishodia S, Faderl S, Harris D, Van Q, Kantarjian HM, et al. Resveratrol blocks interleukin-1beta-induced activation of the nuclear transcription factor NF-kappaB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood. 2003;102:987–995. doi: 10.1182/blood-2002-11-3550. [DOI] [PubMed] [Google Scholar]
- 47.Green AS, Chapuis N, Maciel TT, Willems L, Lambert M, Arnoult C, et al. The LKB1/AMPK signaling pathway has tumor suppressor activity in acute myeloid leukemia through the repression of mTOR-dependent oncogenic mRNA translation. Blood. 2010;116:4262–4273. doi: 10.1182/blood-2010-02-269837. [DOI] [PubMed] [Google Scholar]
- 48.Tolomeo M, Grimaudo S, Di Cristina A, Roberti M, Pizzirani D, Meli M, et al. Pterostilbene and 3′-hydroxypterostilbene are effective apoptosis-inducing agents in MDR and BCR-ABL-expressing leukemia cells. Int J Biochem Cell Biol. 2005;37:1709–1726. doi: 10.1016/j.biocel.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Vakana E, Altman JK, Glaser H, Donato NJ, Platanias LC. Antileukemic effects of AMPK activators on BCR-ABL-expressing cells. Blood. 2011;118:6399–6402. doi: 10.1182/blood-2011-01-332783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem. 1998;273:35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- 51.Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17:113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayerhofer M, Valent P, Sperr WR, Griffin JD, Sillaber C. BCR/ABL induces expression of vascular endothelial growth factor and its transcriptional activator, hypoxia inducible factor-1alpha, through a pathway involving phosphoinositide 3-kinase and the mammalian target of rapamycin. Blood. 2002;100:3767–3775. doi: 10.1182/blood-2002-01-0109. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez-Enriquez S, Torres-Marquez ME, Moreno-Sanchez R. Substrate oxidation and ATP supply in AS-30D hepatoma cells. Arch Biochem Biophys. 2000;375:21–30. doi: 10.1006/abbi.1999.1582. [DOI] [PubMed] [Google Scholar]
- 54.Dworkin MB, Dworkin-Rastl E. Metabolic regulation during early frog development: glycogenic flux in Xenopus oocytes, eggs, and embryos. Dev Biol. 1989;132:512–523. doi: 10.1016/0012-1606(89)90246-7. [DOI] [PubMed] [Google Scholar]
- 55.Rousset M, Zweibaum A, Fogh J. Presence of glycogen and growth-related variations in 58 cultured human tumor cell lines of various tissue origins. Cancer Res. 1981;41:1165–1170. [PubMed] [Google Scholar]
- 56.Fang M, Shen Z, Huang S, Zhao L, Chen S, Mak TW, et al. The ER UDPase ENTPD5 promotes protein N-glycosylation, the Warburg effect, and proliferation in the PTEN pathway. Cell. 2010;143:711–724. doi: 10.1016/j.cell.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 57.McBride A, Ghilagaber S, Nikolaev A, Hardie DG. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab. 2009;9:23–34. doi: 10.1016/j.cmet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melendez-Hevia E, Waddell TG, Shelton ED. Optimization of molecular design in the evolution of metabolism: the glycogen molecule. Biochem J. 1993;295(Pt 2):477–483. doi: 10.1042/bj2950477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pederson BA, Cope CR, Schroeder JM, Smith MW, Irimia JM, Thurberg BL, et al. Exercise capacity of mice genetically lacking muscle glycogen synthase: in mice, muscle glycogen is not essential for exercise. J Biol Chem. 2005;280:17260–17265. doi: 10.1074/jbc.M410448200. [DOI] [PubMed] [Google Scholar]
- 60.Pederson BA, Schroeder JM, Parker GE, Smith MW, DePaoli-Roach AA, Roach PJ. Glucose metabolism in mice lacking muscle glycogen synthase. Diabetes. 2005;54:3466–3473. doi: 10.2337/diabetes.54.12.3466. [DOI] [PubMed] [Google Scholar]
- 61.Irimia JM, Meyer CM, Peper CL, Zhai L, Bock CB, Previs SF, et al. Impaired glucose tolerance and predisposition to the fasted state in liver glycogen synthase knock-out mice. J Biol Chem. 2010;285:12851–12861. doi: 10.1074/jbc.M110.106534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee YC, Chang CJ, Bali D, Chen YT, Yan YT. Glycogen-branching enzyme deficiency leads to abnormal cardiac development: novel insights into glycogen storage disease IV. Hum Mol Genet. 2011;20:455–465. doi: 10.1093/hmg/ddq492. [DOI] [PubMed] [Google Scholar]
- 63.Carretero J, Medina PP, Blanco R, Smit L, Tang M, Roncador G, et al. Dysfunctional AMPK activity, signalling through mTOR and survival in response to energetic stress in LKB1-deficient lung cancer. Oncogene. 2007;26:1616–1625. doi: 10.1038/sj.onc.1209951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.