Abstract
Magnetic resonance imaging (MRI) variables, such as T2* and volume, can predict the healing ligament structural properties. How these MR variables relate to semi-quantitative histology of the healing ACL is yet unknown. We hypothesized that T2* and volume would predict the histological scoring of a healing ACL. Yucatan minipigs underwent ACL transection and received: bridge-enhanced ACL repair or no treatment. The surgical legs were harvested after 52 weeks and imaged using a high resolution 2-echo sequence. For each ligament the volume and median T2* values were determined. The ACL specimens were then histologically analyzed using the advanced Ligament Maturity Index (LMI). The T2* of the healing ligaments significantly predicted the Total LMI score as well as the Cell, Collagen and Vessel sub-scores; R2=0.78, 0.67, 0.65, and 0.60, respectively (p≤0.001). The ligament volume also predicted the Total LMI score, Cell and Collagen sub-scores; R2=0.39, 0.33, 0.37, and 0.60, respectively (p≤0.001). A lower ligament T2* or a higher volume was associated with higher histological scores of the healing ligaments. This study provides a critical step in the development of a noninvasive method to evaluate ligament healing on a microscopic scale.
Keywords: MRI, ACL, Histology, Ligament, Healing
Introduction
Current methods to evaluate ligament healing in animal models, such as histological evaluation or biomechanical testing, are crucial for tracking the healing response. However, these methods require biopsy or destructive testing and are not conducive for in vivo assessment. Using non-invasive MR imaging to evaluate graft maturation biomechanically, significant correlations were found between signal intensity and structural properties of the ACL reconstruction graft in an animal model.1–3 The combination of both ligament/graft volume (amount of tissue) and signal intensity (tissue quality) significantly improved those predictions.1 However, signal intensity is not ideal to document ligament healing as it is dependent on MR specifications, including manufacturer, acquisition parameters, and hardware effects that can vary between scanners.4
T2* relaxation time, another MR variable, correlates with the level of tissue organization,5,6 is well suited for imaging highly organized collagenous structures,5,7,8 and is less sensitive to imaging parameters than signal intensity data.4 Healing ligament volume, as defined by the distributions of T2* relaxation times within that ligament, can provide a predictive model of structural properties, where longer T2* times are associated with lower structural properties.9 Longer T2* values in the meniscus were also found to be associated with worse biomechanical performance7 and degeneration,8 as defined by collagen content accessed via histological grading. While these findings between T2* relaxation time and histological outcomes in the meniscus are promising, the relationship of healing ACL T2* relaxation time with microscopic evidence remained unknown. More specifically, in both the research and clinical settings, it would be advantageous to understand what histological factors contribute to shorter T2* times in the healing ACL.
The Ligament Maturity Index (LMI) has been used to assess histological sections of the early and late stages of healing.10,11 Recently the LMI scoring system was adapted for later stages in “ligamentization”, where proliferation and remodeling of the tissue are more apparent. The adapted LMI assesses a number of complex factors critical to late stage ligament healing. These factors are evaluated with metrics, which form the Cell, Collagen and Vessel sub-scores and are then combined into the Total LMI score (Table 1).11
Table 1.
Criteria used to determine the advanced Ligament Maturity Index (LMI). The five regions from each ligament were separately scored according to the cell, collagen and vessel criteria. The cell, collagen and vessel sub-scores for each ligament were then determined by averaging the scores for each of the five regions using the sub-scores respective criteria. The resulting cell, collagen and vessel sub-scores for each ligament were then summed to determine the total LMI score representing the cumulative indications of healing.
| Cell Sub-Score (total = 8 pts) | Collagen Sub-Score (total = 12 pts) | Vessel Sub-Score (total = 6 pts) | |||
|---|---|---|---|---|---|
| Criteria | pts | Criteria | pts | Criteria | pts |
| Presence of inflammatory cells | Width of bundles | Density of blood vessels | |||
| Necrosis | 0 | No bundles | 0 | None present | −1 |
| Polymorphonuclear cells | 1 | Width less than 50 μm | 2 | more than 200% present | 0 |
| No inflammatory cells | 2 | Width greater than 50 μm | 4 | 150%-200% present | 1 |
| Less than 150% present | 2 | ||||
| Nuclear aspect ratio (NAR) of fibroblasts | Bundle orientation with long axis of ligament | Vessel orientation with long axis of ligament | |||
| No cells | −1 | No bundles | −2 | No vessels oriented | −2 |
| Average NAR less than 2 (round) | 0 | less than 50% oriented | 0 | Less than 30% oriented | −1 |
| Average NAR 2-4 | 1 | 50%-75% oriented | 2 | less than 50% oriented | 0 |
| Average NAR greater than 4 (elongated) | 2 | 75%-100% oriented | 4 | 50%-75% oriented | 1 |
| 75%-100% oriented | 2 | ||||
| Nucleus of fibroblast aligned with fascicles and long axis of ligament | Collagen Crimp | Vessel maturity | |||
| No cells | −2 | No crimp | −2 | No vessels seen | 0 |
| Less than 30% of cells oriented | −1 | less than 25% crimp | 0 | Capillaries only present | 1 |
| 30%-50% oriented | 0 | 25%-75% crimp | 2 | Arterioles present | 2 |
| 50%-75% oriented | 1 | Crimp with normal length present | 4 | ||
| 75%-100% oriented | 2 | ||||
| Number of fibroblasts | |||||
| None | −1 | ||||
| Greater than 2× normal | 0 | ||||
| Between 1.5× and 2× normal | 1 | ||||
| Less than 1.5× normal | 2 | ||||
To date there have been no studies evaluating how T2* relaxation time and volume of a healing ligament can predict histological scoring. The objective of this study was to test a noninvasive method for predicting the histological outcomes of a bridge-enhanced ACL repair or a naturally healing ligament in a porcine model at 52 weeks of healing using T2* and volume. We hypothesized that MR derived measures of T2* and volume would be significant predictors of the histological scoring of a healing ACL after 52 weeks of healing.
Methods
Animal Model
This controlled laboratory experiment was approved by the Institutional Animal Care and Use Committee. Fifteen Yucatan minipigs (approximately 15 months of age) underwent unilateral ACL transection surgery as previously described.12 Immediately following transection, eight of the animals received bridge-enhanced ACL repair with an extracellular matrix-blood composite and seven were left untreated to heal naturally without bridge-enhanced repair (ACL transection).12 It should be noted that these animals were part of another study evaluating the long-term effects of the bridge-enhanced ACL repair.9,11,12 At 52 weeks, all surgical knees were harvested and immediately imaged.
MR Imaging
All image data were acquired of the surgical knees using a 3T Siemens Tim Trio scanner (Erlangen, Germany) using a 20cm volume extremity coil. T2* relaxation time was estimated using high-resolution 3-D gradient echo (FLASH) data sets with parameters: TR=25ms, TE=7.36, 15.24ms (2 echoes), flip angle=12°, FOV=140mm, slice thickness=0.85mm (contiguous slices), reconstruction matrix size=512x512, 3 averages, bandwidth=130Hz/pixel, and scan time=19 minutes.9 The bridge-enhanced repaired and naturally healing transected ligaments were manually segmented from the MR images in both the coronal and sagittal planes using commercially available software (Mimics 13.1, Materialise, Ann Arbor, Michigan). 3-D models of the healing ligaments were then created.1 The total number of ligament voxels was used to determine the volume of the whole ligament (16.1 voxels equaled one mm3).
MR Imaging T2* Estimation
Using previously described methods,9 Matlab (R2012b; MathWorks, Natick, MA) code was used to estimate T2* maps using the signal intensity (SI) relationship: ,9,13 where SI1 and SI are the signal intensities corresponding to the echo times TE1 and TE2 where TE2 > TE1 for each voxel. The ligament specific maps were produced by extracting the voxels corresponding to the ligament from the T2* maps using the 3-D models created from the segmented images. The median T2* value was determined for each ligament volume and then compared to the ligament scoring metrics as described below. The distributions of T2* for each ligament were found to be lognormal and positively skewed. Median T2* was used to represent the central tendency of each ligament as differences in the median value would be representative of the entire ligament distribution.
Histological Scoring
Following imaging, the specimens were frozen and stored at -20 degrees Celsius until mechanical testing. The knees were then prepared and mechanically tested to determine structural properties.11,12 Following biomechanical testing, knees were cut sagittally through the center of the ACL. Bone-ligament-bone sections were formalin fixed, decalcified (DELTA-Cal, Delta Products Group, Aurora, IL), and paraffin embedded. 7 μm thick sections were cut with a microtome, placed onto custom glass slides (Corning 75x50 mm Plain Microscope Slides, Corning Incorporated, Corning, NY), and stored at 4° C until staining with hematoxylin and eosin (H&E) or α-smooth muscle actin antibodies (SMA).11 Cell density, morphology, as well as collagen formation (evaluated under polarized light with a 137nm wavelength filter) were assessed on the H&E slides, while SMA stained sections were used to determine vascularity.
Using the advanced LMI (Table 1), the sagittal sections from the central portions of the ligaments were scored in five regions; 1mm into the ligament from either tibial or femoral insertion site, and three regions in between. When choosing the five regions for histological analysis, the examiner selected regions that were free of synovium and not visibly deformed by biomechanical testing.11 The five regions from each ligament were separately scored according to the cell, collagen and vessel criteria. The cell, collagen and vessel sub-scores for each ligament were then determined by averaging the scores for each of the five regions using the sub-scores respective criteria. The resulting cell, collagen and vessel sub-scores for each ligament were then summed to determine the total LMI score, representing the cumulative effects of healing (Table 1). All investigators were blinded to the treatment group for all histological and MRI evaluations. Additionally, the intraclass correlation coefficient between examiners using the Ligament Maturity Index has been shown to be greater than .90.14
Data Analysis
The repaired and naturally healing transected ligaments were grouped together and analyzed as a single data set. First, single linear regression models were used to separately test the relationships between: 1) ligament median T2* and histological scores and, 2) MR volume and histological scores (Total LMI, Cell sub-score, Collagen sub-score, and Vessel sub-score).
Subsequently, both ligament median T2* and ACL volume were included in a multiple linear regression model to predict the histological scores. For all regression equations, the R-square values and p-values for the models were reported as indicators of the strength of the relationships. Individual p-values of the independent variables in the multiple regression equation were used to assess the relative contribution of ligament median T2* and volume to the prediction. 95% confidence intervals (Figure 1) and standard error (SE) (Tables 2 and 3) of the regression equations were also determined as a means to assess the accuracy of the prediction equations.
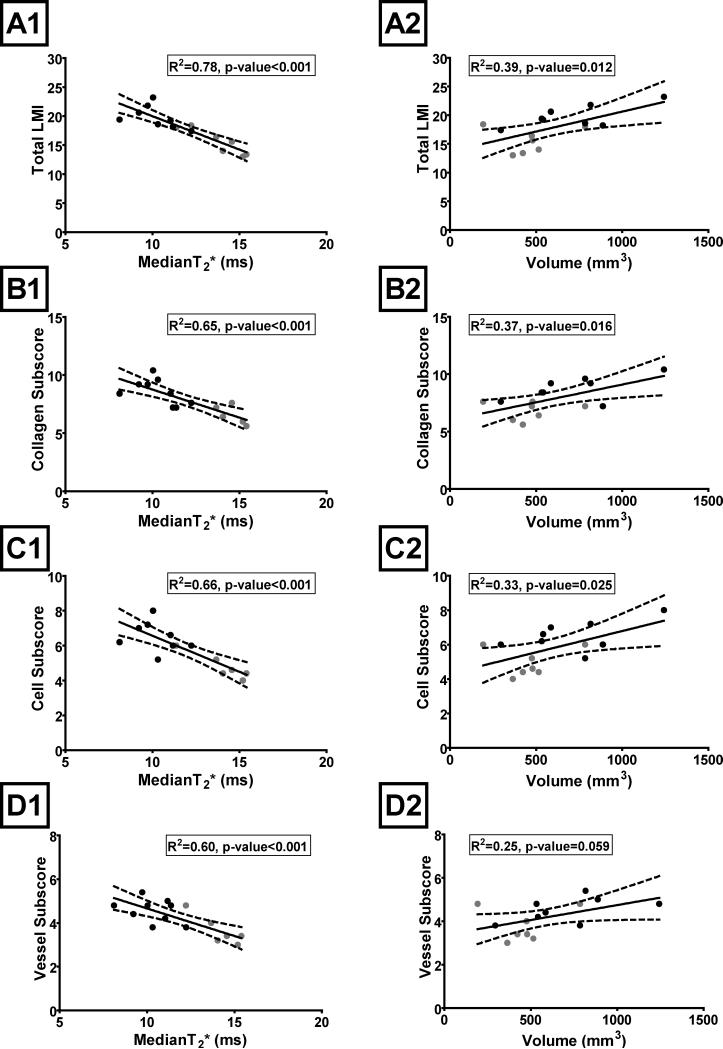
Figure 1.
The healing ligament histology scores (A- Total LMI, B- Collagen Sub-score, C- Cell Sub-score, and D- Vessel Sub-score) as a function of ligament median T2* value (A1, B1, C1, and D1) and volume (A2, B2, C2, and D2) in the linear regression models. Dashed lines represent 95% confidence interval. The ligaments that received bridge-enhanced ACL repair are depicted with black circles and ligaments that were transected and left to heal naturally are depicted with gray circles.
Table 2.
Summary of the healing ligament histology score single linear regression prediction equations as a function of ligament median T2* value or volume.
| Histology Scoring Dependant Variable | Model | MR Independent Variable | Coefficient | p-value | SE of the estimate | R2 |
|---|---|---|---|---|---|---|
| Total LMI | Single Regression | Median T2* | −1.15 | <0.001 | 1.4 | 0.78 |
| Volume | 0.0069 | 0.012 | 2.3 | 0.39 | ||
| Cell Subscore | Single Regression | Median T2* | −0.415 | <0.001 | 0.7 | 0.67 |
| Volume | 0.002 | 0.025 | 1 | 0.33 | ||
| Collagen Subscore | Single Regression | Median T2* | −0.49 | <0.001 | 0.6 | 0.65 |
| Volume | 0.003 | 0.016 | 1.1 | 0.37 | ||
| Vessel Subscore | Single Regression | Median T2* | −0.25 | <0.001 | 0.6 | 0.60 |
| Volume | 0.001 | 0.059 | 0.7 | 0.25 | ||
Table 3.
Summary of the healing ligament histology score multiple linear regression prediction equations as a function of ligament median T2* value and volume.
| Histology Scoring Dependant Variable | Model | MR Independent Variable | Coefficent | Individual p-value | Regression p-value | SE of the estimate | Regression R2 |
|---|---|---|---|---|---|---|---|
| Total LMI | Multi Regress | Median T2* | −0.99 | <0.001 | <0.001 | 0.8 | 0.82 |
| Volume | 0.0027 | 0.106 | |||||
| Cell Subscore | Multi Regress | Median T2* | −0.36 | 0.002 | <0.001 | 0.7 | 0.70 |
| Volume | 0.0009 | 0.254 | |||||
| Collagen Subscore | Multi Regress | Median T2* | −0.40 | 0.003 | <0.001 | 0.8 | 0.71 |
| Volume | 0.0014 | 0.159 | |||||
| Vessel Subscore | Multi Regress | Median T2* | −0.23 | 0.006 | <0.001 | 0.5 | 0.61 |
| Volume | 0.0004 | 0.500 | |||||
Results
The median ligament T2* of bridge-enhanced repaired and naturally healing transected ligaments significantly predicted Total LMI score as well as Cell, Collagen and Vessel sub-scores; R2=0.78, 0.67, 0.65, and 0.60, respectively (all p≤0.001) (Figure 1, Table 2). The MR ligament volume was also a predictor of Total LMI score as well as Cell, and Collagen sub-scores; R2=0.39, 0.33, and 0.37, respectively (p=0.012, p=0.025, and p=0.016) (Figure 1, Table 2). Ligament volume only approached significance for predicting the Vessel sub-score; R2=0.25, p-value=0.059 (Figure 1, Table 2). In general a lower median ligament T2* or a higher ligament volume was associated with indications of healing or higher histological scores of the repaired and transected ligaments (Figures 1, 2, 3).
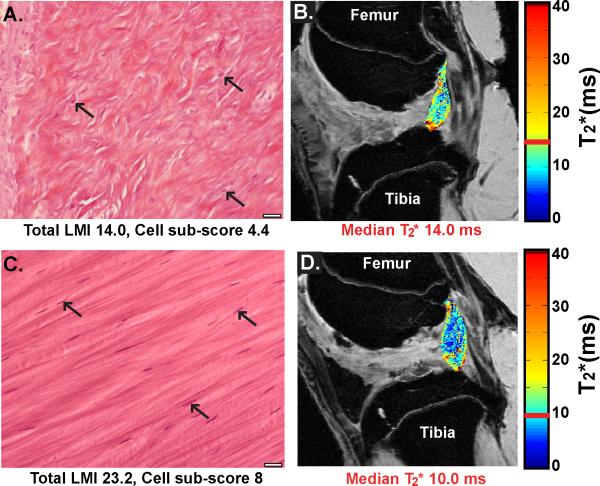
Figure 2.
A) Example ligament histology image with a low total ligament score and cell sub- score (Total LMI 14.0, Cell sub-score 4.4). Arrows indicate cell nuclei not clearly aligned with longitudinal axis of collagen fibers. Also note the collagen fibers lack a distinct longitudinal axis. B) The associated T2* ligament map for the low total LMI histology image overlaid on the original DICOM image. C) Example ligament histology image with a high total ligament score and cell sub-score (Total LMI 23.2, Cell sub-score 8). Arrows indicate cell nuclei aligned with longitudinal axis of collagen fibers. D) The associated T2* ligament map for the high total LMI histology image overlaid on the original DICOM image. Histology images are H&E stained at 40X magnification, scale bar indicates 20μm. The color bars in the T2* maps represent the range of T2* values in the ligament with the median T2* value for the ligament highlighted in red. The MR images are a sagittal view of the femoral notch with the femur at the top of the image and the tibia at the bottom. For the MR images shown TE = 7.36 ms.
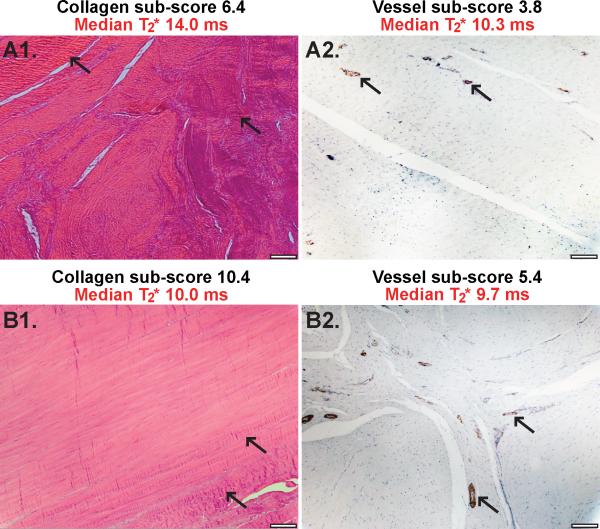
Figure 3.
A1) Example H&E stained polarized image with a collagen sub-score of 6.4 and median ligament T2* of 14 ms. Arrows indicate areas with collagen crimp not distinctly aligned with fiber longitudinal axis. A2) Example SMA stained image with a vessel sub-score of 3.8 and median ligament T2* of 10.3 ms. Arrows indicate smooth muscle like actin rich muscularis layer around arterioles visible in the interfascicular regions. B1) Example H&E stained polarized image with a collagen sub-score of 10.4 and median ligament T2* of 10 ms. Arrows indicate collagen crimp aligned with fiber longitudinal axis. B2) Example SMA stained image with a vessel sub-score of 5.4 and a median ligament T2* of 9.7 ms. Arrows indicate smooth muscle like actin rich muscularis layer around arterioles visible in the interfascicular regions. All histological images at 10X magnification, scale bars indicate 100μm.
Using ligament median T2* in conjunction with volume, the multiple linear regression model predictions for Total LMI score as well as Cell, Collagen and Vessel sub-scores were marginally improved; R2=0.82, 0.70, 0.71, and 0.61, respectively (all p≤0.001) (Table 3).
Discussion
A quantitative tool for assessing the healing of an ACL post-operatively could be valuable to both researchers and clinicians. Ligament T2* and volume has been used to assess structural properties of a healing ligament,9 but has not yet been used to quantitatively assess ligament healing via histology. In this study, the MR derived terms of median ligament T2* and volume significantly predicted the semi-quantitative histological scores in healing bridge-enhanced repaired and natural healing transected ligaments. In general, a shorter median T2* time and larger volume were associated with better histological outcome scores (i.e., improved healing) (Figure 2). As expected, the MR ligament volume was not as strongly associated with histological outcomes as median T2* as indicated by respective p-values and R2 values of the single regressions (Table 2). This difference in prediction strength between median T2* and volume is likely due to the microscopic nature of the histological assessments. Few of the histological evaluation criteria account for gross ligament size, while the majority of the criteria relate to elements of tissue organization and remodeling and it is these factors that have been found to effect T2* times.5,6,8 Furthermore, we found that by combining the median ligament T2* and MR volume in a multiple regression equation (Table 3), the ability to predict the histological outcome scores was only marginally improved as observed by the small R2 increases between the single and the multiple linear regression models. This marginal improvement in the multiple regression model is due to the T2* term dominating the regression prediction and the volume data having a weaker association with the histological outcome criteria, which can be observed by the higher individual p-values for volume from the multiple regression analysis (Table 3).
Using ligament median T2* to predict the Total LMI score (R2=0.78, p-value<0.001) was stronger than the T2* prediction of individual sub-scores (Cell R2=0.67, p-value<0.001; Collagen R2=0.65, p-value<0.001; and Vessel R2=0.60, p-value<0.001)(Table 2). This difference in prediction strength is a result of the cumulative nature of the Total LMI score, which accounts for the complexity of the cellular, collagen and vascular aspects of the healing process for the whole ligament. Furthermore, the Total LMI assesses healing throughout five different regions of the ligament, and as such, we would expect it to relate to the median ligament T2*, a measure representing the whole ligament.
Our findings align with a prior MR investigation looking at signal to noise quotient (a measure of SI) in healing ACL grafts using an ovine model.3 Qualitative histological assessment of graft healing was found to be present with grafts displaying a lower SI and higher structural properties.3 The qualitative nature of the histological assessments in this previous study did not allow for a direct prediction analysis. Building on these results, we found that by using T2* relaxation time we could predict a semi-quantitative histological score (LMI) for assessing ligament healing and maturation. Additionally, the relationship between our MR variable T2* and histological scores tells a similar narrative to the qualitative findings of the previous ovine study. We found low T2* values were associated with greater cell density and collagen organization where the previous study found similar histological results for grafts with low SI.
MR imaging has also been used to find a correlation between T2* and level of collagen organization assessed with semi-quantitative histology in the meniscus. Short T2* times were identified with more densely packed collagen fibers and longer T2* values were associated with less densely packed collagen and meniscal damage.8 While our study used a different means to acquire T2* times, linking collagen structure with shorter T2* values aligns with the findings presented here-in. This further suggests that T2* can serve as a proxy for tissue organization and that remodeling in healing ligaments can be identified with these MR methods.
Using only two echo times adds uncertainty to the determination of T2* by overestimating the relaxation time value.4,15 However, a two echo estimation has been shown to correlate with a six echo determination of T2*, indicating the T2* estimation used here captures the inter-specimen relative differences.16 Additionally, this approach served to limit scan time (19 min) and post-processing time, important factors to consider for eventual clinical translation.13,16,17 Histological scoring was performed after a freeze-thaw cycle, to minimize potential differential effects of freezing on the histological findings of the ligaments all knees were subject to the same freeze thaw protocol. Preceeding histological analysis, mechanical testing was performed.18 However, a quasi-static protocol was used to test the ligaments (ramp speed 20mm/min) allowing the testing to be halted once a drop in load occurred to avoid complete disrupution of the ligament and to minimize irreversible changes during the mechanical testing. All ligaments in this study failed within the midsubtance allowing histological assessment of the areas out side the area of local disruption. It is also reasonable to assume that the failure testing did not alter cellular and vascular content. For this study, we assumed that median graft T2* and volume were the only MR variables that would account for LMI histologic scores. It is possible that other MR analyses, such as diffusion weighted imaging to quantify fiber alignment, could improve correlations to histology. Lastly, the MR images collected in this study were collected postmortem eliminating problems with motion artifact. Future work will evaluate the efficacy of using a T2* imaging method to predict structural properties in vivo for longitudinal studies.
In this study, median ligament T2* and MR volume were found to be predictive of semi-quantitative histological scores assessing healing of the ACL in a porcine model at 52 weeks post operatively. This study provides a critical step in the development of a non-invasive method to predict healing on a microscopic level for bridge-enhanced repair. This technique may prove beneficial as a surrogate outcome measure for healing and may have clinical implications for tracking post-operative changes with ligament healing in patient cohort studies and randomized controlled trials.
Acknowledgements
This publication was made possible by Grant Numbers RO1-AR056834, RO1-AR056834S1, RO1-AR065462, P20-GM104937 from the NIH, and the Lucy Lippitt Endowed Professorship. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The MR images were acquired at the Brown MRI Research Facility (Providence RI). The authors gratefully acknowledge the assistance of Elise Magarian, Patrick Vavken, Carla Haslauer of Children's Hospital Boston; Lynn Fanella, Erika Nixon and Edward Walsh of the Brown MRI Research Facility; David Paller, Ryan Rich, and Sarath Koruprola of the Rhode Island Hospital Orthopaedic Foundation testing facility; and Andrew Rohan of Brown University.
Footnotes
Author Contributions Statement: All authors were involved in the design of the study, the interpretation of the data and the writing of the original manuscript. All authors have read and approved the final content for its submission.
References
- 1.Biercevicz AM, Miranda DL, Machan JT, et al. In Situ, Noninvasive, T2*-Weighted MRI-Derived Parameters Predict Ex Vivo Structural Properties of an Anterior Cruciate Ligament Reconstruction or Bioenhanced Primary Repair in a Porcine Model. Am J Sports Med . 2013 doi: 10.1177/0363546512472978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson K, Seneviratne AM, Izawa K, et al. Augmentation of tendon healing in an intraarticular bone tunnel with use of a bone growth factor. Am J Sports Med. 2001;29(6):689–98. doi: 10.1177/03635465010290060301. [DOI] [PubMed] [Google Scholar]
- 3.Weiler A, Peters G, Mäurer J, et al. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging. A two-year study in sheep. [2011 Dec 9];Am J Sports Med. 2001 29(6):751–761. doi: 10.1177/03635465010290061401. [DOI] [PubMed] [Google Scholar]
- 4.Deoni SCL, Williams SCR, Jezzard P, et al. Standardized structural magnetic resonance imaging in multicentre studies using quantitative T1 and T2 imaging at 1.5 T. [2011 Nov 18];NeuroImage. 2008 40(2):662–671. doi: 10.1016/j.neuroimage.2007.11.052. [DOI] [PubMed] [Google Scholar]
- 5.Chavhan GB, Babyn PS, Thomas B, et al. Principles, Techniques, and Applications of T2*-Based MR Imaging and Its Special Applications. [2012 Mar 14];Radiographics. 2009 29(5):1433–1449. doi: 10.1148/rg.295095034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krasnosselskaia LV, Fullerton GD, Dodd SJ, Cameron IL. Water in tendon: orientational analysis of the free induction decay. Magn Reson Med. 2005;54(2):280–288. doi: 10.1002/mrm.20540. [DOI] [PubMed] [Google Scholar]
- 7.Koff MF, Shah P, Pownder S, et al. Correlation of meniscal T2* with multiphoton microscopy, and change of articular cartilage T2 in an ovine model of meniscal repair. Osteoarthr Cartil. 2013;21(8):1083–1091. doi: 10.1016/j.joca.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams A, Qian Y, Golla S, Chu CR. UTE-T2* mapping detects sub-clinical meniscus injury after anterior cruciate ligament tear. Osteoarthr Cartil. 2012;20(6):486–94. doi: 10.1016/j.joca.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biercevicz AM, Murray Martha M, Walsh EG, et al. T2* MR relaxometry and ligament volume are associated with the structural properties of the healing ACL. J Orthop Res. 2014;32(4):492–9. doi: 10.1002/jor.22563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray MM, Spindler KP, Ballard P, et al. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25(8):1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 11.Proffen BL, Fleming BC, Murray MM. Histological Predictors of Maximum Failure Loads Differ Between the Healing ACL and ACL Grafts After 6 and 12 Months In Vivo. Orthop J Sports Med. 2013;1(6) doi: 10.1177/2325967113512457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41(8):1762–1770. doi: 10.1177/0363546513483446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haacke EM, Brown RW, Thompson MR, Venkatesan . Magnetic resonance imaging: physical principles and sequence design. A John Wiley and Sons; New York, NY: 1999. pp. 648–651. [Google Scholar]
- 14.Fleming BC, Proffen BL, Vavken P, et al. Increased platelet concentration does not improve functional graft healing in bio-enhanced ACL reconstruction. Knee Surg Sports Traumatol Arthrosc . 2014 doi: 10.1007/s00167-014-2932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kingsley PB, Ogg RJ, Reddick WE, Steen RG. Correction of errors caused by imperfect inversion pulses in MR imaging measurement of T1 relaxation times. [2013 Aug 15];Magnetic Resonance Imaging. 1998 16(9):1049–1055. doi: 10.1016/s0730-725x(98)00112-x. [DOI] [PubMed] [Google Scholar]
- 16.Biercevicz AM, Walsh EG, Murray MM, et al. Improving the clinical efficiency of T2-⍰ mapping of ligament integrity. J Biomech. 2014;47(10):2522–2525. doi: 10.1016/j.jbiomech.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn TC, Lu Y, Jin H, et al. T2 Relaxation Time of Cartilage at MR Imaging: Comparison with Severity of Knee Osteoarthritis. Radiology. 2004;232(2):592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank CB. Ligament structure, physiology and function. J Musculoskelet Neuronal Interact. 2004;4(2):199–201. [PubMed] [Google Scholar]