Abstract
Objective
This systematic review describes effects of body temperature alterations defined as fever, controlled normothermia, and spontaneous or induced hypothermia on outcome following traumatic brain injury (TBI) in adults.
Data sources
A search was conducted using PubMed, Cochrane Library database, CINAHL, EMBASE, and ISI Web of Science in July 2013 with no back date restriction except for induced hypothermia (2009).
Study selection
Of 1366 titles identified, 712 were reviewed. Sixteen articles met inclusion criteria: Randomized Controlled Trials (RCT) in hypothermia since 2009 (last Cochrane review) or cohort studies of temperature in TBI; measure core and/or brain temperature; neurologic outcome reporting; primarily adult patients, and English language publications. Exclusion criteria: majority of patients were non-TBI, primarily pediatric patients, case reports, or lab/animal studies.
Data synthesis
The majority of studies found that fever avoidance resulted in positive outcomes including: decreased intensive care unit length of stay, mortality; and incidence of hypertension, elevated intracranial pressure, and tachycardia. Hypothermia on admission correlated with poor outcomes. Controlled normothermia improved surrogate outcomes. Prophylactic induced hypothermia is not supported by the available evidence from RCT.
Conclusion
Setting a goal of normothermia, avoiding fever, and aggressively treating fever may be most important after TBI. Further research is needed to: characterize the magnitude and duration of temperature alteration after TBI; determine if temperature alteration influences or predicts neurologic outcome; determine if rate of temperature change influences or predicts neurologic outcome; and compare controlled normothermia versus standard practice or hypothermia.
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability, contributing to one third of all injury-related deaths in the United States (U.S.) (Faul, Xu, Wald, & Coronado, 2010). The annual economic burden of TBI in the U.S. has been estimated to be $4.5 billion in direct expenses for hospital care, extended care, and other medical services (Barker-Collo & Feigin, 2009). An additional $20.6 billion in injury-related disability and loss of work and $12.7 billion in lost income from premature death are attributed to TBI in the U.S. (Barker-Collo & Feigin, 2009). Poor outcomes from the primary injury and preventable secondary brain injuries result in significant costs to individuals, families, and society. Published guidelines provide limited evidence from interventions intended to reduce secondary insult. One of the more widely studied strategies has been targeted temperature management, which involves the identification of the desired patient temperature with interventions or treatments provided in order to achieve goals. Targeted temperature management (TTM) may occur in the form of fever reduction, controlled normothermia (NT), or induced hypothermia (HT). Prior studies and reviews have focused on these individual forms of targeted temperature management, but none have looked at this body of literature and synthesized the findings regarding the broader range of temperature and outcome in TBI. Disparate findings regarding the effect of temperature alterations have resulted in a lack of clear and robust evidence to guide temperature management in TBI.
Fever
Fever, generally defined as elevation of core body temperature above normal body temperature (37° centigrade [C]), has been identified as a mechanism of secondary insult that can exacerbate primary TBI through multiple cellular mechanisms (Childs et al., 2006; Thompson, Pinto-Martin, & Bullock, 2003). Healthy human brains tolerate increases in metabolism due to fever; however the injured brain does not. Fever exposure has resulted in an increase in ischemic injury and infarct in brain injury as a result of fever exposure, but the same fever exposure in non-injured brain did not result in such findings – nor demonstrate any impact on the integrity of neuronal tissue (1992). A central reason for this damage may be related to a 7 – 13% increase in cerebral metabolism for each increase of 1° C in core body temperature (Thompson et al., 2003; Wong, 2000). To make matters worse, the threshold for ischemia in the injured brain is lower than that of the normal brain, widening the mismatch between cerebral blood flow and metabolic demand (Schroder, Muizelaar, Kuta, & Choi, 1996). Thus, mechanisms to minimize cerebral metabolic demand have been extensively studied with the goal of avoiding or minimizing the extent of secondary insult. Cerebral insults beyond the primary injury have been associated with longer intensive care unit (ICU) and hospital stays, as well as reduce survival and quality of life after injury (Jones et al., 1994; Stocchetti et al., 2002).
Controlled normothermia
Controlled normothermia (NT) is a form of targeted temperature management. The Guidelines for the Management of Severe Traumatic Brain Injury suggest NT for TBI patients (2007). Aberrance of temperature from normal range (fever or HT) is associated with more deaths and poorer neurologic outcomes (Childs et al., 2006; Sacho, Vail, Rainey, King, & Childs, 2010).
Hypothermia on admission
Hypothermia on admission has been found to correlate with poor outcomes in both general trauma literature (Jeremitsky, Omert, Dunham, Protetch, & Rodriguez, 2003; Steinemann, Shackford, & Davis, 1990) and specifically in TBI (Jurkovich, Greiser, Luterman, & Curreri, 1987). This may be related to the established association between HT, coagulopathy, and acidosis. Poor outcomes associated with HT might simply reflect a higher severity of brain tissue injury (e.g., more extensive injury to the hypothalamus) or more severe blood loss related to systemic injuries.
Induced hypothermia
Interest in the effect of temperature on outcome in TBI led to randomized controlled trials (RCTs) testing HT in TBI. Prophylactic induced mild to moderate HT (32 – 35° C), has been studied extensively in ischemic neurologic injury and has been found to be neuroprotective via decrease in excitatory amino acid release, metabolism suppression, and other actions (Adelson, 2009; Adelson et al., 2005; Bayir et al., 2009; G. L. Clifton, 1995b; Fox et al., 2010; J. Y. Jiang, 2009; Shann, 2003). Multiple pilot studies of HT in TBI have resulted in improved outcomes and decreased mortality (G. L. Clifton, 1995a; Marion et al., 1997; Shiozaki et al., 1993). Several single-center trials have demonstrated that moderate HT compared to normothermia led to improvement in survival and outcome (G. L. Clifton et al., 1993; Inamasu et al., 2006; J. Jiang, Yu, & Zhu, 2000). Contrary to those promising pilot results (G. L. Clifton et al., 1993), large multicenter clinical trials (G L Clifton et al., 2001; G. L. Clifton et al., 2011) failed to demonstrate improvement in TBI outcomes with induced HT. While a pilot study in ischemic stroke (Krieger et al., 2001) and an RCT in hypoxic ischemic encephalopathy in newborns (Shankaran et al., 2005) have found benefit from induced moderate HT (Krieger et al., 2001; Shankaran et al., 2005), studies investigating HT in TBI have not consistently demonstrated improved patient outcomes, identified an ideal duration of treatment, nor established an optimal body temperature goal. Recent investigation of 33° C versus 36° C in cardiac arrest patients has demonstrated no difference in neurologic outcome or mortality between groups (Nielsen et al., 2013).
A 2009 Cochrane review (Sydenham, Roberts, & Alderson) included RCTs of HT versus control in patients with blunt TBI. Exclusion of low quality studies, defined as non RCTs, lacking good allocation concealment, high risk of bias, or unclear methods resulted in no significant differences in survival, neurologic outcome, or incidence of pneumonia between the HT groups versus control patients (Sydenham et al., 2009). Sadaka and Veremakis (2012) conducted a systematic review of induced HT (32 – 34° C) specific to management of elevated ICP (> 20 mm Hg) in severe TBI (GCS ≤ 8). The review included 13 RCTs and five observational studies and concluded that induced HT should be included as an option to control ICP. Fox and colleagues (Fox et al., 2010) performed a systematic review of induced HT in TBI, dividing studies into two categories: those with cooling protocols for a short, predetermined period (e.g., 24 – 48 hours) and those that cool for > 48 hours and/or terminate based on normalization of ICP. The review supported the use of early prophylactic induced mild-to-moderate HT in patients with severe TBI with a goal-directed cooling protocol in which cooling was maintained for ≥ 72 hours and/or until normalization of ICP for at least 24 hours was achieved (see Supplementary Table 1).
Purpose
Despite lack of consistent robust evidence, TTM has been a fundamental element of patient care after TBI. The diverse approaches to temperature management after TBI and lack of well-controlled clinical trials of interventions have hindered the development of evidenced-based treatment guidelines. Prior publications have reviewed fever in TBI or in general neurocritical care or induced hypothermia in TBI or a variety of populations. The purpose of this systematic review of the literature is to describe the effects of body temperature alterations defined as fever, controlled NT, HT on admission, or spontaneous or induced HT on outcome following TBI in adults.
Methods
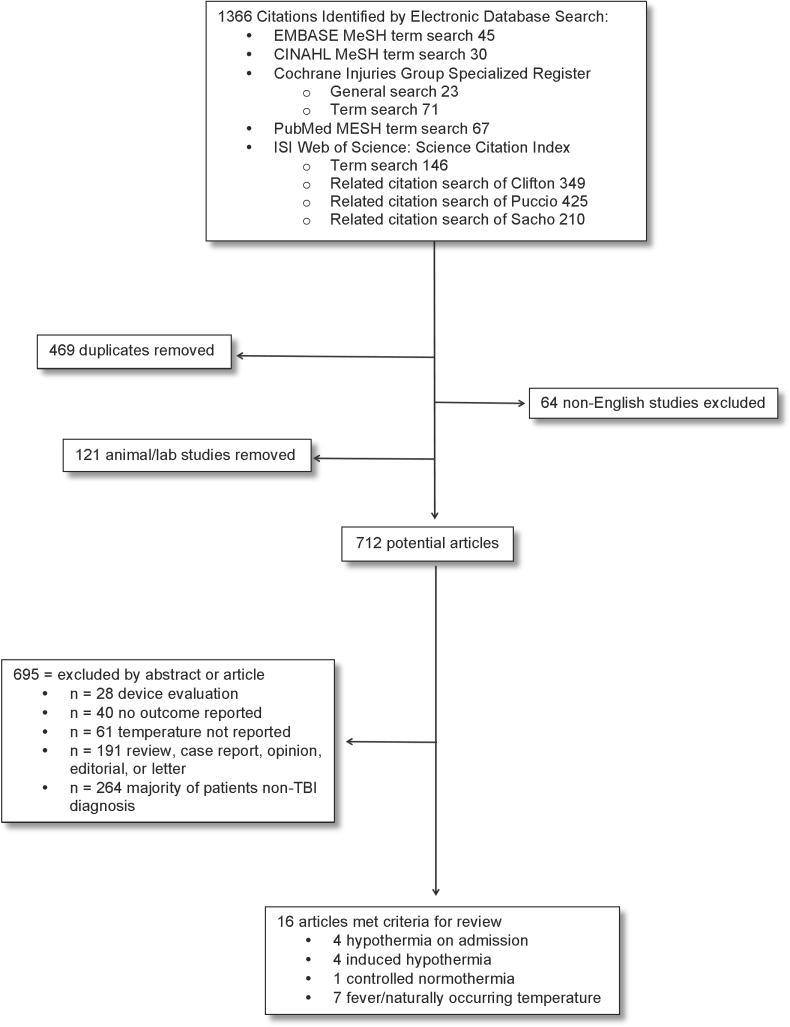
A search was conducted using PubMed, the Cochrane Library database, Cumulative Index to Nursing and Allied Health Literature (CINAHL), EMBASE, and ISI Web of Science. The search was conducted in July 2013 with no back date restriction except for induced HT RCTs (2009 back date, date of last Cochrane review). Of a total of 1366 titles identified, 712 were reviewed (see Figure 1). Sixteen articles met the following criteria: 1) either RCTs in HT made available since 2009 or cohort studies of temperature (‘naturally occurring temperature’, fever, NT, HT on admission, or induced HT) in TBI; 2) measure of core and/or brain temperature (BT); 3) reports of neurologic outcome measures, or for the only NT study, outcome surrogate (e.g., intracranial hypertension burden); 4) primarily adult patients, and 5) English language publications. Exclusion criteria were majority of patients with non-TBI diagnosis, primarily pediatric patients, case reports, or lab/animal studies.
Figure 1.
Flowchart of Search & Selection Strategy
Results
Cohort characteristics
Patients were primarily adults with blunt-force moderate or severe TBI. Some observational cohorts included lower severity of injury. Consistent with epidemiologic reporting of TBI (Faul et al., 2010), males were more prevalent in studies, with some studies including a disproportionate number of males. Due to the heterogeneity of studies reviewed, a brief summary of each study is provided (See Tables 1 – 4). Although methodologically diverse, the studies included reflect the most rigorous and recent studies considering the spectrum of temperature variations after TBI.
Table 1.
Naturally occurring temperature/fever in TBI
| Author | Study Design & Participants | Comparisons/Measures | Outcomes | Main Findings |
|---|---|---|---|---|
| Childs et al. (2006) | n = 36 (32M/4F) Median age 31 (18 – 70) years Moderate & severe TBI |
BT ICU admission temp 48-hours post-injury temp |
3-month mortality 3-month GOS |
|
| Elf et al. (2008) | n = 53 (42M/11F; 79.2% M) Mean age 42.3 (16 – 75 years) Severe TBI Patients with ≥ 54 hours of valid monitoring measures in the first 120 hours post-TBI |
Bladder temp Mean temp over 5 days post-injury: HT: temp < 36° C (adjusted to 36.5° C for analysis between groups) NT: temp 36.5 – 38° C Fever: temp > 38° C ICP, CPP, SBP, MAP & HR |
6-month GOS | 44 experienced fever (> 38° C) and 29 experienced HT (< 36° C) Incidence of fever:
Outcomes:
|
| Geffroy et al. (2004) | n = 101 (83M/18F) Median age 33 (18 – 51) years Severe TBI Early fever = 44 (42M/2F; 82.2% M) No fever = 57 (41M/16F) |
Tympanic temp Early fever = T > 38.5° C during the first 2 days after admission |
6-month GOS | Predictors of early fever occurrence (univariate analysis):
Predictors of no occurrence of early fever:
Strong predictors of early fever (multivariate analysis):
Those with early fever were:
|
| Li and Jiang (2012) | n = 7145 (5427M/1718F; 76% M) Age 1 – 92 years (75.3% adult) Mild TBI (4297) Moderate TBI (1222) Severe TBI (1626) |
Axillary body temp Temp magnitudes
Duration: # of fever days (based on highest temp for 2 consecutive hrs in that day) for first 3 days after injury |
3-month mortality 3-month dichotomized GOS |
Dichotomized GOS
Mortality
|
| Sacho et al. (2010) | n = 67 (52M/15F; 77.6% M) Median age 32 years Severe TBI Admission to ICU |
BT 5 days post injury
Fever = > 39° C BT Details of temp profile not reported |
30-day mortality Dichotomized 3-month GOS-E |
|
| Stocchetti et al. (2002) | n = 110 (93M/17F; 84.5% M) Median age 34 (14 – 83) years Mild to severe TBI (median GCS 7) Admission to ICU |
Core body temp Axillary temp > 38.0° C or core temp > 38.4° C in first 7 days post-injury Classified as number of days at least one fever was detected |
6-month GOS ICU length of stay Elevated ICP & CPP Effects of antipyretic tx on
|
Severe TBI
|
| Yamamoto et al. (2002) | n = 84 Severe TBI NT (49 total; 17 [15M/2F] with barbiturate therapy had GOS assessed) Mild HT (35 total; 22 [16M/6F] with complete data) |
Identify factors predictive of outcome in order to identify indications for HT Prediction modeling for GOOD vs. POOR outcome Mild HT ([BT 33 – 35° C] for 36 – 168 hrs) with rewarm rate ≤ 0.5° C/12 hrs |
Dichotomized 3-month GOS
|
HT
|
Note. AIS-H is abbreviated injury score of the head region, CI is confidence interval, CPP is cerebral perfusion pressure, DI is diabetes insipidus, F is female, GCS is Glasgow Coma Scale, GOS is Glasgow Outcome Scale, GOS-E is extended Glasgow Outcome Scale, HF is high fever, mild TBI is GCS 13 – 15, HR is heart rate, hrs is hours, HT is hypothermia, HTN is hypertension, ICP is intracranial pressure, IICP is increased intracranial pressure, LOS is length of stay, M is male, MAP is mean arterial pressure, MiF is mild fever, moderate TBI is GCS 9 – 12, MoF is moderate fever, NT is normothermia, OR is odds ratio, SBIF is secondary brain injury factors, SBP is systolic blood pressure, severe TBI is GCS 3 – 8, TBI is traumatic brain injury, temp is temperature, tx is treatment, WBC is white blood cell count.
Table 4.
Induced Hypothermia in TBI since 2009
| Author | Study Design & Participants | Interventions/Measures | Outcomes | Main Findings |
|---|---|---|---|---|
| G. L. Clifton et al. (2011) | Initial n = 232 n = 97 ultimately included in entire study (no gender reporting)
Age 16 – 45 years (mean 26 HT, 31 NT) Severe TBI |
Bladder temp 119 cooled to 35° C within 2.6 ± 1.2 hrs of injury
NT group temps > 38° C tx with acetaminophen and cooling blankets |
6 month mortality Dichotomized 6-month GOS |
|
| G. L. Clifton et al. (2012) | NABIS:H I (392 patients) HT: 33° C x 48 hours within 8.4 ± 3 after injury NABIS:H II (97 patients) HT: 35° C x 48 hours within 2.6 ± 1.2 hours and 33° C within 4.4 ± 1.5 hours after injury Subgroup analysis of those requiring surgery (craniotomy for hematoma evacuation) |
Bladder temp Cooling as noted for HT NT group temps > 38° C treated with acetaminophen and cooling blankets Rewarming rate of HT 0.5° C/2 hrs |
Dichotomized 6-month GOS | Poor outcome (dichotomized GOS) in NABIS:H II in 5/15 HT and in 9/13 NT (RR 0.44, 95% CI 0.22 – 0.88; p = 0.02) In NABIS: H I, poor outcome in:
Meta-analysis of pts achieving 35°C ≤ 1.5 hrs of surgery (n=46): significantly decreased rate of poor outcomes (41%) vs. 94 HT pts who did not reach 35° C ≤ 1.5 hrs and NT pts (62%, p = 0.009) |
| Tokutomi et al. (2009) | n = 61 (47M/14F; 77% M)
Age 15 – 69 years
GCS ≤5 (range 3–5; very severe TBI) |
Rectal temp Non-randomized. Tx goal changed from 33° C to 35° C in January 2000. Matched cohorts. All cooled to respective goal temp after ICU admission, then slowly rewarmed after 48 – 72 hours of HT if ICP < 20 mm Hg. If ICP remained > 20 mm Hg or rose during rewarming, continued mild HT up to 48 additional hrs. Rewarm of HT reported as “slow” but rate not reported |
6-month GOS 6-month mortality |
|
| Zhao et al. (2011) | n = 81 (59M/22F)
Age
Severe TBI |
Core (rectal) temp HT cooling blanket set to 33° C for 72 hrs, spontaneous rewarming in room temp (rate not reported) NT maintained at 37° C |
Arterial glucose Lactic acid Dichotomized 3-month GOS
|
|
Note. AIS-H is abbreviated injury score of the head region, CI is confidence interval, CPP is cerebral perfusion pressure, F is female, GCS is Glasgow Coma Scale, GOS is Glasgow Outcome Scale, hrs is hours, HT is hypothermia, ICH is intracerebral hematoma, ICP is intracranial pressure, IICP is increased intracranial pressure, ICU is intensive care unit, LOS is length of stay, M is male, MAP is mean arterial pressure, NABIS:H is National Acute Brain Injury Study: Hypothermia, NT is normothermia, pts is patients, RR is relative risk, severe TBI is GCS 3 – 8, TBI is traumatic brain injury, temp is temperature, tx is treatment.
Quality of studies
Volume of studies to review
According to criteria for Cochrane review (RCT of HT to a maximum of 35° C for ≥ 12 consecutive hours versus control in patients with any closed TBI requiring hospitalization), lower quality studies (poor or unclear allocation concealment) of HT were excluded. Also, studies in which surrogate measures of outcome were used (measures other than GOS or GOS-E) were excluded. Surrogate measures may not consistently correlate with neurologic outcome, so this would also likely confound recommendations. The exception from this is the singular controlled normothermia study (Puccio et al., 2009) that used surrogate measures of outcome that was selected for inclusion, as it is the only study of controlled normothermia in TBI.
Measurement issues
Studies used a variety of temperature sources (bladder, brain, arterial, axillary, rectal). This is one limitation of studies using existing data. Childs convened a consensus group regarding temperature and TBI and advised that BT should be used, as this is the organ of interest (2010). However, studies using BT monitoring to guide management have a significant delay in temperature measurement and interventions. Childs’ initial BT measurement occurred 4 – 41 hours after admission with a mean delay of 10.5 hours. Sacho’s initial BT measurement occurred 6 – 32 hours after admission with a mean time of eleven hours (Childs et al., 2006; Sacho et al., 2010).
While Puccio and colleagues considered secondary insult burden by identifying the percentage of time outside of a normal threshold (intracranial hypertension burden [percent of time, in minutes, for ICP measurements > 25 mm Hg calculated for the initial five days of the monitoring period]), they did not account for the degree (or extent) of difference between the measured temperature value and the threshold temperature value. There may be some effect related to the magnitude of the difference between those two values. This is the same limitation in studies that used mean temperature values or stratified patients according to temperature groups (NT, mild fever or high fever). Consequently, limiting statistical power and internal validity of the findings.
Outcome assessment
The outcome measure most commonly reported was the five-point GOS score at three or six months. The one controlled normothermia study considered a surrogate measure (ICP burden) rather than neurologic outcome. Most studies collapsed the five-point GOS ordinal scale into a binary variable of ‘favorable’ (moderate disability; good recovery) versus ‘unfavorable’ (death; vegetative state; severe disability) or ‘survival’ versus ‘mortality’ outcome (McHugh et al., 2007).
Findings
Naturally occurring temperature
While many studies focused on specific temperatures within a sample, a few observed ‘naturally occurring’ patient temperatures which include fever, NT, and HT during varying time periods after injury (Childs et al., 2006; Elf, Nilsson, Ronne-Engstrom, Howells, & Enblad, 2008; Jeremitsky et al., 2003; Sacho et al., 2010; Yamamoto, Mori, & Maeda, 2002). Childs examined BT and outcomes in 36 patients with moderate to severe TBI (2006) in order to explore the relationship between initial and 48-hour post-injury mean BT and three-month mortality. Initial BT measured shortly after ICU admission did not predict outcome. Depending upon which statistical model was used, patients with higher or lower mean BTs were associated with increased risk of death. Sacho (2010) followed a cohort prospectively with the purpose of exploring the relationship between five-day post-injury BT and 30-day mortality and three-month dichotomized GOS-E (favorable/unfavorable neurologic outcome). Systemic cooling was provided in a tiered fashion for elevated ICP > 25 mm Hg accompanied by fever > 37.5° C or temperature > 39° C regardless of ICP. Antipyretic medications were provided as part of routine care. Brain temperature was independently predictive of 30-day mortality adjusting for some potential confounders. When pupillary reaction was added as a variable, the relationship between BT and outcome was no longer significant (Table 1).
Elf and colleagues (2008) identified 53 patients during the first 120 hours after TBI in order to describe the occurrence of spontaneous fever, HT, and temperature correlates with secondary insults. However, review of bladder temperature and other secondary insult variables revealed a non-significant trend towards better outcome for patients with normal temperature compared to those with aberrant temperatures (Table 1). Both Jeremitsky (2003) and Yamamoto (2002) sought to identify variables that might predict outcome in severe TBI. Jeremitsky identified frequency of occurrence of secondary insults, including HT and fever. Hypotension, hypoglycemia, and HT were associated with an increased mortality rate. Yamamoto looked at variables that were associated with good or poor outcomes in a sample of HT patients compared to a sample of NT who received barbiturates as a part of their therapy. Patients in the HT group fared better than the NT group.
Fever
When Li and Jiang stratified patient temperatures into groups, they found that increasing severity and duration of fever predicted poorer outcomes (Table 1). Normal temperature or low fever and shorter duration of exposure to elevated temperature were associated with more favorable outcomes (Li & Jiang, 2012). Those with severe TBI who had more high fever days had higher mortality (Li & Jiang, 2012). Stochetti also found that the occurrence and duration of fever is significantly associated with severe TBI (2002). Most studies found that avoidance of fever is valuable, decreasing ICU length of stay, mortality, incidence of hypertension, high ICP and tachycardia (Childs et al., 2006; Elf et al., 2008; Jeremitsky et al., 2003; Li & Jiang, 2012; Sacho et al., 2010; Stocchetti et al., 2002). However, these findings are not consistent across studies (Childs et al., 2006; Spiotta et al., 2008). One critical aspect to consider may be timing of fever. Geffroy found that patients who have an early fever are more likely to have a poor outcome. Those with early fever more likely to have a less favorable survival than those without early fever (Geffroy et al., 2004).
Controlled normothermia
Puccio performed the only study of controlled normothermia using a cohort of 21 adult patients with severe TBI treated with routine care matched to 21 patients treated with induced NT via an intravascular cooling catheter (2009). ICP was measured via an external ventricular drain and time duration for ICP > 25 mm Hg was calculated for the initial 72-hour monitoring period (ICP burden). Fever burden was defined as the percentage of time rectal temperature was > 38° C in the first 72 hours. Induced NT via intravascular cooling catheter was effective in reducing both fever burden and intracranial hypertension burden (Table 2).
Table 2.
Normothermia in TBI
| Author | Study Design & Participants | Comparisons/Temperature Measure | Outcomes | Main Findings |
|---|---|---|---|---|
| Puccio et al. (2009) | n = 42 (36M/6F)
GCS median 7 (3 – 8) Age 36.4 mean (21.6 – 51.2) years
|
Rectal temp Induced NT (via intravascular cooling catheter with rectal temp set at 36 –36.5° C) Traditional temp management (treatment for rectal temp ≥ 38° C) |
Surrogate outcomes of: ICP burden
Fever burden
|
NT:
|
Note. F is female, GCS is Glasgow Coma Scale, hrs is hours, ICP is intracranial pressure, M is male, NT is normothermia, severe TBI is GCS 3–8, TBI is traumatic brain injury, temp is temperature.
Hypothermia on admission
Bukur (2012) examined the relationship between admission HT and mortality in patients with moderate to severe TBI and found that admission HT was independently associated with increased mortality in moderate to severe TBI. Similarly, Konstantinidis (2011) sought to identify the influence of admission HT on outcome in patients with isolated severe TBI. All trauma patients admitted to the surgical intensive care unit (SICU) with isolated (no other significant injuries) severe TBI were classified as HT (T ≤ 35° C) or NT (T > 35° C) based on their first core temperature recorded after SICU admission. Patients who were HT on SICU admission were significantly less likely to survive.
Thompson and colleagues (2010) sought to determine the incidence and magnitude of HT in patients on ED admission and the effect of HT and rate of rewarming on patient outcomes in a secondary data analysis of patients admitted to a single Level 1 Trauma Center following severe TBI. The authors found that HT on admission was correlated with worse outcomes (Table 3) and concluded this related to rapid rewarming (increase of 0.25° C/hour) of patients presenting with HT on admission.
Table 3.
Hypothermia on Admission in TBI
| Author | Study Design & Participants | Comparisons/Temperature Measure | Outcomes | Main Findings |
|---|---|---|---|---|
| Thompson et al. (2010) | n = 147
Median age
Severe TBI |
Admission temp to ED (route not specified) HT = Temp < 35° C Rate of rewarming calculated by determining time to ≥ 36.5° C Rapid rewarm = > 0.25° C/h |
Discharge & 6-month GOS-E | HT on admission correlated with
Mortality at D/C based on rewarming rates:
|
| Konstantinidis (Konstantinidis et al., 2011) | n = 1,403 Mean age 38.1 ± 21.2 years Severe TBI Admission to SICU |
HT = first SICU measured core temp ≤ 35° C) NT = first SICU measured core temp ≥ 35° C Rewarming rate of HT not reported |
In-hospital mortality SICU & hospital LOS |
HT on SICU admission
Independent risk factors of HT on SICU admission:
|
| Bukur (Bukur et al., 2012) | n = 1,834 Ages ≥ 14 years Severe TBI (AIS-H ≥ 3, all other <3) |
HT= ≤ 35° C NT= > 35° C Rewarming rate of HT not reported |
Mortality (time frame not identified) | HT (n = 44) NT (n = 1790) Mortality for HT (25% vs. 7%) Admission HT independently associated with increased mortality (AOR 2.5, 95% CI 1.1 – 6.3, p = 0.04) |
| Jeremitsky et al. (2003) | n = 81 Adult Severe TBI Transport time < 2 hours to Level I Trauma Center |
Retrospective review 11 SBIFs in first 24 hours post-injury: hypotension, hypoxia, hypercapnia, hypocapnia, HT, fever, metabolic acidosis, seizures, coagulopathy, hyperglycemia, & IICP SBIF was recorded during 6 time periods: hours 1, 2, 3, 4, 5 to 14, & 16–24 Occurrence of each SBIF then correlated with outcome |
Morality | Increased mortality rate associated with
Mortality independently related to
Survivors (mean 34.0 years) significantly younger than non-survivors (mean 44. 9 years) (p = 0.03) |
Note. AIS-H is abbreviated injury score of the head region, AOR is adjusted odds ratio, CI is confidence interval, D/C is discharge, ED is emergency department, F is female, GCS is Glasgow Coma Scale, GOS-E is extended Glasgow Outcome Scale, HT is hypothermia, ISS is injury severity score, LOS is length of stay, M is male, MOI is mechanism of injury, NT is normothermia, OR is odds ratio, pts is patients, R is Pearson product-moment correlation coefficient, SICU is surgical ICU, TBI is traumatic brain injury, temp is temperature, tx is treatment.
Induced hypothermia
Since the latest Cochrane review in 2009, two RCTs, one observational study comparing two targeted temperature management goals, and one secondary analysis of data from two RCTs have been published (Table 4). Zhao and colleagues (2011) studied the effect of 72 hours of mild HT (32.7° C) on glucose and lactate levels in patients randomized to HT or NT. Hypothermia was identified as an independent predictor for favorable outcome in patients with severe TBI.
Clifton and colleagues (2011) reported on the findings of the National Acute Brain Injury Study: Hypothermia (NABIS:H II), a multicenter RCT of severe TBI treated with NT or HT for 48 hours. The study was stopped for futility at interim analysis, failing to demonstrate that early induction of HT resulted in improved outcomes. Clifton suggested that elevated ICP in the HT treatment group may be related to rebound elevation in ICP during rewarming as well as ICP elevation related to mass lesions prior to evacuation. This led to a post-hoc analysis of patients enrolled in both NABIS:H I and NABIS:H II to identify whether cooling before evacuation of traumatic intracranial hematomas protects the brain from reperfusion injury. Clifton (2012) proposed that induced HT in this population prior to or soon after craniotomy may be associated with improved outcomes.
Tokutomi (2009) attempted to clarify whether cooling to 35° C has the same effect as 33° C in reducing elevated ICP and whether it is associated with fewer complications and improved outcomes in patients with severe TBI. There were no significant differences in ICP, cerebral perfusion pressure (CPP), or in outcomes between groups. The authors concluded that cooling to 35° C is equally effective as 33° C.
Limitations
Temperature measures
Investigators stratified patients by temperature ranges rather than considering temperature as a continuous variable. All studies quantified temperature by the number of days or fever incidence rather than the nuances of temperature during that 24-hour time period such as amount of time temperature was elevated, severity of temperature measurement difference from identified normal range, or timing of a peak temperature. A few studies reported findings as “naturally occurring” temperatures, yet interventions modifying temperature were provided. Delayed temperature measurement from six to 24 hours after injury may have missed important early information (Childs et al., 2006; Sacho et al., 2010; Spiotta et al., 2008). Definitions of fever and treatment thresholds for elevated temperature vary in the literature and in practice. Details of fever dose and impact on outcome are not well described in the literature.
Warming rates
Patients presenting with HT on admission may be passively or actively rewarmed. The rate of rewarming is not reported in most of the HT literature. Thompson considered this issue in a post-hoc analysis of TBI patients presenting to the ED and noted that the rate of rewarming varies and is not well documented (Thompson et al., 2010). Patients who have been cooled for induced HT are also rewarmed at varying rates. In the Clifton RCTs, rewarming was done slowly. When rates have been reported, they vary among trials and may or may not be done according to ICP response. Prior RCT control groups were not purely control groups of usual care since the. In prior RCTs, there were also variations in “control” methods making comparison among results challenging. Further, the negative effect of active rewarming upon randomization to NT may have made NT patient outcomes worse than if they were passively or more slowly rewarmed. In the case of passive rewarming, spontaneous elevations of temperature may result in more rapid rewarming than controlled rewarming (Polderman & Herold, 2009).
Variables influencing temperature
Injury activates an immune response and incites varying degrees of cytokine and interleukin release with white blood cell (WBC) activation resulting in temperature elevation. Injury to the hypothalamus can also cause impaired regulation of temperature. Blood products within the brain tissue and/or ventricular system can elicit temperature elevation. None of these variables (such as serum or CSF cytokine levels, head CT or brain MRI findings) were reported in the studies reviewed, with the exception of WBC values in one study (Geffroy et al., 2004). Barbiturate administration confounded results. Yamamoto found HT who did not receive barbiturates had better survival than NT patients who received barbiturates (2002). Elf found that excluding barbiturate-treated patients, those with HT and NT fared better than those with fever (2008).
Conclusion
Studies investigating targeted temperature management in TBI suggest that mild HT (targeting either 33°C or 35°C) may benefit those patients who have elevated ICP, yet with no statistically significant difference in 6-month GOS between those groups, the evidence is not compelling (Tokutomi et al., 2009). The Guidelines for the Management of Severe Traumatic Brain Injury (Brain Trauma Foundation, 2007) suggest normothermia for TBI patients. While controlled NT improved surrogate outcome measures of ICP burden (Puccio et al., 2009) and was associated with a lower probability of death (Childs et al., 2006), its practice is lacking outcomes-based evidence. Maintenance of NT may be most important for physiologic homeostasis after TBI. Yet, identification of a temperature range that positively impacts patient outcomes has not been determined.
Timing, duration and severity of fever exposure influence patient outcomes. However, many interventions to treat fever are not aggressive (Thompson, Kirkness, & Mitchell, 2007). Recent investigation of 33°C versus 36°C in cardiac arrest patients (Nielsen et al., 2013) supports the hypothesis that fever avoidance may be the highest yield intervention in TTM (Rittenberger & Callaway, 2013), but this is not translatable directly to TBI and requires further investigation in this population.
Hypothermia on admission correlated with poor outcomes. Advanced Trauma Life Support protocols do not clearly direct rates of rewarming trauma patients. Aggressive practices of warming hypothermic patients in order to avoid the “lethal triad” of acidosis, hypothermia, and coagulopathy (Burch et al., 1992) may be detrimental to those patients with TBI. The benefits associated with HT (lowered ICP) are negated when rewarming is done rapidly (Povlishock & Wei, 2009). The rate of temperature change, whether from HT to normal temperature or further toward fever, may be as important as the rate of cooling from fever toward NT or HT. Rate of change, or slopes, of the patient temperature curve are not documented in the literature and may be important in both understanding the process after injury and identifying targets for treatment that may improve outcomes. Slow rewarming of hypothermic trauma patients in the ED with a goal of NT may be a safer approach for management of this patient population.
Current evidence-based targeted temperature management goals are poorly defined. Further research is needed to; (1) characterize the magnitude and duration of temperature alteration after TBI; (2) determine if temperature alteration influences or predicts neurologic outcome; (3) determine if the rate of temperature change influences or predicts neurologic outcome; and (4) compare controlled NT versus standard practice or HT. Setting a goal of NT, avoiding fever, and aggressively treating fever if it occurs, may help achieve the neuroprotective benefits of HT with lower risks.
Supplementary Material
Acknowledgments
Special thanks to reference librarian Bruce Abbott for his assistance in identifying the literature in this review.
Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health (F31NR013813) and the Gordon and Betty Moore Foundation.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Lori Kennedy Madden, Email: lori.madden@ucdmc.ucdavis.edu, PhD Candidate, Betty Irene Moore School of Nursing, Nurse Practitioner, Department of Neurological Surgery, University of California Davis. Work Address: 4860 Y Street, Suite 3740, Sacramento, CA 95817, T 916-734-6518, F 916-703-5006.
Holli A DeVon, Email: hdevon1@uic.edu, Associate Professor, Department of Biobehavioral Health Science, College of Nursing, University of Illinois at Chicago. Work Address: 845 S. Damen Avenue #748 MC 802, Chicago, IL 60612, T 312-413-5362, F 312-996-4979.
References
- Adelson PD. Hypothermia following pediatric traumatic brain injury. J Neurotrauma. 2009;26(3):429–436. doi: 10.1089/neu.2008.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelson PD, Ragheb J, Kanev P, Brockmeyer D, Beers SR, Brown SD, Levin H. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56(4):740–754. doi: 10.1227/01.neu.0000156471.50726.26. discussion 740–754. [DOI] [PubMed] [Google Scholar]
- Barker-Collo SL, Feigin VL. Capturing the spectrum: suggested standards for conducting population-based traumatic brain injury incidence studies. Neuroepidemiology. 2009;32(1):1–3. doi: 10.1159/000170084. [DOI] [PubMed] [Google Scholar]
- Bayir H, Adelson PD, Wisniewski SR, Shore P, Lai Y, Brown D, Kochanek PM. Therapeutic hypothermia preserves antioxidant defenses after severe traumatic brain injury in infants and children. Crit Care Med. 2009;37(2):689–695. doi: 10.1097/CCM.0b013e318194abf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain Trauma Foundation. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007 doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- Bukur M, Kurtovic S, Berry C, Tanios M, Ley EJ, Salim A. Pre-hospital hypothermia is not associated with increased survival after traumatic brain injury. J Surg Res. 2012;175(1):24–29. doi: 10.1016/j.jss.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Burch JM, Ortiz VB, Richardson RJ, Martin RR, Mattox KL, Jordan GL., Jr Abbreviated laparotomy and planned reoperation for critically injured patients. Ann Surg. 1992;215(5):476–483. doi: 10.1097/00000658-199205000-00010. discussion 483-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs C, Vail A, Leach P, Rainey T, Protheroe R, King A. Brain temperature and outcome after severe traumatic brain injury. Neurocrit Care. 2006;5(1):10–14. doi: 10.1385/NCC:5:1:10. [DOI] [PubMed] [Google Scholar]
- Childs C, Wieloch T, Lecky F, Machin G, Harris B, Stocchetti N. Report of a consensus meeting on human brain temperature after severe traumatic brain injury: its measurement and management during pyrexia. Front Neurol. 2010;1:146. doi: 10.3389/fneur.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton GL. Hypothermia and hyperbaric oxygen as treatment modalities for severe head injury. New Horizons: Science and Practice of Acute Medicine. 1995a;(3):474–478. [PubMed] [Google Scholar]
- Clifton GL. Systemic hypothermia in treatment of severe brain injury: a review and update. J Neurotrauma. 1995b;12(5):923–927. doi: 10.1089/neu.1995.12.923. [DOI] [PubMed] [Google Scholar]
- Clifton GL, Allen S, Barrodale P, Plenger P, Berry J, Koch S, Choi SC. A phase II study of moderate hypothermia in severe brain injury. J Neurotrauma. 1993;10(3):263–271. doi: 10.1089/neu.1993.10.263. discussion 273. [DOI] [PubMed] [Google Scholar]
- Clifton GL, Coffey CS, Fourwinds S, Zygun D, Valadka A, Smith KR, Jr, Okonkwo DO. Early induction of hypothermia for evacuated intracranial hematomas: a post hoc analysis of two clinical trials. J Neurosurg. 2012;117(4):714–720. doi: 10.3171/2012.6.JNS111690. [DOI] [PubMed] [Google Scholar]
- Clifton GL, Miller ER, Choi SC, Levin HS, McCauley S, Smith KR, Schwartz M. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344(8):556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- Clifton GL, Valadka A, Zygun D, Coffey CS, Drever P, Fourwinds S, Okonkwo DO. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet Neurology. 2011;10(2):131–139. doi: 10.1016/S1474-4422(10)70300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich WD. The importance of brain temperature in cerebral injury. J Neurotrauma. 1992;9(Suppl 2):S475–485. [PubMed] [Google Scholar]
- Elf K, Nilsson P, Ronne-Engstrom E, Howells T, Enblad P. Temperature disturbances in traumatic brain injury: relationship to secondary insults, barbiturate treatment and outcome. Neurol Res. 2008;30(10):1097–1105. doi: 10.1179/174313208X319125. [DOI] [PubMed] [Google Scholar]
- Faul MD, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta (GA): Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. 2010;2:1–9. [Google Scholar]
- Fox JL, Vu EN, Doyle-Waters M, Brubacher JR, Abu-Laban R, Hu Z. Prophylactic hypothermia for traumatic brain injury: a quantitative systematic review. CJEM. 2010;12(4):355–364. doi: 10.1017/s1481803500012471. [DOI] [PubMed] [Google Scholar]
- Geffroy A, Bronchard R, Merckx P, Seince PF, Faillot T, Albaladejo P, Marty J. Severe traumatic head injury in adults: which patients are at risk of early hyperthermia? Intensive Care Med. 2004;30(5):785–790. doi: 10.1007/s00134-004-2280-y. [DOI] [PubMed] [Google Scholar]
- Inamasu J, Saito R, Nakamura Y, Horiguchi T, Kuroshima Y, Ichikizaki K. Therapeutic hypothermia for severely head-injured patients with acute subdural haematoma. J Clin Neurosci. 2006;13(7):733–737. doi: 10.1016/j.jocn.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Jeremitsky E, Omert L, Dunham CM, Protetch J, Rodriguez A. Harbingers of poor outcome the day after severe brain injury: hypothermia, hypoxia, and hypoperfusion. J Trauma. 2003;54(2):312–319. doi: 10.1097/01.TA.0000037876.37236.D6. [DOI] [PubMed] [Google Scholar]
- Jiang J, Yu M, Zhu C. Effect of long-term mild hypothermia therapy in patients with severe traumatic brain injury: 1-year follow-up review of 87 cases. J Neurosurg. 2000;93(4):546–549. doi: 10.3171/jns.2000.93.4.0546. [DOI] [PubMed] [Google Scholar]
- Jiang JY. Clinical study of mild hypothermia treatment for severe traumatic brain injury. J Neurotrauma. 2009;26(3):399–406. doi: 10.1089/neu.2008.0525. [DOI] [PubMed] [Google Scholar]
- Jones PA, Andrews PJ, Midgley S, Anderson SI, Piper IR, Tocher JL, et al. Measuring the burden of secondary insults in head-injured patients during intensive care. J Neurosurg Anesthesiol. 1994;6(1):4–14. [PubMed] [Google Scholar]
- Jurkovich GJ, Greiser WB, Luterman A, Curreri PW. Hypothermia in trauma victims: an ominous predictor of survival. J Trauma. 1987;27(9):1019–1024. [PubMed] [Google Scholar]
- Konstantinidis A, Inaba K, Dubose J, Barmparas G, Talving P, David JS, Demetriades D. The impact of nontherapeutic hypothermia on outcomes after severe traumatic brain injury. J Trauma. 2011;71(6):1627–1631. doi: 10.1097/TA.0b013e3182159e31. [DOI] [PubMed] [Google Scholar]
- Krieger DW, De Georgia MA, Abou-Chebl A, Andrefsky JC, Sila CA, Katzan IL, Furlan AJ. Cooling for acute ischemic brain damage (cool aid): an open pilot study of induced hypothermia in acute ischemic stroke. Stroke. 2001;32(8):1847–1854. doi: 10.1161/01.str.32.8.1847. [DOI] [PubMed] [Google Scholar]
- Li J, Jiang JY. Chinese Head Trauma Data Bank: effect of hyperthermia on the outcome of acute head trauma patients. J Neurotrauma. 2012;29(1):96–100. doi: 10.1089/neu.2011.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion DW, Penrod LE, Kelsey SF, Obrist WD, Kochanek PM, Palmer AM, DeKosky ST. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336(8):540–546. doi: 10.1056/NEJM199702203360803. [DOI] [PubMed] [Google Scholar]
- McHugh GS, Butcher I, Steyerberg EW, Lu J, Mushkudiani N, Marmarou A, Murray GD. Statistical approaches to the univariate prognostic analysis of the IMPACT database on traumatic brain injury. J Neurotrauma. 2007;24(2):251–258. doi: 10.1089/neu.2006.0026. [DOI] [PubMed] [Google Scholar]
- Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Investigators TTMT. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369(23):2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med. 2009;37(3):1101–1120. doi: 10.1097/CCM.0b013e3181962ad5. [DOI] [PubMed] [Google Scholar]
- Povlishock JT, Wei EP. Posthypothermic rewarming considerations following traumatic brain injury. J Neurotrauma. 2009;26(3):333–340. doi: 10.1089/neu.2008.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccio AM, Fischer MR, Jankowitz BT, Yonas H, Darby JM, Okonkwo DO. Induced normothermia attenuates intracranial hypertension and reduces fever burden after severe traumatic brain injury. Neurocrit Care. 2009;11(1):82–87. doi: 10.1007/s12028-009-9213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberger JC, Callaway CW. Temperature management and modern post-cardiac arrest care. N Engl J Med. 2013;369(23):2262–2263. doi: 10.1056/NEJMe1312700. [DOI] [PubMed] [Google Scholar]
- Sacho RH, Vail A, Rainey T, King AT, Childs C. The effect of spontaneous alterations in brain temperature on outcome: a prospective observational cohort study in patients with severe traumatic brain injury. J Neurotrauma. 2010;27(12):2157–2164. doi: 10.1089/neu.2010.1384. [DOI] [PubMed] [Google Scholar]
- Sadaka F, Veremakis C. Therapeutic hypothermia for the management of intracranial hypertension in severe traumatic brain injury: a systematic review. Brain Inj. 2012;26(7–8):899–908. doi: 10.3109/02699052.2012.661120. [DOI] [PubMed] [Google Scholar]
- Schroder ML, Muizelaar JP, Kuta AJ, Choi SC. Thresholds for cerebral ischemia after severe head injury: relationship with late CT findings and outcome. J Neurotrauma. 1996;13(1):17–23. doi: 10.1089/neu.1996.13.17. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF Human Development Neonatal Research N. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Shann F. Hypothermia for traumatic brain injury: how soon, how cold, and how long? The Lancet. 2003;362(9400):1950–1951. doi: 10.1016/s0140-6736(03)15083-0. [DOI] [PubMed] [Google Scholar]
- Shiozaki T, Sugimoto H, Taneda M, Yoshida H, Iwai A, Yoshioka T, Sugimoto T. Effect of mild hypothermia on uncontrollable intracranial hypertension after severe head injury. Journal of Neurosurgery. 1993;79(3):363–368. doi: 10.3171/jns.1993.79.3.0363. [DOI] [PubMed] [Google Scholar]
- Spiotta AM, Stiefel MR, Heuer GG, Bloom S, Maloney-Wilensky E, Yang W, Le Roux PD. Brain hyperthermia after traumatic brain injury does not reduce brain oxygen. Neurosurgery. 2008;62(4):864–872. doi: 10.1227/01.neu.0000310763.45510.10. [DOI] [PubMed] [Google Scholar]
- Steinemann S, Shackford SR, Davis JW. Implications of admission hypothermia in trauma patients. J Trauma. 1990;30(2):200–202. doi: 10.1097/00005373-199002000-00011. [DOI] [PubMed] [Google Scholar]
- Stocchetti N, Rossi S, Zanier ER, Colombo A, Beretta L, Citerio G. Pyrexia in head-injured patients admitted to intensive care. Intensive Care Med. 2002;28(11):1555–1562. doi: 10.1007/s00134-002-1513-1. [DOI] [PubMed] [Google Scholar]
- Sydenham E, Roberts I, Alderson P. Hypothermia for traumatic head injury. Cochrane Database of Systematic Reviews. 2009;(2) doi: 10.1002/14651858.CD001048.pub4. [DOI] [Google Scholar]
- Thompson HJ, Kirkness CJ, Mitchell PH. Intensive care unit management of fever following traumatic brain injury. Intensive Crit Care Nurs. 2007;23(2):91–96. doi: 10.1016/j.iccn.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson HJ, Kirkness CJ, Mitchell PH. Hypothermia and rapid rewarming is associated with worse outcome following traumatic brain injury. J Trauma Nurs. 2010;17(4):173–177. doi: 10.1097/JTN.0b013e3181ff272e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson HJ, Pinto-Martin J, Bullock MR. Neurogenic fever after traumatic brain injury: an epidemiological study. J Neurol Neurosurg Psychiatry. 2003;74(5):614–619. doi: 10.1136/jnnp.74.5.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokutomi T, Miyagi T, Takeuchi Y, Karukaya T, Katsuki H, Shigemori M. Effect of 35 degrees C hypothermia on intracranial pressure and clinical outcome in patients with severe traumatic brain injury. J Trauma. 2009;66(1):166–173. doi: 10.1097/TA.0b013e318157dbec. [DOI] [PubMed] [Google Scholar]
- Wong FW. Prevention of secondary brain injury. Crit Care Nurse. 2000;20(5):18–27. [PubMed] [Google Scholar]
- Yamamoto T, Mori K, Maeda M. Assessment of prognostic factors in severe traumatic brain injury patients treated by mild therapeutic cerebral hypothermia therapy. Neurol Res. 2002;24(8):789–795. doi: 10.1179/016164102101200906. [DOI] [PubMed] [Google Scholar]
- Zhao QJ, Zhang XG, Wang LX. Mild hypothermia therapy reduces blood glucose and lactate and improves neurologic outcomes in patients with severe traumatic brain injury. J Crit Care. 2011;26(3):311–315. doi: 10.1016/j.jcrc.2010.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.