Abstract
Objectives
Head CT after out-of-hospital cardiac arrest is often obtained to evaluate intracranial pathology. Among children admitted to the PICU following pediatric out-of-hospital cardiac arrest, we hypothesized that loss of gray-white matter differentiation and basilar cistern and sulcal effacement are associated with mortality and unfavorable neurologic outcome.
Design
Retrospective, cohort study.
Setting
Single, tertiary-care center PICU.
Patients
Seventy-eight patients less than 18 years old who survived out-of-hospital cardiac arrest to PICU admission and had a head CT within 24 hours of return of spontaneous circulation were evaluated from July 2005 through May 2012.
Interventions
None.
Measurements and Main Results
Median time to head CT from return of spontaneous circulation was 3.3 hours (1.0, 6.0). Median patient age was 2.3 years (0.4, 9.5). Thirty-nine patients (50%) survived, of whom 29 (74%) had favorable neurologic outcome. Nonsurvivors were more likely than survivors to have 1) loss of gray-white matter differentiation (Hounsfield unit ratios, 0.96 [0.88, 1.07] vs 1.1 [1.07, 1.2]; p < 0.001), 2) basilar cistern effacement (93% vs 7%; p = 0.001; positive predictive value, 94%; negative predictive value, 59%), and 3) sulcal effacement (100% vs 0%; p ≤ 0.001; positive predictive value, 100%; negative predictive value, 68%). All patients with poor gray-white matter differentiation or sulcal effacement had unfavorable neurologic outcomes. Only one patient with basilar cistern effacement had favorable outcome.
Conclusions
Loss of gray-white matter differentiation and basilar cistern effacement and sulcal effacement are associated with poor outcome after pediatric out-of-hospital cardiac arrest. Select patients may have favorable outcomes despite these findings.
Keywords: basilar cistern effacement, cardiac arrest, children, computed tomography, gray white matter, reversal sign, sulcal effacement
Up to 6,000 children in the United States suffer an out-of-hospital cardiac arrest (OHCA) every year (1). Between 10% and 30% of pediatric OHCA patients treated in the field have sustained return of spontaneous circulation (ROSC) and survive to a PICU (2). Of these, 40–62% survive to hospital discharge and more than half have neurologic sequelae (1, 3).
Assessment of neurologic function and neuroprognostication are important issues for families and healthcare providers. Tools for these assessments include neurologic examination, early electroencephalography, head CT, biomarkers, and historical information specific to the cardiac arrest (4, 5). None of these modalities is 100% sensitive and specific for neurologic outcome in the first 24 hours post–cardiac arrest. Early head CT is often obtained following successful initial resuscitation to diagnose potential cardiac arrest etiology (e.g., intracranial hemorrhage, hydrocephalus, trauma, and tumor) and to assess the presence and severity of post–cardiac arrest cerebral ischemia and edema (6, 7). Early CT loss of gray-white matter (GWM) differentiation is associated with worse survival and neurologic outcomes following adult OHCA (8–10). One study of children who suffered drowning, with a small subset having had a cardiac arrest, also demonstrated an association of loss of GWM with worse outcome (11).
We sought to evaluate the association of early CT findings of brain injury after pediatric OHCA with discharge mortality and neurologic outcome. We hypothesized that the loss of GWM and basilar cistern effacement and sulcal effacement on CT within 24 hours of cardiac arrest would be associated with increased mortality and unfavorable neurologic outcomes.
MATERIALS AND METHODS
We performed a retrospective study of patients less than 18 years old presenting to the PICU at The Children’s Hospital of Philadelphia from July 1, 2005, through May 31, 2012. Patients were included for analysis if they had an OHCA, received chest compressions, had sustained ROSC greater than 20 minutes, and had a head CT performed at the discretion of the care team within 24 hours of ROSC. Patients were excluded if the CT images were unavailable or they had a prearrest Pediatric Cerebral Performance Category (PCPC) score of greater than 3 (moderate neurologic dysfunction) or they had accidental head trauma such as those in a motor vehicle accident. Patients with abusive head trauma (shaken baby syndrome) were not excluded because they often present in cardiac arrest and the diagnosis of abuse if frequently determined in the days after their hospital presentation. The study was approved with a waiver of informed consent granted by the Children’s Hospital of Philadelphia Institutional Review Board.
Patients were prospectively identified from September 2009 to May 2012 and entered into a cardiac arrest database. Patients from January 2006 through September 2009 were identified from existing hospital cardiac arrest databases. Search terms were “code,” “arrest,” “cardiac arrest,” “CPR,” “prior CPR,” “hanging,” “drowning,” “near drowning,” “SIDS,” and “ALTE.” All identified patients underwent chart review to verify if they had received chest compressions and qualified as an OHCA.
Prehospital and inpatient medical records were reviewed by two trained investigators. Data extracted included prearrest patient demographics and characteristics, cardiac arrest event details, postarrest processes of care, and discharge survival and neurologic outcome. The interpretation of head CT scan was performed by a single neuroradiologist who was blinded to all patient data, outcomes, and previous CT interpretations.
Head CT characteristics were determined a priori. Head CT scans were evaluated for the presence of intracranial hemorrhage, midline shift, loss of GWM differentiation, sulcal effacement, and basilar cistern effacement (Fig. 1). Reversal sign was determined by the attenuation of the cerebellum being higher than that of the supratentorial brain (Fig. 2). GWM differentiation was determined both qualitatively and quantitatively at the level of the corpus callosum and posterior limb of the internal capsule for white matter and caudate nucleus head and putamen for gray matter (12). Hounsfield unit ratios (HUrs) were used for quantitative measurement. An abnormal head CT was defined as the presence of parenchymal lesions or abnormal fluid collections, midline shift, mass effect, presence of intracranial hemorrhage, basilar cistern effacement or sulcal effacement, loss of GWM, or reversal sign. A normal CT was defined as normal size and configuration of ventricles and sulci with absence of abnormalities (Fig. 3). Six patients had a prearrest CT scan, one was normal and five had volume loss. None had loss of GWM, intracranial hemorrhage, basal cistern or sulcal effacement, or reversal sign. All were included in the analysis.
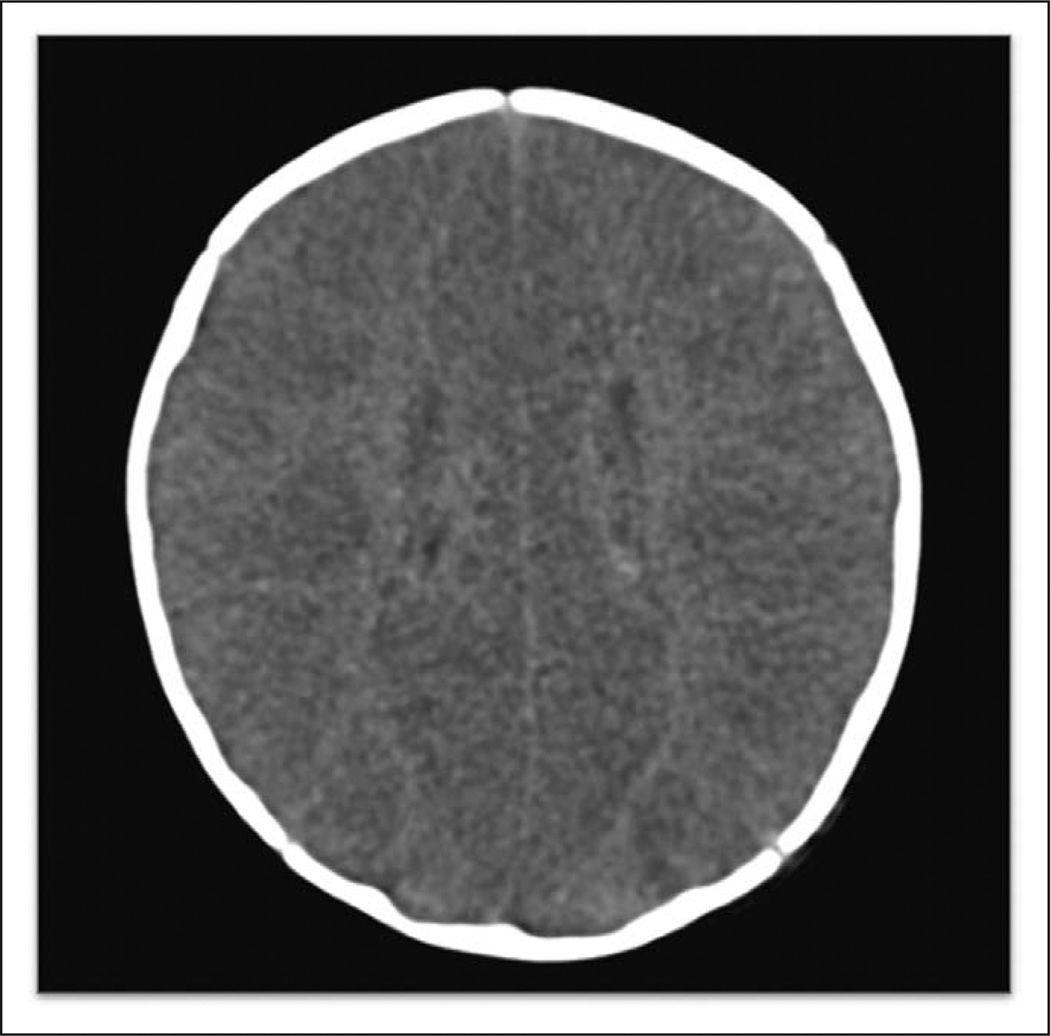
Figure 1.
This head CT demonstrates severe loss of gray-white matter differentiation and sulcal effacement due to severe cerebral edema. This patient was 2 months old and previously healthy with sudden infant death syndrome. The Hounsfield unit ratio was 0.75.
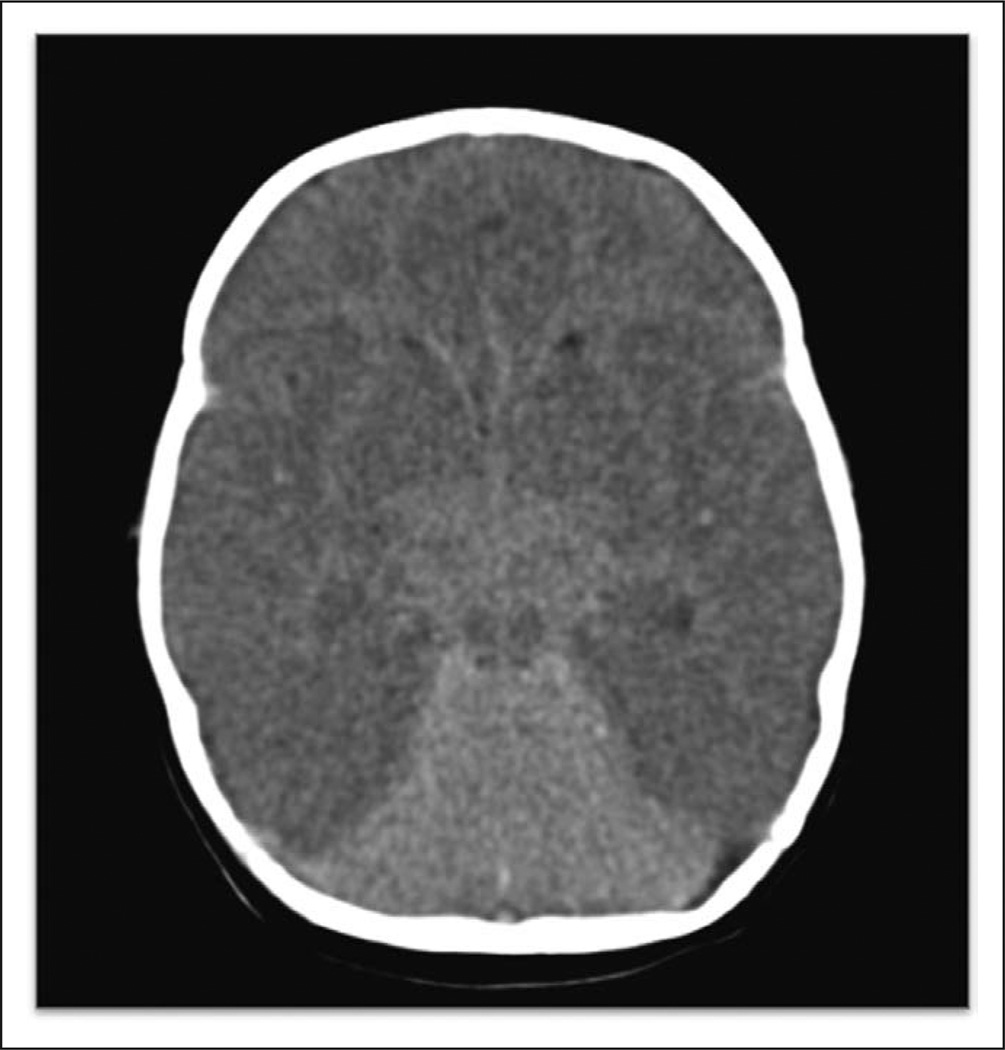
Figure 2.
This head CT demonstrates reversal sign with a bright cerebellum and dark cortex. There is also loss of gray-white mater differentiation, sulcal effacement, and basilar cistern effacement. This patient was 2 months old and previously healthy with sudden infant death syndrome. The Hounsfield unit ratio was 0.75.
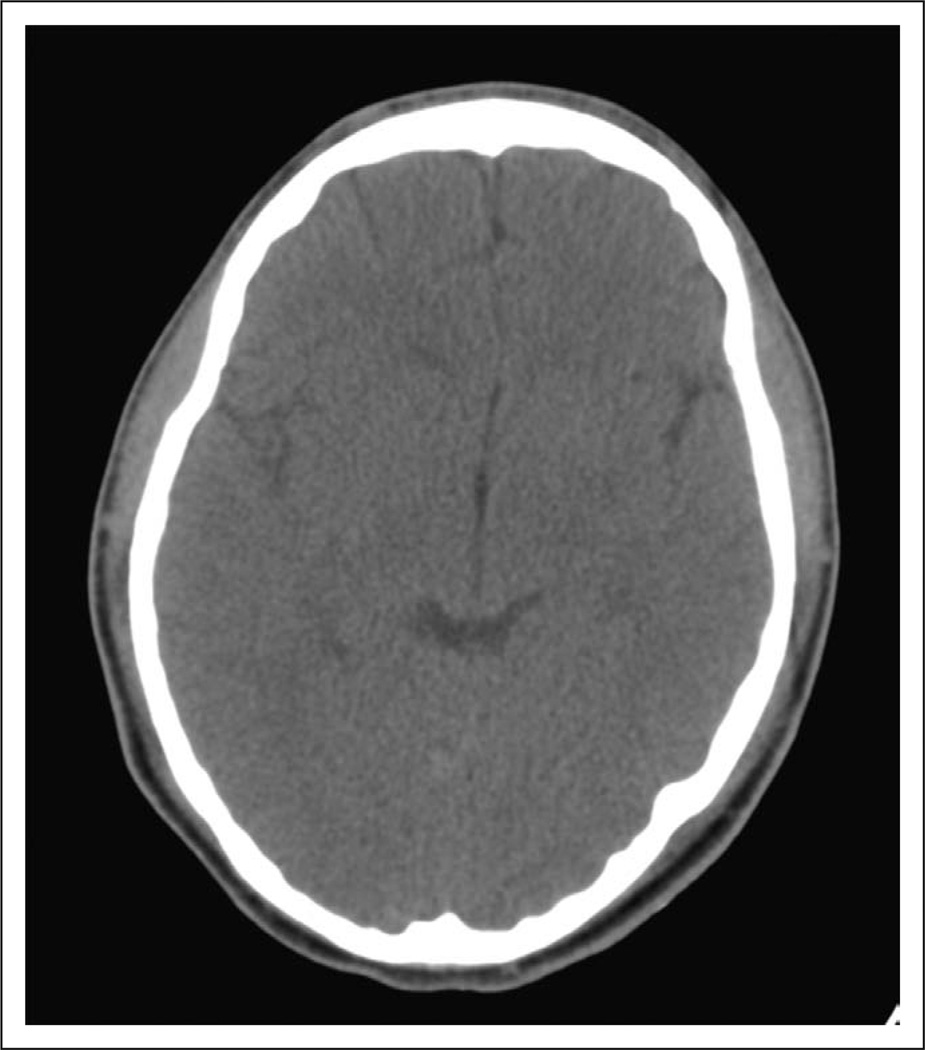
Figure 3.
This head CT demonstrates normal parenchyma and ventricles.
The primary outcome was discharge mortality. The secondary outcome was discharge neurologic outcome, determined by the PCPC. The PCPC is a six-point classification system to define cognitive function: 1 = normal; 2 = mild disability; 3 = moderate disability; 4 = severe disability; 5 = coma or vegetative state; and 6 = death (13). Favorable neurologic outcome was defined as a PCPC score of 1, 2, or 3 at hospital discharge or no change from prearrest to hospital discharge (14). Unfavorable neurologic outcome was defined as a discharge PCPC score of 4, 5, or 6 or a change from prearrest PCPC score greater than or equal to 1. Patients with a prearrest PCPC score greater than 3 were excluded because of the higher likelihood of baseline abnormal neuroimaging.
Summary statistics are reported as median and interquartile ranges (25th–75th percentiles) for continuous variables and proportions and percentages for categorical variables. Fisher exact tests or Wilcoxon rank-sum tests were used to determine differences between groups. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were performed for each CT finding. All statistical analyses were conducted using Stata 13 (StataCorp, College Station, TX).
RESULTS
One hundred sixty-six patients had an OHCA during the study period, of whom 88 met inclusion criteria. After applying exclusion criteria, 78 patients had a CT within 24 hours and a prearrest PCPC score of 1, 2, or 3 (Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/PCC/A155).
The median age of the patients was 2.3 years (0.4, 9.5). Forty-nine patients (63%) were male, 62 patients (80%) had a prearrest PCPC score of 1, and 42 (54%) had a preexisting condition (Supplemental Table 1, Supplemental Digital Content 2, http://links.lww.com/PCC/A156). The most common causes of cardiac arrest were respiratory failure, 16 (21%); drowning, 14 (18%); trauma, 12 (18%); and acute life-threatening event/sudden infant death syndrome, 13 (17%). Twenty-nine patients (37%) had a witnessed arrest and 45 (58%) received bystander cardiopulmonary resuscitation. The most common initial documented rhythm was pulseless electrical activity/asystole. Twenty-six patients (33%) were treated with induced hypothermia. Fifty-seven patients (80%) had an admission Glasgow Coma Scale (GCS) score of 3.
Thirty-nine patients (50%) died. Of the 39 patients who survived, 29 (74%) had a favorable neurologic outcome. Twenty of 57 patients (35%) who had an admission GCS of 3 survived, whereas 13 of 14 patients (93%) who had an admission GCS greater than 3 survived to discharge (p < 0.001). The cause of death was brain death in 19 (49%), withdrawal of technologic support in 18 (46%), and repeat recurrent cardiac arrest without ROSC in 2 (5%). In-hospital mortality was associated with duration of CPR greater than 20 minutes (p < 0.001), non-white race (p = 0.008), initial rhythm (p < 0.001), and received greater than or equal to two doses of epinephrine (p < 0.001). Cause of arrest and treatment with induced hypothermia were not associated with survival outcome (p = 0.81).
Head CT Characteristics
Head CT scans were obtained in a median of 3.3 hours (1.0, 6.0) following ROSC. Twenty-eight patients (36%) had a normal head CT. Of the 28 patients with an initially normal head CT, only two had a second CT that was normal, both survived with a favorable neurologic outcome. Fifty patients (64%) had a head CT with at least one abnormality. Of the 50 who had an abnormal head CT, 24 had a repeat head CT; only 1 of 24 had a normal head CT and that patient survived with a favorable neurologic outcome. Head CT findings included intracranial hemorrhage, 7 (9%); loss GWM differentiation, 32 (41%); sulcal effacement, 21 (27%); basilar cistern effacement, 14 (18%); and reversal sign, 10 (13%). There were no patients with midline shift. Patients who had CT findings of qualitative loss of GWM differentiation (70%), sulcal effacement (87%), basilar cistern effacement (90%), and reversal sign (87%) were significantly more likely to have received greater than or equal to 20 minutes of CPR (p < 0.02). Normal CT scans were associated with shorter duration of CPR (12 [2.5, 23] vs 21 [15, 45] min; p = 0.006), fewer doses of epinephrine administered (p < 0.001), or drowning (p < 0.001).
Of the seven patients with intracranial hemorrhage, all had subdural hemorrhage and one also had a parenchymal hemorrhage. None had epidural or subarachnoid hemorrhages. The one patient with parenchymal hemorrhage died. All patients with subdural hemorrhages were diagnosed with abusive head trauma based on review of official diagnoses. One patient underwent nonacute neurosurgical intervention with bilateral subdural shunts. None of the patients received an externalized ventricular drain. The presence of intracranial hemorrhage was not associated with mortality or neurologic outcome (p = 0.635 and p = 1.000, respectively).
The presence of a normal head CT was associated with survival and favorable neurologic outcome (p < 0.001) (Table 1). The presence of at least one head CT abnormality was associated with hospital mortality 35 (70%) versus 15 (30%) (p < 0.001) and unfavorable neurologic outcome 42 (84%) versus 8 (16%) (p < 0.001). Qualitative loss of GWM differentiation, basilar cistern effacement, sulcal effacement, and reversal sign were all associated with mortality and unfavorable neurologic outcome. All patients with sulcal effacement or reversal sign died. One patient with basilar cistern effacement had a favorable neurologic outcome. Quantitative loss of GWM differentiation measured in HUr was significantly associated with mortality and unfavorable neurologic outcome: death (0.96; HUr, 0.88, 1.07) versus survival (1.14; HUr, 1.1, 1.2) (p < 0.001) and unfavorable (1.03; HUr, 0.92, 11) versus favorable (1.16; HUr, 1.11, 1.2) (p < 0.001). Using a cutpoint of 1.2 HUr, the sensitivity of qualitative loss of GWM differentiation for survival was 27% and the specificity was 92%. The area under the curve (AUC) was 0.84. To account for cerebral myelination, patients were stratified into age groups of less than 2 years old and 2 years old or older. To achieve a 100% specificity for survival, for children less than 2 years old, a HUr cutpoint of 1.09 resulted in a sensitivity of 75% (AUC, 0.96) and for children 2 years old or older, a HUr cutpoint of 1.28 resulted in a sensitivity of 11.7% (AUC, 0.83).
TABLE 1.
The Association of Head CT Findings by Survival Status and Neurologic Outcomes
| CT Findings | Dead (n = 39) |
Alive (n = 39) |
p | Unfavorable (n = 29) |
Favorable (n = 49) |
p |
|---|---|---|---|---|---|---|
| Normal | 4 (14) | 24 (86) | < 0.001 | 7 (25) | 21 (75) | < 0.001 |
| Abnormal | 35 (70) | 15 (30) | < 0.001 | 42 (84) | 8 (16) | < 0.001 |
| Intracranial hemorrhage | 4 (57) | 3 (43) | 1.000 | 5 (71) | 2 (29) | 1.000 |
| Qualitative loss of GWM differentiation | 29 (91) | 3 (9) | < 0.001 | 32 (100) | 0 (0) | < 0.001 |
| Quantitative loss of GWM differentiation (Hounsfield unit ratio) | 0.96 [0.88, 1.07] |
1.1 [1.07, 1.2] |
< 0.001 | 1.03 [0.92, 1.11] |
1.16 [1.11,1.2] |
< 0.001 |
| Basilar cistern effacement | 13 (93) | 1 (7) | 0.001 | 13 (93) | 1 (7) | 0.013 |
| Sulcal effacement | 21 (100) | 0 (0) | < 0.001 | 21 (100) | 0 (0) | < 0.001 |
| Reversal sign | 10 (100) | 0 (0) | 0.001 | 10 (100) | 0 (0) | 0.011 |
GWM = gray-white matter.
Values are represented asn (%) or median [25th–75th percentile].
A normal head CT had a sensitivity of 62% for survival and specificity of 90%, PPV of 86%, and NPV of 70%. The sensitivity, specificity, PPV, and NPV for head CT findings and mortality are presented in Table 2 and for unfavorable neurologic outcomes are presented in Table 3. The AUC for qualitative loss of GWM differentiation was 0.84 for mortality and 0.82 for unfavorable neurologic outcome.
TABLE 2.
Predictive Values of Head CT Findings for In-Hospital Mortality
| CT Results | Sensitivity (%) |
Specificity (%) |
Positive Predictive Value (%) |
Negative Predictive Value (%) |
Area Under the Curve |
|---|---|---|---|---|---|
| Intracranial hemorrhage | 10 | 92 | 57 | 51 | 0.54 |
| Loss of gray-white matter differentiation | 74 | 92 | 91 | 78 | 0.84 |
| Basilar cistern effacement | 33 | 97 | 93 | 59 | 0.76 |
| Sulcal effacement | 54 | 100 | 100 | 69 | 0.84 |
| Reversal sign | 26 | 100 | 100 | 57 | 0.78 |
TABLE 3.
Predictive Values of Head CT Findings for Discharge With Unfavorable Neurologic Outcome
| CT Results | Sensitivity (%) |
Specificity (%) |
Positive Predictive Value (%) |
Negative Predictive Value (%) |
Area Under the Curve |
|---|---|---|---|---|---|
| Intracranial hemorrhage | 10 | 93 | 71 | 38 | 0.55 |
| Loss of gray-white matter differentiation | 65 | 100 | 100 | 63 | 0.82 |
| Basilar cistern effacement | 27 | 97 | 93 | 44 | 0.68 |
| Sulcal effacement | 43 | 100 | 100 | 51 | 0.75 |
| Reversal sign | 20 | 100 | 100 | 43 | 0.71 |
DISCUSSION
These data demonstrate that the presence of abnormal head CT findings, specifically loss of gray-white matter differentiation and cistern effacement, early after resuscitation from out-of-hospital pediatric cardiac arrest were associated with and highly specific for mortality and unfavorable neurologic outcome. All patients with sulcal effacement and reversal sign died. By contrast, a normal head CT was associated with survival and favorable neurologic outcome, but with low sensitivity.
In cardiac arrest, a global cerebral ischemic insult and/or subsequent reperfusion with return of circulation may result in various degrees of cellular injury. This global process can lead to cytotoxic edema and cellular neuronal necrosis or apoptosis within hours following reperfusion (15–18). Loss of GWM differentiation on CT can be explained by the preferential vulnerability of gray matter over white matter with resultant cytotoxic edema and the subsequent radiographic loss of distinction between the two regions (17). The presence of basilar cistern effacement and sulcal effacement is due to cerebral edema and limited intracranial capacitance, thus compromising the cerebrospinal fluid space.
Reversal sign is seen on CT as a diffuse decrease in the density of the cerebral hemispheres, with a relative increase in the density of the thalami, brain stem, and cerebellum. This may occur because of distention of the deep medullary veins due to partial venous outflow obstruction because of increased intracranial pressure from cerebral edema or preferential posterior circulation blood flow. Once again shown with our data, reversal sign is associated with poor outcome (19). The 10 children with reversal sign in our study all died.
Twenty-nine of the 32 patients (91%) with loss of GWM died, and the three patients who survived had an unfavorable neurologic outcome. In our cohort, the PPV of qualitative loss of GWM differentiation for death was 91% (AUC, 0.84) and unfavorable outcome was 100% (AUC, 0.82). The median time to imaging in our cohort was 3 hours, thus CT signs of swelling were demonstrable early after cardiac arrest.
Our findings are consistent with adult cardiac arrest data. Loss of GWM differentiation is associated with poor outcome and death regardless of arrest etiology (8, 20). By contrast, there is a paucity of published pediatric data. Rafaat et al (11) evaluated 156 children following drowning episodes, of whom 101 (65%) received CPR. Twenty-eight percent of the children (28 of 101) had an initial abnormal post–cardiac arrest head CT and all 28 died.
We specifically quantified the ratio of Hounsfield units between gray matter and white matter and found that patients who died or had unfavorable neurologic outcome had lower HUr. Choi et al (10) evaluated 28 adults resuscitated from OHCA who had head CT scan within 24 hours of resuscitation and found that the GWM ratio was significantly lower in patients in a vegetative state or death (20). When we applied a specific HUr cutpoint for poor prognosis of 1.2, the AUC was 84%; however, we had a low sensitivity but a high specificity for death. Several adult studies have sought to determine cutpoints for prognostication of poor outcome based on quantitative GWM ratios, most resulting in low sensitivity but high specificity and PPVs (10). These threshold values have ranged from 1.14 to 1.22 to be 100% predictive of poor outcome in retrospective cohorts (10, 17, 21, 22). Recent approaches to validate a prospective cutpoint of 1.2 among adults post–cardiac arrest had a sensitivity of 52–56% for poor outcome and a PPV of 81–85% for poor outcome. When the selected cutpoint was decreased to 1.1, the sensitivity for poor outcome dropped to 13–19%, whereas the PPV increased to 88–100% (23). In our cohort to achieve a specificity of 100%, a cutpoint of 1.28 HUr would be required. Because myelination occurs during the first 2 years of development, we stratified our age groups and found that to achieve 100% specificity for survival a cutpoint of 1.09 would be required for those younger than 2 and 1.28 for those older than 2. These differences may be important for future evaluation of qualitative cutpoints for outcome in pediatric populations with developing brains.
All patients with sulcal effacement and 13 of 14 patients with basilar effacement died. Both of these radiographic findings are due to cerebral edema. Our data indicate that their presence early after hypoxic ischemic injury represent severe brain injury. The one patient with basilar cistern effacement who survived with a favorable outcome had no other abnormal CT findings and had a spina bifida, a Chiari type II malformation, and crowding of soft tissue at the foramen magnum on multiple head CT evaluations. Therefore, basilar effacement without other signs of cerebral edema and anoxic injury must be carefully considered. The duration of CPR only mattered if the early head CT was abnormal, but not if the CT was normal. This is likely because patients who had abnormal early head CT scan had a more prolonged low flow or no flow state, which led to more severe injury. Inamasu et al (8) demonstrated that adults who received greater than or equal to 20 minutes CPR were more likely to have sulcal effacement and no patient who had sulcal effacement survived with a favorable neurologic outcome.
Children who had a normal head CT within the first 24 hours following resuscitation were significantly more like to survive and survive with a favorable neurologic outcome. Rafaat et al (11) evaluated 73 children who had a normal CT following CPR for drowning, of which 24 were reported to have an abnormal repeat CT and only one of 24 (4%) survived with a favorable neurological outcome. The outcomes for 59 patients with an initially normal head CT and no repeat CT were not documented (11).
Clinicians caring for the pediatric postarrest patient should incorporate these data into their diagnosis and treatment; however, like all early determinants of neurologic injury, CT findings are not 100% reliable to predict outcome and the use of quantitative cutpoints using HUr is premature (24). Although an abnormal early CT scan may portend a dismal prognosis, a normal early CT scan does not guarantee a full recovery. Combining CT imaging with clinical examination may improve the ability to prognosticate, but may not be able to do so until at least 72 hours (25). In the era of aggressive postresuscitation care, it is unclear whether the association of early head CT findings and neurologic outcome may change.
This study had several limitations. It was a retrospective study and therefore CT scans were obtained based on clinician preference and therefore may have selected out a cohort of patients who had concerning neurologic examinations, were at higher risk for brain injury, or had an unclear precipitating cause of arrest. Eighteen children (49%) had withdrawal of support or “do-not-resuscitate” orders. Preexisting bias (“self-fulfilling prophecy”) may have led to withdrawal of therapy based on physician interpretation of the head CT. However, more than half of the 39 children who died in our cohort had brain death or inability to achieve ROSC on a subsequent cardiac arrest. Clinician opinion would not have impacted outcome for these children. A standardized targeted postresuscitation care bundle only became available in the last year of the study period, but was not available from 2005 to 2011. Nevertheless, decisions regarding brain CT imaging remain at the discretion of the clinician. Therefore, temperature management, treatment of hypotension, and timing of head imaging were variable. We omitted patients with a baseline PCPC of 4 and 5 because of our concern that there would have been baseline abnormal imaging that may have confounded our results. Finally, our measure of neurologic outcome, the PCPC score, is a gross estimation of neurologic function, so favorable neurological outcome does not preclude significant functional deficits (26).
CONCLUSIONS
In this large retrospective cohort of children who suffered an out-of-hospital cardiac arrest, loss of GWM differentiation, sulcal effacement, basilar cistern effacement, and reversal sign on head CT performed early after resuscitation were associated with mortality and unfavorable neurologic outcome. Clinicians may consider using early head CT findings in conjunction with the patient’s history and clinical findings to guide their management and counseling of families.
Supplementary Material
Acknowledgments
Dr. Topjian is funded by a National Institutes of Health career development award (K23NS075363 and U01HL094345).
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Atkins DL, Everson-Stewart S, Sears GK, et al. Resuscitation Outcomes Consortium Investigators: Epidemiology and outcomes from out-of-hospital cardiac arrest in children: The Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Circulation. 2009;119:1484–1491. doi: 10.1161/CIRCULATIONAHA.108.802678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nitta M, Iwami T, Kitamura T, et al. Utstein Osaka Project: Agespecific differences in outcomes after out-of-hospital cardiac arrests. Pediatrics. 2011;128:e812–e820. doi: 10.1542/peds.2010-3886. [DOI] [PubMed] [Google Scholar]
- 3.Moler FW, Donaldson AE, Meert K, et al. Pediatric Emergency Care Applied Research Network: Multicenter cohort study of out-of-hospital pediatric cardiac arrest. Crit Care Med. 2011;39:141–149. doi: 10.1097/CCM.0b013e3181fa3c17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wijdicks EF, Hijdra A, Young GB, et al. Quality Standards Subcommittee of the American Academy of Neurology: Practice parameter: Prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203–210. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 5.Abend NS, Licht DJ. Predicting outcome in children with hypoxic ischemic encephalopathy. Pediatr Crit Care Med. 2008;9:32–39. doi: 10.1097/01.PCC.0000288714.61037.56. [DOI] [PubMed] [Google Scholar]
- 6.Naples R, Ellison E, Brady WJ. Cranial computed tomography in the resuscitated patient with cardiac arrest. Am J Emerg Med. 2009;27:63–67. doi: 10.1016/j.ajem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Cocchi MN, Lucas JM, Salciccioli J, et al. The role of cranial computed tomography in the immediate post-cardiac arrest period. Intern Emerg Med. 2010;5:533–538. doi: 10.1007/s11739-010-0403-8. [DOI] [PubMed] [Google Scholar]
- 8.Inamasu J, Miyatake S, Suzuki M, et al. Early CT signs in out-of-hospital cardiac arrest survivors: Temporal profile and prognostic significance. Resuscitation. 2010;81:534–538. doi: 10.1016/j.resuscitation.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Inamasu J, Miyatake S, Tomioka H, et al. Cardiac arrest due to food asphyxiation in adults: Resuscitation profiles and outcomes. Resuscitation. 2010;81:1082–1086. doi: 10.1016/j.resuscitation.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 10.Choi SP, Park HK, Park KN, et al. The density ratio of grey to white matter on computed tomography as an early predictor of vegetative state or death after cardiac arrest. Emerg Med J. 2008;25:666–669. doi: 10.1136/emj.2007.053306. [DOI] [PubMed] [Google Scholar]
- 11.Rafaat KT, Spear RM, Kuelbs C, et al. Cranial computed tomographic findings in a large group of children with drowning: Diagnostic, prognostic, and forensic implications. Pediatr Crit Care Med. 2008;9:567–572. doi: 10.1097/PCC.0b013e31818c8955. [DOI] [PubMed] [Google Scholar]
- 12.Gentsch A, Storm C, Leithner C, et al. Outcome prediction in patients after cardiac arrest: A simplified method for determination of gray-white matter ratio in cranial computed tomography. Clin Neuroradiol. 2015;25:49–54. doi: 10.1007/s00062-013-0281-3. [DOI] [PubMed] [Google Scholar]
- 13.Fiser DH, Long N, Roberson PK, et al. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 14.Nadkarni VM, Larkin GL, Peberdy MA, et al. National Registry of Cardiopulmonary Resuscitation Investigators: First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295:50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 15.Deshpande JK, Siesjö BK, Wieloch T. Calcium accumulation and neuronal damage in the rat hippocampus following cerebral ischemia. J Cereb Blood Flow Metab. 1987;7:89–95. doi: 10.1038/jcbfm.1987.13. [DOI] [PubMed] [Google Scholar]
- 16.Martins E, Inamura K, Themner K, et al. Accumulation of calcium and loss of potassium in the hippocampus following transient cerebral ischemia: A proton microprobe study. J Cereb Blood Flow Metab. 1988;8:531–538. doi: 10.1038/jcbfm.1988.93. [DOI] [PubMed] [Google Scholar]
- 17.Torbey MT, Selim M, Knorr J, et al. Quantitative analysis of the loss of distinction between gray and white matter in comatose patients after cardiac arrest. Stroke. 2000;31:2163–2167. doi: 10.1161/01.str.31.9.2163. [DOI] [PubMed] [Google Scholar]
- 18.Schuier FJ, Hossmann KA. Experimental brain infarcts in cats. II. Ischemic brain edema. Stroke. 1980;11:593–601. doi: 10.1161/01.str.11.6.593. [DOI] [PubMed] [Google Scholar]
- 19.Han BK, Towbin RB, De Courten-Myers G, et al. Reversal sign on CT: Effect of anoxic/ischemic cerebral injury in children. AJNR Am J Neuroradiol. 1989;10:1191–1198. [PMC free article] [PubMed] [Google Scholar]
- 20.Inamasu J, Miyatake S, Nakatsukasa M, et al. Loss of gray-white matter discrimination as an early CT sign of brain ischemia/hypoxia in victims of asphyxial cardiac arrest. Emerg Radiol. 2011;18:295–298. doi: 10.1007/s10140-011-0954-7. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Choi SP, Park KN, et al. Early brain computed tomography findings are associated with outcome in patients treated with therapeutic hypothermia after out-of-hospital cardiac arrest. Scand J Trauma Resusc Emerg Med. 2013;21:57. doi: 10.1186/1757-7241-21-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metter RB, Rittenberger JC, Guyette FX, et al. Association between a quantitative CT scan measure of brain edema and outcome after cardiac arrest. Resuscitation. 2011;82:1180–1185. doi: 10.1016/j.resuscitation.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cristia C, Ho ML, Levy S, et al. The association between a quantitative computed tomography (CT) measurement of cerebral edema and outcomes in post-cardiac arrest—A validation study. Resuscitation. 2014;85:1348–1353. doi: 10.1016/j.resuscitation.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn DK, Geocadin RG, Greer DM. Quality of evidence in studies evaluating neuroimaging for neurologic prognostication in adult patients resuscitated from cardiac arrest. Resuscitation. 2014;85:165–172. doi: 10.1016/j.resuscitation.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 25.Wu O, Batista LM, Lima FO, et al. Predicting clinical outcome in comatose cardiac arrest patients using early noncontrast computed tomography. Stroke. 2011;42:985–992. doi: 10.1161/STROKEAHA.110.594879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollack MM, Holubkov R, Funai T, et al. Relationship between the functional status scale and the pediatric overall performance category and pediatric cerebral performance category scales. JAMA Pediatr. 2014;168:671–676. doi: 10.1001/jamapediatrics.2013.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.