Abstract
Alpha-syntrophin (SNTA) is an adaptor protein which regulates several signaling pathways. To analyze expression of SNTA immunoblot assays have to be performed. Here, the specificity of four commercially available SNTA antibodies has been evaluated in immunoblot experiments using liver tissues of wild type and SNTA deficient mice. While one of the antibodies reacts with SNTA, two antibodies specifically recognize beta 2 syntrophin (SNTB2). The antigen detected by the forth antibody has not been identified but is different from SNTA and SNTB2. Therefore, only one of the four tested antibodies is appropriate to analyze SNTA protein levels by immunoblot.
Keywords: Immunoblot, Knock-out mice, Liver
The molecular adaptor protein alpha syntrophin (SNTA) interacts with various signaling proteins such as neuronal nitric oxide synthase, ion and water channels, adrenergic receptors, G-proteins, G alpha subunits and ATP-binding cassette transporter A1 (ABCA1) (1; 2; 3; 4; 5; 6). SNTA can form complexes via its Drosophila disks-large protein (Dlg), and the Zona Occludens 1 (ZO-1) protein (PDZ) domain, its two pleckstrin homoloy (PH) domains, and two proline-rich regions that interact with src homology 3 domains (1). The C-terminally located syntrophin unique domain participates in the interaction with dystrophin and utrophin (1).
SNTA is a component of the dystrophin associated glycoprotein complex in skeletal muscle and has a prominent role in muscle and neuromuscular development (1). SNTA is also involved in cardiac pathologies like long-QT syndrome and sudden infant death syndrome (1). Altered expression of SNTA in esophageal, stomach, lung, colon, rectal, and breast cancerous tissues suggests a function in carcinogenesis (1). SNTA has been further shown to stabilize ABCA1 which is a central regulator of lipid metabolism (7). ABCA1 and SNTA are expressed in the liver (7; 8) but hepatic ABCA1 is not reduced in mice lacking SNTA and beta 2 syntrophin (SNTB2) (9). To find out whether SNTA has any role in the liver we intend to study hepatic SNTA expression in diseased liver tissues by immunoblot analysis. To test for the specificity of commercially available SNTA antibodies liver tissue of C57BL/6 mice and SNTA deficient mice (10) was used. Liver tissue was solubilized in radioimmunoprecipitation assay lysis buffer (50 mM Tris HCl, pH 7.5, 150 mM NaCl, 1% v/v Nonidet P-40, 0.5% v/v sodium desoxycholate and 0.1% v/v SDS). Protein (20 μg) was separated by SDS-polyacrylamide gel electrophoresis (15 % acrylamide) and transferred to PVDF membranes (Bio-Rad, Munich, Germany). Incubations with the primary antibodies were performed in 1.5% BSA in TBS, 0.1% Tween at 4°C overnight. Secondary antibodies were from Dianova (Hamburg, Germany) and were diluted 1:5000 fold for anti-rabbit and anti-mouse immunoglobulins and 1:1000 fold to detect goat antibodies. Incubations were performed in 5% low-fat milk powder in TBS, 0.1% Tween at RT for 1 h. Detection of the immune complexes was carried out with the ECL Western blot detection system (Amersham Pharmacia, Deisenhofen, Germany).
For the generation of SNTA deficient mice the first exon and part of the promoter had been removed and it is very unlikely that any truncated syntrophin is expressed in these knock-out animals (10). The recently described SNTA antibody (11) recognizes a peptide sequence (RQPSSPGPQPRNLSEA) in the PH1b domain and was raised in rabbits. This antibody (1:1000 fold diluted) does not generate a band in the liver of SNTA−/− mice (Fig. 1A, B). The polyclonal SNTA antibody from Abcam (ab11187; Cambridge, UK) was raised in rabbits using the peptide described above (amino acids 191-206 of mouse SNTA) as immunogen. The antibody was tested at a dilution of 1:2000 as suggested by the company. This antibody detects a protein of about 60 kDa in the liver of wild type but not SNTA−/− animals (Fig. 1A). A SNTA antibody raised in goat was ordered from Thermo Fisher Scientific Pierce (PA1-9107). The immunogen was a synthetic peptide corresponding to the N terminal amino acids ASGRRAPRTGLLE of SNTA. For immunoblot analysis 1.5 μg/ml antibody was used. A band of about 60 kDA was detected in the liver of wild type and SNTA−/− mice (Fig. 1A). Next monoclonal antibodies were tested. Monoclonal antibodies were purchased from Sigma-Aldrich (SAB4200213) and Life Span BioScience (LS-C89921). The immunogens used were Torpedo electric organ membranes and whole purified syntrophin from Torpedo californica electric organ postsynaptic membrane, respectively (data sheets provided by the companies). Both antibodies were used at a concentration of 1 μg/ml. A band of about 60 kDA was detected in the liver of wild type and SNTA−/− mice by both antibodies (Fig. 1B).
Fig. 1.
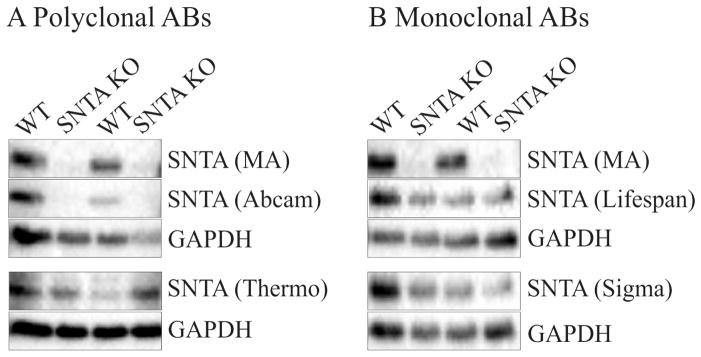
Analysis of SNTA in the liver of two SNTA−/− and two wild type (WT) mice. A) Detection with polyclonal antibodies described by Peters et al. (11) (MA), Abcam and Thermo Fisher Scientific Pierce. B) Detection with the polyclonal antibody described by Peters et al. (11) (MA) and the monoclonal antibodies from Life Span BioScience and Sigma-Aldrich.
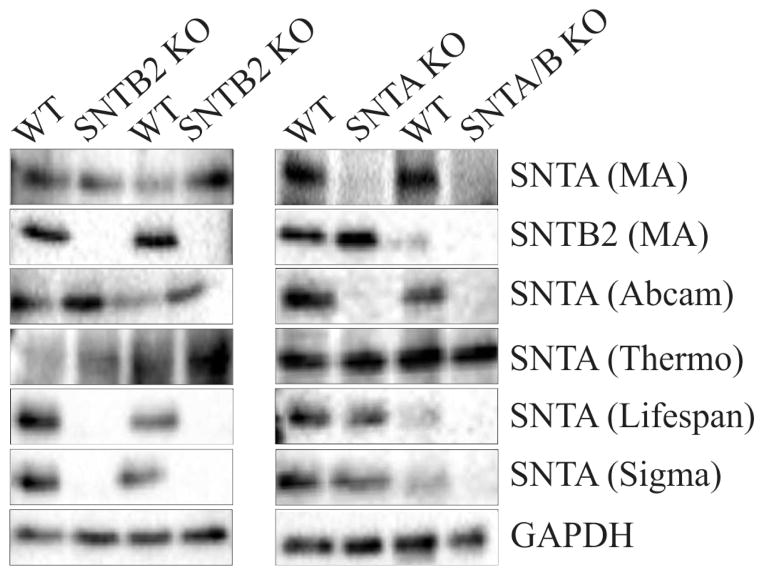
At the amino acid level murine SNTA and SNTB2 are nearly 50% identical (1). Further, murine SNTB2 is only 21 amino acids longer than SNTA. Therefore, it was tested whether these antibodies may detect SNTB2. Immunoblot analysis was performed in liver lysates of wild type mice and mice deficient for SNTA, SNTB2 or both syntrophins (12). Using recently described non-commercial antibodies (11) it was confirmed that SNTA was not expressed in the livers of SNTA−/− and SNTA/B2−/− mice and SNTB2 was only detected in wild type and SNTA−/− mice (Fig. 2). The antibody from Abcam recognized SNTA (Fig. 2). The antibodies from Sigma-Aldrich and Life Span BioScience gave no signals in liver tissues where SNTB2 had been knocked-out indicating that these antibodies specifically detect SNTB2. The antibody from Thermo Fisher Scientific Pierce recognized proteins in all of the liver samples with similar intensities (Fig. 2).
Fig. 2.
Analysis of SNTA in the liver of SNTA−/−, SNTB2−/−, SNTA/B2−/− mice and wild type (WT) mice. Immunoblot analysis of SNTA and SNTB2 using antibodies described by Peters et al. (11) and four commercially available antibodies purchased from Abcam, Thermo Fisher Scientific Pierce, Life Span BioScience and Sigma-Aldrich.
Current findings show that three of the four commercially available SNTA antibodies tested are not suitable to analyze SNTA protein. Although only liver tissue has been used herein it is highly unlikely that these antibodies react with SNTA in other tissues even when using different conditions. Indeed, two of the four antibodies raised against Torpedo electric organ membranes and whole purified syntrophin from Torpedo californica electric organ postsynaptic membrane react with SNTB2. Therefore, it seems reasonable to recommend that the specificity of SNTA antibodies has to be proven even when using other tissues and experimental protocols. This can be easily analyzed by using tissues of SNTA knock-out mice or SNTA siRNA transfected cells.
All of the antibodies tested detect a band of about 60 kDA in liver of mice deficient for SNTA. Although it can not be completely excluded that a truncated SNTA is produced in these animals the molecular weight of such a shorter form would be much smaller than 60 kDa. The SNTA specific antibodies described in the literature (11) and the antibody from Abcam which specifically react with SNTA have been produced using the identical peptide sequence. Two of the antibodies tested and claimed to be specific for SNTA reacted with SNTB2 which is highly homologous to SNTA (1). The antigen detected by the antibody from Thermo Fisher Scientific Pierce is unknown. BLAST search (http://blast.ncbi.nlm.nih.gov) using the peptide sequence ASGRRAPRTGLLE as query did not identify suitable antigens beside SNTA. Therefore, caution is recommended on the use of commercially available SNTA antibodies suggesting that it is convenient to conduct experiments to validate the specificity of the different antibodies.
Acknowledgments
Funding
The study was supported by a grant of the German Research Foundation (BU 1141/8-1) and the NIH (R01NS33145).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhat HF, Adams ME, Khanday FA. Syntrophin proteins as Santa Claus: role(s) in cell signal transduction. Cell Mol Life Sci. 2012;70:2533–2554. doi: 10.1007/s00018-012-1233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 3.Gee SH, Madhavan R, Levinson SR, Caldwell JH, Sealock R, Froehner SC. Interaction of muscle and brain sodium channels with multiple members of the syntrophin family of dystrophin-associated proteins. J Neurosci. 1998;18:128–137. doi: 10.1523/JNEUROSCI.18-01-00128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyssand JS, DeFino MC, Tang XB, Hertz AL, Feller DB, Wacker JL, Adams ME, Hague C. Blood pressure is regulated by an alpha1D-adrenergic receptor/dystrophin signalosome. J Biol Chem. 2008;283:18792–18800. doi: 10.1074/jbc.M801860200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munehira Y, Ohnishi T, Kawamoto S, Furuya A, Shitara K, Imamura M, Yokota T, Takeda S, Amachi T, Matsuo M, Kioka N, Ueda K. Alpha1-syntrophin modulates turnover of ABCA1. J Biol Chem. 2004;279:15091–15095. doi: 10.1074/jbc.M313436200. [DOI] [PubMed] [Google Scholar]
- 6.Neely JD, Amiry-Moghaddam M, Ottersen OP, Froehner SC, Agre P, Adams ME. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc Natl Acad Sci U S A. 2001;98:14108–14113. doi: 10.1073/pnas.241508198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buechler C, Bauer S. ATP binding cassette transporter A1 (ABCA1) associated proteins: potential drug targets in the metabolic syndrome and atherosclerotic disease? Curr Pharm Biotechnol. 2012;13:319–330. doi: 10.2174/138920112799095365. [DOI] [PubMed] [Google Scholar]
- 8.Neumeier M, Krautbauer S, Schmidhofer S, Hader Y, Eisinger K, Eggenhofer E, Froehner SC, Adams ME, Mages W, Buechler C. Adiponectin receptor 1 C-terminus interacts with PDZ-domain proteins such as syntrophins. Exp Mol Pathol. 2013;95:180–186. doi: 10.1016/j.yexmp.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hebel T, Eisinger K, Neumeier M, Rein-Fischboeck L, Pohl R, Meier EM, Boettcher A, Froehner SC, Adams ME, Liebisch G, Krautbauer S, Buechler C. Lipid abnormalities in alpha/beta2-syntrophin null mice are independent from ABCA1. Biochim Biophys Acta. 2015;1851:527–536. doi: 10.1016/j.bbalip.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams ME, Kramarcy N, Krall SP, Rossi SG, Rotundo RL, Sealock R, Froehner SC. Absence of alpha-syntrophin leads to structurally aberrant neuromuscular synapses deficient in utrophin. J Cell Biol. 2000;150:1385–1398. doi: 10.1083/jcb.150.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters MF, Kramarcy NR, Sealock R, Froehner SC. beta 2-Syntrophin: localization at the neuromuscular junction in skeletal muscle. Neuroreport. 1994;5:1577–1580. [PubMed] [Google Scholar]
- 12.Adams ME, Kramarcy N, Fukuda T, Engel AG, Sealock R, Froehner SC. Structural abnormalities at neuromuscular synapses lacking multiple syntrophin isoforms. J Neurosci. 2004;24:10302–10309. doi: 10.1523/JNEUROSCI.3408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]