Abstract
Seasonal changes play roles in the transmission success of fish-borne zoonotic trematodes (FZT). This study examined the seasonal transmission patterns of Opisthorchis viverrini sensu lato (s.l.) and a virgulate cercaria (family Lecithodendriidae) in the snail intermediate host, Bithynia siamensis goniomphalos in northeast Thailand. Snail samples were collected monthly during the rainy, cool, and hot seasons during 2012–2013 to determine the prevalence and intensity of larval trematode infections. The prevalence of O. viverrini s.l. varied significantly with season, being 0.31%, 1.05%, and 0.37% in the rainy, cool, and hot seasons, respectively (P < 0.05). Similarly, the prevalence of virgulate cercariae was 3.11%, 6.80%, and 1.64% in the rainy, cool, and hot seasons, respectively (P < 0.05). The intensity of larval trematode infections also varied between seasons and peaked in the hot season (P < 0.05) in both species. The snails infected with O. viverrini s.l. were significantly smaller (P < 0.05) and those infected with virgulate cercariae were significantly larger (P < 0.05) than uninfected snails. Seasonal variation and the different sizes of B. s. goniomphalos parasitized by O. viverrini s.l. and virgulate trematodes indicate complex host–parasite interactions with important implications for the epidemiology of O. viverrini s.l.
Introduction
The liver fluke Opisthorchis viverrini sensu lato (s.l.) is a zoonotic fish-borne trematode that is prevalent in the Mekong area of southeast Asia, including, Thailand, Lao PDR, Vietnam, and Cambodia.1 Evidence from various sources indicates that O. viverrini represents a species complex.2,3 Human infection with O. viverrini s.l. is contracted by the consumption of raw or partially cooked fish, and chronic infection leads to hepatobiliary diseases and potentially to bile duct cancer (cholangiocarcinoma [CCA]).4,5 O. viverrini s.l. is classified as a group 1 carcinogenic parasite together with Clonorchis sinensis and Schistosoma haematobium.6 The life cycle of O. viverrini s.l. requires freshwater snails as the first intermediate hosts, and cyprinid fish as the second intermediate hosts, as well as a human definitive host or other reservoir host to complete its cycle.
In Thailand and Lao PDR, three species or subspecies of Bithynia, namely B. siamensis goniomphalos, B. s. siamensis, and B. funiculata, act as intermediate hosts. These Bithynia snails play a crucial role in the life cycle of O. viverrini s.l. as the amplification point from definitive and reservoir hosts to fish intermediate host. The prevalence of O. viverrini s.l. in Bithynia snails, particularly in B. s. goniomphalos, is typically low, although it is also variable (0.03–8.37% in infected populations), in contrast to the high prevalence found in fish and human hosts.6–11
In addition to O. viverrini s.l., several groups of trematodes can be found concurrently in B. s. goniomphalos. These often have several fold higher prevalences of infection than for O. viverrini s.l.12 The xiphidiocercariae group, for example, has been subdivided into virgulate, ornatae, and armatae cercariae based on morphological differences. Virgulate cercariae are the most commonly found cercariae in B. s. goniomphalos. They belong to the family Lecithodendriidae, which are parasites of amphibians, reptiles, birds, and mammals.13
For O. viverrini s.l., data on host–parasite interactions in B. s. goniomphalos are limited, with most coming from cross-sectional surveys of the prevalence of infections. These rarely consider the effects of seasonal factors, intensity of infection, and host size selection. The only longitudinal study on transmission dynamics of O. viverrini s.l. in B. s. goniomphalos was conducted more than 30 years ago.7 Subsequently, laboratory studies indicated that susceptibility of B. s. goniomphalos to O. viverrini s.l. infection was related to size and age of the snails,14 as well as to water temperature.15 Such information is not available for virgulate xiphidiocercariae, which also use B. s. goniomphalos as an intermediate host in the same habitat. Current data on the transmission dynamics of the liver flukes as well as B. s. goniomphalos in relation to seasonal changes during a time of climate and land use changes are important to understand the transmission of O. viverrini s.l. from human and other animal definitive hosts to the snail hosts.
This study, therefore, describes variation in the prevalence and intensity of snail infection, as well as host size distribution on the transmission dynamics of O. viverrini s.l. and virgulate cercariae in B. s. goniomphalos in the same endemic locality.
Materials and Methods
Study area and snail collection.
The snail collections were performed monthly in the same rice paddy field with an area of 1,534 m2 in Phang Khon district, Sakon Nakhon Province, northeast Thailand, which is a known endemic area of O. viverrini s.l.2 In addition to rain water, the area is irrigated with water from the Nam Un Dam through a network of irrigation canals. Consequently, irrigated water is available particularly in the dry season, allowing the planting two crops of rice per year.16 From November to January and June to July most of the paddy fields had dried out and snails were sampled from areas with shallow water near inlet canals. The rice paddy habitat was chosen because previous studies detected B. s. goniomphalos at high densities there.17,18
A minimum of 500 snails were collected by handpicking and scooping from 10 to 12 sites with shallow water (< 10 cm deep) in one paddy field for 10 minutes/site. Snail samples were collected monthly during the rainy (June–October), cool (November–February), and hot (March–May) seasons during 2012–2013. Snails collected were pooled and transported to the laboratory where B. s. goniomphalos were separated according to Brandt19 and Upatham and others20 from other snails, predominantly Filopaludina and Pomacea species.
Trematode infection in B. s. goniomphalos was examined by cercarial shedding,11 where individual snails were placed in small plastic containers (3 cm in diameter and 2.5 cm high) half filled with dechlorinated water (5 mL) and exposed to a 60 W light for 5 hours. The emerged cercariae were identified by morphology according to the keys published by Schell.21 After a primary screening to determine the presence of trematode infection, the snails were separated into three groups according their infection status. These could also be divided into three groups according to size as small, medium, and large virgulate. For the small virgulate, the measurement (min–max [average] in μm) of the body width and length was 48–62 (54) × 61–93 (74) and the tail 11–19 (15) × 37–77 (61). For the medium virgulate, the body width and length was 65–87 (76) × 96–123 (108) and the tail 15–18 (17) × 79–107 (92). For the large virgulate, the body width and length was 80–108 (103) × 124–188 (165) and the tail 21–35 (29) × 42–96 (73), with small cercariae being the most common (89.6%). Snails infected exclusively by the most common small virgulate cercariae were used in this study. The snails infected with other trematodes or mixed infections with other types of cercariae (0.08%) were not included. Group 1 were trematode-free snails (uninfected controls) as confirmed by the crushing method22 and microscopy. Group 2 snails were infected with O. viverrini s.l. exclusively, and group 3 was infected with virgulate cercariae exclusively. Three types of virgulate cercariae infecting the snails were also identified by specific polymerase chain reaction (PCR) primers, ITS2 and 28s rRNA (Kiatsopit N and Sithithaworn P, unpublished data).
Enumeration of sporocyst, redia, and cercaria.
Infected snails with either O. viverrini s.l. or virgulate cercariae were allowed to acclimatize for 24 hours under laboratory conditions before being exposed to light from 06.00 am to 6.00 pm. The number of cercariae released was counted. Positive cercariae shedding snails were individually crushed and dissected to enumerate sporocysts, rediae, and cercariae following the methods by Sunita and Singh.23 Identification of the immature stages of O. viverrini s.l. was based on the morphology of developing cercariae from rediae to cercariae.8,24 In the case of virgulate cercariae, the anterior stylet in the oral sucker and the presence of well-developed penetration glands were used to identify cercariae that were found within the sporocysts.25
Measurements of size of snails.
Of the B. s. goniomphalos collected each month, 30 uninfected and 30 virgulate-infected snails were randomly chosen for size measurements. For O. viverrini s.l. the number of infected snails examined per month varied (1–51 snails) because the prevalence of O. viverrini s.l. infection in snails is normally low.7,8,10,26–28 The snails were measured under a light microscope with digital calipers (length of shell from apex to aperture).
Statistical analyses.
Data were assessed for normality prior to performing statistical tests. Only data for length were normally distributed while all data for intensity of trematode infection had an aggregated distribution. Statistical tests were done using SPSS Statistics V21.0 (IBM Corporation, Armonk, NY). Independent t tests and analysis of variance (ANOVA) were used for normally distributed data to assess size differences between groups of infected and uninfected snails. Mann–Whitney U tests were used to compare the intensity of larval trematode infection between snails collected from different seasons. χ2 tests were used to compare differences between prevalence in different seasons, and Kendall's tau was used to determine whether there was a correlation between intensity of larval trematode infection and the size of snails. Multivariate linear regression on log-transformed data was used to assess the contribution of seasonal factors and size of snails to the intensity of trematode infection, that is, sporocysts, rediae, and cercariae. The statistical tests were considered significant when P < 0.05.
Results
Seasonal prevalence of trematode cercariae in B. s. goniomphalos.
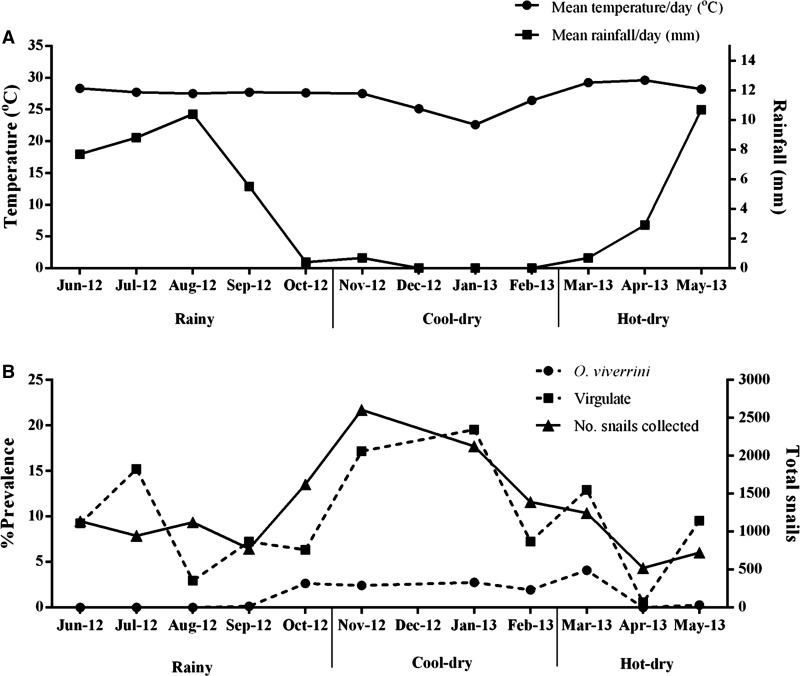
A total of 14,183 B. s. goniomphalos snails were analyzed. O. viverrini s.l. was found in 1.73% and small virgulate cercariae in 11.55% of the snails. The infected snails collected during the three seasons contained medium virgulate (0.51%), large virgulate (0.82%), monostome (0.80%), cystophorous (0.39%), furcocercous (0.37%), lophocercous (0.22%), and mutabile (0.04%) cercariae. Data on average temperature, rainfall, and the prevalence of O. viverrini s.l. and virgulate cercariae are shown in Table 1. For O. viverrini s.l., the prevalence during the rainy season was significantly lower than for the cool–dry and hot–dry seasons (P < 0.05), but the rates in the cool–dry were not different from those in the hot–dry season (P > 0.05). Detailed prevalence profiles (Figure 1) show that a higher prevalence of O. viverrini s.l. occurred after rainfall and that this diminished in the hot–dry season. In virgulate cercariae, the infection rate for cool–dry was significantly higher than in the rainy season (P < 0.05), but was not significantly different for the hot–dry season. The prevalence profiles of virgulate cercaria were similar to those for O. viverrini s.l. but with significantly higher levels that peaked in the cool–dry season after a drop in temperature (Figure 1).
Table 1.
Infection status of Opisthorchis viverrini sensu lato (s.l.) and virgulate cercariae in Bithynia siamensis goniomphalos over three consecutive seasons from the study area in northeast Thailand
| Seasons | Mean daily temperature (°C) Mean ± SD | Mean daily rainfall (mm) Mean ± SD | Number of snails | O. viverrini | Virgulate cercariae | ||
|---|---|---|---|---|---|---|---|
| Number of infected snails | Prevalence (%) | Number of infected snails | Prevalence (%) | ||||
| Rainy | 27.8 ± 0.3 | 6.5 ± 3.8 | 5,601 | 44 | 0.31 | 441 | 3.11 |
| Cool–dry | 25.4 ± 2.1 | 0.2 ± 0.3 | 6,102 | 149 | 1.05 | 965 | 6.80 |
| Hot–dry | 29.0 ± 0.7 | 4.8 ± 5.2 | 2,480 | 53 | 0.37 | 233 | 1.64 |
| Total | − | − | 14,183 | 246 | 1.73 | 1,639 | 11.55 |
Figure 1.
Variation in the pattern of mean daily temperature (°C) (A) and mean rainfall/day (mm) (B) and the seasonal prevalence of Opisthorchis viverrini sensu lato (s.l.) and virgulate cercariae in Bithynia siamensis goniomphalos over three consecutive seasons.
Effect of season on intensity of larval trematode infection in B. s. goniomphalos.
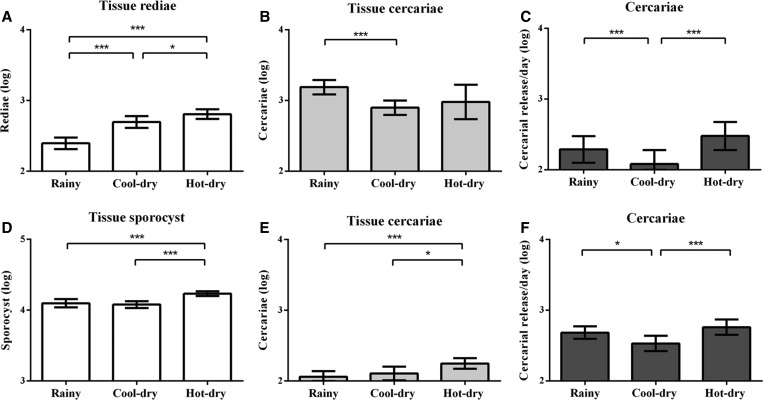
Intensities of O. viverrini s.l. and virgulate immature stages during the different seasons are shown in Figure 2. The intensity of infection with O. viverrini s.l. immature stages (tissue rediae, tissue cercariae, and released cercariae) varied significantly with season (P < 0.001). The intensity of tissue rediae in the rainy season was lower than in the hot–dry and cool–dry seasons (P < 0.05) (Figure 2A). When both shell length and season were analyzed as independent variables, both significantly influenced the intensity of rediae, but only shell length had a statistically significant effect on the intensity of tissue infection and released cercariae (P < 0.05). The intensity of tissue infection with cercariae was comparable in the rainy and hot–dry seasons. This was greater than that in the cool–dry season (P < 0.05) (Figure 2B). Cercarial release was higher in the hot–dry season, than during the rainy season, and became lowest in the cool–dry season (Figure 2C). No sporocysts of O. viverrini s.l. were detected in the snails examined.
Figure 2.
Intensity of infection of immature trematode stages in Bithynia siamensis goniomphalos during different seasons. The mean intensity ± standard deviation (SD; log transformed) for Opisthorchis viverrini sensu lato (s.l.) (A–C) and for virgulate (D–F) are shown. *P < 0.05, **P < 0.01, ***P < 0.001 by Mann–Whitney U test. (A) Tissue rediae; (D) tissue sporocyst; (B, E) tissue cercariae; and (C, F) cercarial release per day. SD = standard deviation.
For virgulate cercariae, the intensity of sporocyst and cercarial infection also varied significantly with season (P < 0.01). The intensity of sporocyst infection was highest in the hot–dry (P < 0.05) compared with the rainy season followed by the lowest intensity in the cool–dry season (Figure 2D). The intensity of tissue cercariae was also highest in the hot–dry season but was different to sporocysts in that the lowest intensity was in the rainy season (Figure 2E). The number of cercariae released per day had the same pattern as that for tissue cercariae (Figure 2F) but the lowest intensity was in the cool–dry season. When both shell length and season were considered together, only shell length had a statistically significant effect on the intensity of sporocyst infection and cercariae released (P < 0.05).
Effect of immature trematode infection and season on the size of snails.
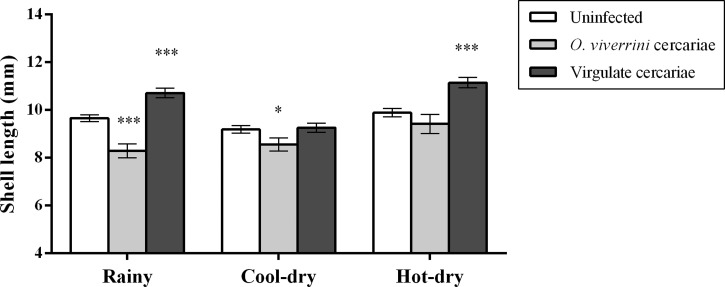
A sample of 839 snails was selected for size comparisons. Of them, 360 were uninfected, 360 were infected by virgulate cercariae, and 119 were infected by O. viverrini s.l. The shell length was higher in the hot–dry and rainy seasons than in the cool–dry season (Figure 3). The shell length was significantly influenced by season and infection status (ANOVA, P < 0.001).
Figure 3.
Comparisons of shell length of Bithynia siamensis goniomphalos with different infection statuses and seasons. Data shown are mean ± SD of shell length for uninfected (white), Opisthorchis viverrini sensu lato (s.l.) infected (gray) and virgulate cercariae infected (black). *P < 0.05, **P < 0.01, ***P < 0.001 by Mann–Whitney U test. SD = standard deviation.
The shell length of uninfected snails was greatest in the hot–dry season, followed by the rainy season and was smallest in the cool–dry season with a range of 7.30–12.12 mm overall three seasons. Snails infected with O. viverrini s.l. were significantly smaller than uninfected snails in the rainy and the cool–dry seasons (P < 0.001) but were not different in the hot–dry season. The shell length overall 3 seasons ranged from 6.84 to 11.11 mm and 31.93% were small snails (< 8 mm), 52.10% were prereproductive and in medium (8–10 mm) and (15.97%) were reproductive, large snails (> 10 mm). For virgulate cercariae, the infected snails were larger than uninfected snails and snails infected with O. viverrini s.l. in the rainy and hot–dry season (P < 0.001), but not in the cool–dry season. The shell length overall three seasons ranged between 7.17 and 13.43 mm, and 37.50%, 58.33%, and 4.17% were medium, large, and small snails, respectively.
Correlation between the number of immature trematode stages and snail size.
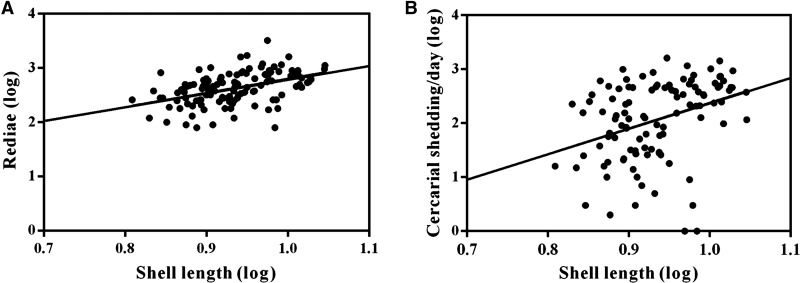
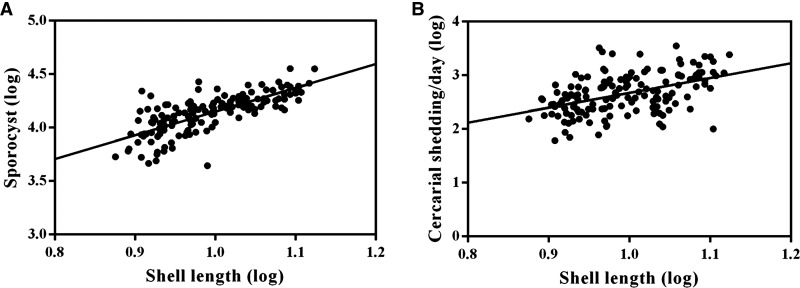
A total of 269 snails were examined for O. viverrini s.l. and virgulate immature stages, with 119 infected with O. viverrini s.l. rediae and tissue and shedding cercariae and 150 infected with virgulate sporocysts and tissue and shedding cercariae. The numbers of rediae, tissue and shedding cercariae of O. viverrini s.l. were significantly correlated with shell length of snails (Figure 4 ). The correlation between the number of sporocysts and cercarial shedding and shell length are shown in Figure 5 .
Figure 4.
Correlation between Opisthorchis viverrini sensu lato (s.l.) rediae (A) and cercariae (B) with shell length of Bithynia siamensis goniomphalos (N = 119). For rediae (A) the solid line represents the line of best fit (r = 0.304, P < 0.000) and for cercariae (B) (r = 0.172, P = 0.006).
Figure 5.
Correlation between virgulate sporocysts (A) and cercariae (B) with shell length of Bithynia siamensis goniomphalos (N = 150). For sporocyst (A) the solid line represents the line of best fit (r = 0.584, P < 0.000) and for cercariae (B) (r = 0.315, P < 0.001).
Discussion
Within the overall prevalence of trematode cercarial infections in B. s. goniomphalos found in this study (16.58%), the majority, 11.55%, were virgulate cercariae and only 1.73% comprised O. viverrini s.l. The prevalences of both trematodes varied with season and became highest in the cool–dry season compared with the rainy season and hot–dry season. The high prevalence during the cool–dry season may be attributed to the reduced water volume accompanied by increased density of snail hosts and potentially the accumulation of eggs, increasing the chance of infection.29 In addition, fluke eggs shed into the environment are more likely to be washed into water bodies during the rainy season,9 and this corresponds to the fecal bacterial contamination being higher during the rainy season (April–October).30 In snails, the miracidia hatch and develop further through the stages of sporocysts, rediae, and cercariae in 6–8 weeks.31 This results in the accumulation of infected snails in the cool–dry season. Upatham and Sukhapanth26 found that cercarial infection occurs all year round, with the highest infection rate in snails being found during the rainy season. Brockelman and others,7 working at a different site, found that the percentage of native snails infected with O. viverrini s.l. was low (0.11%), but prevalence was higher in the cool–dry season than other seasons. This seasonal pattern also fits our data in spite of major environmental and land use changes having taken place in the northeast of Thailand since Brockelman's study.
The intensity of intra-molluscan stages, that is, tissue rediae, tissue cercariae, and cercariae, in O. viverrini s.l., as well as virgulate cercaria-infected snails, was highest in the hot–dry season. This indicates that temperature is one of the crucial factors for snails, as well as for larval growth and development. Prasopdee and others15 found that infectivity of O. viverrini s.l. in B. s. goniomphalos was correlated with temperature and that a temperature of 34°C was optimal for obtaining the highest infection rate. This is important given the predicted increase in temperatures because of global climatic change.32 Alternatively, small body size may affect the number of rediae and sporocysts produced, as the redial burden has been shown to be related to the size of Galba truncatula snails infected with Fasciola hepatica.33 For both O. viverrini s.l. and virgulate cercariae, significantly fewer cercariae were released by snails in the cool–dry season with a peak being reached in the hot–dry season, which could also be related to temperature, snail size, or other environmental factors.
Size-related parameters showed that O. viverrini s.l. infects smaller prereproductive snails, whereas the virgulate species infected larger, reproductively active snails. In case of O. viverrini s.l., a possible explanation involves parasite-induced growth retardation, which reduces growth rate, potentially causing the low number of O. viverrini s.l. infecting reproductively larger snails.14 Prereproductive Bithynia snails (shell length < 8 mm) were reported to be more susceptible to O. viverrini s.l. infection,14,15 as enhanced trematode cercarial development has been shown to occur in juvenile snails because they have a weaker defense system.34–36 In addition, O. viverrini s.l. may be more pathogenic to snails than the virgulate species leading to higher death rate of larger infected snails.14 On the other hand, parasite-induced gigantism and the fact that larger snails are behaviorally more active37 may explain why the virgulate species is predominantly found infecting larger snails. For snail–trematode interactions, gigantism is often postulated to benefit the parasite as a larger host provides a greater volume for parasite occupation and reproduction.38,39 Indeed, results from our work support this idea as larger infected B. s. goniomphalos contained higher numbers of virgulate sporocysts than smaller individuals. Further, miracidia are generally attracted to larger-size snails, as they are more active and have more of the body exposed as a target for miracidia.40
There are data indicating that the snail growth rate increases or decreases depending on the biological features of the trematodes developing in the snail. In the case of O. viverrini s.l., infected snails were significantly smaller than uninfected individuals. Studies by Chanawong and Waikagul14 and Prasopdee and others15 found that the small snails had higher infection rates than larger snails. Therefore, shell size influences the susceptibility of B. s. goniomphalos infection and may be interpreted as a consequence of age variation.
The prevalence of O. viverrini s.l. in snails can be influenced by mixed infections when two trematode species occur within one snail. The first trematode species is usually able to interfere with the development of the second (interspecific competition). Low prevalence rates of O. viverrini s.l. in snails might therefore be the result of inter-trematode competition, with the virgulate species being substantially more common. Double species infection by trematode larvae in snails is usually infrequent.41–43
This study considered the intra-molluscan development of O. viverrini s.l. and virgulate cercariae infected B. s. goniomphalos as natural infections in snails collected in the field. These are always more intensively infected with greater numbers of rediae and of cercariae than under laboratory conditions.44 However, the lifespan of the naturally infected snails could not be elucidated in the field, since the date on which the snails became infected was not known. Laboratory experiments are required to clarify and extend these field results.
To conclude, in O. viverrini s.l. the prevalence peaked in the cool–dry season while the intensity of immature stages was greatest in the hot–dry season. Medium and large snails, or reproductive sizes, were less commonly infected than small prereproductive snails. Compared with uninfected snails, O. viverrini s.l.–infected snails were smaller during all seasons. For virgulate cercariae, the prevalence was highest in cool–dry season and the intensity of immature stages was greatest in hot–dry season. Snails infected with virgulate cercariae were larger in size than uninfected snails, as well as those snails infected with O. viverrini s.l. This pattern of seasonal infection and intensity, and the size/age of B. s. goniomphalos snails influence parasite epidemiology throughout their three host life. Different snail size selection by the trematodes suggests possible resource partitioning to avoid competition, or size-related pathology and susceptibility to infection.
ACKNOWLEDGMENTS
We acknowledge the support of the Cholangiocarcinoma Screening and Care Program (CASCAP), Khon Kaen University, Thailand.
Footnotes
Financial support: This work was supported by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Center of Excellence in Specific Health Problems in Greater Mekong Subregion Cluster (SHeP-GMS), Project no. NRU 54205 and the Invitation Research fund (I 57102) from the Faculty of Medicine, Khon Kaen University, Thailand.
Authors' addresses: Jutamas Namsanor, Paiboon Sithithaworn, Kulthida Kopolrat, Nadda Kiatsopit, Opal Pitaksakulrat, and Smarn Tesana, Department of Parasitology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand, and Liver Fluke and Cholangiocarcinoma Research Center (LFCRC), Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand, E-mails: jutamas.namsanor@gmail.com, paibsit@gmail.com, kulthida_kop@yahoo.com, nd_tg_na@yahoo.com, opioid81_vm16@hotmail.com, and smarn_te@kku.ac.th. Ross H. Andrews, CASCAP, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand, and Imperial College London, Faculty of Medicine, St. Mary's Campus, South Wharf Street, London, United Kingdom, E-mail: rhandrews@gmail.com. Trevor N. Petney, Department of Ecology and Parasitology, Karlsruhe Institute of Technology, Karlsruhe, Germany, and CASCAP, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand, E-mail: trevor.petney@kit.edu.
References
- 1.Sithithaworn P, Andrews R, Nguyen V, Wongsaroj T, Sinuon M, Odermatt P, Nawa Y, Liang S, Brindley P, Sripa B. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol Int. 2012;61:10–16. doi: 10.1016/j.parint.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saijuntha W, Sithithaworn P, Wongkham S, Laha T, Pipitgool V, Tesana S, Chilton NB, Petney TN, Andrews RH. Evidence of a species complex within the food-borne trematode Opisthorchis viverrini and possible co-evolution with their first intermediate hosts. Int J Parasitol. 2007;37:695–703. doi: 10.1016/j.ijpara.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laoprom N, Saijuntha W, Sithithaworn P, Wongkham S, Laha T, Ando K, Andrews RH, Petney TN. Biological variation within Opisthorchis viverrini sensu lato in Thailand and Lao PDR. J Parasitol. 2009;95:1307–1313. doi: 10.1645/GE-2116.1. [DOI] [PubMed] [Google Scholar]
- 4.Sripa B, Bethony J, Sithithaworn P, Kaewkes S, Mairiang E, Loukas A, Mulvenna J, Laha T, Hotez P, Brindley P. Opisthorchiasis and Opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop. 2011;120:158–168. doi: 10.1016/j.actatropica.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sithithaworn P, Yongvanit P, Duenngai K, Kiatsopit N, Pairojkul C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2014;21:301–308. doi: 10.1002/jhbp.62. [DOI] [PubMed] [Google Scholar]
- 6.IARC A review of human carcinogens: biological agents, Opisthorchis viverrini and Clonorchis sinensis. IARC Monogr Eval Carcinog Risks Hum. 2011;100:351–376. [Google Scholar]
- 7.Brockelman W, Upatham E, Viyanant V, Ardsungnoen S, Chantanawat R. Field studies on the transmission of the human liver fluke, Opisthorchis viverrini, in northeast Thailand: population changes of the snail intermediate host. Int J Parasitol. 1986;16:545–552. doi: 10.1016/0020-7519(86)90091-3. [DOI] [PubMed] [Google Scholar]
- 8.Adam R, Arnold H, Pipitgool V, Sithithaworn P, Hinz E, Storch V. Studies on lophocercous cercariae from Bithynia siamensis goniomphalos (Prosobranchia: Bithyniidae) Southeast Asian J Trop Med Public Health. 1993;24:697–700. [PubMed] [Google Scholar]
- 9.Sithithaworn P, Haswell-Elkins M. Epidemiology of Opisthorchis viverrini. Acta Trop. 2003;88:187–194. doi: 10.1016/j.actatropica.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Ngern-klun R, Sukontason K, Tesana S, Sripakdee D, Irvine K, Sukontason K. Field investigation of Bithynia funiculata, intermediate host of Opisthorchis viverrini in northern Thailand. Southeast Asian J Trop Med Public Health. 2006;37:662–672. [PubMed] [Google Scholar]
- 11.Kiatsopit N, Sithithaworn P, Saijuntha W, Boonmars T, Tesana S, Sithithaworn J, Petney T, Andrews R. Exceptionally high prevalence of infection of Bithynia siamensis goniomphalos with Opisthorchis viverrini cercariae in different wetlands in Thailand and Lao PDR. Am J Trop Med Hyg. 2012;86:464–469. doi: 10.4269/ajtmh.2012.11-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tesana S, Thabsripair P, Suwannatrai A, Haruay S, Piratae S, Khampoosa P, Thammasiri C, Prasopdee S, Kulsantiwong J, Chalorkpunrut P, Jones M. Parasite surveys and environmental management for prevention of parasitic infection in cultivated Barbonymus gonionotus (Cyprinidae) in fishponds, in an opisthorchiasis endemic area of northeast Thailand. Aquaculture. 2014;428–429:54–60. [Google Scholar]
- 13.Brooks D, O'Grady R, Glen D. Phylogenetic analysis of the Digenea (Platyhelminthes: Cercomeria) with comments on their adaptive radiation. Can J Zool. 1985;63:411–443. [Google Scholar]
- 14.Chanawong A, Waikagul J. Laboratory studies on host-parasite relationship of Bithynia snails and the liver fluke, Opisthorchis viverrini. Southeast Asian J Trop Med Public Health. 1991;22:235–239. [PubMed] [Google Scholar]
- 15.Prasopdee S, Kulsantiwong J, Piratae S, Khampoosa P, Thammasiri C, Suwannatrai A, Laha T, Grams R, Loukas A, Tesana S. Temperature dependence of Opisthorchis viverrini infection in first intermediate host snail, Bithynia siamensis goniomphalos. Acta Trop. 2015;141:112–117. doi: 10.1016/j.actatropica.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Kanokkanjana K, Garivait S. Alternative rice straw management practices to reduce field open burning in Thailand. Int J Environ Sci Dev. 2013;4:119–123. [Google Scholar]
- 17.Petney T, Sithithaworn P, Andrews R, Kiatsopit N, Tesana S, Grundy-Warr C, Ziegler A. The ecology of the Bithynia first intermediate hosts of Opisthorchis viverrini. Parasitol Int. 2012;61:38–45. doi: 10.1016/j.parint.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Wang YC, Ho RC, Feng CC, Namsanor J, Sithithaworn P. An ecological study of Bithynia snails, the first intermediate host of Opisthorchis viverrini in northeast Thailand. Acta Trop. 2015;141:244–252. doi: 10.1016/j.actatropica.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Brandt R. The non-marine aquatic Mollusca of Thailand. Arch für Molluskenkd. 1974;105:1–423. [Google Scholar]
- 20.Upatham E, Sornmani S, Kitikoon V, Lohachit C, Bruch J. Identification key for fresh-brackish water snails of Thailand. Malacol Rev. 1983;16:107–132. [Google Scholar]
- 21.Schell S. How to Know the Trematode. Dubuque, IA: WMC Brown Company Publishers; 1970. p. 355. [Google Scholar]
- 22.Caron Y, Rondelaud D, Losson B. The detection and quantification of a digenean infection in the snail host with special emphasis on Fasciola sp. Parasitol Res. 2008;103:735–744. doi: 10.1007/s00436-008-1086-1. [DOI] [PubMed] [Google Scholar]
- 23.Sunita K, Singh D. Fascioliasis control: in vivo and in vitro phytotherapy of vector snail to kill Fasciola larva. J Parasitol Res. 2011;240807 doi: 10.1155/2011/240807. doi:10.1155/2011/240807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaewkes S. Taxonomy and biology of liver flukes. Acta Trop. 2003;88:177–186. doi: 10.1016/j.actatropica.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Cheng T. General Parasitology. New York, NY: Academic Press; 1973. p. 965. [Google Scholar]
- 26.Upatham E, Sukhapanth N. Field studies on the bionomics of Bithynia siamensis siamensis and the transmission of Opisthorchis viverrini in Bangna, Bangkok, Thailand. Southeast Asian J Trop Med Public Health. 1980;11:355–358. [PubMed] [Google Scholar]
- 27.Lohachit C. Ecological Studies of Bithynia siamensis goniomphalos A Snail Intermediate Host of Opisthorchis viverrini in Khon Kaen Province, Northeast Thailand. PhD thesis. Bangkok, Thailand: Mahidol University; 2001. [Google Scholar]
- 28.Sri-Aroon P, Butraporn P, Limsomboon J, Kerdpuech Y, Kaewpoolsri M, Kiatsiri S. Freshwater mollusks of medical importance in Kalasin Province, northeast Thailand. Southeast Asian J Trop Med Public Health. 2005;36:653–657. [PubMed] [Google Scholar]
- 29.Suwannatrai A, Suwannatrai K, Haruay S, Piratae S, Thammasiri C, Khampoosa P, Kulsantiwong J, Prasopdee S, Tarbsripair P, Suwanwerakamtorn R, Sukchan S, Boonmars T, Malone J, Kearney M, Tesana S. Effect of soil surface salt on the density and distribution of the snail Bithynia siamensis goniomphalos in northeast Thailand. Geospat Health. 2011;5:183–190. doi: 10.4081/gh.2011.170. [DOI] [PubMed] [Google Scholar]
- 30.Kaewkes W, Kaewkes S, Tesana S, Laha T, Sripa B. Fecal bacterial contamination in natural water reservoirs as an indicator of seasonal infection by Opisthorchis viverrini in snail intermediate hosts. Parasitol Int. 2012;61:49–51. doi: 10.1016/j.parint.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Harinasuta C, Harinasuta T. Opisthorchis viverrini: life cycle, intermediate hosts, transmission to man and geographical distribution in Thailand. Arzneimittelforschung. 1984;34:1164–1167. [PubMed] [Google Scholar]
- 32.Poulin R. Global warming and temperature-mediated increases in cercarial emergence in trematode parasites. Parasitology. 2006;132:143–151. doi: 10.1017/S0031182005008693. [DOI] [PubMed] [Google Scholar]
- 33.Rondelaud D, Barthe D. Fasciola hepatica L.: the productivity of a sporocyst as a function of the size of Lymnaea truncatula Muller. Parasitol Res. 1987;74:155–160. doi: 10.1007/BF00536027. [DOI] [PubMed] [Google Scholar]
- 34.Richards C. Schistosoma mansoni: susceptibility reversal with age in the snail host Biomphalaria glabrata. Exp Parasitol. 1977;42:165–168. doi: 10.1016/0014-4894(77)90074-1. [DOI] [PubMed] [Google Scholar]
- 35.Dikkeboom R, Van der Knapp W, Meuleman E, Sminia T. Differences between blood cells of juvenile and adult specimens of the pond snail Lymnaea stagnalis. Cell Tissue Res. 1984;238:43–47. [Google Scholar]
- 36.Dreyfuss G, Vignoles P, Rondelaud D. Variability of Fasciola hepatica infection in Lymnaea ovata in relation to snail population and snail age. Parasitol Res. 2000;86:69–73. doi: 10.1007/s004360050013. [DOI] [PubMed] [Google Scholar]
- 37.Seppälä O, Karvonen A, Kuosa M, Haataja M, Jokela J. Are sick individuals weak competitors? Competitive ability of snails parasitized by a gigantism-inducing trematode. PLoS ONE. 2013;8:e79366. doi: 10.1371/journal.pone.0079366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandland G, Minchella D. Effects of diet and Echinostoma revolutum infection on energy allocation patterns in juvenile Lymnaea elodes snails. Oecologia. 2003;134:479–486. doi: 10.1007/s00442-002-1127-x. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy H, Fitzpatrick S, Irwin S. Parasite alteration of host shape: a quantitative approach to gigantism helps elucidate evolutionary advantages. Parasitology. 2004;128:7–14. doi: 10.1017/s0031182003004190. [DOI] [PubMed] [Google Scholar]
- 40.Lim H, Heyneman D. Intramolluscan inter-trematode antagonism: a review of factors influencing the host-parasite system and its possible role in biological control. Adv Parasitol. 1972;10:191–268. doi: 10.1016/s0065-308x(08)60175-x. [DOI] [PubMed] [Google Scholar]
- 41.Sousa W. Interspecific antagonism and species coexistence in a diverse guild of larval trematode parasites. Ecol Monogr. 1993;63:103–128. [Google Scholar]
- 42.Kuris A, Lafferty K. Community structure: larval trematodes in snail hosts. Annu Rev Ecol Syst. 1994;25:189–217. [Google Scholar]
- 43.Lafferty K, Sammond D, Kuris A. Analysis of larval trematode communities. Ecology. 1994;75:2275–2285. [Google Scholar]
- 44.ErhardovaÌ-KotrlaÌ B. The Occurrence of Fascioloides magna (Bassi, 1875) in Czechoslovakia. Prague, Czech Republic; Academia: 1971. p. 155. [Google Scholar]