Abstract
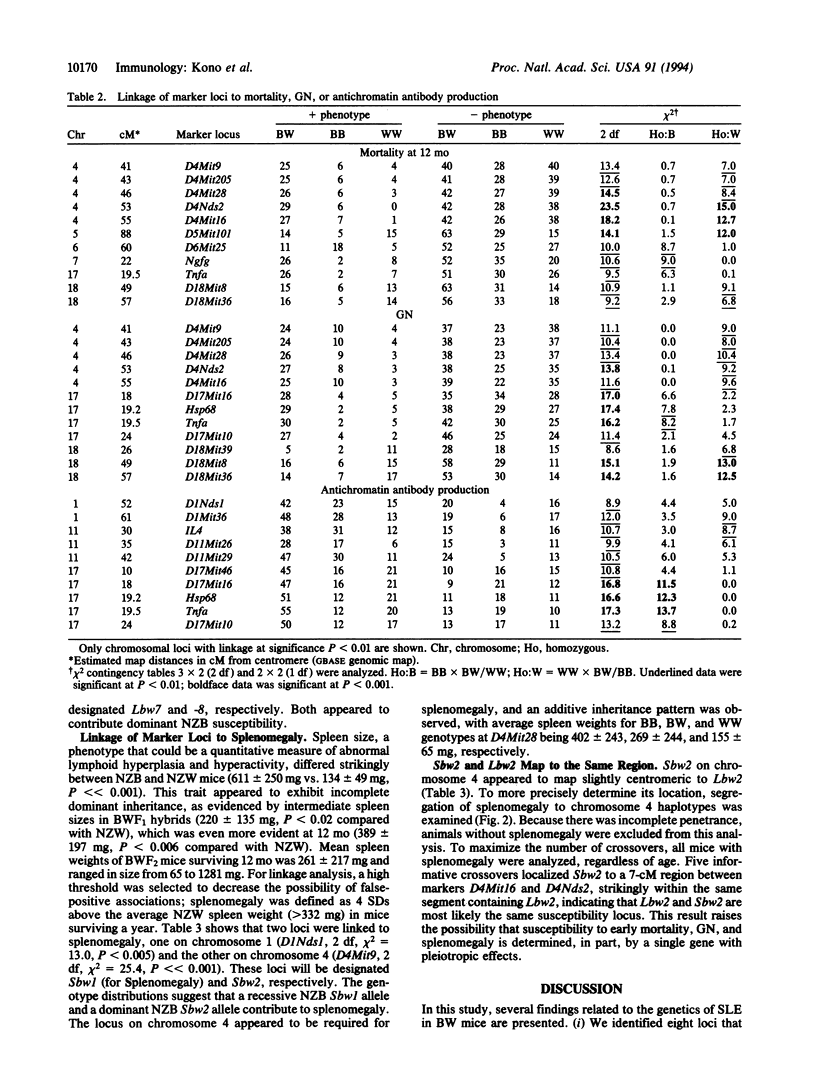
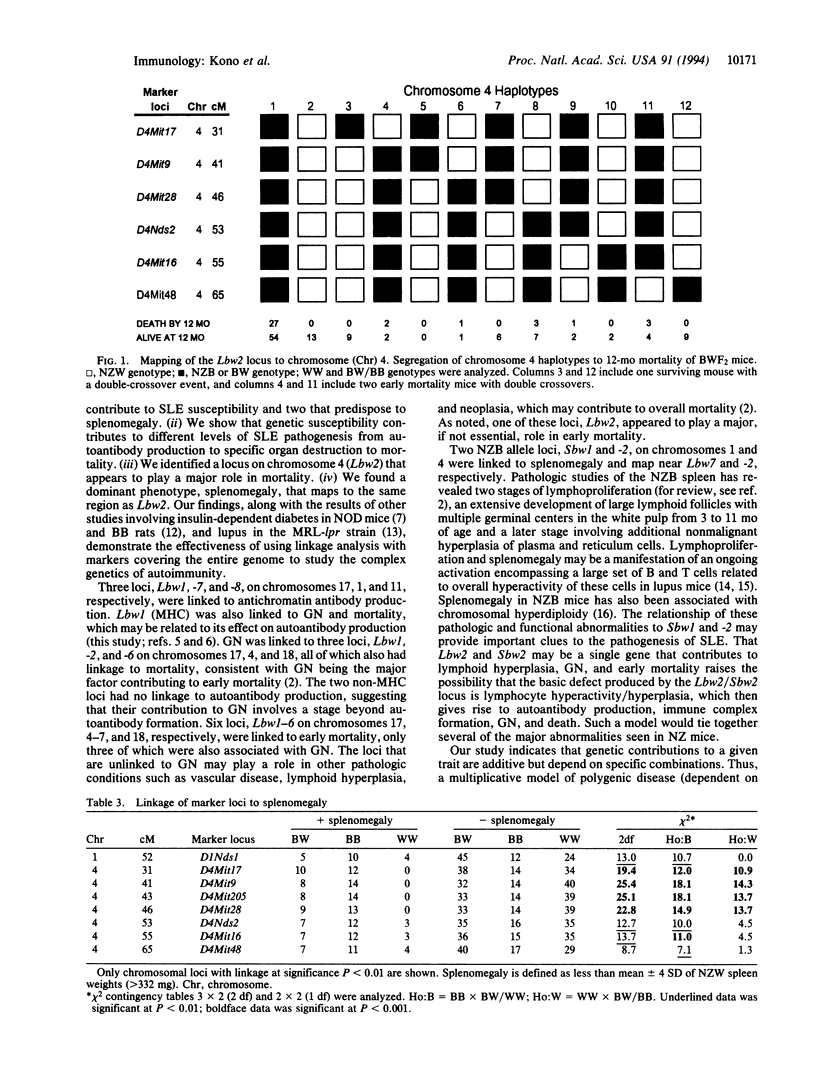
Susceptibility to systemic lupus erythematosus has been unequivocally established to be an inherited trait, but the exact genes and how they confer susceptibility remain largely unknown. In this study of (NZB x NZW)F2 intercross mice, we used linkage analysis of markers covering > 90% of the autosomal genome and identified eight susceptibility loci (Lbw1 to -8, chromosomes 17, 4-7, 18, 1, 11, respectively) associated with antichromatin autoantibody production, glomerulonephritis, and/or mortality. Only one locus, the major histocompatibility complex, was linked to all three traits. Two other loci were associated with both glomerulonephritis and mortality, whereas the remaining loci were linked to one of the above traits. Two additional loci (Sbw1 and -2) that contributed to splenomegaly were also identified. The Sbw2 locus mapped to the identical region as Lbw2, a locus on chromosome 4 linked to glomerulonephritis and mortality, suggesting a single locus with pleiotropic effects. The results indicate that the immunopathologic features of lupus are affected by distinct, but additive, genetic contributions. Studies to determine the nature of the genes associated with these loci should help define the genetic mechanisms involved in this systemic autoimmune disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlingame R. W., Rubin R. L., Balderas R. S., Theofilopoulos A. N. Genesis and evolution of antichromatin autoantibodies in murine lupus implicates T-dependent immunization with self antigen. J Clin Invest. 1993 Apr;91(4):1687–1696. doi: 10.1172/JCI116378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich W., Katz H., Lincoln S. E., Shin H. S., Friedman J., Dracopoli N. C., Lander E. S. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992 Jun;131(2):423–447. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake C. G., Babcock S. K., Palmer E., Kotzin B. L. Genetic analysis of the NZB contribution to lupus-like autoimmune disease in (NZB x NZW)F1 mice. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):4062–4066. doi: 10.1073/pnas.91.9.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Palmer S. M., Rodrigues N. R., Cordell H. J., Hearne C. M., Cornall R. J., Prins J. B., McShane P., Lathrop G. M., Peterson L. B. Polygenic control of autoimmune diabetes in nonobese diabetic mice. Nat Genet. 1993 Aug;4(4):404–409. doi: 10.1038/ng0893-404. [DOI] [PubMed] [Google Scholar]
- Hirose S., Sekigawa I., Ozaki S., Sato H., Shirai T. Genetic regulation of hypergammaglobulinaemia and the correlation to autoimmune traits in (NZB X NZW) F1 hybrid. Clin Exp Immunol. 1984 Dec;58(3):694–702. [PMC free article] [PubMed] [Google Scholar]
- Hirose S., Tokushige K., Kinoshita K., Nozawa S., Saito J., Nishimura H., Shirai T. Contribution of the gene linked to the T cell receptor beta chain gene complex of NZW mice to the autoimmunity of (NZB x NZW)F1 mice. Eur J Immunol. 1991 Mar;21(3):823–826. doi: 10.1002/eji.1830210343. [DOI] [PubMed] [Google Scholar]
- Hirose S., Ueda G., Noguchi K., Okada T., Sekigawa I., Sato H., Shirai T. Requirement of H-2 heterozygosity for autoimmunity in (NZB X NZW)F1 hybrid mice. Eur J Immunol. 1986 Dec;16(12):1631–1633. doi: 10.1002/eji.1830161226. [DOI] [PubMed] [Google Scholar]
- Jacob H. J., Pettersson A., Wilson D., Mao Y., Lernmark A., Lander E. S. Genetic dissection of autoimmune type I diabetes in the BB rat. Nat Genet. 1992 Sep;2(1):56–60. doi: 10.1038/ng0992-56. [DOI] [PubMed] [Google Scholar]
- Klinman D. M. Polyclonal B cell activation in lupus-prone mice precedes and predicts the development of autoimmune disease. J Clin Invest. 1990 Oct;86(4):1249–1254. doi: 10.1172/JCI114831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight J. G., Adams D. D. Three genes for lupus nephritis in NZB x NZW mice. J Exp Med. 1978 Jun 1;147(6):1653–1660. doi: 10.1084/jem.147.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzin B. L., Palmer E. The contribution of NZW genes to lupus-like disease in (NZB x NZW)F1 mice. J Exp Med. 1987 May 1;165(5):1237–1251. doi: 10.1084/jem.165.5.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E. S., Green P., Abrahamson J., Barlow A., Daly M. J., Lincoln S. E., Newberg L. A., Newburg L. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987 Oct;1(2):174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Ledbetter J. A. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- Miyamoto M., Fujita T., Kimura Y., Maruyama M., Harada H., Sudo Y., Miyata T., Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988 Sep 9;54(6):903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- Prud'homme G. J., Balderas R. S., Dixon F. J., Theofilopoulos A. N. B cell dependence on and response to accessory signals in murine lupus strains. J Exp Med. 1983 Jun 1;157(6):1815–1827. doi: 10.1084/jem.157.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveche E. S., Novotny E. A., Hansen C. T., Tjio J. H., Steinberg A. D. Genetic studies in NZB mice. V. Recombinant inbred lines demonstrate that separate genes control autoimmune phenotype. J Exp Med. 1981 May 1;153(5):1187–1197. doi: 10.1084/jem.153.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N., Ghosh S., Todd J. A. Statistical evaluation of multiple-locus linkage data in experimental species and its relevance to human studies: application to nonobese diabetic (NOD) mouse and human insulin-dependent diabetes mellitus (IDDM). Am J Hum Genet. 1993 Sep;53(3):702–714. [PMC free article] [PubMed] [Google Scholar]
- Sidman C. L., Marshall J. D., Beamer W. G., Nadeau J. H., Unanue E. R. Two loci affecting B cell responses to B cell maturation factors. J Exp Med. 1986 Jan 1;163(1):116–128. doi: 10.1084/jem.163.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Whittingham S., Vaux D. L., Bath M. L., Adams J. M., Cory S., Harris A. W. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M. L., Rao J. K., Gilkeson G. S., Ruiz P., Eicher E. M., Pisetsky D. S., Matsuzawa A., Rochelle J. M., Seldin M. F. Genetic analysis of MRL-lpr mice: relationship of the Fas apoptosis gene to disease manifestations and renal disease-modifying loci. J Exp Med. 1992 Dec 1;176(6):1645–1656. doi: 10.1084/jem.176.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]



