Abstract
Human granulocytic anaplasmosis is an acute, febrile illness transmitted by the ticks Ixodes scapularis and Ixodes pacificus in the United States. We present a summary of passive surveillance data for cases of anaplasmosis with onset during 2008–2012. The overall reported incidence rate (IR) was 6.3 cases per million person-years. Cases were reported from 38 states and from New York City, with the highest incidence in Minnesota (IR = 97), Wisconsin (IR = 79), and Rhode Island (IR = 51). Thirty-seven percent of cases were classified as confirmed, almost exclusively by polymerase chain reaction. The reported case fatality rate was 0.3% and the reported hospitalization rate was 31%. IRs, hospitalization rates, life-threatening complications, and case fatality rates increased with age group. The IR increased from 2008 to 2012 and the geographic range of reported cases of anaplasmosis appears to have increased since 2000–2007. Our findings are consistent with previous case series and recent reports of the expanding range of the tick vector I. scapularis.
Introduction
Anaplasma phagocytophilum is the etiologic agent of human granulocytic anaplasmosis, an acute, tick-borne zoonosis.1 Symptoms of anaplasmosis are nonspecific: fever, chills, headache, myalgia, and malaise; laboratory findings of leukopenia, thrombocytopenia, and elevated liver enzymes are common.2,3 Although anaplasmosis is potentially severe and even fatal when untreated, early treatment with doxycycline typically resolves symptoms within 48 hours.2,4,5 Doxycycline is the recommended treatment for patients of all ages.6
The dynamics of the enzootic cycle of A. phagocytophilum drive the epidemiology of anaplasmosis in the United States. The tick Ixodes scapularis is the primary vector of A. phagocytophilum, as well as other agents of human disease including Borrelia burgdorferi (Lyme disease) and Babesia microti (babesiosis).7,8 The tick Ixodes pacificus transmits A. phagocytophilum in the West, where it is also implicated in the transmission of B. burgdorferi.9,10 Small mammals, such as the white-footed mouse (Peromyscus leucopus), are the primary reservoirs for A. phagocytophilum and hosts of immature I. scapularis in the midwest and northeast.11–14 Immature I. scapularis also feed on small reptiles, such as the eastern glass lizard (Ophisaurus ventralis) in the south, but this life cycle does not maintain A. phagocytophilum.15,16 As adults, I. scapularis prefers large mammal hosts such as the white-tailed deer (Odocoileus virginianus).11
Because A. phagocytophilum infects granulocytes, microscopic examination of peripheral blood smears from acutely ill cases may reveal morulae, which are clusters of these organisms within the cytoplasm of infected cells; although, this method is not sensitive—the proportion of infected granulocytes may be very low—and may not be able to distinguish between Ehrlichia and Anaplasma species.4,17,18 For severely ill cases, polymerase chain reaction (PCR) is highly sensitive for testing DNA extracted from whole blood specimens.19 Cases presenting early in the clinical course with less severe disease may have fewer organisms circulating and while PCR is highly specific, its sensitivity may be low (70%).4,20 A. phagocytophilum may also be cultured from whole blood using specialized techniques, although the intracellular nature of A. phagocytophilum makes culture impractical for routine diagnostics.21 Serologic methods using paired acute and convalescent sera have good negative predictive value and are recommended for all suspected cases of anaplasmosis.6,22 A 4-fold or greater increase in immunoglobulin G (IgG) titer using indirect immunofluorescence antibody (IFA) assay between an acute serum collected at initial presentation and a convalescent serum collected 2–4 weeks later confirms anaplasmosis.20 Because serum from the first week of illness is typically negative by IFA and because background seroprevalence may be elevated, a single acute IFA result may be uninformative when negative or low positive; comparison with a convalescent serum is required to attribute illness to A. phagocytophilum.20,23 The clinical presentation and epidemiologic information should always be considered when interpreting results from these diagnostic assays.6,20,22
Since 2000, anaplasmosis has been a nationally notifiable disease.24 In states where the disease is notifiable, cases are reported from the diagnostic laboratory or the health-care provider to the state or local public health department.6 The state and local health departments gather further clinical, epidemiological, and laboratory information related to the report. Then, state and local health officials report these data to the Centers for Disease Control and Prevention (CDC) as a case report to two surveillance systems: the Nationally Notifiable Diseases Surveillance System (NNDSS) and directly to the Rickettsial Zoonoses Branch on case report forms (CRFs) (http://www.cdc.gov/ticks/forms/2010_tbrd_crf.pdf). The NNDSS collects basic demographics and additional information is submitted on CRFs. A new case definition became effective in 2008, which updated nomenclature and altered criteria for laboratory evidence.25 Here, we present a summary of reports of both surveillance systems with onset of symptoms during 2008–2012, the first 5 years after the new case definition was in effect.
Methods
Case definition.25
Meeting the case definition of A. phagocytophilum infection requires both clinical evidence and laboratory evidence. Criteria for clinical evidence include fever and at least one of the following symptoms: headache, myalgia, malaise, anemia, leukopenia, thrombocytopenia, or elevated hepatic transaminases. Laboratory evidence must be supportive or confirmatory. Supportive evidence includes serological evidence of elevated IgG or IgM titers or visualization of morulae in the cytoplasm of neutrophils or eosinophils. Confirmatory evidence includes serological evidence of at least a 4-fold change in IgG titer by IFA between paired serum samples (acute serum taken during first week of illness and convalescent sample taken 2–4 weeks later), detection of DNA by PCR, demonstration of antigen in a biopsy or autopsy by immunohistochemical (IHC) methods, and isolation by culture from a clinical specimen. A confirmed case of anaplasmosis has clinical evidence and confirmatory laboratory evidence; a probable case has clinical evidence, but only supportive laboratory evidence. Cases not meeting the case definition for either a confirmed case or a probable case were not included for analysis.
Surveillance systems.
Reports collected by NNDSS include whether a case is confirmed or probable as well as demographics: sex, race, ethnicity, age, and county of residence. The date of onset of symptoms is reportable, but when missing, the earliest date of diagnosis, the date of collection of specimen for laboratory testing, or the date of report is used as a proxy. Data collected by CRFs, but not by NNDSS, include whether hospitalized or not, whether died or survived, any life-threatening complications due to anaplasmosis, the presence of preexisting immunosuppressive condition, and any diagnostic laboratory results. Because we believe cases reported through CRFs generally represent a subset of the cases reported via NNDSS, IRs are calculated using NNDSS data. The CRFs data are used for describing outcomes, risk factors for severe disease, and trends in laboratory diagnostics.
Analysis.
To calculate reported IRs, person-time at risk was calculated using U.S. Census Bureau population estimates.26,27 For years when anaplasmosis was notifiable within a given state, the person-years at risk are the population estimates. When anaplasmosis was not notifiable, the person-years at risk are zero for the following states and years: Alabama (2008), Alaska (2008–2012), Arkansas (2008), California (2008), Colorado (2008–2012), District of Columbia (2008–2012), Hawaii (2008–2012), Idaho (2008–2012), Iowa (2008–2012), Montana (2011–2012), Nevada (2008–2010), New Mexico (2008–2012), North Dakota (2008–2010), Vermont (2009), and Washington (2008–2009). Because reported cases are not generalizable to unreported cases or other periods, no statistical tests were performed and no confidence intervals were calculated. All calculations were performed using SAS software (Cary, NC) or R software (Vienna, Austria).28,29 IRs were not calculated for race and ethnic groups due to a large proportion of cases with missing race and ethnicity data.
Results
NNDSS.
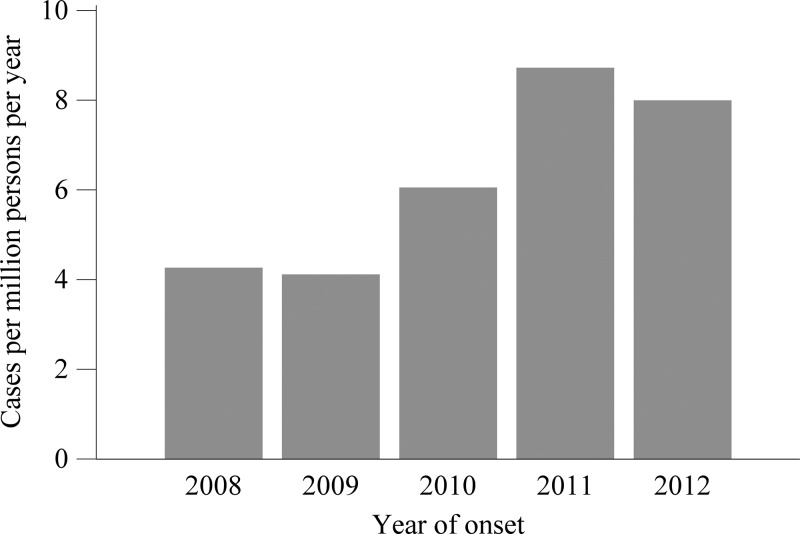
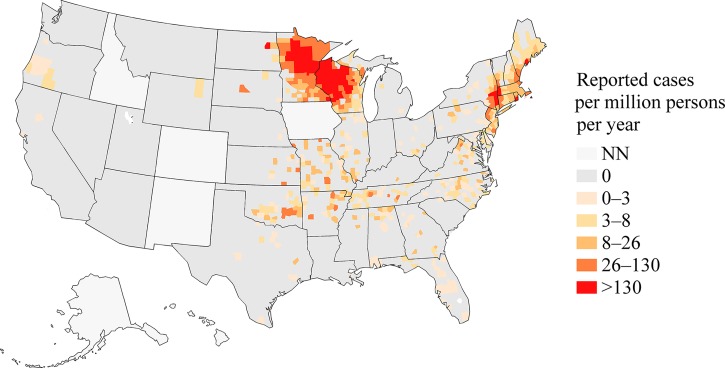
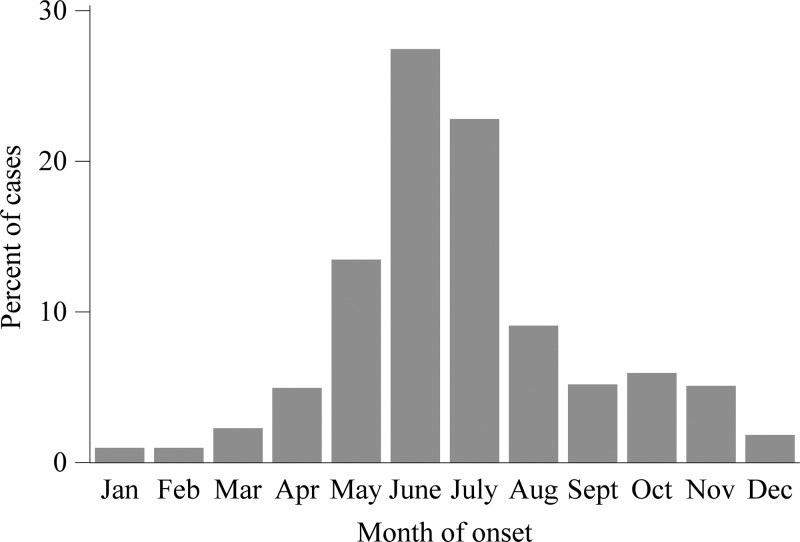
A total of 8,896 cases of anaplasmosis were reported with onset dates during 2008–2012, yielding an IR of 6.3 cases per million person-years. The annual IR ranged from 4.1 to 8.7 (Figure 1). The county level IR was highest along the seaboard of New England and within a large area of Minnesota and Wisconsin (Figure 2). Cases were reported from 38 states and from New York City, with the highest reported IR in Minnesota (IR = 97), Wisconsin (IR = 79), and Rhode Island (IR = 51, Table 1). Monthly incidence peaked May through August, and a second, smaller peak occurred in October (Figure 3).
Figure 1.
A chart of the annual incidence rate, the number of incident cases per million persons at risk, of anaplasmosis vs. the year of onset of symptoms, 2008–2012. The number of incident cases is from the Nationally Notifiable Diseases Surveillance System, and the number of person-years at risk is from the U.S. Census Bureau.
Figure 2.
A map of reported incidence rates, the number of incident cases per million persons at risk per year, of anaplasmosis in the counties of the United States, 2008–2012. States where the disease was not notifiable for the duration of 2008–2012 are shaded with the “NN” category. The number of incident cases is from the Nationally Notifiable Diseases Surveillance System, and the number of person-years at risk is from the U.S. Census Bureau.
Table 1.
The number of reported cases and the reported incidence rate (IR) per million persons per year by state, 2008–2012
| State | Cases (IR) |
|---|---|
| Alabama | 24 (1.3) |
| Alaska | NN |
| Arizona | 0 (0) |
| Arkansas | 27 (2.3) |
| California | 2 (0.01) |
| Colorado | NN |
| Connecticut | 404 (22.6) |
| Delaware | 12 (2.7) |
| District of Columbia | NN |
| Florida | 24 (0.3) |
| Georgia | 19 (0.4) |
| Hawaii | NN |
| Idaho | NN |
| Illinois | 41 (0.6) |
| Indiana | 0 (0) |
| Iowa | NN |
| Kansas | 15 (1.1) |
| Kentucky | 1 (0.05) |
| Louisiana | 1 (0.04) |
| Maine | 127 (19.1) |
| Maryland | 32 (1.1) |
| Massachusetts | 674 (20.6) |
| Michigan | 10 (0.2) |
| Minnesota | 2,586 (97.3) |
| Mississippi | 4 (0.3) |
| Missouri | 66 (2.2) |
| Montana | 0 (0) |
| Nebraska | 4 (0.4) |
| Nevada | 0 (0) |
| New Hampshire | 135 (20.5) |
| New Jersey | 457 (10.4) |
| New Mexico | NN |
| New York City | 93 (2.3) |
| New York State | 1,313 (23.5) |
| North Carolina | 75 (1.6) |
| North Dakota | 6 (4.3) |
| Ohio | 13 (0.2) |
| Oklahoma | 57 (3) |
| Oregon | 6 (0.3) |
| Pennsylvania | 19 (0.3) |
| Rhode Island | 269 (51.1) |
| South Carolina | 1 (0.04) |
| South Dakota | 4 (1) |
| Tennessee | 35 (1.1) |
| Texas | 9 (0.1) |
| Utah | 0 (0) |
| Vermont | 19 (7.6) |
| Virginia | 59 (1.5) |
| Washington | 0 (0) |
| West Virginia | 2 (0.2) |
| Wisconsin | 2,250 (79.1) |
| Wyoming | 1 (0.4) |
States where anaplasmosis was not notifiable for the duration of 2008–2012 are marked “NN.” The number of incident cases is from the Nationally Notifiable Diseases Surveillance System, and the number of person-years at risk is from the U.S. Census Bureau.
Figure 3.
A chart of the percent of cases of anaplasmosis vs. month of onset, 2008–2012. The data are from the Nationally Notifiable Diseases Surveillance System.
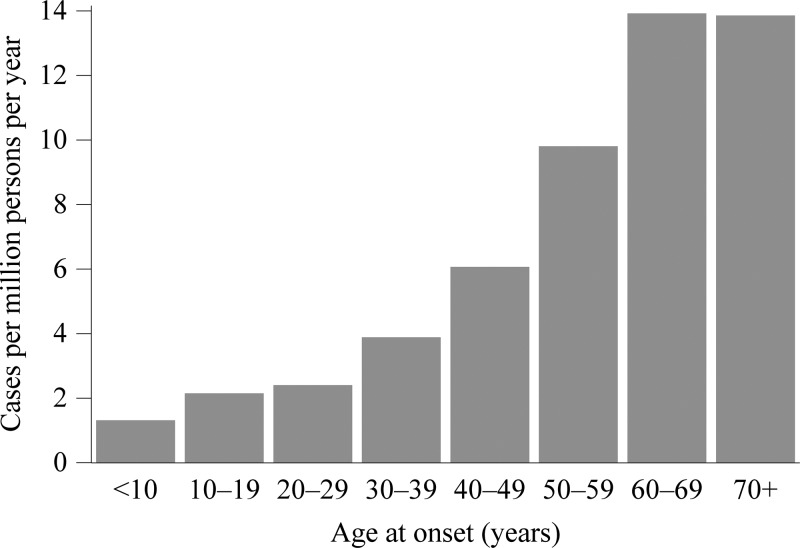
The IR increased with age group, ranging from 1.3 in children under 10 years of age to 13.8 in people of 70 years of age and older (Figure 4). Race was not reported for 3,694 cases (42%) and ethnicity was not reported for 4,626 cases (52%, Table 2). The ratio of male cases to female cases was 1.4:1. During 2008–2012, 3,233 cases (36%) were reported as confirmed and 5,661 cases (64%) were reported as probable.
Figure 4.
A chart of the incidence rate of anaplasmosis vs. age group at onset of symptoms, 2008–2012. The number of incident cases is from the Nationally Notifiable Diseases Surveillance System, and the number of person-years at risk is from the U.S. Census Bureau.
Table 2.
Demographics of cases of anaplasmosis as reported through case report forms (CRFs) and the Nationally Notifiable Diseases Surveillance System (NNDSS), 2008–2012
| Demographic | CRFs, N = 7,849 n (%) | NNDSS, N = 8,896 n (%) |
|---|---|---|
| Sex | ||
| Male | 4,439 (56.6) | 5,161 (58) |
| Female | 3,008 (38.3) | 3,602 (40.5) |
| Unknown | 402 (5.1) | 133 (1.5) |
| Race | ||
| White | 5,385 (68.6) | 4,939 (55.5) |
| Black | 36 (0.5) | 45 (0.5) |
| American Indian | 80 (1) | 106 (1.2) |
| Asian† | 33 (0.4) | 44 (0.5) |
| Pacific Islander† | 5 (0.1) | |
| Other* | 68 (0.8) | |
| Unknown | 2,310 (29.4) | 3,694 (41.5) |
| Ethnicity | ||
| Hispanic | 73 (0.9) | 97 (1.1) |
| Nonhispanic | 4,672 (59.5) | 4,173 (46.9) |
| Unknown | 3,104 (39.5) | 4,626 (52.0) |
| Age group | ||
| Under 10 | 218 (2.8) | 240 (2.7) |
| 10–19 | 343 (4.4) | 414 (4.7) |
| 20–29 | 398 (5.1) | 467 (5.2) |
| 30–39 | 632 (8.1) | 707 (7.9) |
| 40–49 | 1,064 (13.6) | 1,200 (13.5) |
| 50–59 | 1,724 (22) | 1,873 (21.1) |
| 60–69 | 1,738 (22.1) | 1,870 (21.0) |
| 70+ | 1,663 (21.2) | 1,779 (20.0) |
| Unknown | 69 (0.9) | 346 (3.9) |
For CRFs, “Other” is not a reportable race.
For NNDSS, Asian and Pacific Islander are combined into a single category.
CRFs.
A total of 8,154 reports were received through CRFs. Of these, 7,849 were unique cases (not a duplicate of another report in the CRFs) meeting the case definition. The number of cases generally increased with age group (Table 2). Race was not reported for 2,310 cases (29%) and ethnicity was not reported for 3,104 cases (40%). The ratio of male cases to female cases was 1.5:1. A total of 4,099 cases (46%) reported whether a preexisting immunosuppressive condition was present or not, and 464 of these cases (11%) reported an immunosuppressive condition. The most frequently reported conditions were diabetes (N = 145), immunosuppressive medications (N = 52), asplenia (N = 24), and arthritis (N = 21). The prevalence of an immunosuppressive condition was 11% among whites, 14% among blacks, 20% among American Indians, 25% among Asians, and 25% among Pacific Islanders.
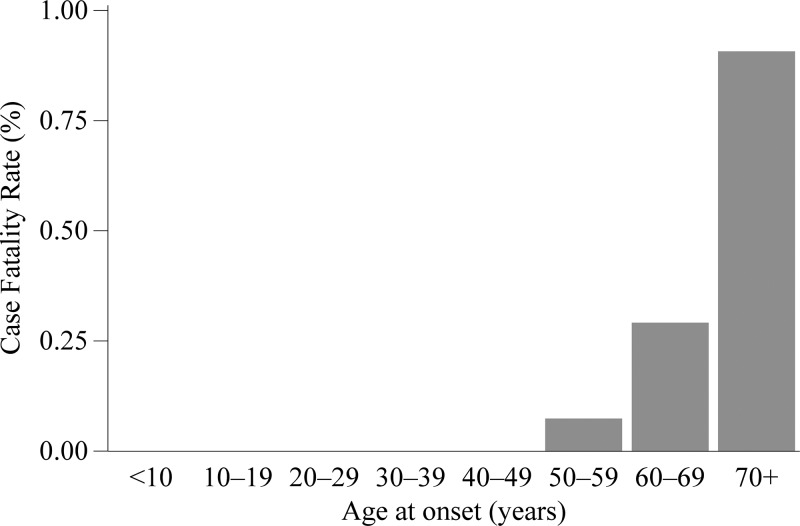
Whether a case survived or died was reported for 6,076 cases (77%). Among these cases with known outcome, 18 were fatal, yielding a case fatality rate (CFR) of 0.3%. There were four fatal cases among the 416 cases reporting an immunosuppressive condition with known outcome. Whereas, there were two fatal cases among the 3,305 cases reporting no immunosuppressive conditions. The risk of fatal outcome for those reporting an immunosuppressive condition was 16 relative to those without such a condition. There were no fatalities among cases less than 50 years and the CFR increased with age group (Figure 5). There were no fatal cases reported among Hispanics, blacks, American Indians or Alaskan Natives, Asians, or Pacific Islanders. There were 12 fatal cases among males (CFR = 0.3%) and six fatal cases among females (CFR = 0.3%); yielding a relative risk (RR) of 1.3.
Figure 5.
A chart of the case fatality rate vs. age group at onset, 2008–2012. The data are from case report forms.
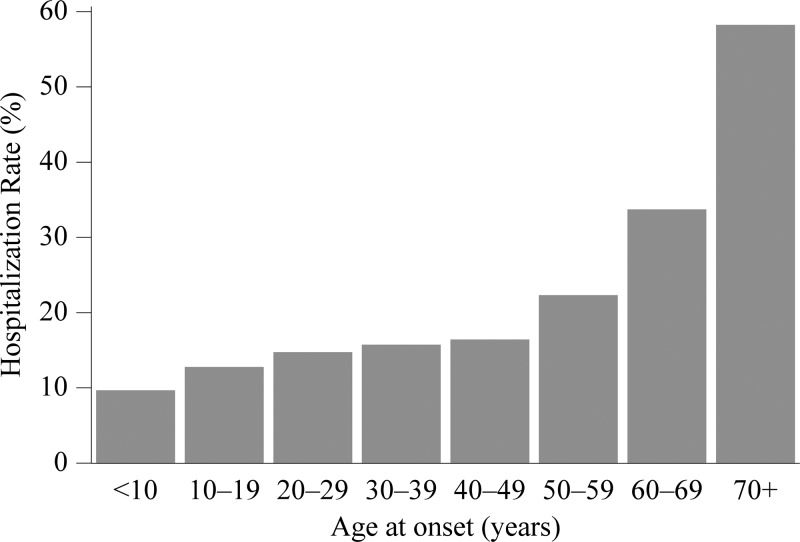
Whether a case was hospitalized was reported for 5,937 cases (76%). Among these cases, 1,827 were hospitalized, yielding a hospitalization rate (HR) of 31%. The HR among those reporting an immunosuppressive condition was 55% (236/433); whereas, cases not reporting such a condition had an HR of 26% (863/3321, RR = 2.1). The HR increased with age (Figure 6). The HR among American Indians and Alaskan Natives was 33% and that among whites was 31% (RR = 1.0). Using whites as a reference, the HR was higher among the following race groups: blacks (RR = 1.8), Asians (RR = 1.1), and Pacific Islanders (RR = 2.1). The HR was higher among Hispanics relative to non-Hispanics (RR = 1.5). The HR was 31% among males and 29% among females (RR = 1.0).
Figure 6.
A chart of the hospitalization rate vs. age group at onset, 2008–2012. The data are from case report forms.
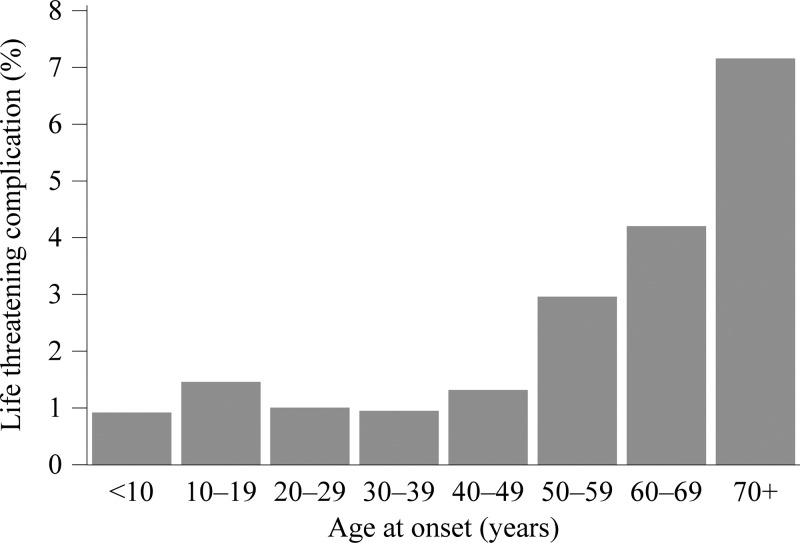
Whether there were any life-threatening complications during the clinical course was reported for 2,825 cases (36%). Among these cases, there were 85 reports (3%) of renal failure, 28 reports (1%) of adult respiratory distress, 25 reports (0.9%) of meningitis or encephalitis, 15 reports (0.5%) of pneumonia, 11 reports (0.4%) of disseminated intravascular coagulopathy, and 9 (0.3%) reports of sepsis. The reported proportion of cases with a life-threatening complication generally increased with age group (Figure 7). Among the 461 cases reporting an immunosuppressive condition and reporting whether a life-threatening complication arose, 61 cases reported a life-threatening complication; whereas, among the 3,635 cases reporting no immunosuppressive conditions, 162 cases (4%) reported a life-threatening complication (RR = 3.0).
Figure 7.
A chart of the proportion of cases reporting a life-threatening complication vs. age group at onset, 2008–2012. The data are from case report forms.
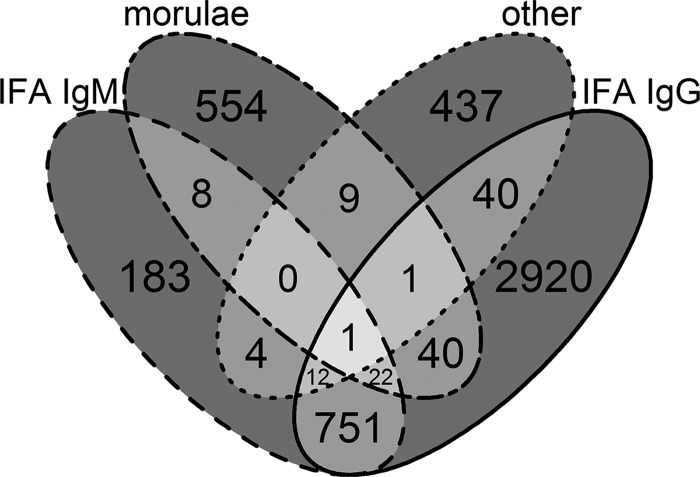
A total of 2,867 cases (37%) were confirmed cases. Among these, 2,839 cases (99%) were PCR positive, 15 cases (0.5%) demonstrated a seroconversion on IFA IgG, 17 cases (0.6%) were positive by IHC, and 4 cases (0.1%) were culture positive. The median time between onset and specimen collection was 4 days (inter-quartile range [IQR] = 1–10 days) for the 634 PCR positive cases with known dates. Among cases with PCR positive results, 92 cases (3%) also had visualization of morulae, and 170 cases (6%) had at least one positive serology result. An additional 93 probable cases demonstrated a 4-fold or higher change in IgG titer by IFA; however, the timing of the sera collections did not conform to the confirmatory evidence criteria of the acute serum taken during the first week of illness and the convalescent serum taken 2–4 weeks later. Among the 4,982 probable cases (64%), 3,787 cases (76%) had positive IFA IgG, 981 cases (20%) had positive IFA IgM, 635 cases (13%) had visualization of morulae, and 504 (10%) had positive serology other than IFA (Figure 8). The median time between onset of symptoms and collection of the earliest clinical specimen was 7 days (IQR = 2–18) among the 4,285 probable cases with known dates.
Figure 8.
A Venn diagram of supportive laboratory evidence among the 4,982 probable cases: positive immunoglobulin M (IgM) by indirect immunofluorescence assay (IFA IgM), morulae visualization in granulocytes by microscopy (morulae), positive results from serological assays other than IgM or IgG IFAs (other), and positive IgG by IFA IgG. Data are from case report forms.
Discussion
The IR of anaplasmosis has increased since becoming notifiable in 2000: the incidence was 2.0 cases per million person-years in 2000–2007, with an increase from 1.4 in 2000 to 3.0 in 2007.30 In this surveillance summary, we found an IR of 6.3 during 2008–2012, with an increase from 4.3 in 2008 to 8.0 in 2012. IRs in Minnesota, Wisconsin, and Rhode Island were all above 50 cases per million person-years. With increasing age group, cases were more likely to occur, more likely to have a life-threatening complication, more likely to be hospitalized, and more likely to die of anaplasmosis. In addition, cases reporting an underlying immunosuppressive condition were also more likely to have a severe or fatal clinical course. These findings are consistent with previous case series.4,20
The range of I. scapularis is reportedly expanding. From surveillance of white-tailed deer, the range of I. scapularis in Wisconsin is increasing to the southeast.31 Similarly, increasing ranges for I. scapularis have been documented along the Hudson River Valley, Michigan, and Virginia.32–34 Comparing the maps presented here with our previous surveillance summary from 2000 to 2007, anaplasmosis appears to be increasing in geographic range as well as in IR, consistent with changes in the range of its tick vector.30 More cases were reported during 2008–2012 than during 2000–2007; and, analysis of human data in tandem with ecological data may elucidate changing risk of exposure to human populations.30
In our previous report, 21% of reported cases of anaplasmosis were confirmed.30 In this report, the proportion of confirmed cases has increased to 37%, largely due to an increased proportion of cases with positive results from PCR. Only 15 reported cases, a very small minority, were confirmed by demonstrating a seroconversion with appropriately timed serum collections for the case definition. The laboratory evidence for reported cases is nearly bimodal: cases confirmed by PCR (N = 2,839) and cases with a single positive result on IFA IgG as the only laboratory evidence (N = 2,920) account for almost 3/4 of the reported cases (N = 7,849). Acute serology—and PCR on whole blood—may be negative in cases of anaplasmosis; and, a convalescent serology often provides the only evidence of infection.4,20 As clinical suspicion of anaplasmosis and paired serology for IFA IgG is the most sensitive diagnostic method, the CDC recommends paired serology for all suspected cases of anaplasmosis.6 In anaplasmosis cases with negative results from acute samples, results from a convalescent serum may be the only evidence to count these cases with passive surveillance.
The results presented here are subject to several limitations. Although anaplasmosis is likely underreported, the degree to which it is underreported relates to the severity of illness, the level of endemic disease, and the resources available to clinicians and public health authorities. Less severe cases may be less likely to present and receive laboratory testing, so our results may be biased toward more severe cases. Suspicion for anaplasmosis may be lower in nonendemic areas, and health-care providers in highly endemic areas may be accustomed to treating anaplasmosis empirically without ordering laboratory tests. Rare outcomes, especially death, are based on small numbers and the comparisons of CFR between two subgroups may not be accurate. Race and ethnicity were largely missing in both NNDSS and CRFs datasets, making it difficult to draw conclusions on the potential role of race and ethnicity in the role of illness. While the reported prevalence of diabetes in our results was 3.5%, the national prevalence of diabetes has increased to over 8% in the same period.35 Although comorbidities like diabetes may limit activities that may result in tick exposure, this result suggests that our data on immunosuppressive conditions may not be reliable. Similarly, medical terms may not be reported accurately as information may flow from a health-care provider to the patient and to a family member before being captured in surveillance by an investigator; for example, reports of sepsis may represent systemic inflammatory response syndrome with infection. The relationships between race, ethnicity, the prevalence of immunosuppressive conditions, and age group with hospitalization, life-threatening complications, and case fatality are all potentially confounded; but, the large proportion of missing data preclude assessing whether these associations are confounded. Results from passive surveillance are not generalizable to unreported cases, and we do not know how accurately surveillance reflects the underlying trends of anaplasmosis. Although only confirmed and probable cases are included here, this information was not collected for several cases in the NNDSS data because of a technical issue that is now resolved. Despite these limitations, our results are useful for a basic understanding of incidence, distribution, and severity of human anaplasmosis.
Anaplasmosis is a potentially fatal disease, but clinical suspicion and early treatment with doxycycline leads to good outcomes. Our results suggest that the geographic range of anaplasmosis is widening while incidence is also increasing in endemic areas. Health-care providers in previously unaffected areas may begin to see anaplasmosis cases. Similarly, previously unaffected state and local public health departments may begin receiving positive laboratory results. In addition, health-care providers should consider patient travel history to endemic areas, especially when patients live in an area where the tick vectors for A. phagocytophylum are not endemic. Systematic evaluation of the presence of A. phagocytophilum within a state may help guide prevention messaging and public health policy decisions, such as whether to add anaplasmosis to the state's list of notifiable diseases.
ACKNOWLEDGMENTS
We kindly thank the health-care providers, laboratorians, and public health partners whose dedication and work is indispensable to rickettsial disease surveillance. We also thank Eric Mandel, John Krebs, and Jennifer McQuiston for their work in designing and building this data, and Holly Biggs for her careful review.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. All authors report no potential conflicts of interest.
Footnotes
Financial support: This study was supported by the Centers for Disease Control and Prevention. This research was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC.
Authors' addresses: F. Scott Dahlgren, Kristen Nichols Heitman, Naomi A. Drexler, Robert F. Massung, and Casey Barton Behravesh. Rickettsial Zoonoses Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: iot0@cdc.gov, wwd7@cdc.gov, isj3@cdc.gov, rfm2@cdc.gov, and dlx9@cdc.gov.
References
- 1.Chen SM, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakken JS, Dumler JS, Chen SM, Eckman MR, Vanetta LL, Walker DH. Human granulocytic ehrlichiosis in the Upper Midwest United States: a new species emerging? JAMA. 1994;272:212–218. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Human granulocytic ehrlichiosis—New York, 1995. MMWR Morb Mortal Wkly Rep. 1995;44:593–595. [PubMed] [Google Scholar]
- 4.Bakken JS, Krueth J, Wilson-Nordskog C, Tilden RL, Asanovich K, Dumler JS. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. [PubMed] [Google Scholar]
- 5.Hardalo CJ, Quagliarello V, Dumler JS. Human granulocytic ehrlichiosis in Connecticut: report of a fatal case. Clin Infect Dis. 1995;21:910–914. doi: 10.1093/clinids/21.4.910. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichiosis, and anaplasmosis—United States. MMWR Recomm Rep. 2006;55:1–27. [PubMed] [Google Scholar]
- 7.Pancholi P, Kolbert CP, Mitchell PD, Reed KD, Jr, Dumler JS, Bakken JS, Telford SR, 3rd, Persing DH. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- 8.Des Vignes F, Fish D. Transmission of the agent of human granulocytic ehrlichiosis by host-seeking Ixodus scapularis (Acari:Ixodidae) in southern New York State. J Med Entomol. 1997;34:379–382. doi: 10.1093/jmedent/34.4.379. [DOI] [PubMed] [Google Scholar]
- 9.Richter PJ, Jr, Kimsey RB, Madigan JE, Barlough JE, Dumler JS, Brooks DL. Ixodes pacificus (Acari: Ixodidae) as a vector of Ehrlichia equi (Rickettsiales: Ehrlichieae) J Med Entomol. 1996;33:1–5. doi: 10.1093/jmedent/33.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Gewirtz AS, Cornbleet PJ, Vugia DJ, Traver C, Niederhuber J, Kolbert CP, Persing DH. Human granulocytic ehrlichiosis: report of a case in northern California. Clin Infect Dis. 1996;23:653–654. doi: 10.1093/clinids/23.3.653. [DOI] [PubMed] [Google Scholar]
- 11.Keirans JE, Hutcheson HJ, Durden LA, Klompen JSH. Ixodes (Ixodes) scapularis (Acari: Ixodidae): redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J Med Entomol. 1996;33:297–318. doi: 10.1093/jmedent/33.3.297. [DOI] [PubMed] [Google Scholar]
- 12.Telford SR, 3rd, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walls JJ, Greig B, Neitzel DF, Dumler JS. Natural infection of small mammal species in Minnesota with the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:853–855. doi: 10.1128/jcm.35.4.853-855.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholson WL, Muir S, Sumner JW, Childs JE. Serologic evidence of infection with Ehrlichia spp. in wild rodents (Muridae: Sigmodontinae) in the United States. J Clin Microbiol. 1998;36:695–700. doi: 10.1128/jcm.36.3.695-700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliver JH, Cummins GA, Joiner MS. Immature Ixodes scapularis (Acari, Ixodidae) parasitizing lizards from the southeastern USA. J Parasitol. 1993;79:684–689. [PubMed] [Google Scholar]
- 16.Durden LA, Oliver JH, Jr, Banks CW, Vogel GN. Parasitism of lizards by immature stages of the blacklegged tick, Ixodes scapularis (Acari, Ixodidae) Exp Appl Acarol. 2002;26:257–266. doi: 10.1023/a:1021199914816. [DOI] [PubMed] [Google Scholar]
- 17.Bakken JS, Aguero-Rosenfeld ME, Tilden RL, Wormser GP, Horowitz HW, Raffalli JT, Baluch M, Riddell D, Walls JJ, Dumler JS. Serial measurements of hematologic counts during the active phase of human granulocytic ehrlichiosis. Clin Infect Dis. 2001;32:862–870. doi: 10.1086/319350. [DOI] [PubMed] [Google Scholar]
- 18.Buller RS, Arens M, Hmiel SP, Paddock CD, Sumner JW, Rikihisa Y, Unver A, Gaudreault-Keener R, Manian FA, Liddell AM, Schmulewitz N, Storch GA. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 19.Walls JJ, Caturegli P, Bakken JS, Asanovich KM, Dumler JS. Improved sensitivity of PCR for diagnosis of human granulocytic ehrlichiosis using epank1 genes of Ehrlichia phagocytophila-group ehrlichiae. J Clin Microbiol. 2000;38:354–356. doi: 10.1128/jcm.38.1.354-356.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakken JS, Haller I, Riddell D, Walls JJ, Dumler JS. The serological response of patients infected with the agent of human granulocytic ehrlichiosis. Clin Infect Dis. 2002;34:22–27. doi: 10.1086/323811. [DOI] [PubMed] [Google Scholar]
- 21.Goodman JL, Nelson C, Vitale B, Madigan JE, Dumler JS, Kurtti TJ, Munderloh UG. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N Engl J Med. 1996;334:209–215. doi: 10.1056/NEJM199601253340401. [DOI] [PubMed] [Google Scholar]
- 22.Dumler JS, Brouqui P. Molecular diagnosis of human granulocytic anaplasmosis. Expert Rev Mol Diagn. 2004;4:559–569. doi: 10.1586/14737159.4.4.559. [DOI] [PubMed] [Google Scholar]
- 23.Bakken JS, Goellner P, Van Etten M, Boyle DZ, Swonger OL, Mattson S, Krueth J, Tilden RL, Asanovich K, Walls J, Dumler JS. Seroprevalence of human granulocytic ehrlichiosis among permanent residents of northwestern Wisconsin. Clin Infect Dis. 1998;27:1491–1496. doi: 10.1086/515048. [DOI] [PubMed] [Google Scholar]
- 24.Council of State and Territorial Epidemiologists Position Statements 2000 ID-03: Changes in the Case Definition for Human Ehrlichiosis, and Addition of a New Ehrlichiosis Category as a Condition Placed Under Surveillance According to the National Public Health Surveillance System (NPHSS) 2000. http://www.cste.org/ps/2000/2000-id-03.htm Available at. Accessed December 8, 2009.
- 25.Council of State and Territorial Epidemiologists Position Statements 2007 ID-03: Revision of the National Surveillance Case Definition for Ehrlichiosis (Ehrlichiosis/Anaplasmosis) 2007. http://www.cste.org/ps/2007ps/2007psfinal/id/07-id-03.pdf Available at. Accessed December 8, 2009.
- 26.U.S. Census Bureau, Population Division Intercensal Estimates of the Resident Population for Counties and States: April 1, 2000 to July 1, 2010. 2011. http://www.census.gov/popest/data/intercensal/county/county2010.html Available at. Accessed July 23, 2014.
- 27.U.S. Census Bureau, Population Division Annual Resident Population Estimates, Estimated Components of Resident Population Change, and Rates of the Components of Resident Population Change for States and Counties: April 1, 2010 to July 1, 2013. 2014. https://www.census.gov/popest/data/counties/totals/2013/CO-EST2013-alldata.html Available at. Accessed July 23, 2014.
- 28.SAS Institute . SAS System for Windows [computer program]. Version 9.3. Cary, NC: SAS Institute; 2012. [Google Scholar]
- 29.R Core Team . R: A Language and Environment for Statistical Computing [computer program]. Version 3.1.1. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 30.Dahlgren FS, Mandel EJ, Krebs JW, Massung RF, McQuiston JH. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000–2007. Am J Trop Med Hyg. 2011;85:124–131. doi: 10.4269/ajtmh.2011.10-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee X, Hardy K, Johnson DH, Paskewitz SM. Hunter-killed deer surveillance to assess changes in the prevalence and distribution of Ixodes scapularis (Acari: Ixodidae) in Wisconsin. J Med Entomol. 2013;50:632–639. doi: 10.1603/me12234. [DOI] [PubMed] [Google Scholar]
- 32.Hamer SA, Tsao JI, Walker ED, Hickling GJ. Invasion of the Lyme disease vector Ixodes scapularis: implications for Borrelia burgdorferi endemicity. EcoHealth. 2010;7:47–63. doi: 10.1007/s10393-010-0287-0. [DOI] [PubMed] [Google Scholar]
- 33.Prusinski MA, Kokas JE, Hukey KT, Kogut SJ, Lee J, Backenson PB. Prevalence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in Ixodes scapularis (Acari: Ixodidae) collected from recreational lands in the Hudson Valley Region, New York State. J Med Entomol. 2014;51:226–236. doi: 10.1603/me13101. [DOI] [PubMed] [Google Scholar]
- 34.Herrin BH, Zajac AM, Little SE. Confirmation of Borrelia burgdorferi sensu stricto and Anaplasma phagocytophilum in Ixodes scapularis, southwestern Virginia. Vector Borne Zoonotic Dis. 2014;14:821–823. doi: 10.1089/vbz.2014.1661. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention Increasing prevalence of diagnosed diabetes—United States and Puerto Rico, 1995–2010. MMWR Morb Mortal Wkly Rep. 2012;61:918–921. [PubMed] [Google Scholar]