Abstract
Dengue is of public health importance in tropical and sub-tropical regions. Dengue virus (DENV) transmission dynamics was studied in Kamphaeng Phet Province, Thailand, using an enhanced spatiotemporal surveillance of 93 hospitalized subjects with confirmed dengue (initiates) and associated cluster individuals (associates) with entomologic sampling. A total of 438 associates were enrolled from 208 houses with household members with a history of fever, located within a 200-m radius of an initiate case. Of 409 associates, 86 (21%) had laboratory-confirmed DENV infection. A total of 63 (1.8%) of the 3,565 mosquitoes collected were dengue polymerase chain reaction positive (PCR+). There was a significant relationship between spatial proximity to the initiate case and likelihood of detecting DENV from associate cases and Aedes mosquitoes. The viral detection rate from human hosts and mosquito vectors in this study was higher than previously observed by the study team in the same geographic area using different methodologies. We propose that the sampling strategy used in this study could support surveillance of DENV transmission and vector interactions.
Introduction
Dengue illness is a disease of increasing public health importance.1 Available data and modeling estimate that there are 390 million dengue virus (DENV) infections annually with 96 million manifesting clinically.2 International travel, population growth, increasing urbanization, and a changing global ecology foster an increasingly favorable environment for the expanding dengue endemic areas and the peridomestic Aedes aegypti, which transmit the viruses.3 There are currently no licensed drugs or vaccines to treat or prevent dengue. When applied properly, vector control and personal protective measures have successfully disrupted epidemic and endemic DENV transmission.4,5 Unfortunately, successful vector control programs have been the exception and difficult to sustain.6 The strategic use of safe and efficacious dengue vaccines in combination with appropriately targeted and sustained vector control measures is increasingly being considered as the optimal approach to produce a sustained reduction in dengue's global burden.7
Once a vaccine is available, numerous questions will remain about how to most effectively target and co-implement vaccination and vector control programs. The prospect of implementing large-scale, control programs raises a number of questions:
-
1)
How will vaccination and vector control affect the complex, dynamic, and evolving interactions between vector, virus, and host occurring at the macro (i.e., country or region) and micro (i.e., province, district, village, or neighborhood) spatial scales?
-
2)
How will vaccination and vector control affect the complex, dynamic, and evolving interactions between vector, virus, and host at the population (i.e., Aedes species, DENV serotypes and genotypes, and people of various ethnic backgrounds) level?
-
3)
How will “herd immunity” be affected and how will this influence DENV evolution at the micro- and macro-population levels and the associated observed clinical phenotypes?
-
4)
What effect, if any, will existing herd immunity (due to vaccination or natural infection) to non-dengue flaviviruses (e.g., Japanese encephalitis and yellow fever viruses) have on DENV transmission and the observed clinical phenotypes following infection?
The overarching study objective was to explore DENV transmission dynamics and virus–vector–host interactions prior to, during, and following the introduction of dengue vaccines into central Thailand. The authors pursued this objective by building on observations from previous prospective studies such as the focality of DENV transmission, presence or history of fever increasing the likelihood of isolating virus from a household, and the significance of year-round DENV transmission.8–10 Study methods were modified in an effort to maximize DENV isolation rates from human hosts and mosquito vectors and further explore earlier observations, which included:
-
1)
Enrolling initiate and associate cases throughout the year (i.e., high dengue season and low dengue season) to explore trends in seasonal and spatial DENV transmission.
-
2)
Only enrolling DENV polymerase chain reaction positive (PCR+) initiate cases to increase the likelihood of capturing active transmission.
-
3)
Only enrolling associates with fever or a history of fever within the last 7 days or sharing a household with someone meeting these criteria to increase the likelihood of identifying associates with recent infection.
-
4)
Reassessing associates for the occurrence of fever between the acute and convalescent blood collection to capture additional viremic cases.
-
5)
Expanding the age of enrollment to include children above the age of 6 months and adults to improve understanding of transmission inside the home.
-
6)
Extending the enrollment of associates and mosquito collection from 100 to 200 m radius around the initiate.
In this report, the authors describe initiation of the baseline phase (i.e., prior to vaccine introduction) and include detailed accounts of study methodology and the entomologic, clinical, epidemiologic, virologic, serologic, and molecular characterization of human cases captured by active and passive surveillance methods between November 2009 and December 2010.
Methods
Ethics statement.
The study protocol was approved by the Institutional Review Boards (IRBs) of the Thai Ministry of Public Health (MOPH), Walter Reed Army Institute of Research (WRAIR), and the State University of New York (SUNY), Upstate Medical University. The IRBs of the University of California, Davis (UCD), University of Rhode Island (URI), and University at Buffalo established relying agreements with WRAIR IRB.
All study subjects engaged in documented informed consent or assent process, as applicable, prior to participating in any study activities. In the event the subject was unable to participate in the informed consent/assent process, a recognized health-care proxy represented them in the process and documented consent. From this point forward, when the authors discuss consent, assent is also implied as applicable.
Role of the funding source.
Funding sources for this project included National Institutes of Health grants R01 GM083224-01 and P01 AI034533. Additional funding was provided by the U.S. Military Infectious Diseases Research Program. The funding sources had no involvement in study design, data collection, analysis or interpretation, report writing, or publication submission. The corresponding author had full access to all study data and final responsibility for the decision for publication.
Study location.
The study was conducted in Kamphaeng Phet (KPP) Province in northcentral Thailand. There were 725,846 registered residents of KPP in 2009 (Thailand, Department of Provincial Administration, 2010). The KPP Provincial Hospital (KPPPH) is located in the province's central district. The Armed Forces Research Institute of Medical Sciences (AFRIMS), Department of Virology field site (KPP AFRIMS Virology Research Unit [KAVRU]) is located on the KPPPH grounds. The Department of Entomology, AFRIMS field site is located a short distance from KPPPH.11
Demographics of house residents were collected and house spatial coordinates were identified using a geographic positioning system (GPS) handheld unit (Trimble® GeoXH™, GeoExplorer® 2008 series; Trimble Navigation Limited, ASC Scientific Carlsbad, CA) and geo-coded into a geographic information system (GIS) database (CorporationArcMap™, version 9.1; ESRI, Redlands, CA).
Study definitions.
The authors recognize the potential confusion using terms such as “index case,” “contacts,” and “cluster investigations.” As the index case may not be the true first infection in space and time and the contact may not be a true infection resulting from DENV transmission from the initiating case, we have attempted to more accurately define the relationships investigated in this study without making claims of causation. The hospitalized dengue cases serving as the initiator of community transmission investigations are referred to as the “initiating case,” or “initiate.” The enrolled individuals residing within the initiate's home or within 200 m of the initiate are referred to as “associates” and if found to have DENV infection, as “associate cases.” The combination of the initiate, associate, and associate cases is referred to as a “spatiotemporal group.”.
Symptomatic DENV infection in either initiating or associated cases are defined as any febrile illness (reported or measured fever) paired with a confirmatory molecular or serologic assay run on the acute or acute and convalescent blood sample pair, respectively. Subclinical DENV infections are defined as associated cases (all initiating cases were symptomatic and hospitalized) with a positive molecular or serologic result, but without reported or measured fever during the period between the acute and convalescent blood sample collection (0–14 days).
Subjects are serologically classified as having an “acute” DENV infection if their acute and/or convalescent blood sample pair (or day 0 and/or day 14 blood samples in associates) was dengue immunoglobulin M (IgM) positive; or if IgM is negative, immunoglobulin M (IgG) is positive with rising titer. Subjects are classified as “recent” DENV infection if IgM was negative and IgG was positive with declining titer. These are further categorized as “primary” infection if the IgM to IgG ratio is ≥ 1.8 and “secondary” infection if the ratio is < 1.8.12
Initiate case identification and evaluation.
KAVRU provides the KPPPH dengue diagnostic research assays for patients presenting with fever or history of fever and dengue-like symptoms as outlined in the World Health Organization (WHO) guidelines for the diagnosis and treatment of dengue.13 Hospital staff members identified suspected dengue cases, completed and documented the informed consent process allowing the testing of a blood sample, and then sent the sample to KAVRU for reverse transcriptase PCR (RT-PCR) testing. Virus RNA was extracted from human serum or mosquito suspension using Qiagen Viral RNA Extraction kits. Serotype-specific DNA fragments from each unknown sample were amplified by TaqDNA polymerase through RT-PCR performed at KAVRU following modifications to the Lanciotti protocol.14 Suspected cases > 6 months of age, that provided a sample collected within the prior 24 hours, and had detectable DENV RNA by RT-PCR were provided the opportunity for study enrollment as initiate cases. Following acquisition of informed consent, the acute blood sample was accessed and served as the baseline sample. Demographic and clinical laboratory information was collected. Initiate case house spatial coordinates were recorded and geo-coded into a GIS database. Mosquitoes inside and outside the initiate home were collected by aspiration and processed as described in preparation for DENV RNA detection RT-PCR.10 In approximately 14 days, a second blood sample was collected from the initiating case for serologic testing by in-house IgM/IgG enzyme immunoassay (EIA).
Associate case identification and evaluation.
Residents > 6 months of age sharing the initiate household as their primary residence were provided the opportunity for study enrollment. Residents previously enrolled as an associate within the past 6 months were excluded in an attempt to improve geographic diversity and the likelihood of isolating DENV (i.e., reduce chances of recent infection and lingering homo- or heterotypic immunity). Following the informed consent process, clinical information and a blood sample were collected. In approximately 14 days, a second blood sample was collected for serologic testing. General well-being of the associates was tracked by active surveillance (i.e., combination of self-reporting and outreach by staff via phone or daily home visit by village health worker over the 14-day period). If, between the day of enrollment and the day of convalescent blood sampling, the associate develops fever, a second acute blood sample was collected and the 14-day “clock” started again. Therefore, it was possible an associate might have two acute samples, one triggered as an associate of the initiating case (acute sample 1) and one triggered by the development of illness (acute sample 2), as well as one convalescent sample.
Individuals not residing within the same house as the initiate case, but living in a home within a 200-m radius of the initiate case were also considered for enrollment as associates. If anyone within the home reported an active fever or history of fever (temperature ≥ 38°C) within the past 7 days, all residents of the home, > 6 months of age, were eligible for enrollment. Following the informed consent process, residents had clinical and demographic information and an acute blood sample collected. Associates were followed and blood collected as detailed above.
House mapping, associate case home identification, and entomologic sampling.
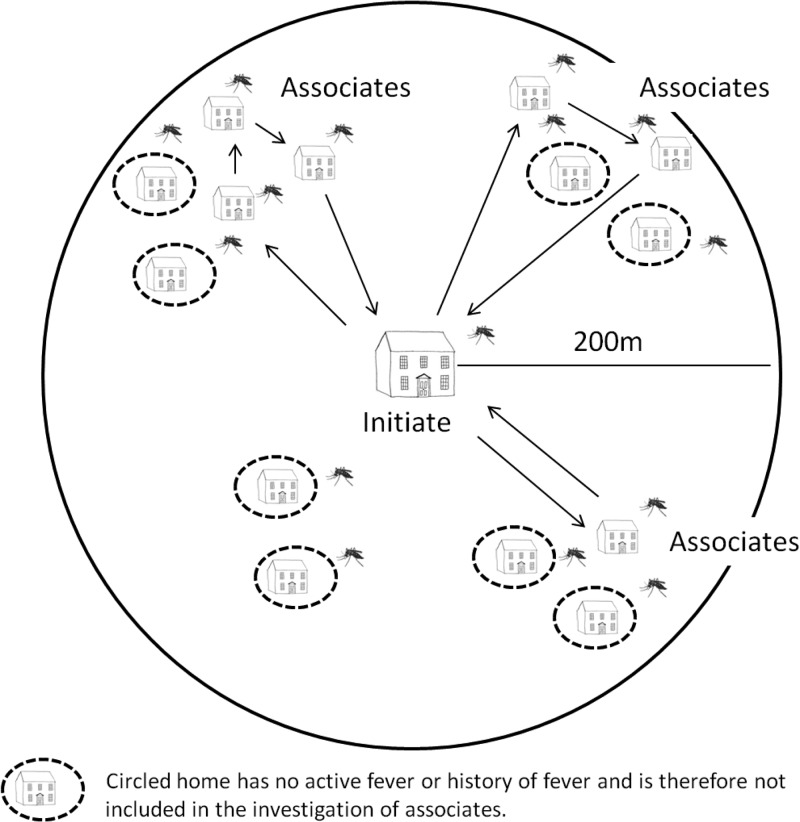
GPS mapped initiate households were used to construct a digital map, enabling the team to precisely identify houses located within a 200-m radius of the initiate case.10,15 Study nurses visited households starting closest to the initiate house and moving in sequential fashion to the periphery of the area and back to the initiate house, then back out again along a different line until the circle was complete or 25 eligible associates were enrolled, whichever occurred first (Figure 1).
Figure 1.
Method of identifying and enrolling associates around an initiating case. The clinical research team would begin at the initiate's home, identify a collection of homes within 200 m of the initiate's home, identify homes with an occupant having active fever or history of fever within the past 7 days, and enroll associates only from those homes while bypassing homes without active fever or history of fever. The entomology research team, meanwhile, collected mosquitoes inside and outside the home from all homes within a 200-m radius of the initiating case home regardless of fever history. Once investigations and enrollment of associates were completed in one grouping, the teams would return to the initiating case's home and then identify, using the aerial map, the next grouping of homes moving in a clockwise manner.
On day 1 of each initiate/associate case investigation, adult Ae. aegypti were collected using standard backpack aspirators from inside and within the immediate vicinity of each potential associate's house. The end of each aspirator tube was fitted with a 1-pint cardboard cage. After completing the collection for each home, the cage was labeled and stored on dry ice for transportation to the laboratory where the chilled mosquitoes were examined, speciated, and processed for DENV detection. A thorough adult aspiration collection usually requires ∼10–15 minutes per house. It was estimated that approximately 25% of the adult Ae. aegypti were captured in a single pass through the house (T. W. Scott, unpublished data). All specimens were identified to species by entomology field supervisors to ensure speciation accuracy and quality control. As previously mentioned, mosquitoes inside and outside of the initiate home were collected by aspiration and processed as described with serotype-specific DENV RNA detection RT-PCR performed on each individual mosquito.10
Statistical analyses.
Data were analyzed using SPSS (SPSS for Windows version 19) and R (The R Project for Statistical Computing 2.12). Demographic, clinical, and laboratory parameters were analyzed at the initiate, associate, and house levels. Student's t test and analysis of variance (ANOVA) were used to test for differences in continuous variables. Fisher's exact test and Pearson's χ2 were used to examine associations between categorical variables.
Results
Enrollment of initiates and associates.
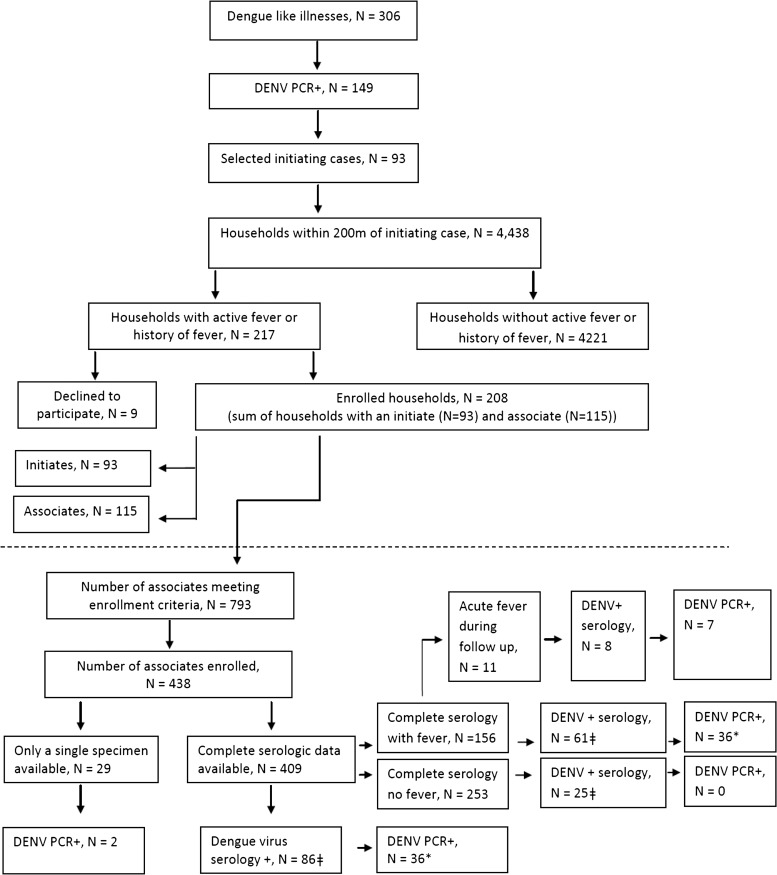
Figure 2 depicts enrollment of initiates and associates and results of serologic and molecular testing for DENV infection. Approximately 49% (149/306) of patients hospitalized with suspected dengue were PCR+ and of those 62% (93/149) were enrolled in the study. Of the 4,438 households within a 200-m radius of the 93 initiate homes, 217 (4.8%) had someone with active fever or history of fever; 115 of these associate households had volunteers who consented to enroll. Initiate and associate households contained 793 individuals meeting enrollment criteria, and 438 (55%) consented to enroll as associates. Complete serologic data were available on 93% of the enrolled associates and of these 21% had a positive dengue serology. Of the 86 associates with positive serology, 42% also were PCR+. All PCR+ associates had fever at the time of enrollment. Eleven associates who enrolled without fever developed fever following their first acute blood sampling; of these eight were serology positive and seven were PCR+.
Figure 2.
Flow diagram of initiate and associate enrollment and testing outcome.
Of note, logistic limitations guided enrollment on days with heavy dengue patient census accounting for the difference between the 149 PCR+ cases meeting inclusion criteria and the 93 ultimately enrolled. In these instances, the study team randomly selected which initiate cases would lead to an associate case investigation using a random numbers table.
Characteristics of the initiating cases.
Each of the 93 initiate cases was a hospitalized acute, PCR+ DENV infection (Table 1). Infections with DENV-2 were most numerous followed by DENV-3 and then DENV-1; there were no DENV-4 infections. Most cases were acute secondary DENV infections (85%). Approximately half of all cases were DF (48%) and half dengue hemorrhagic fever (DHF) (52%); there were no deaths among the initiates.
Table 1.
Initiate and associate group demographic data and infection characterization
| Initiate (N = 93) | Associate | |||
|---|---|---|---|---|
| Available serology (N = 409) | Available serology + fever (N = 156) | Dengue + serology (N = 86) | ||
| Age (years) | ||||
| Minimum | 2.6 | 0.58 | 0.58 | 0.83 |
| Maximum | 56.0 | 94.2 | 77.0 | 82.0 |
| Mean | 18.7 | 31.4 | 20.1 | 23.1 |
| Sex (%) | ||||
| Female | 48 | 54 | 57 | 55 |
| Male | 52 | 46 | 43 | 45 |
| Total case count = 93 | Acute sample not available, no clinical symptoms. | Total case count = 86 | ||
| Infecting DENV type (% of total typed cases) | ||||
| DENV-1 | 16 | 1 | ||
| DENV-2 | 60 | 16 | ||
| DENV-3 | 24 | 24 | ||
| DENV-4 | 0 | 0 | ||
| Not detected | 58 | |||
| Serology results (% of total results) | ||||
| Acute primary | 2 | 3 | 6 | 16 |
| Acute secondary | 85 | 17 | 25 | 80 |
| Recent secondary | 2 | 1 | 1 | 3 |
| No serologic diagnosis | 8 | 79 | 67 | |
| JE infection | 0.2 | |||
DENV = dengue virus; JE = Japanese encephalitis.
Associated cases available for enrollment.
The number of associate houses within 200 m of an initiating case ranged from 1 to 232 with a mean of 47.7 (standard deviation [SD] 42 houses). The number of households enrolled ranged from 1 to 9 with a mean of 2.2 (SD 1.5 households). For each spatiotemporal group, there was a range of associates enrolled from 0 to 18 people with a mean of 4.7 (SD 4.0 people).
Characteristics of the associated cases.
The majority (79%) of 409 enrolled associates had no serologic evidence of infection, 20% had evidence of an acute DENV infection, and there was one case with evidence of Japanese encephalitis (JE) infection (IgM+, no encephalitis; Table 1). There was complete concordance between the DENV serotype of the initiating case and the DENV serotypes detected in associate cases in that spatiotemporal unit. Nested PCR results among associates revealed that DENV-3 was the most common infection followed by DENV-2 and then DENV-1.
There was a significant difference in the reporting of nausea between primary and secondary DENV infections and significant variation in reporting of headache, rhinorrhea, cough, and retro-orbital pain among the age groups (Tables 2 and 3). There was a statistically significant difference in the probability of an associate experiencing a DENV infection based on the DENV type infecting the initiate. DENV-3 infection in the initiate carried the highest probability of associate infection (Table 4).
Table 2.
Symptom complex in primary versus secondary associate infections
| Symptom | Primary (N = 14) | Secondary (N = 72) | P value |
|---|---|---|---|
| Fever | 11 (0.79) | 50 (0.69) | 0.749 |
| Headache | 5 (0.36) | 37 (0.51) | 0.384 |
| Rhinorrhea | 3 (0.21) | 16 (0.22) | 1.000 |
| Anorexia | 5 (0.36) | 27 (0.38) | 1.000 |
| Cough | 4 (0.29) | 27 (0.38) | 0.762 |
| Nausea | 0 (0.00) | 25 (0.35) | 0.008 |
| Drowsiness | 0 (0.00) | 12 (0.17) | 0.202 |
| Muscle or joint pain | 3 (0.21) | 36 (0.50) | 0.077 |
| Abdominal pain | 2 (0.14) | 15 (0.21) | 0.727 |
| Retro-orbital pain | 3 (0.21) | 22 (0.31) | 0.749 |
| Rash | 4 (0.29) | 10 (0.14) | 0.231 |
| Diarrhea | 2 (0.14) | 14 (0.19) | 1.000 |
| Bleeding | 0 (0.00) | 5 (0.07) | 0.586 |
| Hospitalized | 1 (0.07) | 6 (0.05) | 1.000 |
Table 3.
Clinical spectrum of EIA positive associates by age groups
| Symptom | 0–9 Years (N = 22) | 10–19 Years (N = 31) | 20–29 Years (N = 10) | > 30 Years (N = 23) | P value |
|---|---|---|---|---|---|
| Fever | 18 (0.82) | 24 (0.77) | 8 (0.80) | 11 (0.48) | 0.052 |
| Headache | 6 (0.27) | 20 (0.65) | 7 (0.70) | 9 (0.39) | 0.021 |
| Rhinorrhea | 8 (0.36) | 7 (0.23) | 3 (0.30) | 1 (0.04) | 0.043 |
| Anorexia | 9 (0.41) | 11 (0.35) | 6 (0.60) | 6 (0.26) | 0.325 |
| Cough | 13 (0.59) | 11 (0.35) | 4 (0.40) | 3 (0.13) | 0.012 |
| Nausea | 4 (0.18) | 12 (0.39) | 5 (0.50) | 4 (0.17) | 0.101 |
| Drowsiness | 2 (0.09) | 7 (0.23) | 1 (0.10) | 2 (0.09) | 0.519 |
| Muscle or joint pain | 7 (0.32) | 16 (0.52) | 7 (0.70) | 9 (0.39) | 0.189 |
| Abdominal pain | 3 (0.14) | 8 (0.26) | 2 (0.20) | 4 (0.17) | 0.741 |
| Retro-orbital pain | 1 (0.05) | 15 (0.48) | 4 (0.40) | 5 (0.22) | 0.002 |
| Rash | 5 (0.23) | 6 (0.19) | 2 (0.20) | 1 (0.04) | 0.282 |
| Diarrhea | 2 (0.09) | 8 (0.26) | 3 (0.30) | 3 (0.13) | 0.285 |
| Bleeding | 2 (0.09) | 2 (0.06) | 1 (0.10) | 0 (0.00) | 0.440 |
| Hospitalized | 1 (0.05) | 3 (0.10) | 2 (0.20) | 1 (0.04) | 0.432 |
EIA = enzyme immunoassay.
Table 4.
Probability of dengue virus infection in associates according to the initiating case infecting DENV serotype
| Serologic diagnosis | Infecting DENV of initiate | ||
|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | |
| EIA negative | 43 | 191 | 88 |
| EIA positive | 4 (0.09) | 44 (0.19) | 38 (0.30) |
DENV = dengue virus; JE = Japanese encephalitis; EIA = enzyme immunoassay.
Numbers in parentheses are the proportions EIA positive. The infecting DENV type is taken from the initiating case. The differences are significant (Fisher's exact test, P value = 0.003).
Spatial distribution of associate case households.
Closer proximity of a household to the initiate was correlated with an increased associate household enrollment (Table 5). Households further from the initiate household had a lower rate of DENV infection among associate household residents (Table 6). This pattern of transmission was not gradual with distance. In an associate from a household with fever, for example, the rate of infection was similarly high (around 0.3) within 120 m and very low in households with fever beyond 120 m. This would indicate that spatiotemporal transmission in this investigation was primarily limited to 120 m and extending the radius from 100 to 200 m did not substantially increase the efficiency of detecting dengue cases or viremia.
Table 5.
Spatial distribution of households and enrolled households
| Distance from initiate household (m) | |||||
|---|---|---|---|---|---|
| > 0–40 | > 40–80 | > 80–120 | > 120–160 | > 160–200 | |
| Total households | 539 | 814 | 971 | 1,051 | 1,063 |
| Enrolled households | 103 (0.191) | 53 (0.065) | 32 (0.033) | 13 (0.012) | 7 (0.007) |
Numbers in parentheses indicate proportions of houses enrolled for each distance category. Differences are significant, P value < 0.001 by Fisher's exact test.
Table 6.
Spatial distribution of associates and associates with DENV infection by serology
| Initiate household | Distance from initiate household | |||||
|---|---|---|---|---|---|---|
| > 0–40 | > 40–80 | > 80–120 | > 120–160 | > 160–200 | ||
| Total associates with available serology | 187 | 41 | 67 | 51 | 38 | 25 |
| EIA positive associates | 42 (0.225) | 14 (0.341) | 12 (0.179) | 16 (0.314) | 0 (0.000) | 2 (0.080) |
DENV = dengue virus; JE = Japanese encephalitis; EIA = enzyme immunoassay.
Numbers in parentheses indicate proportions of associates that were EIA positive within the initiate house and for each distance category. Differences are significant, P value < 0.001 by Fisher's exact test.
DENV infection in Ae. aegypti collected in the homes of human dengue cases.
A total of 3,565 Ae. aegypti were collected from 4,438 households (i.e., all households). A total of 233 (6.5%) mosquitoes were from initiate households, of which 23 (9.9%) were DENV PCR+. There was a greater likelihood that one or more collected mosquitoes were DENV PCR+ within the initiate house or a house closer to the initiate's house (Table 7).
Table 7.
Spatial distribution of female Aedes aegypti within houses and PCR+ mosquitoes within houses
| Initiate household | Distance to initiate household | |||||
|---|---|---|---|---|---|---|
| > 0–40 | > 40–80 | > 80–120 | > 120–160 | > 160–200 | ||
| Total mosquitoes | 233 | 328 | 545 | 733 | 745 | 981 |
| PCR+ mosquitoes | 23 (0.099) | 10 (0.030) | 7 (0.013) | 13 (0.018) | 7 (0.009) | 3 (0.003) |
Numbers in parentheses indicate proportions of mosquitoes that were PCR+ for each distance category. Differences are significant, P value < 0.001 by Fisher's exact test.
PCR+ = polymerase chain reaction positive.
There were 162 initiate households with at least one associate resident who had complete serology and 212 associate households with at least one associate with complete serology. Of the initiate households, female Ae. aegypti were collected in 54.9% and 58.5% of the associate households had collected female Ae. aegypti. Of the female mosquitoes collected in initiate households 29% of households had a DENV PCR+ mosquito, whereas 6.5% of associate households had a DENV PCR+ mosquito. There was a higher likelihood of finding a DENV PCR+ mosquito in an initiate household (Table 8). Of the 31 initiate households with a DENV serology and an associate resident, 54.8% of the houses had female Ae. aegypti captured and 64.7% of these households had a DENV PCR+ mosquito. Of the 42 associate households with a DENV serology + associate, 57.1% of households had a female Ae. aegypti mosquito and one (4.1%) of these households had a DENV PCR+ mosquito. Despite a similar likelihood of finding female Ae. aegypti in households with a DENV+ serology resident, there was a higher likelihood of finding DENV PCR+ mosquitoes in the initiate household (Table 8). In total, there were 46 households with a DENV PCR+ mosquito and an associate residing with complete serology; 80.4% were initiate households (Table 8). There was a high degree of concordance between the isolated DENV serotypes from mosquitoes within a cluster and the infecting serotype of the index case (Table 9). In 2010, only one cluster (10-077) was found discordant from the mosquito (DENV-2) as compared with the index case (DENV-1).
Table 8.
Associate infection rates by infection status of female Aedes aegypti captured in households
| Initiate houses | Associate houses | |||||
|---|---|---|---|---|---|---|
| PCR+ female Ae. aegypti | Females captured Ae. aegypti | No females captured Ae. aegypti | PCR+ females Ae. aegypti | Females captured Ae. aegypti | No females captured Ae. aegypti | |
| Total associates with available serology | 26 | 88 | 71 | 8 | 125 | 90 |
| Serology positive associates | 11 (0.423) | 17 (0.193) | 14 (0.197) | 1 (0.125) | 25 (0.200) | 18 (0.200) |
PCR+ = polymerase chain reaction positive.
Numbers in parentheses indicate proportions of associates that were serology positive within the initiate house and for each category.
Table 9.
Isolated DENV serotypes from Aedes aegypti mosquitoes obtained in clusters
| Years | 2009–2010 |
|---|---|
| Number of Ae. aegypti samples | 3,545 |
| DENV-1 | 5 |
| DENV-2 | 36 |
| DENV-3 | 22 |
| DENV-4 | 0 |
DENV = dengue virus; JE = Japanese encephalitis.
Discussion
Our current study further characterizes the complex transmission dynamics and virus–vector–host interactions in a well-characterized spatial area around an infected viremic inpatient in Central Thailand. We demonstrated a significant relationship between spatial proximity to the initiate case and likelihood of detecting DENV from associate cases and A. aegypti with higher than anticipated virus detection from both human hosts and mosquito vectors. We propose that the sampling strategy described is valuable for ongoing surveillance of DENV transmission during and after field studies and the introduction of dengue vaccines.
The design of this study was built on observations from previous prospective cohort studies of DENV transmission conducted with and without initiate case and associate (contact/cluster) investigations.8–10,16,17 Modifications were made to the study design in an attempt to increase the detection of virus, symptomatic and subclinical associates of initiate cases, and infected mosquito vectors. The result was a demographically diverse group of DENV infected people representing a broad virologic, serologic, and clinical spectrum. Substantial virus detection rates were observed in both human associate cases and mosquitoes.
The age range of initiate cases was surprisingly wide (2.6–56 years), and the mean age was higher than expected at 18.7 years.18 This observation is consistent with unpublished data from the authors across additional dengue seasons and data from other sources. For example, in 2010, the Thailand MOPH reported that the highest case rate for DHF was in the 10–14-year age group, and that the 5–9- and 15–24-year age groups had the second and third highest rates, respectively. These findings are in contrast to decades of data where the mean age of hospitalized dengue was in the age range of 5–9 years.19–22 One explanation for the shift is that smaller birth cohorts had reduced number of susceptibles, thereby impacting the force of infection and the time in a person's life when they acquire their first and second DENV infection, the latter being more often associated with clinically significant disease in Thailand.18
We also enrolled associates across a wide age range (7 months–94.2 years) with a mean age almost double the initiate cases (31.4 versus 18.7 years). Serologic evidence of DENV infection and symptomatic DENV infection was observed in a number of subjects aged 40 years and older. Most of these were secondary (i.e., post-primary) DENV infections, but we also detected primary DENV infections, defined serologically, in the 50–59- and 85+-year age groups. Dengue occurs throughout the year with all four serotypes circulating with high rates of infection and transmission (hyperendemic) in KPP. Pediatric cohort studies have demonstrated high DENV infection attack rates (combined symptomatic and subclinical) in the range of 2.2–7.9% per year (average incidence 5.8%) over at least the preceding 10 years.9 Based on these observations, it was assumed that lifelong KPP residents have experienced multiple DENV infections by their late 20s. A modified plaque reduction neutralization assay (single dilution neutralization assay, 1:30 dilution, 70% viral plaque reduction) completed on a cohort of children enrolled in a KPP prospective study from 1998 to 2004 revealed a gradually increasing prevalence of neutralizing antibodies to at least one DENV type from 45% among 4-year-olds to 91% among 13-year-olds (I. K. Yoon, unpublished data).10,16,23 Our finding that all PCR+ associate cases had fever at the time of enrollment may also underscore the increasing infection burden in adults as they are more likely to experience symptomatic DENV infections compared with primary infection in children. Our observation of symptomatic dengue in older individuals merits reconsideration of traditional views on the force of infection in hyperendemic areas and the durability of homotypic and heterotypic immunity.
There was complete concordance between the infecting DENV serotypes in the initiate case and the corresponding associate cases. This finding supports the concept that DENV transmission is focal and serotype-conserved in space and time.17,24 The authors' acknowledge that the assumption that each initiating case represents the true index (i.e., first) infection in each spatiotemporal group may be incorrect. Because most DENV infections are asymptomatic or not clinically severe enough to drive health-care-seeking behavior, it is possible the initiating case simply represents the first clinically overt infection in that defined geographic area.
The ratio of symptomatic to subclinical infection among primary infections was 1:0.2; the ratio in secondary infections was 1:0.4 and similar (1:0.4) among the 86 serologically positive associates. These ratios are consistent with previous work by our group and others, including previous cluster and cohort studies, which reported symptomatic to subclinical ratios ranging from 1:0.2 to 1:18.9,10,25–28 The differences in results are most likely associated with the study's surveillance focus in and around symptomatic, PCR+ initiating cases. Previous studies conducted routine biologic sampling to capture subclinical cases and cluster studies exploring houses with or without active or a recent history of fever.9
The probability of an associate becoming infected was associated with a number of factors.29–31 As mentioned above, a significant association existed between the DENV serotype infecting the initiating case and the proportion of associates subsequently infected with the same DENV serotype (P < 0.001). Associates in samples initiated by a DENV-3 infection had a 30% chance of being serologically positive. The likelihood of infection was 19% for associates in DENV-2 samples and 9% in DENV-1 samples. Taken by itself, this may point to viral properties allowing for more efficient transmission, such as higher titer viremia in the human host or mosquito vector, a longer duration of viremia increasing the potential window for transmission to vectors and/or shorter incubation period in the mosquito. Another significant association was between the distance a potential associate lived from an initiating case and the likelihood of becoming infected (negative correlation, P < 0.001). It is reasonable to assume the factors culminating in an initiate infection (i.e., convergence of susceptible host and infected vector in space and time) would also drive efficiency of transmission and associate infections until a geographic barrier was introduced (i.e., next susceptible beyond the dispersal distance of the vector) or other factors limited transmission (i.e., protective herd immunity of associates). We did not define the relative roles of humans and mosquitoes in moving virus from one house to another within clusters, although in general, humans tend to move more often and greater distances than Ae. aegypti.29–31
Approximately, 29% of all households had female Ae. aegypti and 1.8% of the females collected were infected with DENV. Although initiating case houses and associate houses with a PCR+ contact had similar rates of female Ae. aegypti infestation (55% and 59%, respectively), the percentage of PCR+ mosquitoes was higher in the initiate case households (9.9%) compared with noninitiate households with and without a DENV positive contact, 1.2% and 1.1%, respectively. There was a significant association (P < 0.001) between the distance of a home from the initiating case and the proportion of PCR+ mosquitoes found within the house. These observations are concordant with previous reports and intuitive; once a clinical case of dengue is identified there is a high likelihood of finding infected vector(s) co-residing in the same geographic location as the ill human.
Initiate cases represented the full spectrum of DENV infections clinically, serologically, and virologically. We consider the finding of more than one in five associates of an initiating case having serologic and, in some cases, clinical evidence of an acute DENV infection significant from an epidemiologic and transmission dynamics standpoint. These results represent the initial phase of a multi-year prospective study. The study design used improved the efficiency of capturing DENV in associates and vector populations compared with previous efforts by focusing surveillance in areas and households where DENV transmission was most likely to be occurring. Our observations emphasize the focal spread of DENV and its spatial restriction associated with the mosquito vector and suggests that initiate cases were important in this study (ill and PCR+) in transmitting DENV to mosquitoes and thus to others living near the initiate. Previous studies support the observation that patient DENV viremia was a marker of human infectiousness and blood meals containing high concentrations of DENV were positively associated with the prevalence of infectious mosquitoes.32 Our results and study design may be useful when designing experiments to study fine scale patterns of DENV transmission and to increase detection of case contacts with inapparent infections.
ACKNOWLEDGMENTS
This research would not have been possible without outstanding support of the staff of the Kamphaeng Phet Provincial Hospital and the excellent research capabilities of those at the Kamphaeng Phet AFRIMS Virology Research Unit. We specifically acknowledge the efforts of Chaleaw Saengchan and Rungkarn Hangsuwan for clinical research coordinator services, and Udom Kijchalao and Arissara Pongsiri for their work in the field investigations. We thank Dr. Angkana Uppapong and Dr. Kamchai Rungsimunpaiboon for expert medical consultation and leadership, and Dr. Nuttaporn Wongsutthipakorn for the support of the provincial health office. We are especially grateful to the citizens of Kamphaeng Phet for their willingness to participate in this study and their support of over three decades of biomedical research in their community.
Disclaimer: The authors declare that no competing financial interests exist. The opinions or assertions contained herein are the private views of the authors and are not to be construed as reflecting the official views of the United States Army, the United States Department of Defense, or the National Institutes of Health.
Footnotes
Authors' addresses: Stephen J. Thomas and Richard G. Jarman, Viral Diseases Branch, Walter Reed Army Institute of Research, Silver Spring, MD, E-mails: stephen.j.thomas3.mil@mail.mil and richard.g.jarman.mil@mail.mil. Jared Aldstadt, Department of Geography, University at Buffalo, Buffalo, NY, E-mail: geojared@buffalo.edu. Darunee Buddhari, Department of Virology, United States Army Medical Component, Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand, E-mail: DaruneeT@afrims.org. In-Kyu Yoon and Robert V. Gibbons, Department of Virology, Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand, E-mails: In-Kyu.Yoon@afrims.org and robert.v.gibbons2.mil@mail.mil. Jason H. Richardson, Armed Forces Pest Management Board, Silver Spring, MD, and Walter Reed Army Institute of Research, Entomology Branch, Silver Spring, MD, E-mail: jason.h.richardson.mil@mail.mil. Alongkot Ponlawat, Entomology Branch, Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand, E-mail: AlongkotP@afrims.org. Sopon Iamsirithaworn, Ministry of Public Health, Disease Control Sciences, Nonthaburi, Thailand, E-mail: iamsiri@yahoo.com. Thomas W. Scott, Department of Entomology, University of California, Davis, Davis, CA, E-mail: twscott@ucdavis.edu. Alan L. Rothman, Institute for Immunology and Informatics, University of Rhode Island, Providence, RI, E-mail: alan_rothman@mail.uri.edu. Louis Lambrechts, Department of Genomes and Genetics, Institut Pasteur, Centre National de la Recherche Scientifique, Paris, France, E-mail: lambrechts.louis@gmail.com. Timothy P. Endy, Department of Infectious Diseases, State University of New York, Syracuse, NY, E-mail: endyt@upstate.edu.
References
- 1.Morens DM, Fauci AS. Dengue and hemorrhagic fever: a potential threat to public health in the United States. JAMA. 2008;299:214–216. doi: 10.1001/jama.2007.31-a. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diaz FJ, Farfan-Ale JA, Olson KE, Lorono-Pino MA, Gubler DJ, Blair CD, Black WC, 4th, Beaty BJ. Genetic variation within the premembrane coding region of dengue viruses from the Yucatan peninsula of Mexico. Am J Trop Med Hyg. 2002;67:93–101. doi: 10.4269/ajtmh.2002.67.93. [DOI] [PubMed] [Google Scholar]
- 4.Ooi EE, Goh KT, Gubler DJ. Dengue prevention and 35 years of vector control in Singapore. Emerg Infect Dis. 2006;12:887–893. doi: 10.3201/10.3201/eid1206.051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goh KT. Dengue—a re-emerging infectious disease in Singapore. Ann Acad Med Singapore. 1997;26:664–670. [PubMed] [Google Scholar]
- 6.Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med. 2008;5:e68. doi: 10.1371/journal.pmed.0050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas SJ. The necessity and quandaries of dengue vaccine development. J Infect Dis. 2011;203:299–303. doi: 10.1093/infdis/jiq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endy TP, Nisalak A, Chunsuttiwat S, Libraty DH, Green S, Rothman AL, Vaughn DW, Ennis FA. Spatial and temporal circulation of dengue virus serotypes: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:52–59. doi: 10.1093/aje/kwf006. [DOI] [PubMed] [Google Scholar]
- 9.Endy TP, Chunsuttiwat S, Nisalak A, Libraty DH, Green S, Rothman AL, Vaughn DW, Ennis FA. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 10.Mammen MP, Pimgate C, Koenraadt CJ, Rothman AL, Aldstadt J, Nisalak A, Jarman RG, Jones JW, Srikiatkhachorn A, Ypil-Butac CA, Getis A, Thammapalo S, Morrison AC, Libraty DH, Green S, Scott TW. Spatial and temporal clustering of dengue virus transmission in Thai villages. PLoS Med. 2008;5:e205. doi: 10.1371/journal.pmed.0050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbons RV, Nisalak A, Yoon IK, Tannitisupawong D, Rungsimunpaiboon K, Vaughn DW, Endy TP, Innis BL, Burke DS, Mammen MP, Jr, Scott RM, Thomas SJ, Hoke CH., Jr A model international partnership for community-based research on vaccine-preventable diseases: the Kamphaeng Phet-AFRIMS Virology Research Unit (KAVRU) Vaccine. 2013;31:4487–4500. doi: 10.1016/j.vaccine.2013.07.082. [DOI] [PubMed] [Google Scholar]
- 12.Innis BL, Nisalak A, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, Puttisri P, Hoke CH. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg. 1989;40:418–427. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 13.Anonymous . Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. 2nd edition. Geneva: World Health Organization; 1997. p. 84. [Google Scholar]
- 14.Klungthong C, Gibbons RV, Thaisomboonsuk B, Nisalak A, Kalayanarooj S, Thirawuth V, Nutkumhang N, Mammen MP, Jr, Jarman RG. Dengue virus detection using whole blood for reverse transcriptase PCR and virus isolation. J Clin Microbiol. 2007;45:2480–2485. doi: 10.1128/JCM.00305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul A, Harrington LC, Zhang L, Scott JG. Insecticide resistance in Culex pipiens from New York. J Am Mosq Control Assoc. 2005;21:305–309. doi: 10.2987/8756-971X(2005)21[305:IRICPF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Yoon IK, Getis A, Aldstadt J, Rothman AL, Tannitisupawong D, Koenraadt CJ, Fansiri T, Jones JW, Morrison AC, Jarman RG, Nisalak A, Mammen MP, Jr, Thammapalo S, Srikiatkhachorn A, Green S, Libraty DH, Gibbons RV, Endy T, Pimgate C, Scott TW. Fine scale spatiotemporal clustering of dengue virus transmission in children and Aedes aegypti in rural Thai villages. PLoS Negl Trop Dis. 2012;6:e1730. doi: 10.1371/journal.pntd.0001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarman RG, Holmes EC, Rodpradit P, Klungthong C, Gibbons RV, Nisalak A, Rothman AL, Libraty DH, Ennis FA, Mammen MP, Jr, Endy TP. Microevolution of dengue viruses circulating among primary school children in Kamphaeng Phet, Thailand. J Virol. 2008;82:5494–5500. doi: 10.1128/JVI.02728-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cummings DA, Iamsirithaworn S, Lessler JT, McDermott A, Prasanthong R, Nisalak A, Jarman RG, Burke DS, Gibbons RV. The impact of the demographic transition on dengue in Thailand: insights from a statistical analysis and mathematical modeling. PLoS Med. 2009;6:e1000139. doi: 10.1371/journal.pmed.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Rothman AL, Ennis FA, Nisalak A. Dengue in the early febrile phase: viremia and antibody responses. J Infect Dis. 1997;176:322–330. doi: 10.1086/514048. [DOI] [PubMed] [Google Scholar]
- 20.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, Nisalak A. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 21.Nimmannitya S. Clinical spectrum and management of dengue haemorrhagic fever. Southeast Asian J Trop Med Public Health. 1987;18:392–397. [PubMed] [Google Scholar]
- 22.Nimmannitya S. Dengue haemorrhagic fever in Thailand. Southeast Asian J Trop Med Public Health. 1987;18:291–294. [PubMed] [Google Scholar]
- 23.Yoon IK, Rothman AL, Tannitisupawong D, Srikiatkhachorn A, Jarman RG, Aldstadt J, Nisalak A, Mammen MP, Jr, Thammapalo S, Green S, Libraty DH, Gibbons RV, Getis A, Endy T, Jones JW, Koenraadt CJ, Morrison AC, Fansiri T, Pimgate C, Scott TW. Underrecognized mildly symptomatic viremic dengue virus infections in rural Thai schools and villages. J Infect Dis. 2012;206:389–398. doi: 10.1093/infdis/jis357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabaa MA, Klungthong C, Yoon IK, Holmes EC, Chinnawirotpisan P, Thaisomboonsuk B, Srikiatkhachorn A, Rothman AL, Tannitisupawong D, Aldstadt J, Nisalak A, Mammen MP, Gibbons RV, Endy TP, Fansiri T, Scott TW, Jarman RG. Frequent in-migration and highly focal transmission of dengue viruses among children in Kamphaeng Phet, Thailand. PLoS Negl Trop Dis. 2013;7:e1990. doi: 10.1371/journal.pntd.0001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 26.Balmaseda A, Standish K, Mercado JC, Matute JC, Tellez Y, Saborío S, Hammond SN, Nuñez A, Avilés W, Henn MR, Holmes EC, Gordon A, Coloma J, Kuan G, Harris E. Trends in patterns of dengue transmission over 4 years in a pediatric cohort study in Nicaragua. J Infect Dis. 2010;201:5–14. doi: 10.1086/648592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porter KR, Beckett CG, Kosasih H, Tan RI, Alisjahbana B, Rudiman PI, Widjaja S, Listiyaningsih E, Ma'Roef CN, McArdle JL, Parwati I, Sudjana P, Jusuf H, Yuwono D, Wuryadi S. Epidemiology of dengue and dengue hemorrhagic fever in a cohort of adults living in Bandung, West Java, Indonesia. Am J Trop Med Hyg. 2005;72:60–66. [PubMed] [Google Scholar]
- 28.Endy TP, Yoon IK, Mammen MP. Prospective cohort studies of dengue viral transmission and severity of disease. Curr Top Microbiol Immunol. 2010;338:1–13. doi: 10.1007/978-3-642-02215-9_1. [DOI] [PubMed] [Google Scholar]
- 29.Harrington LC, Scott TW, Lerdthusnee K, Coleman RC, Costero A, Clark GG, Jones JJ, Kitthawee S, Kittayapong P, Sithiprasasna R, Edman JD. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg. 2005;72:209–220. [PubMed] [Google Scholar]
- 30.Stoddard ST, Morrison AC, Vazquez-Prokopec GM, Paz Soldan V, Kochel TJ, Kitron U, Elder JP, Scott TW. The role of human movement in the transmission of vector-borne pathogens. PLoS Negl Trop Dis. 2009;3:e481. doi: 10.1371/journal.pntd.0000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoddard ST, Forshey BM, Morrison AC, Paz-Soldan VA, Vazquez-Prokopec GM, Astete H, Reiner RC, Jr, Vilcarromero S, Elder JP, Halsey ES, Kochel TJ, Kitron U, Scott TW. House-to-house human movement drives dengue virus transmission. Proc Natl Acad Sci USA. 2013;110:994–999. doi: 10.1073/pnas.1213349110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyet MN, Duong TH, Trung VT, Nguyen TH, Tran CN, Long VT, Dui le T, Nguyen HL, Farrar JJ, Holmes EC, Rabaa MA, Bryant JE, Nguyen TT, Nguyen HT, Nguyen LT, Pham MP, Nguyen HT, Luong TT, Wills B, Nguyen CV, Wolbers M, Simmons CP. Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proc Natl Acad Sci USA. 2013;110:9072–9077. doi: 10.1073/pnas.1303395110. [DOI] [PMC free article] [PubMed] [Google Scholar]