Abstract
We used Demographic and Health Survey data to describe the 2-week period prevalence of fever, cough, and diarrhea among children aged < 5 years in low-resources areas. We then explored the relationship between prevalence of isolated fever and national malaria risk. Fever and isolated fever occurred in 26.7% and 7.0% of children, respectively, and was not fully explained by malaria.
Introduction
Diarrhea and pneumonia are widely recognized to be major contributors to illness among infants and children in low-resource areas.1 Investments in etiology research are yielding important insights into the major causes of diarrhea2 and pneumonia,3 providing valuable information to improve disease management and prevention efforts. However, fever is also common among infants and children in low-resource areas in the tropics.4 For patients with fever presenting without localizing features, clinical diagnosis is difficult and malaria may be the default diagnosis. More widespread use of malaria diagnostic tests has unmasked the problem of malaria over diagnosis.5 In addition to clinical misdiagnosis in life, the use of verbal autopsies to estimate malaria-attributable deaths lacks specificity,6 contributing to overestimation of the prevalence of malaria and misclassification of febrile deaths in burden of disease estimates. Apparent declines in malaria over the past decade mean that clinicians now face a growing proportion of febrile children without malaria. Sparse clinical microbiology services and limited comprehensive fever etiology research7 mean that the local epidemiology of causes of fever is seldom well understood.
Efforts to address fever in low-resource areas will require wider awareness of its prevalence and a robust understanding of the contribution of malaria relative to other causes. To provide a picture of the community prevalence of fever alone and with cough or diarrhea among children, we analyzed national Demographic and Health Survey (DHS) data. To explore the contribution of malaria to fever prevalence, we studied the relationship between fever prevalence and national proportion of population for the age 0–4 years at risk for malaria.
Methods and Materials
Community prevalence of fever, cough, and diarrhea.
We sought data from all available DHS from all years and countries.8 DHS surveys are conducted at the household level and seek information about socioeconomic characteristics and health indicators such as maternal and child health, malaria, nutrition, human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), and family planning. The main objective of the DHS is to collect a range of data using standardized procedures and methodologies to enable comparisons across countries and over time. Three core questionnaires are administered to each household selected for a standard DHS: a household questionnaire, a women's questionnaire, and a men's questionnaire.
From the women's questionnaire, administered to all 15- to 49-year-old women who spent the night before the visit in the surveyed household, we extracted responses to three questions about the health of children aged < 5 years: 1) has the child had diarrhea in the past 2 weeks?, 2) has the child been ill with fever at any time in the past 2 weeks?, and 3) has the child had an illness with a cough at any time in the past 2 weeks? In addition, we extracted the country code and year of study.
National malaria risk.
We used the Malaria Atlas Project (MAP) data9 for the proportion of the population per 100,000 at risk for Plasmodium falciparum, 2010, as an indicator of national malaria risk. The main objective of the MAP is to produce maps and estimates that support planning of national and international malaria programs. For our analysis, estimates for the age group 0–4 years from countries with corresponding DHS data on the community prevalence of fever were obtained.
Statistical analyses.
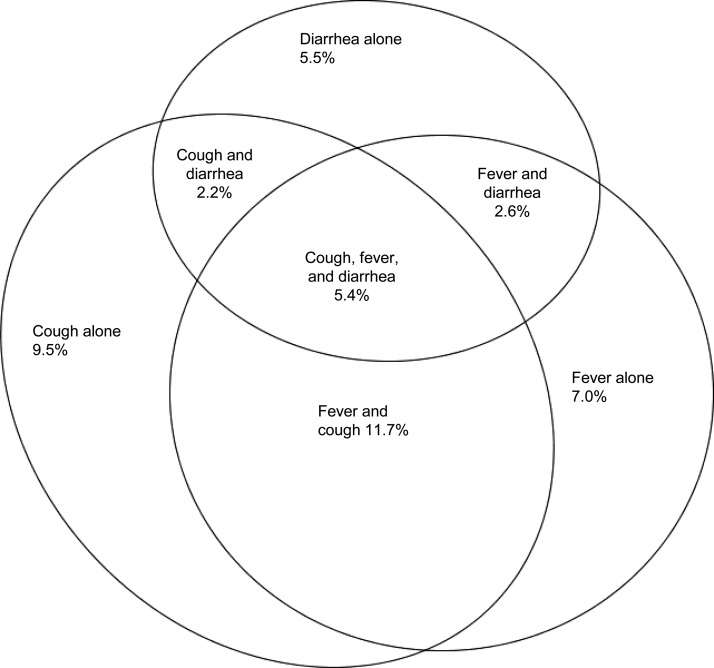
Responses to the three DHS symptom questions were aggregated to develop the combined 2-week period prevalence of fever, cough, and diarrhea, and of combinations of these symptoms. A Venn diagram illustrating the proportion of children experiencing these symptoms during a 2-week period was drawn using eulerAPE software (University of Kent, Canterbury, United Kingdom).10
Country-level MAP data for estimates of the population in age group 0–4 years at risk of P. falciparum malaria per 100,000 in the year 2010 were merged with fever prevalence data from the most recent corresponding DHS surveys to optimize matching of data in time. The relationship between population at risk of P. falciparum malaria per 100,000 and the 2-week period prevalence of fever in each country in our study was examined using a logistic regression model. Because of the skewness, a log scale was used for the fraction of the population under 5 years at risk of malaria. In addition, we examined regional differences in the relationship between population at risk of P. falciparum malaria per 100,000 and the 2-week period prevalence of fever. Analyses were carried out in Stata (StataCorp LP, College Station, TX).
Results
Two-week period prevalence of fever, cough, and diarrhea were available from 182 standard DHS surveys from 73 countries. The median (range) year of survey initiation was 1999 (1986–2012). In total, 1,468,396 children were surveyed with a median (range) of 6,070 (1,127–51,555) children per survey. Of children included, 794,088 (54.1%) were from the African region and 429,638 (29.3%) from the Asian region.
Of the 182 surveys, 158 (86.8%) included 1,200,986 (81.8%) children who were surveyed for all three symptoms. Across the standard DHS for the period 1986–2012, the 2-week period prevalence of diarrhea among children aged < 5 years was 205,547 (15.7%) of 1,305,863; cough was 352,161 (28.8%) of 1,223,220; and fever was 330,538 (26.7%) of 1,236,411. Isolated fever, or fever not associated with diarrhea or cough, occurred in 83,605 (7.0%) of 1,200,986 children. Figure 1 provides a scaled visual representation of all three symptoms and their overlaps among children surveyed for all three symptoms.
Figure 1.
Two-week period prevalence of diarrhea, cough, and fever, and combinations of these in children aged < 5 years surveyed in standard Demographic and Health Surveys (DHS), 1986–2012, N = 1,200,986.
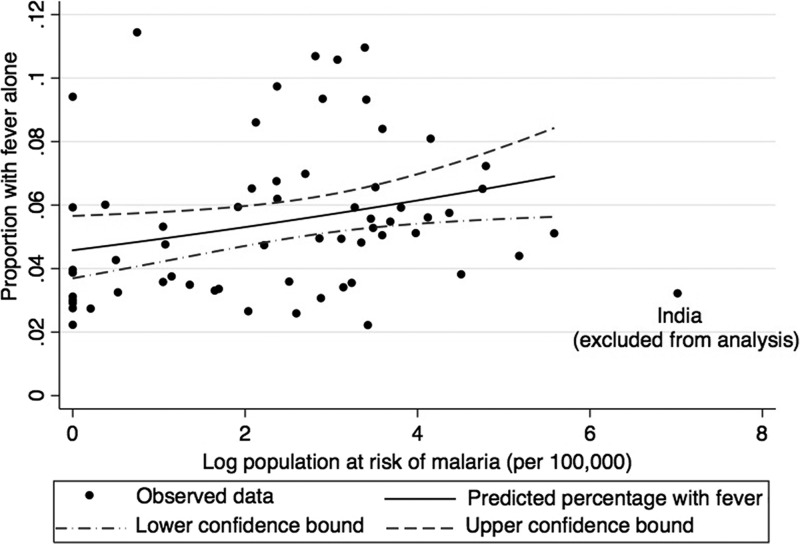
Of surveys, the 64 (35.2%) most contemporary with a median (range) year of 2008 (1995–2012) were merged with MAP data for the investigation of the relationship between isolated fever prevalence and national malaria risk. We hypothesized that the prevalence of isolated fever would increase with increasing national risk of malaria, and that we could estimate the proportion of fever that was due to causes other than malaria from the countries where the malaria risk was very low. In the logistic regression model, both linear and quadratic terms in log of the fraction of population at risk were statistically significant (P = 0.006 and P = 0.005, respectively), suggesting that isolated fever prevalence increased with increasing proportion of population at risk until around 50/100,000, then decreased. However, one country, India, had a much higher fraction of the population at risk of malaria (113.8/100,000 compared with the next highest of 265/100,000). A model excluding India suggested a 20% increase in prevalence of isolated fever with every 10-fold increase in fraction of the population at risk (P = 0.025) (Figure 2). Regional analysis showed a statistically significant quadratic relationship between log population at risk of P. falciparum malaria per 100,000 and the 2-week period prevalence of isolated fever for Africa (N = 37 countries, P = 0.01 for both the linear and squared terms). However, there was no evidence of a relationship for Asia, both with or without India. There were insufficient data to formally test whether the relationship was different in the Africa region compared with the Asia region.
Figure 2.
Population aged 0–4 years at risk of Plasmodium falciparum per 100,000 in 2010 vs. isolated 2-week period prevalence of fever, in children aged 0–5, by country excluding India, 1990–2012.
Discussion
We found that fever was reported almost as often as cough and more often than diarrhea among children aged < 5 years in the low-resource areas included in this study. Although there is considerable overlap in the occurrence of fever, cough, and diarrhea; 7% of children experienced an isolated episode of fever during a 2-week period. We found a weak but statistically significant relationship between 2-week isolated fever prevalence and one measure of national malaria risk, indicating that isolated fever prevalence did increase with national malaria risk. However, there was substantial variation around the line of best fit, suggesting that other predictors of fever prevalence may be more important.
Global burden of disease estimates of morbidity, measured by disability-adjusted life years (DALYs), and mortality have a major influence on resource allocation, targeting of disease-control efforts, and establishment of research priorities. For estimates of morbidity and mortality associated with infectious diseases, the syndromes of diarrhea and respiratory infection account for a large proportion of global DALYs; 3.6% and 4.6%, respectively.1 Because of the structure of disease burden estimates, the contribution of the syndrome of fever as a whole is currently unmeasured. Our findings suggest that fever, including isolated fever, is sufficiently common at the community level to warrant a coordinated approach to understanding and addressing its causes. Furthermore, malaria does not appear to be the major driver of fever among children highlighting the importance of building a clearer picture of causes of non-malaria fever to inform both patient management and prevention efforts. In this structure of burden of disease estimates, the various conditions causing febrile illness are distributed across a range of disorders and diseases with some grouped together in the “other” or “neglected tropical disease” category. Aggregation of these disorders and diseases would help to focus attention on the syndrome of fever as a public health problem. Although our study was not designed to determine the severity or outcomes of fever, cough, or diarrhea at the community level; severe non-malaria fever is known to carry case fatality ratios that equal or exceed malaria in low-resource areas.5
Our study has a number of limitations. DHS data rely on information gathered from caregivers, and responses may be colored by widely varying beliefs about and perceptions of symptoms and their causes.11 The use of a 2-week recall period for illness history has been shown to lead to an underestimation of disease incidence, and may have affected our findings.12 Because DHS and malaria data were aggregated at the national level, we were unable to explore potentially important subnational relationships. Furthermore, national malaria data were not always exactly contemporaneous with DHS surveys. In addition, the relationship between malaria infection and disease, including fever, is complex, influenced by transmission intensity, age, and other factors not accounted for in our analysis. Finally, since symptom histories covered a 2-week period, symptom overlaps do not necessary indicate simultaneous occurrence or the same underlying cause for overlapping symptoms.
Despite the limitations, our analysis provides a valuable insight into the prevalence of three major infectious disease-associated symptoms. We propose that fever without localizing features be addressed as a syndrome in burden of disease efforts. Multicenter research is needed in low-resource areas to define the major treatable and preventable causes of non-malaria febrile illness.
ACKNOWLEDGMENTS
We thank the Demographic and Health Surveys Program and the Malaria Atlas Project for providing publicly available data for this project.
Disclaimer: The study funders had no role in the study design, implementation, analysis, manuscript preparation, or decision to submit this article for publication.
Footnotes
Financial support: JAC was supported by the joint U.S. National Institutes of Health–National Science Foundation Ecology of Infectious Disease program (R01TW009237), the U.K. Biotechnology and Biological Sciences Research Council (BBSRC) (BB/J010367/1) the U.K. BBSRC Zoonoses and Emerging Livestock Systems Initiative (BB/L017679, BB/L018926/1 and BB/L018845).
Authors' addresses: Namrata Prasad and John A. Crump, Centre for International Health, University of Otago, Dunedin, New Zealand, E-mails: nam.p@me.com and john.crump@otago.ac.nz. Katrina J. Sharples, Department of Preventive and Social Medicine, Dunedin School of Medicine, University of Otago, Dunedin, New Zealand, E-mail: katrina.sharples@otago.ac.nz. David R. Murdoch, Department of Pathology, University of Otago, Christchurch, Canterbury, New Zealand, E-mail: david.murdoch@otago.ac.nz.
References
- 1.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 3.Levine OS, O'Brien KL, Deloria-Knoll M, Murdoch DR, Feikin DR, DeLuca AN, Driscoll AJ, Baggett HC, Brooks WA, Howie SR. The Pneumonia Etiology Research for Child Health Project: a 21st century childhood pneumonia etiology study. Clin Infect Dis. 2012;54:S93–S101. doi: 10.1093/cid/cir1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feikin DR, Olack B, Bigogo GM, Audi A, Cosmas L, Aura B, Burke H, Njenga MK, Williamson J, Breiman RF. The burden of common infectious disease syndromes at the clinic and household level from population-based surveillance in rural and urban Kenya. PLoS ONE. 2011;6:e16085. doi: 10.1371/journal.pone.0016085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, Saganda K, Shao J, Kitua A, Olomi R. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ. 2004;329:1212. doi: 10.1136/bmj.38251.658229.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todd J, De Francisco A, O'dempsey T, Greenwood BM. The limitations of verbal autopsy in a malaria-endemic region. Ann Trop Paediatr. 1993;14:31–36. doi: 10.1080/02724936.1994.11747689. [DOI] [PubMed] [Google Scholar]
- 7.Crump JA. Time for a comprehensive approach to the syndrome of fever in the tropics. Trans R Soc Trop Med Hyg. 2014;108:61–62. doi: 10.1093/trstmh/trt120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Demographic and Health Surveys Program http://dhsprogram.com/ Available at. Accessed April 29, 2014.
- 9.Malaria Atlas Project (MAP) http://www.map.ox.ac.uk/ Available at. Accessed April 29, 2014.
- 10.Micallef L, Rodgers P. eulerAPE: drawing area-proportional 3-Venn diagrams using ellipses. PLoS One. 2014;9:e101717. doi: 10.1371/journal.pone.0101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hertz JT, Munishi OM, Sharp JP, Reddy EA, Crump JA. Comparing actual and perceived causes of fever among community members in a low malaria transmission setting in northern Tanzania. Trop Med Int Health. 2013;18:1406–1415. doi: 10.1111/tmi.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feikin DR, Audi A, Olack B, Bigogo GM, Polyak C, Burke H, Williamson J, Breiman RF. Evaluation of the optimal recall period for disease symptoms in home-based morbidity surveillance in rural and urban Kenya. Int J Epidemiol. 2010;39:450–458. doi: 10.1093/ije/dyp374. [DOI] [PMC free article] [PubMed] [Google Scholar]