Abstract
The zebrafish’s potential as a model for human neurobehavioral research appears nearly limitless despite its relatively recent emergence as an experimental organism. Since the zebrafish has only been part of the research community for a handful of decades, pathogens from its commercial origins continue to plague laboratory stocks. One such pathogen is Pseudoloma neurophilia, a common microparasite in zebrafish laboratories world-wide that generally produces subclinical infections. Given its high prevalence, its predilection for the host’s brain and spinal cord, and the delicate nature of neurobehavioral research, the behavioral consequences of subclinical P. neurophilia infection must be explored. Fish infected via cohabitation were tested for startle response habituation in parallel with controls in a device that administered ten taps over ten minutes along with taps at 18 and 60 minutes to evaluate habituation extinction. After testing, fish were euthanized and evaluated for infection via histopathology. Infected fish had a significantly smaller reduction in startle velocity during habituation compared to uninfected tankmates and controls. Habituation was eliminated in infected and control fish at 18 minutes, whereas exposed negative fish retained partial habituation at 18 minutes. Infection was also associated with enhanced capture evasion: Despite the absence of external symptoms, infected fish tended to be caught later than uninfected fish netted from the same tank. The combination of decreased overall habituation, early extinction of habituation compared to uninfected cohorts, and enhanced netting evasion indicates that P. neurophilia infection is associated with a behavioral phenotype distinct from that of controls and uninfected cohorts. Because of its prevalence in zebrafish facilities, P. neurophilia has the potential to insidiously influence a wide range of neurobehavioral studies if these associations are causative. Rigorous health screening is therefore vital to the improvement of the zebrafish as a translational model for human behavior.
Keywords: Pseudoloma neurophilia, zebrafish, habituation, hypervigilance, startle, behavior
Introduction
1.1 The Zebrafish: a burgeoning model organism
The use of zebrafish in neurobehavioral studies has increased exponentially since their inception as an experimental animal in the 1970s [1]. In this short time, these studies have come to utilize a variety of stimuli including, but not limited to, genetic manipulations, pharmaceutical products, and environmental toxins. As the reliance on zebrafish in neurobehavioral studies has increased, so too has their utility as a model organism for such diverse human behavioral traits as stress, memory, and learning. Zebrafish are also being developed as models for such complex human neurological diseases as schizophrenia, autism, and Parkinson’s disease [2–12]
Like any relatively new laboratory animal, the zebrafish comes with its own menagerie of infectious diseases that range in severity from the merely problematic to the completely devastating. Paradoxically, the severest can be the easiest to monitor: laboratories maintain intense vigilance against highly virulent organisms such as Edwardsiella ictaluri, Mycobacterium marinum and M. haemophilum, which can decimate entire stocks [13]. Because of their pathogenicity, these diseases generally produce visible symptoms and sick fish can be excluded from behavioral experiments. More sinister are the subclinical diseases of zebrafish [14]. From a pure husbandry standpoint, a low-virulence ‘background’ disease may not seem like an important concern. However, as zebrafish are increasingly used in sensitive experiments across the scientific spectrum, infection-associated, non-protocol induced variation is a rising threat.
Pseudoloma neurophilia is one of the most common pathogens identified in many zebrafish facilities with infections present in up to 74% of all facilities submitting zebrafish to the ZIRC diagnostic service between 2006 and 2010 (Zebrafish International Resource Center. Eugene, Oregon). [13]. Currently, the only identified symptoms of infection are fairly nonspecific and include weight loss, decreased fecundity, and increased mortality [15–17]. P. neurophilia’s lack of pathogenicity compared to more florid organisms makes it nearly invisible to researchers without intensive monitoring, and subtle behavioral consequences of infection have not yet been identified.
1.2 Pseudoloma neurophilia
Members of the phylum Microsporidia are intracellular, fungus-like parasites that infect a wide range of host phyla [18]. They are particularly prevalent and pathogenic in fishes [19]. P. neurophilia is spread primarily through the consumption of environmentally-resistant spores, either through scavenging of infected carcasses or through consumption of free spores released with eggs during spawning. Alternatively, spores can be transmitted vertically, as infections have been observed in both eggs and newly-hatched larvae [20–21]. P. neurophilia spores are a particular problem for zebrafish facilities because they can survive bleaching at a concentration of 25–50ppm, which is the standard concentration used in most facilities for embryo sterilization [22]. This makes transmission of spores between facilities via embryonated eggs a risk [23].
Many zebrafish researchers are either unconcerned or uninformed about underlying P. neurophilia in their animals, because most infections are subclinical and many facilities lack screening protocols [24]. There is currently only one zebrafish facility that is specific pathogen free (SPF) for P. neurophilia [25]. Hence, zebrafish are usually obtained from non-SPF facilities. Simultaneously, there is almost universally no mention of pathogen screening in in zebrafish-based neurobehavioral studies. Even laboratories maintaining in-house zebrafish populations tend to have a fairly high incidence of P. neurophilia infection [23–26].
1.3 Zebrafish neuroanatomy and potential consequences of infection
In order to explore the potential consequences of subclinical infection, our group performed a retrospective study of P. neurophilia cases submitted to the ZIRC zebrafish diagnostic service between the years 1999 and 2013 [26]. In most chronic neural infections, P. neurophilia forms non-membrane-bound, intra-axonal aggregates of spores and pre-sporogonic stages termed parasite clusters (PCs) [26, 27]. P. neurophilia has a strikingly specific tropism for certain neural structures and by studying the most common anatomic locations of infection, we can hypothesize which behaviors might be altered by the parasite.
PCs were found most frequently in spinal nerve roots and spinal white matter [26]. PCs in these locations would most likely affect motor function [28]. In the hindbrain, PCs were most frequently located in descending white matter tracts, (the dorsal and ventral medial longitudinal fasciculi) which transmit signals from the brain to the spinal cord and then to the rest of the body [26]. Lesions in these areas could also affect motor function [28]. The dorsal medial longitudinal fasciculus contains the Mauthner axon, which runs the entire length of the spinal cord and plays a major role in coordinating the startle response. Since we found that PCs in the hindbrain and the spinal cord frequently impinge upon the Mauthner axon, it is likely that the startle response would be altered by infection [26, 28–33].
PCs located in rhombencephalic gray matter were frequently observed in the griseum centrale and the reticular system [26]. Anxiety and fear-learning in mammals are generally associated with the amygdala [6, 34]. Although cyprinids lack an amygdala, the medial habenula of the telencephalon has been implicated in anxiety and aversion learning and it has descending connections that associate with the griseum centrale. Because many zebrafish-based behavioral experiments utilize avoidance learning and the memory of noxious stimuli (either directly or indirectly), it is possible that griseum centrale lesions could influence these experiments [6, 34]. The reticular formation contains arousal circuitry and acts with the Mauthner neurons to integrate the startle response [32–35].
Based on the frequent presence of PCs in anatomic structures involved with motor function, anxiety, fear-learning, and the startle response, any experimental protocol that evaluates or involves one or more of these features could be unduly influenced by P. neurophilia infection. Startle response habituation tests, therefore, should be highly sensitive to alteration by neural microsporidiosis.
1.4 Startle response habituation
In this study, we explored the potential of P. neurophilia infection to influence a common neurobehavioral assay. We accomplished this by comparing the performances of infected and uninfected adult zebrafish to a progressive tap test for startle response habituation and habituation extinction. We evaluated the effects of P. neurophilia infection using the progressive tap test because we felt that this assay had the highest potential to be affected by neural microsporidiosis based on the parasite’s anatomic tropisms as explained in section 1.3. The progressive tap test for adult fish was used with protocols based largely on those described in Eddins et al. 2010 [4].
2. Materials and methods
2.1 Fish
Zebrafish of the 5D strain were used and reared to an age of two months. These fish were obtained from the Sinnheuber Aquatic Research Laboratory (SARL, Corvallis, OR). The 5D strain is an outbred strain derived from zebrafish reared for the ornamental fish industry and the SARL is SPF for P. neurophilia [25]. At 70 days (d) of age, approximately 250 fish from the SARL facility were transported to our laboratory where they were separated into two equally-numbered groups. Fish were reared in the same room on the same lighting schedule and were exposed to the same system water at the same temperature (27 °C). Both groups of fish were housed in 28 L tanks on a flow-through system and fed twice daily with the same artificial commercial diet.
Test fish were exposed to P. neurophilia in the following manner: To optimize infection rates, and to allow enough time for the development of chronic infections, naïve fish were placed into water that had previously housed infected fish. The contaminated water containing naïve fish was then supplemented wih effluent water from a tank containing infected adult 5D fish for 24d beginning upon their arrival from the SARL facility (70d old). The “feeder” tank containing adult infected fish was placed on a shelf above the exposure tank and an outflow tube was fed from the upper tank into the exposure tank. Effluent water was allowed to flow into the exposure tank by gravity. In order to maintain appropriate water quality levels, the exposure tank also received supplementary system water at a rate approximately twice that of the effluent flow rate. Water quality values were comparable between the two tanks and considered within acceptable limits. At the end of the 24 d exposure period, neural infection of test fish (and, simultaneously, the non- infected status of control fish) was confirmed via histopathology. At this time, there was no histopathologic evidence of chronic exposure to poor quality water (proliferative branchitis, etc.). Exposure to effluent was chosen as the method of infection in this experiment in order to mimic conditions in an actual zebrafish facility where infections would be transmitted by exposure of naïve fish to spore-laden water.
Approximately half of the fish in the test tank died by 24 d post-exposure, most likely due to the stress of transfer of young fish combined with exposure to parasite-laden water. This mortality rate far exceeded that of the control fish, and so at the end of the 24 d period (fish aged 94 d) the tank of control fish was divided in half, leaving both tanks at a stocking density of approximately 40–60 fish per liter. This was done in order to eliminate differences in survival rates and stocking densities as potential complicating variables.
2.2 Tap test
The startle response is a “fast start” response in which a loud noise or a sudden, frightening stimulus causes a fish to turn and swim rapidly away from the source [29–32]. The intensity of the startle response can be quantified in adult zebrafish by measuring swimming velocity following a stimulus (e.g., tapping on the aquarium wall). Habituation occurs when the post-startle response decreases over serial stimuli [4, 6, 37] and the degree of habituation can be measured by evaluating the overall reduction in startle velocity during the habituation period [37]. The progressive tap test for startle response habituation combines multiple neural structures and behavioral circuits, and the results are best interpreted as an integration of multiple fear and anxiety responses resulting in a quantifiable motor response [6].
We used a testing apparatus similar to that described by Eddins et al (2010) [4]. It consisted of an aluminum frame measuring 52×42×220cm which held the arenas on top of a flat plastic board. Located beneath the board were four solenoid coil-driven pistons (Guardian brand Model TP6x12 Push-Type DC Tubular Solenoid). These were placed along the longitudinal midline of the board at regular spaces directly between each pair of arenas so that the vibrations from each strike would be distributed evenly throughout the arenas (Fig 1). The solenoid pistons were connected by wire to a push-button for activation. Video was captured using a Sony Handycam model HDR-CX240. The camera was attached to the frame at a height of 180cm above the upper rims of the arenas and was positioned to record all eight simultaneously from the top-down.
Figure 1. Testing Apparatus.
Video still showing a top-down view of eight testing arenas containing fish. X= location of solenoid piston beneath the arenas.
Opaque cylindrical white plastic arenas were used, each 12cm tall and 8cm in diameter at the base (Fig 1). These were filled with 250 mL of water from the laboratory aquaculture system (27°C). These containers and the water volume were chosen so individual fish could not see each other and to prevent fish from leaping out of their containers during the experiment. Water in the arenas was replaced and the arenas themselves were rinsed with system water between testing sessions in order to minimize the potential effect of alarm substance released by fish during testing. Tests were performed over the course of 4d, beginning when the fish were aged 171d (101 d after initial experimental exposure). Testing occurred between 0800 and 1700 hours (h); during standard daylight activity times for tested fish. Three to five tests were performed per day, beginning at 0800, 1000, 1200, 1400, and/or 1600 h where applicable. Each testing session utilized eight fish. While fish from both control and exposed groups were available, four fish from each group were used during each testing session in order to both maintain similar stocking densities and to minimize the effect of individual session variation. The location of each fish (control or exposed) within rig arenas was randomized via coin toss to reduce locational variation. For each test, four fish were captured from the two tanks (exposure and control) using similar square nylon mesh nets measuring 10×25cm. The same investigator performed each netting session.
The investigator was located in another room and separated from the testing apparatus by a closed door. The moment of door closing was used as the start of the ten minute acclimation period during which no taps were given. Using a stopwatch, the investigator manually pressed a button to activate tapping solenoids on the rig. Taps occurred once every sixty seconds over 10 minutes (min) after the acclimation period. These 10 taps over 10 min were used to assess the fish’s habituation to the startle response. After these stimuli, two more taps were administered; at 8 min and 50 min following the completion of the first set of taps. This was conducted to evaluate the extinction of the adaptation response. Eddins et al. (2010) utilized taps at 8 minutes and 50 minutes following the last tap of the 10 minute habituation series to evaluate the extinction of the habituation response [4]. This can be measured by comparing the post-tap startle velocities between tap 1 and taps at minute 18 and minute 60. Retention of habituation is indicated by later post-tap velocities being reduced compared to the starting post-tap velocity: a larger difference indicates a greater degree of retention. If there is no difference in post-tap velocity between tap 1 and the later taps, then the habituation response developed during the initial ten minute series is said to be extinct [4].
2.3 Histopathology
Fish were euthanized by exposure to ice water, an approved protocol in the guidelines for the use of zebrafish in the NIH intramural research program [36]. The abdomen of the each fish was opened with a longitudinal cut, and fish were preserved whole, fixed in Dietrich’s fixative, and decalcified using Cal-Ex II. Fish were then processed for histology. Fish were sectioned longitudinally in midline sections so that slides contained the brain and spinal cord, and sections were with hematoxylin and eosin. Fish with granulomas were further stained with Kinyoun’s Acid Fast, Luna, and Hall’s bile stains using standard methods. Severity of infection and associated inflammatory changes were scored as described by Spagnoli et al. (meninxitis and encephalitis both on a scale of 0–3) (2015) [26].
2.4 Video Analysis
Video was analyzed using Ethovision XT 10.1 software (Noldus, The Netherlands). The average swimming velocity of each fish over five seconds post-tap was used as an indicator of startle response strength. The length of each fish was measured using still images from video recordings and ‘length’ was defined as the longitudinal measurement of the fish from the midpoint between the eyes to the thinnest visible portion of the caudal peduncle.
2.5 Statistical Analysis
We applied the logarithm transformation to startle velocity to stabilize the variance and transform the positively defined variable to the entire real line. We modeled the logarithm of startle velocity as a linear function of tap number, but allowed different interceptors and slopes for three different exposure types (control, exposed negative, and infected), and accounted for the effects of bile duct hyperplasia, hepatic granulomas, fish length, presence of PCs in notochord remnants, total PC number, encephalitis score, and meninxitis score. Both session number and fish identification were considered as random effects to incorporate possible correlation of measurements taken on the same fish and in the same session. Along with the slope of the logarithmic linear function, the difference in startle velocities between tap1 and tap10 was used to quantify the degree of habituation over ten taps. A linear mixed model was used to quantify the effects of sex and exposure/infection status on fish habituation while controlling for bile duct hyperplasia, hepatic granulomas, fish length, presence of PCs in notochord remnants, total PC number, encephalitis score, and meninxitis score. The session was considered as a random effect to incorporate potential correlations within the same sessions into the model. Two similar linear mixed models were also used, where with logarithms of the 18 and 50 minute time points were treated as response variables, respectively. These models evaluate the effects of exposure/infection and sex on startle velocity at these time points, while controlling for the effects of other variables. Differences in startle velocities between tap1 and tap18, and between tap1 and tap50 were used to quantify the degree of extinction of the habituation. A similar linear mixed model as above was used to assess the effects of exposure/infection and sex on the degree of extinction of the habituation, while controlling for the effects of other variables.
At each tap, the sample variances of the startle velocity were calculated for fish of the same sex and exposure type. A linear regression model was then used to compare the variability of startle velocity for fish with different sex and exposure types.
A one-way analysis of variance (ANOVA) with session as the response variable and exposure type as the explanatory variable was used to investigate the difference of capture times of fish with different exposure type.
All analyses were conducted using PROC GLM, SAS Insititute 2015.
3. Results
Approximately half of the fish in the test tank died by 24 d post-exposure, most likely due to the stress of transfer of young, small fish to a tank full of parasite spores. This mortality rate far exceeded that of the control fish.
3.1 Histopathology
Fish in the two separate tanks were divided into three categories based on exposure and infection status: Control (n=60) in one tank and exposed positive/infected (n=40) and exposed negative (n=31) in the other tank. Infections were generally mild, with a median of 2 intraneural PCs per fish (standard deviation (SD) = 2.29). Central nervous system PCs in infected fish ranged in number from 0–11. The vast majority of PCs in infected fish were observed in the spinal cord white matter or in the nerve roots (Fig 2). Three of these fish had parasite clusters in notochord remnant cells and one of these had PCs only in notochord remnant cells and in no other anatomic structure. The fish with PCs only in the notochord remnant was counted as an infected fish with 0 intraneural PCs (Fig 2). Of the 40 infected fish, only 7 had PCs in the medial longitudinal fasciculus (descending white matter tract) in the hindbrain. No fish had PCs in any rhombencephalic gray matter structure. Myositis was not observed in any infected fish.
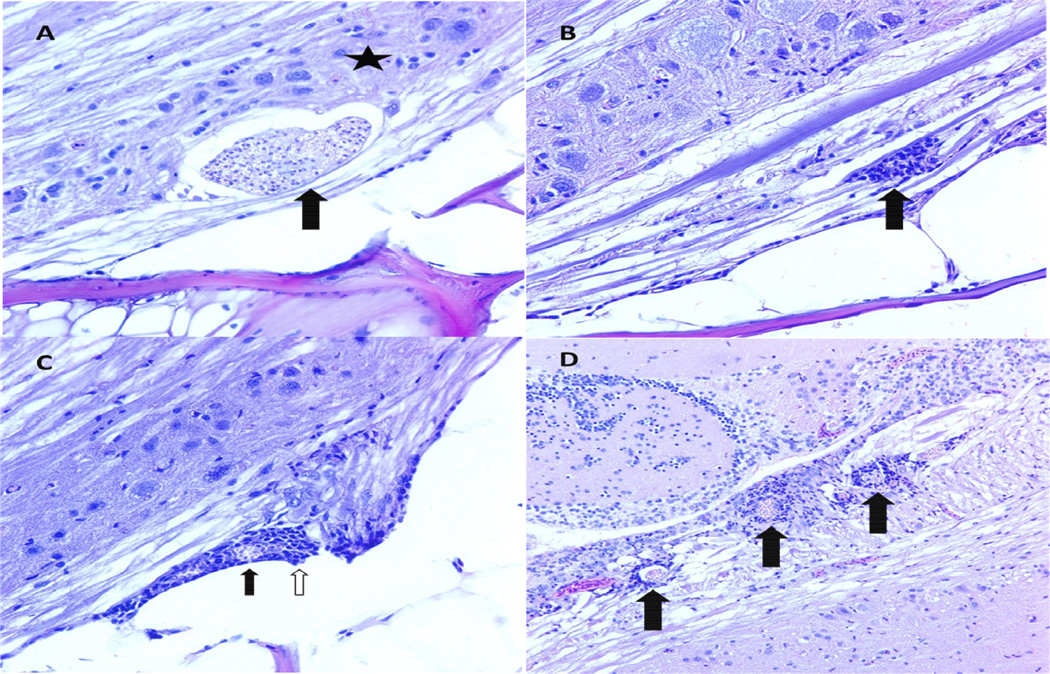
Figure 2. General features of infection.
A. Parasite clusters (PCs) were most commonly observed in the ventral white matter of the spinal cord. In this image, a PC in white matter (arrow) impinges of the gray matter (star). Photomicrograph. H&E. 400× magnification. B. A common feature of infection was myelitis, or inflammation of the spinal cord neuropil. Myelitis in these fish was generally multifocal and composed of inflammatory cells that are most likely a combination of granulocytes and microglial cells (arrow). Photomicrograph. H&E. 400× magnification. C. Meninxitis is inflammation of the perineural membranes of the teleost central nervous system, so called because they do not have a true set of meninges as in mammals. Here, granulocytes (white arrow) surround a ruptured PC (black arrow) at the base of a nerve root. D. Encephalitis, inflammation of the brain, was associated with PCs (black arrows) in the medial longitudinal fasciculus, a descending white matter tract in the rhombencephalon. Photomicrograph. H&E. 200× magnification.
Inflammation was uncommon among the 40 infected fish, with encephalitis or myelitis observed in only 12 fish and meninxitis observed in 13. These were graded according to the scheme outlined in Spagnoli et al. (2015) [26]. Scores for both encephalitis/myelitis and meninxitis ranged from 0–3 on a scale of 0–3 for both inflammation categories. Encephalitis scores had a mean of 0.5 and a standard deviation of 0.9. Meninxitis scores had a mean of 0.5 and a standard deviation of 0.8. Four fish in the exposed tanks had grossly visible skeletal deformities. Of these, three were infected and one was uninfected. One fish in the control tank had a skeletal deformity but it was confirmed negative for infection by histopathology.
Over half of all fish in all exposure groups (control, exposed positive, and exposed negative) had some combination of biliary hyperplasia or hepatic granulomas (Fig 2). These lesions were negative for mycobacteria with Kinyoun’s acid fast stain, negative for P. neurophilia spores with the Luna stain, and negative for bile via Hall’s bile stain.
3.2.1 Habituation testing
Log habituation slopes are listed in table 1. When the log habituation slopes are compared to each other, statistical differences between the groups were as follows: Exposed positive versus control, significant (p = 0.04); exposed negative versus control, not significant (p=0.4); exposed positive versus exposed negative, not significant (p=0.3). This indicates that infected fish habituated significantly more slowly than control fish. There was no significant difference in log slope between infected and exposed negative fish. Slopes for control and exposed negative fish were quite similar and did not differ significantly. R2 values were low, indicating that the data were a poor fit for the linear model, however, the negative slope of habituation velocity was sufficient to support the use of T1–T10 post-tap velocity difference as a quantitative measure of habituation.
Table 1.
Slopes of log-transformed post-tap velocities
| Exposure Group | Slope of log transformed post-tap velocity |
p-value | r2 |
|---|---|---|---|
| Control | −0.105 | <0.0001 | 0.096 |
| Exposed Negative | −0.0815 | 0.0005 | 0.081 |
| Infected | −0.0502 | 0.0182 | 0.045 |
Slopes of log transformed post-tap velocities for all three exposure groups. All three slopes were negative and statistically significant, indicating that habituation occurred in all three groups.
Because all three exposure groups had negative velocity slopes on the log scale and because there was no significant difference between T1 post-tap velocities between groups, the reduction in post-tap velocity between tap 1 and tap 10 was used to quantify and compare the overall degree of habituation between groups. The reduction in post-tap velocity between tap 1 and tap 10 for the control and exposed negative groups did not differ significantly (8.193 and 7.45cm/sec, respectively; p=0.9). However, the reduction for exposed positive fish was 4.047 cm/sec, which was about half that of the exposed negative and control groups. This difference was statistically significant (p=0.03).
Evaluation of post-tap velocities between the three groups at specific, individual time points, including the initial tap and taps given at 18 and 50 minutes showed no statistical differences (linear mixed model, p>0.05). There were no significant effects attributable to parasite cluster number, severity of meningitis or encephalitis, hepatic lesions, or parasite cluster location (linear mixed model, p>0.05 for all parameters).
3.2.2 Extinction of the Habituation Response
The difference in mean post-tap velocity between the tap at one minute and the tap at 18 minutes (8 minutes after the last tap of the 10 tap habituation series) was significantly different only for exposed negative fish (Table 2). There were no significant differences in mean post-tap velocity between the tap at one minute and the tap at 60 minutes (50 minutes after the last tap of the 10 tap habituation series) for any group.
Table 2.
Habituation Extinction
| Group | Velocity T1-T18 | p-value | Velocity T1-T60 | p-value |
|---|---|---|---|---|
| Control | 5.86 | 0.06 | 0.25 | 0.9 |
| Exposed Negative | 8.63 | 0.01 | 2.37 | 0.4 |
| Infected | 2.93 | 0.3 | −0.73 | 0.7 |
Only exposed negative fish retained habituation at minute 18. No fish retained habituation at minute 60.
3.2.2 Variability
There were high levels of interfish variability within groups (Fig 3). However, startle velocity variability was significantly different between control, exposed positive and exposed negative groups (p=0.01). Controls (SD estimate = 5.633) were more variable than exposed positive fish (SD estimate =5.148), and the latter were more variable than exposed negative fish (SD estimate=5.0264). Whereas mean startle velocities did not differ based on sex, males had significantly greater variability than females (males SD estimate = 5.453, females SD estimate = 5.0854, p=0.03)
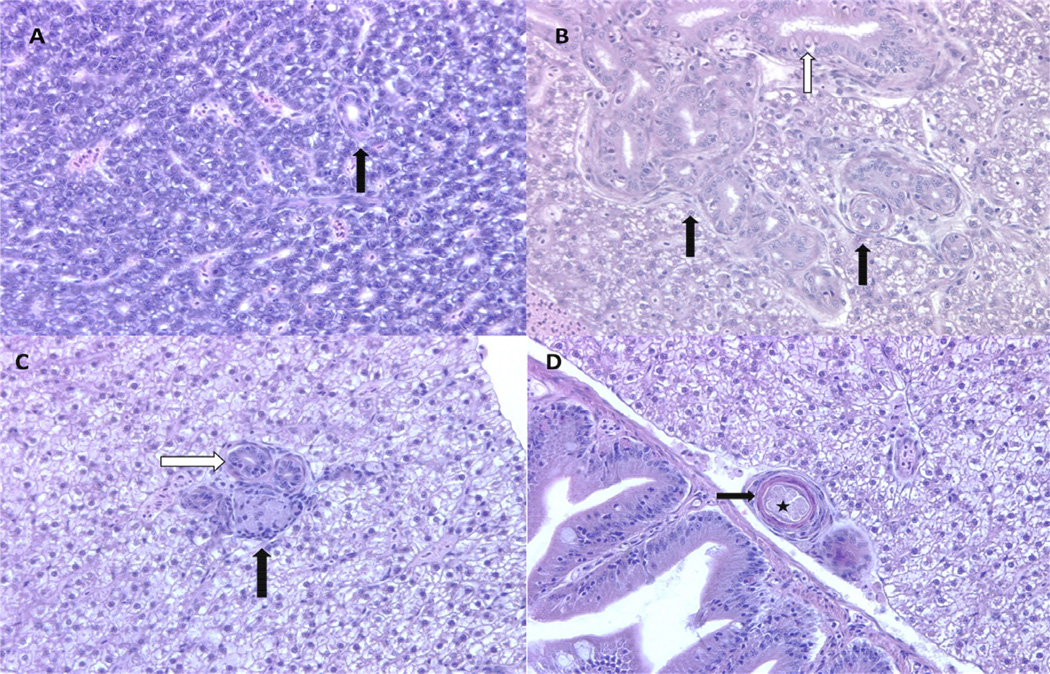
Figure 3. Biliary hyperplasia and granulomas.
A Less than half of the fish in all groups examined had normal-appearing livers. Normal zebrafish bile ducts (arrow) are lined by a single layer of low cuboidal epithelium. Photomicrograph. H&E. 400× magnification. B. In the most severe cases, biliary hyperplasia was characterized by well-differentiated, tortuous bile ducts of varying size with prominent basement membranes (black arrows). Ducts were lined by tall simple to pseudostratified columnar epithelial cells that were occasionally infiltrated by varying numbers of rodlet cells (white arrow). Photomicrograph. H&E. 400× magnification. C. The vast majority of visceral granulomas in these animals were observed in the liver. They were commonly observed in association with hyperplastic bile ducts (white arrow). Some granulomas were relatively poorly organized with a central area of flocculent, acellar material admixed with and partially lined by lymphocytes and macrophages (arrow). Photomicrograph. H&E. 400× magnification. D. Granulomas frequently had central acellular flocculent material (star) surrounded by what appeared to be lamellar keratin developed from stratified squamous epithelium (black arrow). Photomicrograph. H&E. 400× magnification.
3.3 Capture Avoidance
Within the exposure tank, infection was associated with the netting session in which a fish was caught (ANOVA, p=0.006). Although it was not possible to visually differentiate between infected and uninfected fish, infected fish were caught, on average, approximately four sessions later than exposed negative fish captured from the same tank (ANOVA, p<0.001).
4. Discussion
4.1 P. neurophilia infections in experimental animals
Approximately half of the larval fish in the exposure tank died during the exposure period. Because water quality values were similar to control tank values and because surviving exposed fish had no histologic lesions associated with poor water quality, the most likely explanation for the high mortality was transport stress combined with early exposure to parasites. Ramsey et al (2009) established that elevated cortisol levels increase parasite load in stressed fish [15]. Coupled with the age of the fish, this fact provides one possible explanation for the high mortality.
Of the several reports on P. neurophilia, only Ramsay et al. (2009) provided data on prevalence relating to time after exposure. The 56% infection rate in the exposure tank at 14 wk post-exposure was lower than expected given that Ramsay et al. (2009), who observed greater than 80% infection at 13 weeks post-exposure. Left for a long enough time, 100% infection rates within a tank are achievable [15], however the rate of spread through a population varies from tank to tank. Anecdotally, infection rates observed on routine examination of tanks in contaminated facilities range from 40–60% and in these cases, the time of initial contamination is always unknown. The possibility also exists that different infection rates may be caused by different strains of P. neurophilia or different strains of the host. In this case, it is possible that the high early mortality rates selected for more resistant fish, resulting in an overall lower infection rate and relatively light infections among infected fish.
Our study resulted in two control groups, a separate tank of unexposed fish and negative fish within the exposed tank. The presence of uninfected fish along with infected fish in the exposure tank provided us with an internal control group, avoiding the confounding factor of tank conditions between control and exposed groups. Nevertheless, we believe that the while in a separate tank, the unexposed fish were also an appropriate control to use in our analyses as they were from the same stock of fish and held under otherwise identical conditions.
4.2 Startle response habituation
The slope of the log habituation line for infected fish was significantly shallower than that of control fish. Although there was no statistically significant difference between habituation slopes for control and exposed negative fish, there was also no significant difference between exposed negative and exposed positive fish. This could have been due to the fact that exposed negative and infected fish had been reared in the same tank, making their behavioral phenotypes more similar than those of the fish in the control tank. Also, the R2 value of all groups was low, likely due to the high interfish variability. Even though the linear model was likely not a good fit for the data, the negative value of the slopes proves that there was an overall reduction in startle velocity during the habituation period and supports the use of the T1–T10 startle velocity reduction as an overall measure of habituation.
There were no statistically significant differences between post-tap velocities at any individual time point, including the first tap, so any increased reduction in startle velocity between tap 1 and tap 10 corresponds to an increased degree of habituation. The use of the overall reduction in startle response to quantify the habituation response is similar to methods used in some rodent studies [37,38]. The difference between velocities at tap 1 and tap 10 for infected fish was approximately half that of the control and exposed negative groups. This was statistically significant, indicating that infected fish had less of an overall habituation response than either control group. Moreover, differences for control and exposed negative groups did not differ significantly.
The overall reduced velocity for infected fish during the habituation period indicates that fish infected with P. neurophilia remain excitable despite repeated startling stimuli. A second possibility is that infected fish may simply have a higher baseline velocity than uninfected cohorts. Further research using an increased number of taps to discover a baseline velocity could determine whether the observation of overall reduced velocity was due to hyper-excitability or to an elevated baseline velocity in infected fish.
4.3 Extinction of the habituation response
Only exposed negative fish retained a degree of habituation at the 18 minute tap. Habituation was extinct in control and infected fish at the 18 minute tap, and in all groups at the 60 minute tap (Table 2). Potential reasons for this finding include exposed negative fish having enhanced recall or reduced anxiety compared to control and infected fish. Alternatively, exposed negative fish could have simply had a lower baseline velocity than control or infected fish, meaning it might take longer for their post-tap velocity to return to starting levels. Regardless of the mechanism underlying this finding, it provides further evidence for a difference in behavioral phenotypes between infected fish and uninfected tankmates as well as potentially different phenotypes between exposed negative fish and control fish.
4.4 Variability
The greatest variation was observed among control fish, followed by infected fish, followed by exposed negative fish. While it is difficult (and impossible from this experiment alone) to determine why these differences occurred, some features of zebrafish behavior might lend themselves to a possible explanation. Individual variation within populations of zebrafish has been well-documented as has the formation of intrashoal hierarchies, [39] with more aggressive fish dominating and chasing less aggressive fish. Interestingly, the variation in response to the tap test was greater for infected fish than for exposed negative fish held in the same tank. Again, we see a difference between behavioral responses of infected and uninfected fish in the same tank as well as differences between infected fish, uninfected fish, and controls.
The formation of dominance hierarchies between fish of different sexes is unlikely to be the cause of the reduced variation of females compared to males. Spence et al. (2008) found that sex is not associated with the assumption of dominant or submissive roles [40]. Also, even though size might contribute to the formation of dominance hierarchies, various studies have shown conflicting results with regards to whether size is positive or negatively associated with rank [40]. Interestingly, the reduced variability of females compared to males may be an intrinsic facet of female zebrafish behavior. Tran and Gerlai (2013) demonstrated that females had more consistent behavior patterns than males during both passive observation and an open field task [41]—this behavioral consistency could extend to habituation and the startle response.
4.5 Netting observations
The observation that infected fish tended to be caught in later sessions than uninfected fish from the same tank may be related to the results of the tap test. Fish that habituate poorly (have a smaller velocity reduction during habituation) may be particularly excitable and more apt to flee from a net. Alternatively, fish with a higher baseline velocity may escape netting for more sessions by virtue of their speed. While more research is needed to repeat the experiment using a pre-designed protocol, this particular experiment indicates that infected fish may be better at avoiding netting than uninfected fish. The difference in netting avoidance between infected and uninfected fish in the same tank provides more evidence for a difference in behavioral phenotypes between these groups.
4.6 Synthesis
The behavioral pattern of infected fish, highlighted by the reduction in startle velocity during habituation compared to controls and the enhanced netting avoidance, suggests that that infection is associated with a hyper-alert behavioral syndrome or an increased baseline swimming velocity. Further research is necessary to determine the factors underlying these observations, however, we have shown that P. neurophilia infection was associated with a behavioral phenotype distinct from those of control fish and uninfected fish reared in the same tank.
The most intriguing explanation for the distinct behavioral pattern is that P. neurophilia infection alters the behavior of infected fish. Because the observed behavioral differences involve the startle response, habituation, and motor function, the tropism of P. neurophilia for neuroanatomical sites connected to these behaviors makes a causative relationship eminently plausible [26, 28–35]. The complex behavioral consequences of neural parasitism are well-documented in numerous species, particularly in trophic organisms (parasites that require the consumption of the intermediate host by a predator) [42, 43]. A widely publicized example of a trophic parasite is the apicomplexan Toxoplasma gondii, which forms small lesions in its bradyzoite stage with almost no inflammation in chronic infections. Pertinent to our study, these stages infect the central nervous system similar to P. neurophilia. Despite the noninflammatory nature of most chronic T. gondii infections, there is ample evidence from rodent experiments that the parasite suppresses predator avoidance behaviors, presumably as an evolutionary tactic to facilitate consumption by the final feline host [44]. There is also precedent for behavioral effects of parasitism on fish: metacercariae of Diplostomum phoxini, a trophic parasite, in Eurasian minnows (Phoxinus phoxinus) infects the brain and may induce subtle personality changes (measured by exploratory boldness) in the host [45]. Although it is unclear from an evolutionary standpoint how, or even if, D. phoxini facilitates predation by the final host, the fact that a neural parasite in fish produces behavioral changes provides an interesting precedent to our own findings.
Another possible explanation for our observations is that pre-existing behavioral phenotypes may increase or decrease a fish’s susceptibility to infection rather than infection itself causing behavioral changes. Different behavioral syndromes between animals can be associated with different immune responses and susceptibility to disease [46]. Furthermore, behaviors such as increased egg and carcass consumption could increase the risk of infection, as stress and aggression have been suggested as the reason for higher infection rates among males compared to females [24].
If we further consider the idea of behavioral phenotypes influencing infection susceptibility, we may find a more subtle causative explanation for our observations. We observed high mortalities among naïve fish initially exposed to parasite-laden water. It is possible that the dead fish represented a third group of animals with a behavioral syndrome that made them particularly susceptible to fatal infections. If this was the case, then early infection could have selected for a population of adult fish with the behavioral patterns observed in our study. In this case, early P. neurophilia exposure could result in a population of adults with a particular set of behavioral traits even among uninfected fish. In this study, exposed negative fish had reduced variability and failed to abolish habituation at 18 minutes compared to controls. This distinct behavioral phenotype could be due to parasite-based selection or to interactions with their infected tankmates. Even if infection does not directly alter fish behavior, early infections present in contaminated facilities rearing their own fish could select for animals with altered behavioral phenotypes.
There were no statistically significant effects of PC number (a measure of parasite burden), PC location, encephalitis, or meninxitis on responses to the tap test. This could be explained by the relative lightness of infection patterns observed among fish in this study. Alternatively, the high mortality rate among young fish could have selected for a group of highly resistant adults, explaining the resulting combination of uninfected and mildly infected fish. A more intriguing explanation is that any P. neurophilia infection, no matter how mild, could produce similar effects across hosts due to molecular crosstalk with the parasite. Therefore, the parasite probably has more effects on the host than simply occupation of tissue spaces Toxoplasma gondii is an excellent example of this, as mentioned previously [44]. The finding that disease severity was not significantly associated with any differences in behavioral responses coincides with the findings of our retrospective study: PC number, inflammation severity, and PC location were not associated with clinical disease [26].
4.7 Startle response habituation in rodents: Using established models to improve zebrafish behavioral models
In order to improve the zebrafish as a powerful neurobehavioral model, we should look to established models as a guide for its development. The acoustic startle response has been well-characterized in rodents for decades. Although similar to the fast start response in zebrafish, startling auditory stimuli elicit a whole body flinch in rodents that does not result in a ‘run’ stage as we have quantified in our study [3,4,37,38]. Also, because rodents lack a lateral line, the perceptive aspect of their startle response is almost entirely auditory, whereas zebrafish also perceive pressure waves [5]. As we described in our study, habituation is considered a decrease of response magnitude in the face of repeated stimuli [37]. Reduced habituation inhibition and pre-pulse inhibition (the reduction of a startle response following exposure to a non-startling stimulus) are observed in human schizophrenia along with a number of other psychological disorders [37, 38, 47, 48] and for this reason, habituation inhibition and pre-pulse inhibition have been thoroughly studied in rodent models. Auditory startle response habituation inhibition has been documented in adenosine A2A receptor knockout mice, seratonin1B receptor knockout mice, and transgenic mice overexpressing corticotropin-releasing hormone among others [37, 38, 47, 48]. Rodent facilities providing animals for such sensitive studies generally use extensive biosecurity protocols, and rightly so: The development of rodent models for human behavior, particularly those utilizing genetic knockouts, is both time-consuming and costly. It is nearly unthinkable that researchers would intentionally use rodents with viral encephalitis or chronic T. gondii infections in the experiments mentioned above, and zebrafish investigators should be similarly reluctant to use fish infected with P. neurophilia in their own studies [49].
4.8 Potential impacts of P. neurophilia on neurobehavioral research
Whereas further research is needed to determine precisely how P. neurophilia induces behavioral changes or whether certain behavioral syndromes result in elevated infection susceptibility, our study shows that P. neurophilia is associated with a distinct behavioral phenotype in infected fish as well as in uninfected cohorts. If infection causes behavioral changes in a population, whether directly by molecular crosstalk with the animal’s brain, or indirectly by mortality-based selection for particular behavioral syndromes, we can hypothesize what types of studies might be influenced by its presence.
The test most likely to be influenced by P. neurophilia is the one performed in this study: the serial tap test for startle response habituation, as well as any study that involves habituation to the startle response. This test is widely used as an indicator of anxiety, fear, stress, and psychomotor response in zebrafish [3–6]. Habituation inhibition may also be particularly useful in the study of human PTSD [2]. If the zebrafish does become a model for PTSD, it will be vital for investigators to avoid P. neurophilia.
Although more research must be performed in order to determine precisely which facets of behavior may be influenced by P. neurophilia, the underlying traits potentially responsible for our findings could influence a wide range of neurobehavioral protocols if the association is causative. The high baseline velocity or hyper-alert behavioral phenotype observed in our infected fish may be an emergent property of several neurobehavioral components, the most likely of which are motor function, stress, fear, anxiety, and arousal [3–6]. These traits are involved in the vast majority of zebrafish behavioral studies and they are the basis for innumerable protocols including the conditioned alarm reaction, shuttle box avoidance, inhibitory avoidance, open field testing, novel tank testing, novel object exploration, scototaxis (preference for dark over light areas), and Schrecksreaktion (place avoidance) [5,6].
If P. neurophilia infection causes reliable behavioral changes in a population, its potential to confound research is augmented by its highly variable infection rates and uneven distribution of infection severity. Indeed, as with most other chronic parasite infections, P. neurophilia infections occur with a negative binomial distribution [26] in which only a few fish have heavy infections and most have light or no infecitons.
Consider a theoretical neurobehavioral experiment in a contaminated facility: If control and experimental groups had equal numbers of infected animals, the influence might be equal between the two groups. However, it is unlikely that all tanks in an affected facility would be contaminated. While no surveys have been performed to directly measure the number of infected tanks within a contaminated facility (since an unintentionally contaminated facility would be unlikely to agree to such a survey), consider that in our own experiment, the control tank was maintained in a room full of contaminated tanks without becoming contaminated itself. While special precautions were taken to ensure that contamination did not occur, most facilities undertake similar precautions to reduce transmission of infectious diseases between tanks [23]. Anecdotally, no facility contaminated with P. neurophilia has been observed with a 100% tank infection rate. If control and test fish in the theoretical neurobehavioral experiment are derived from two separate rearing tanks, which is especially likely in chronic exposure studies, it would be possible for one group to have infections but not the other, thus introducing an unintended bias. Furthermore, infection rates in contaminated tanks are rarely 100%. Even in this study, we achieved only a 56% infection rate after heavy exposure at an early age. Even if both tanks in the theoretical study were infected, it is unlikely that they would have the same rate of infection even if they were contaminated at exactly the same time with exactly the same dose of infectious material. If infection reliably produces a consistent behavioral phenotype, the varying infection rates between the two groups could adversely influence study data.
Future research should focus on the interactions between P. neurophilia infection and various experimental manipulations. Certain drugs, genetic knockouts, or toxicants could either enhance or reduce the effects of the parasite. In a similar vein, any alteration to a fish’s immune system, stress included, could be associated with an increase or decrease in infection rates. Until this research is performed, this study should act as a red flag for investigators to screen their fish for this potentially damaging parasite.
Conclusion
P. neurophilia is a common contaminating parasite of laboratory zebrafish that preferentially infects the central nervous system. Based on this study, infection is associated with altered startle response habituation and potentially enhanced netting evasion. While further research is necessary to definitively prove a direct or indirect causal relationship between P. neurophilia infection and altered behavioral phenotypes, our observations indicate that this is a strong possibility. Because zebrafish are used in a wide variety of sensitive behavioral studies that may share fundamental traits affected by P. neurophilia, the potential influence of infection on fish behavior along with unpredictable intertank infection rates makes it vitally important to screen fish prior to experimentation.
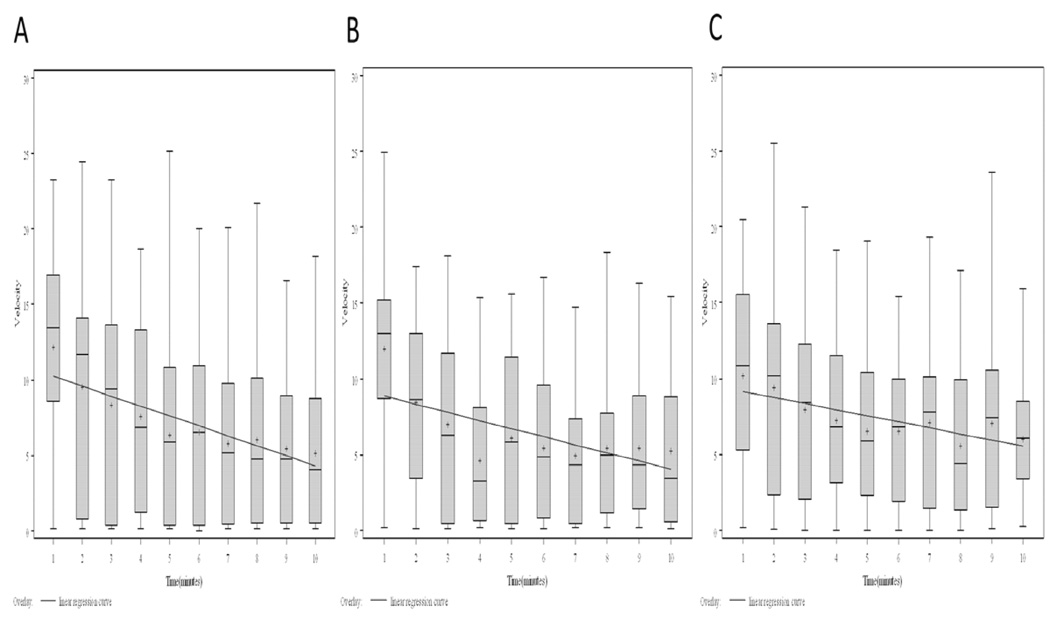
Figure 4.
Mean post-tap velocity (cm/sec) for control fish during habituation period (one tap per minute over ten minutes). Key: Box=Interquartile range, Whiskers=Range, Line=Median, Plus sign=Mean. Y-Axis: Tap/minute number. A. Control B. Exposed negative C. Infected
Highlights.
We compare Pseudoloma neurophilia-infected zebrafish with uninfected fish.
We examine startle response habituation and habituation extinction.
Infected fish have a behavioral phenotype distinct from uninfected fish.
P. neurophilia could influence neurobehavioral research.
Facilities must screen for P. neurophilia to avoid experimental biases.
Acknowledgements
This research was funded in part by NIH grants T32 RR023917: Training of veterinarians in aquatic animal research and 2R24OD010998-11 to Michael L. Kent. Thanks to Thomas Sharpton, Stevan J. Arnold, John Sproule, Emily Hatfield-Kirk, Rhea Hanselmann for editing and revisions. Thanks to Robert Tanguay and SARL for use of their facilities, equipment, and software. Thanks to Andrea Knecht for Ethovision training.
Abbreviations
- PC
Parasite cluster
- MA
Mauthner axon
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Grunwald D, Eisen J. Headwaters of the Zebrafish—Emergence of a new model vertebrate. Nat Rev: Genetics. 2002;3:717–724. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- 2.Caramillo EM, Khan KM, Collier AD, Echevarria DJ. Modeling PTSD in the zebrafish: are we there yet? Behav Brain Res. 2015;276:151–160. doi: 10.1016/j.bbr.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Chanin S, Fryar C, Varga D, Raymond J, Kyzar E, Enriquez J, Bagawandoss S, Gaikwad S, Roth A, Pham M, Zapolsky I, Bruce I, Hester J, Green J, Desmond D, Stewart AM, Kalueff A. Assessing startle responses and their habituation in adult zebrafish. In: Kalueff AV, Stewart AM, editors. Zebrafish protocols for neurobehavioral research. New York: Springer; 2012. pp. 287–300. [Google Scholar]
- 4.Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: Comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicol Teratol. 2010;32:99–108. doi: 10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalueff AV, Stewart AM, editors. Zebrafish Protocols for neurobehavioral research. New York: Springer; 2012. [Google Scholar]
- 6.Maximino C, de Brito TM, da Silva Batista AW, Herculano AM, Morato S, Gouveia A., Jr Measuring anxiety in zebrafish: a critical review. Behav Brain Res. 2010;214:157–171. doi: 10.1016/j.bbr.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Morris JA. Zebrafish: a model system to examine the neurodevelopmental basis of schizophrenia. Prog Brain Res. 2009;179:97–106. doi: 10.1016/S0079-6123(09)17911-6. [DOI] [PubMed] [Google Scholar]
- 8.Pittman JT, Lott CS. Startle response memory and hippocampal changes in adult zebrafish pharmacologically-induced to exhibit anxiety/depression-like behaviors. Physiol Behav. 2014;123:174–179. doi: 10.1016/j.physbeh.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Stewart A, Gaikwad S, Kyzar E, Green J, Roth A, Kalueff AV. Modeling anxiety using adult zebrafish: A conceptual review. Neuropharmacol. 2012;62:135–143. doi: 10.1016/j.neuropharm.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart A, Wong K, Cachat J, Gaikwad S, Kyzar E, Wu N, Hart P, Piet V, Utterback E, Elegante M, Tien D, Kalueff AV. Zebrafish models to study drug abuse-related phenotypes. Rev Neurosci. 2011;22:95–105. doi: 10.1515/RNS.2011.011. [DOI] [PubMed] [Google Scholar]
- 11.Stewart AM, Nguyen M, Wong K, Poudel KW, Kalueff AV. Developing zebrafish models of autism spectrum disorder (ASD) Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:27–36. doi: 10.1016/j.pnpbp.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Willemsen R, Hasselaar W, van der Linde H, Bonifati V. Zebrafish as a new model organism for Parkinson’s disease. Proc Meas Behav. 2008:50–51. [Google Scholar]
- 13.Kent ML, Spitsbergen JM, Matthews JM, Fournie JW, Murray KN, Westerfield M. Diseases of Zebrafish in Research facilities. ZIRC Health Services Zebrafish Disease Manual. 2012 Online only: http://zebrafish.org/zirc/health/diseaseManual.php. [Google Scholar]
- 14.Kent ML, Harper C, Wolf JC. Documented and potential research impacts of subclinical diseases in zebrafish. ILAR J. 2012;53:126–134. doi: 10.1093/ilar.53.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramsay JM, Watral V, Schreck CB, Kent ML. Pseudoloma neurophilia infections in zebrafish Danio rerio: effects of stress on survival, growth, and reproduction. Dis Aquat Organ. 2009;88:69–84. doi: 10.3354/dao02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders GE. Zebrafish housing, husbandry, health and care: IACUC considerations. ILAR J. 2012;53:205–207. doi: 10.1093/ilar.53.2.205. [DOI] [PubMed] [Google Scholar]
- 17.Sanders JS, Watral V, Kent ML. Microsporidiosis in zebrafish research facilities colonies. ILAR J. 2012;53:106–113. doi: 10.1093/ilar.53.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cali A, Kent M, Sanders J, Pau C, Takvorian PM. Development, ultrastructural pathology, and taxonomic revision of the Microsporidial genus, Pseudoloma and its type species Pseudoloma neurophilia, in skeletal muscle and nervous tissue of experimentally infected zebrafish Danio rerio. J Eukaryot Microbiol. 2012;59:40–48. doi: 10.1111/j.1550-7408.2011.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kent ML, Shaw RW, Sanders J. Fish Micorsporidia. In: Weiss LM, Becnel JJ, editors. Microsporidia: Pathogens of Opportunity. Chapter 20. Wiley Enterprise; 2014. pp. 493–520. [Google Scholar]
- 20.Sanders J, Watral V, Clarkson K, Kent ML. Verification of intraovum transmission of a microsporidium of vertebrates: Pseudoloma neurophilia infecting the zebrafish, Danio rerio. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0076064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders J, Peterson T, Kent M. Early development and tissue distribution of Pseudoloma neurophilia in the zebrafish, Danio rerio. J Eukaryot Microbiol. 2014;61:238–246. doi: 10.1111/jeu.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson J, Watral V, Schwindt A, Kent ML. Spores of two fish Microsporidia (Pseudoloma neurophilia and Glugea anomola) are highly resistant to chlorine. Dis. Aquat. Org. 2007;76:205–214. doi: 10.3354/dao076205. [DOI] [PubMed] [Google Scholar]
- 23.Murray KN, Dreska M, Nasiadka A, Rinne M, Matthews JL, Carmichael C, Bauer J, Varga ZM, Westerfield M. Transmission, diagnosis, and recommendations for control of Pseudoloma neurophilia infections in laboratory zebrafish (Danio rerio) facilities. Comp Med. 2011;61:322–329. [PMC free article] [PubMed] [Google Scholar]
- 24.Chow FW, Xue L, Kent ML. Retrospective study of the prevalence of Pseudoloma neurophilia shows male gender bias. Zebrafish. doi: 10.1111/jfd.12328. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kent ML, Buchner C, Watral VG, Sanders JL, Lad J, Peterson TS, Tanguay RL. Development and maintenance of a specific pathogen free (SPF) zebrafish research facility for Pseudoloma neurophilia. Dis Aquat Organ. 2011;95:73–79. doi: 10.3354/dao02333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spagnoli S, Xue L, Murray KM, Chow F, Kent ML. Pseudoloma neurophilia: A retrospective and descriptive study of nervous system and muscle infections, with new implications for pathogenesis and behavioral phenotypes. Zebrafish. 2015;12:189–201. doi: 10.1089/zeb.2014.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kent ML, Bishop-Stewart JK. Transmission and tissue distribution of Pseudoloma neurophilia (Microsporidia) of zebrafish Danio rerio. J Fish Dis. 2003;26:1–4. doi: 10.1046/j.1365-2761.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- 28.Wulliman MF, Barbara R, Reichert H. Neuroanatomy of the Zebrafish Brain: A Topological Atlas. Boston: Birkhaüser; 1996. [Google Scholar]
- 29.Eaton RC, Bombardieri RA, Meyer DL. The Mauthner-initiated startle response in teleost fish. J Exp Biol. 1977;66:65–81. doi: 10.1242/jeb.66.1.65. [DOI] [PubMed] [Google Scholar]
- 30.Hatta K, Korn H. Physiological properties of the Mauthner system in the adult zebrafish. J Comp Neurol. 1998;395:493–509. [PubMed] [Google Scholar]
- 31.Liu DW, Westerfield M. Function of identified motoneurones and coordination of primary and secondary motor systems during zebrafish swimming. J Physiol. 1988;403:73–89. doi: 10.1113/jphysiol.1988.sp017239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu KS, Fetcho JR. Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron. 1999;23:325–335. doi: 10.1016/s0896-6273(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 33.Zottoli SJ, Newma BC, Rieff HI, Winters DC. Decrease in occurrence of fast startle responses after selective Mauthner cell ablation in goldfish (Carassius auratus) J Comp Physiol A. 1999;184:207–218. doi: 10.1007/s003590050319. [DOI] [PubMed] [Google Scholar]
- 34.Mathuru AS, Jesuthusasan S. The medial habenula as a regulator of anxiety in adult zebrafish. Front Neural Circuits. 2013;7:1–3. doi: 10.3389/fncir.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Malley DM, Kao Y-H, Fetcho JR. Imaging the functional organization of zebrafish hindbrain segments during escape behaviors. Neuron. 1996;17:1145–1155. doi: 10.1016/s0896-6273(00)80246-9. [DOI] [PubMed] [Google Scholar]
- 36.Guidelines for use of Zebrafish in the NIH intramural research program. NIH; 2013. Online only. http://oacu.od.nih.gov/ARAC/documents/Zebrafish.pdf. [Google Scholar]
- 37.Valsamis B, Schmid S. Habituation and prepulse inhibition of acoustic startle in rodents. J Vis Exp. 2011;55 doi: 10.3791/3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dulawa SC, Hen R, Scaerce-Levie K, Geyer MA. Serotonin1B receptor modulation of startle reactivity, habituation, and prepulse inhibition in wild-type serotonin1B knockout mice. Psychopharm. 1997;132:125–134. doi: 10.1007/s002130050328. [DOI] [PubMed] [Google Scholar]
- 39.Toms CN, Echevarria DJ. Back to basics: Searching for a comprehensive framework for exploring individual differences in zebrafish (Danio rerio) behavior. Zebrafish. 2014;11:325–340. doi: 10.1089/zeb.2013.0952. [DOI] [PubMed] [Google Scholar]
- 40.Spence R, Gerlach G, Lawrence C, Smith C. The behavior and ecology of the zebrafish, Danio rerio. Biol Rev Camb Philos Soc. 2008;83:13–34. doi: 10.1111/j.1469-185X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- 41.Tran S, Gerlai R. Individual differences in activity levels in zebrafish (Danio rerio) Behav Brain Res. 2013;257:224–229. doi: 10.1016/j.bbr.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barber I, Hoare D, Krause J. Effects of parasites on fish behavior: a review and evolutionary perspective. Rev Fish Biol and Fisheries. 2000;10:131–165. [Google Scholar]
- 43.Thomas F, Adamo S, Moore J. Parasitic manipulation: where are we and where should we go? Behav Processes. 2005;68:185–199. doi: 10.1016/j.beproc.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Gatkowska J, Wieczorek M, Dziadek B, Dzitko K, Dlugonska H. Behavioral changes in mice caused by Toxoplasma gondii invasion of brain. Parasit Res. 2012;111:53–58. doi: 10.1007/s00436-011-2800-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kekalainen J, Lai Y-T, Vainikka A, Sirkka I, Kortet R. Do brain parasites alter host personality?— Experimental study in minnows. Behav Ecol Sociobiol. 2014;68:197–204. [Google Scholar]
- 46.Koolhaas JM. Coping style and immunity in animals: making sense of individual variation. Brain Behav Immun. 2008;22:662–667. doi: 10.1016/j.bbi.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Dirks A, Groenink L, Schipholt MI, van der Gugten J, Hijzen TH, Geyer MA, Oliver B. Reduced startle reactivity and plasticity in transgenic mice overexpressing corticotrophin-releasing hormone. Soc Bio Psych. 2002;51:583–590. doi: 10.1016/s0006-3223(01)01323-3. [DOI] [PubMed] [Google Scholar]
- 48.Wang JH, Short J, Ledent C, Lawrence AJ, van den Buuse M. Reduced startle habituation and prepulse inhibition in mice lacking the adenosine A2A receptor. Behav Brain Res. 2003;143:201–207. doi: 10.1016/s0166-4328(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 49.Percy DH, Barthold SW, editors. Pathology of laboratory rodents and rabbits. third edition. Blackwell, Iowa: 2007. [Google Scholar]