Abstract
Attention deficit/hyperactivity disorder (ADHD) is a risk factor for tobacco use and dependence. This study examines the responsiveness to nicotine of an adolescent model of ADHD, the spontaneously hypertensive rat (SHR). The conditioned place preference (CPP) procedure was used to assess nicotine-induced locomotion and conditioned reward in SHR and the Wistar Kyoto (WKY) control strain over a range of nicotine doses (0.0, 0.1, 0.3 and 0.6 mg/kg). Prior to conditioning, SHRs were more active and less biased towards one side of the CPP chamber than WKY rats. Following conditioning, SHRs developed CPP to the highest dose of nicotine (0.6 mg/kg), whereas WKYs did not develop CPP to any nicotine dose tested. During conditioning, SHRs displayed greater locomotor activity in the Nicotine-Paired compartment than in the Saline-Paired compartment across conditioning trials. SHRs that received nicotine (0.1, 0.3, 0.6 mg/kg) in the Nicotine-Paired compartment showed an increase in locomotor activity between conditioning trials. Nicotine did not significantly affect WKY locomotor activity. These findings suggest that the SHR strain is a suitable model for studying ADHD-related nicotine use and dependence, but highlights potential limitations of the WKY control strain and the CPP procedure for modeling ADHD-related nicotine reward.
Keywords: Attention deficit/hyperactivity disorder (ADHD), spontaneously hypertensive rat (SHR), nicotine, reward, locomotor behavior, adolescence
Vulnerability to tobacco dependence is heightened during adolescence [1]. Several factors are associated with increased risk of adolescent smoking, including low socioeconomic status, peer smoking, parental smoking, risk taking, and comorbid psychopathology [1]. Psychopathologies associated with adolescent smoking include anxiety disorders, depressive disorders, conduct disorders and attention-deficit hyperactivity disorder (ADHD) [2].
ADHD is a developmental neurobehavioral disorder characterized by excessive levels of hyperactivity, inattention, and impulsivity [3], and is a risk factor for tobacco use and dependence [4]. The prevalence of cigarette smoking among adolescents and adults with ADHD is nearly double that of their non-diagnosed peers [5–7]. Adolescent smokers with ADHD first experiment with tobacco and progress to regular use at a younger age than non-ADHD adolescent smokers [8]. Furthermore, adolescent smokers with ADHD are more likely to become tobacco dependent and to continue smoking into adulthood than non-ADHD adolescent smokers [5, 7, 8]. Once dependent, individuals with ADHD have greater difficulty quitting and exhibit more severe withdrawal symptoms than non-ADHD smokers [9, 10].
The subjective experience of smoking and in the motivational factors that contribute to smoking appear to covary with ADHD status [11, 12]. Specifically, smokers with ADHD report that smoking is more calming, reinforcing, and provides greater cognitive enhancement; they also report greater satisfaction and liking of cigarette puffs [12]. Smokers with ADHD report experiencing smoking-related cues more frequently than non-ADHD smokers, which may indicate that those cues acquire greater motivational or attentional salience for ADHD-smokers [11]. These studies suggest that ADHD is linked to a heightened sensitivity to smoking-related reward and smoking-related cues. The present study experimentally tested this hypothesis using an animal model of ADHD, the spontaneously hypertensive rat (SHR).
The SHR is the most validated animal model of ADHD, displaying symptoms of inattentiveness [13], impulsivity [14], and hyperactivity [15, 16]. These symptoms have been established by comparing SHR behavior against the behavior of its progenitor control, the Wistar Kyoto (WKY) rat [17]. Thus, inferences regarding ADHD-related behaviors drawn from SHRs ideally require comparison to the WKY reference strain. Prior studies have shown that SHRs self-administer nicotine at a higher rate than many other rat strains, including the WKY [18]. The present study aimed at providing converging evidence of heightened sensitivity to the rewarding effects of nicotine in SHR using the conditioned place preference procedure (CPP), a Pavlovian measure of conditioned drug reward [19]. Thus, nicotine-induced locomotion and preference for a nicotine-paired context served as measures of sensitivity to nicotine [20, 21].
Ninety-two experimentally naïve adolescent male spontaneously hypertensive rats (SHR/NCrl; Charles River Laboratories, US; n =44) and Wistar Kyoto rats (WKY/NHsd; Harlan Laboratories, US; n = 48) were used. SHR weights ranged from 57–83 g and WKY weights ranged from 56–87 g upon arrival. All rats arrived in the colony room on postnatal day (PND) 24 and were immediately pair-housed and provided ad libitum access to food and water in their home cages. All animals were handled for at least 2 minutes per day prior to the beginning of experimentation. Animals were housed in a colony room maintained on a 12:12 hr light:dark cycle with lights on at 1900 h. Behavioral testing was conducted only during the dark phase. All procedures were conducted in accordance with the guidelines described in the 8th edition of the Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee at Arizona State University.
The experiment was conducted in three identical place-conditioning chambers (Med Associates, St. Albans, VT) (53.3 × 34.3 × 1.3 cm), which were interfaced to a PC computer that recorded animal activity and location in the apparatus. Test chambers were divided into two equally-sized compartments separated by an manual acrylic guillotine door. Compartments were equipped with stainless steel grid floors and white stimulus lights located on the outside walls of both compartments. Exterior walls of the apparatus were made of clear Plexiglas. Black and white striped paper was arranged in opposite directions (vertical vs. horizontal stripes) on the outside of the walls to make the interior of the compartments visually distinct. A stainless steel pan was located beneath the grid floor of the chamber. Sanichip bedding was placed on one side of the pan and was separated from corncob bedding placed on the opposite side to provide distinct olfactory cues. Locomotor activity was monitored by 16 pairs of photobeams (8 per side) spaced evenly along opposite Plexiglas walls. Time spent in each compartment began recording immediately at the start of each session. A rat was considered to have switched sides if the second closest and the closest infrared beams relative to the center door on one side were broken, in that order, followed by the occlusion of the same beams on the alternate side but in the opposite order. After a rat was considered to have switched sides, the recording of amount of time spent on the newly occupied side commenced.
Nicotine hydrochloride tartrate (Sigma, St. Louis, MO, USA) was dissolved in saline (0.9% NaCl) and adjusted to a pH of 7.2. Both nicotine and saline were administered subcutaneously (s.c.) in a volume of 1 ml/kg. Nicotine doses (0.0 (saline), 0.1, 0.3, and 0.6 mg/kg) were expressed as the freebase weight. Doses were chosen based upon previous studies showing that they supported nicotine CPP in adolescent male rats [19].
The CPP procedure consisted of four phases: acclimation, baseline, conditioning, and test. During acclimation (Day 1; PND 29), rats were placed in the CPP chamber with the guillotine door raised and allowed to explore both compartments for 20 min. Rats were initially placed randomly on either the left or right side, with half of each treatment group placed on either side. Two acclimation sessions were conducted on Day 1, separated by 4 h; rats were placed back in their home cage and returned to the colony room between acclimation sessions. During the second acclimation session, rats were placed on the side opposite of where they were initially placed for the first acclimation session.
During baseline assessment (Day 2), rats received saline injections (s.c.) immediately prior to each of two 10-min sessions. In all other respects, baseline sessions were identical to acclimation sessions. The amount of time spent in each compartment pooled across baseline sessions was used to determine potential side bias and subsequent drug-side pairing assignments. Rats that spent less than 350 s (out of 1200 s) in either compartment during baseline were excluded from further testing and their baseline data were excluded from analysis. Using this criterion, 1 SHR and 5 WKY rats were excluded from analysis.
During conditioning (Days 3–6), rats were injected (s.c.) with either nicotine (0.1, 0.3 or 0.6 mg/kg; n = 10–12/dose/strain) or saline once daily, and confined to one compartment for 20 min. A biased design was used, such that nicotine or saline (0.0 mg/kg) was paired with the compartment that was less preferred during baseline (Nicotine-Paired compartment), and saline was always paired with the compartment that was preferred during baseline (Saline-Paired compartment). Rats underwent 4 conditioning sessions (2 Nicotine-Paired and 2 Saline-Paired) alternating across 4 days in a counterbalanced order.
During a single 20-min test session (Day 7), rats received no injections prior to being placed in the CPP chamber. Half of the rats in each strain were initially placed in the preferred compartment and half were placed in the non-preferred compartment. The guillotine door was raised and movement within each compartment was recorded.
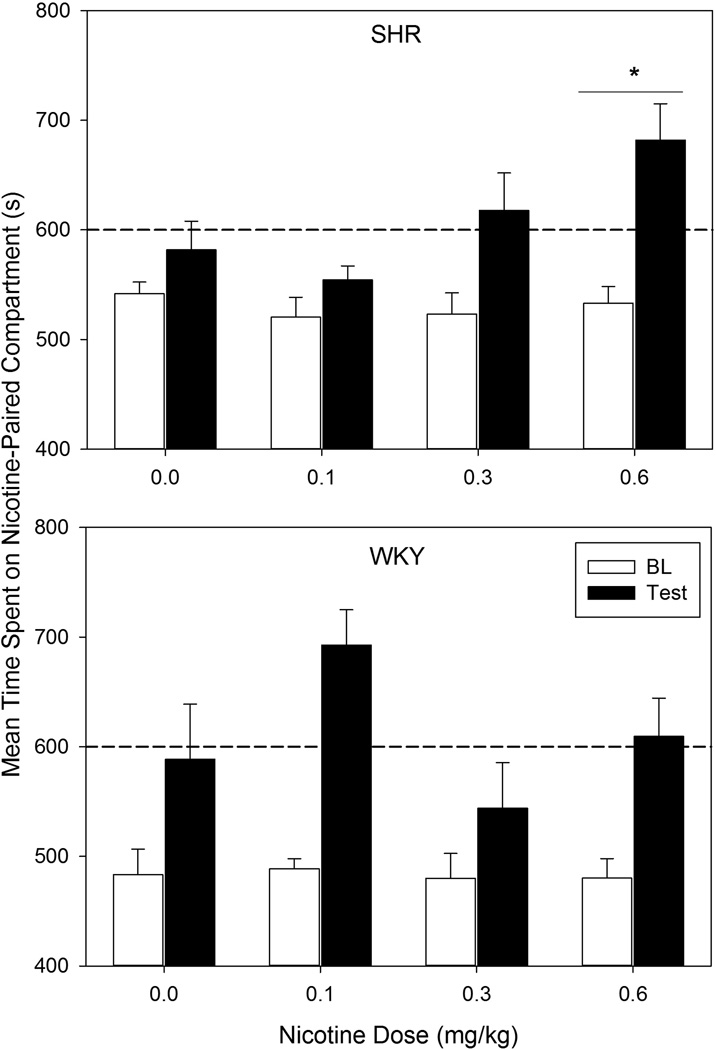
Difference scores (time spent on the non-preferred side during test minus time spent on the non-preferred side during baseline) were calculated for each animal. Nicotine CPP was defined as difference scores that were significantly greater for the groups receiving nicotine injections than for the group receiving saline throughout conditioning. Because an independent t-test conducted on pre-test data revealed a significant strain effect in time spent in the Nicotine-Paired compartment (t (42) = 3.18, p = 0.003; Figure 1), difference scores were analyzed separately for each strain in order to ensure that baseline differences did not interfere with the ability to detect positive results. Dunnett’s multiple comparisons tests [21] were implemented to compare difference scores for all groups within each strain.
Figure 1.
Mean amount of time spent on the Nicotine-Paired compartment during baseline (white bars) and test (black bars) by each SHR (top panel) and WKY (bottom panel) nicotine dose group. Error bars represent SEM. The dashed horizontal line indicates half of the session duration. The asterisk (*) indicates difference scores (test – baseline) significantly greater than those of the 0.0-mg/kg (saline) group (p < 0.05). n = 10–11 rats per dose per strain.
SHR rats in the 0.6 mg/kg group had significantly greater difference scores than SHRs receiving saline (p = 0.036) (Figure 1), indicating that SHRs developed nicotine CPP to the highest dose tested. In contrast, WKYs did not demonstrate nicotine CPP at any dose tested (all p’s > 0.05).
These CPP data should be interpreted with caution. Difference scores, which were the primary measure of CPP, are directly affected by pre-test compartment biases, which differed significantly between strains. SHRs and WKYs that received saline spent nearly the same amount of time in the Nicotine-Paired compartment during test (582 s and 589 s, respectively), yet WKY difference scores were 2.5 times greater than SHR difference scores, because of strain differences in baseline time spent in the Nicotine-Paired compartment. WKYs receiving nicotine also demonstrated a strong baseline compartment bias, which interfered with the interpretation of results by reducing confidence that increases in time spent in the Nicotine-Paired compartment were drug-induced and not due to a regression-to-the-mean effect. Because the SHRs showed a lower baseline compartment bias, regression-to-the-mean interpretations were less of a concern for this strain.
In WKYs, the lowest nicotine dose (0.1 mg/kg) induced the largest difference scores and the largest amount of time spent in the Nicotine-Paired compartment during post-test of any group tested. Although this evidence suggests that WKYs are sensitive to the incentive properties of a low nicotine dose, difference scores were not significantly greater than those produced by saline (p > 0.05). These findings are consistent with previous investigations showing that WKY rats do not develop nicotine CPP [22].
Locomotor activity was assessed by calculating the number of beam breaks on the Nicotine-Paired and Saline-Paired compartments during conditioning sessions. Because an independent t-test revealed that baseline locomotor activity was significantly higher for SHRs than for WKYs (t (85) = 3.48, p < 0.001), locomotor activity was analyzed separately for each strain. Locomotor activity was compared across strains during conditioning using two 2 (Compartment) × 2 (first vs. second Training Day on a particular compartment) × 4 (Dose) ANOVAs. For all analyses, the significance criterion was set at p = 0.05. Follow up t-tests were only conducted on significant effects.
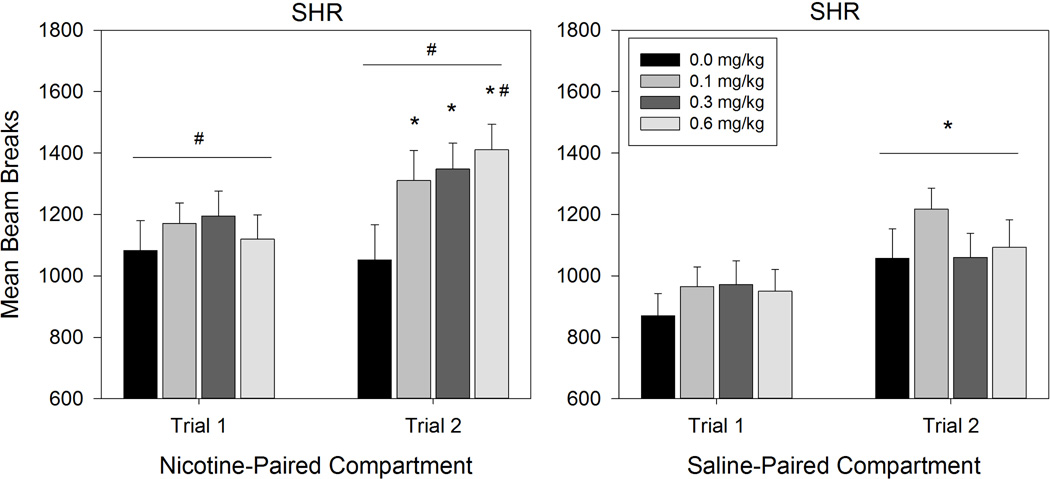
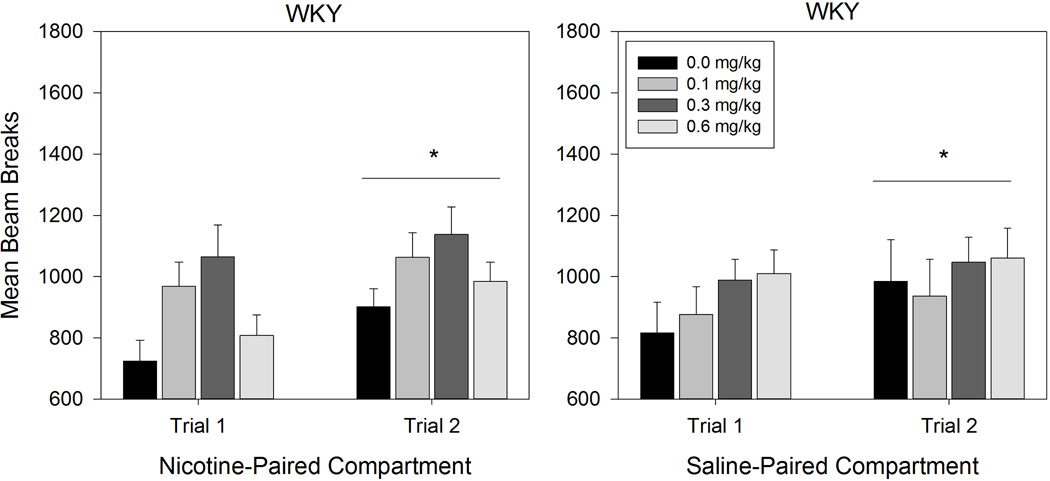
ANOVA conducted on SHR beam breaks revealed a significant Compartment × Day × Dose interaction effect (F (3,39) = 2.997, p = 0.042; Figure 2). Follow-up 2 (Compartment) × 4 (Dose) ANOVAs conducted on each conditioning day revealed that SHRs made significantly more beam breaks in the Nicotine-Paired compartment than in the Saline-Paired compartment across Day 1 (F (1,39) = 12.966, p = .001) and Day 2 (F (1,39) = 8.586, p = .006). Follow-up 2 (Day) × 2 (Compartment) ANOVAs conducted at each Dose level revealed a significant main effect of Day for SHRs receiving the 0.1 mg/kg (F (1,10) = 18.609, p = .002) and 0.3 mg/kg (F (1,10) = 6.339, p = .031) doses of nicotine, with more beam breaks on Day 2 than Day 1 (0.1 mg/kg: p = .002; 0.3 mg/kg: p = .031). For SHRs receiving the 0.6 mg/kg dose, there was a significant main effect of Compartment (F (1,9) = 5.634, p = .042) and Day (F (1,9) = 13.534, p = .005). Pairwise comparisons showed that SHRs in the 0.6 mg/kg group made significantly more beam breaks in the Nicotine-Paired compartment (p = .042) and on Day 2 (p = .005). Follow-up 2 (Day) × 4 (Dose) ANOVAs conducted for each compartment revealed a significant Day × Dose interaction effect (F (3,39) = 3.626, p = .021) in the Nicotine-Paired compartment. Paired samples t-tests comparing Nicotine-Paired compartment beam breaks between Day 1 and Day 2 at each dose revealed no significant difference across days in SHRs receiving saline (t (10) = .357, p = .729). However, Nicotine-Paired compartment beam breaks increased significantly from Day 1 to Day 2 for SHRs receiving the 0.1 mg/kg (t (10) = 2.620, p = .026), 0.3 mg/kg (t (10) = 2.427, p = .036), and 0.6 mg/kg (t (9) = 4.473, p = .002) nicotine doses. The ANOVA conducted on Saline-Paired compartment trials revealed a significant main effect of Day (F (1,39) = 18.768, p < .001) with significantly more beam breaks occurring in the Saline-Paired compartment on Day 2 than on Day 1 (p < .001). ANOVA conducted on WKY beam breaks only revealed a significant main effect of Day (F (1,39) = 5.941, p = 0.019) with more beam beaks occurring on the second conditioning trial than the first (p = .019) (see Figure 3).
Figure 2.
Mean beam breaks of SHR nicotine dose groups on the Nicotine-Paired compartment (left panel) and the Saline-Paired compartment (right panel). Error bars represent SEM. # denotes a significant effect of compartment. * denotes a significant effect of conditioning trial.
Figure 3.
Mean beam breaks of WKY nicotine groups on the Nicotine-Paired compartment (left panel) and Saline-Paired compartment (right panel). Error bars represent SEM. * denotes a significant effect of conditioning trial.
Consistent with previous investigations, the present locomotor data suggest that adolescent SHRs are significantly more active than WKYs [15]. SHRs are also differentially sensitive to the locomotor stimulatory properties of nicotine. During conditioning, SHRs receiving the highest nicotine dose (0.6 mg/kg) were more active in the Nicotine-Paired compartment following nicotine injections than in the Saline-Paired compartment. Although generalized locomotion (in both Nicotine-Paired and Saline-Paired compartments) increased significantly for both strains between conditioning days 1 and 2, for SHRs this increase was selective to rats exposed to nicotine. Because the effect of conditioning day on locomotion was not specific to the Nicotine-Paired compartment or to the WKY rats that were exposed to nicotine, the change in WKY locomotion across days may reflect acclimation to conditioning contexts. In contrast, the selective effect of conditioning day on nicotine-exposed SHR rats suggests the strengthening of a nicotine-context association that may have partially generalized across contexts.
This study replicates strain effects in the locomotor behavior of adolescent rats, and suggests that SHRs display enhanced nicotine-induced place preference and locomotion. These results are consistent with a heightened responsiveness to the rewarding properties of nicotine in an animal model of ADHD, thus indicating a potential model for ADHD-related increased prevalence of tobacco use and dependence. This interpretation of the data is also consistent with reports from clinical cases [11, 12] and self-administration data from SHR rats [18]. Nonetheless, this interpretation should be considered preliminary. Differences in baseline place preference and locomotion between strains may have contributed to differences in nicotine effects. It is also likely that pseudo-conditioning to saline obscured nicotine-induced place preference in WKYs. Finally, CPP performance is not solely dependent on the rewarding properties of the drug; for instance, place preference may be confounded with other behavior induced by the context-drug association [24]. In short, although the present results suggest that an animal model of ADHD shows an enhanced responsiveness to nicotine, further confidence in such inference requires converging evidence from other behavioral paradigms, as well as other animal models of ADHD.
Adolescent SHRs are more hyperactive than WKY controls
SHRs develop CPP to a moderately high nicotine dose
SHRs are sensitive to nicotine’s locomotor-enhancing effects
Acknowledgments
This research was supported by a seed grant from the College of Liberal Arts and Sciences, Arizona State University, and two grants from the National Institutes of Health (DA032632, MH094562). The authors thank Janet Neisewander and Laurie Chassin for providing feedback to early drafts of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wittchen HU, Behrendt S, Höfler M, Perkonigg A, Lieb R, Bühringer G, Beesdo K. What are the high risk periods for incident substance use and transitions to abuse and dependence? Implications for early intervention and prevention. Int J Methods Psychiatr Res. 2008;17:S16–S29. doi: 10.1002/mpr.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lasser K. Smoking and Mental Illness: A Population-Based Prevalence Study. J Am Med Assoc. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition (DSM-5) 2013. [Google Scholar]
- 4.McClernon FJ, Kollins SH. ADHD and smoking: from genes to brain to behavior. Ann N Y Acad Sci. 2008;1141:131–147. doi: 10.1196/annals.1441.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert NM, Hartsough CS. Prospective Study of Tobacco Smoking and Substance Dependencies Among Samples of ADHD and Non-ADHD Participants. J Learn Disabil. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- 6.Molina BSG, Pelham WE., Jr Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J. Abnorm Psychol. 2003;112 doi: 10.1037/0021-843x.112.3.497. 497–50. [DOI] [PubMed] [Google Scholar]
- 7.Breyer JL, Lee S, Winters KC, August GJ, Realmuto GM. A longitudinal study of childhood ADHD and substance dependence disorders in early adulthood. Psychol Addict Behav. 2014;28:238–246. doi: 10.1037/a0035664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang Z, Lichtenstein P, Larsson H. The effects of childhood ADHD symptoms on early-onset substance use: A Swedish twin study. J Abnorm Child Psychol. 2012;40:425–435. doi: 10.1007/s10802-011-9575-6. [DOI] [PubMed] [Google Scholar]
- 9.Humfleet GL, Prochaska JJ, Mengis M, Cullen J, Muñoz R, Reus V, Hall SM. Preliminary evidence of the association between the history of childhood attention-deficit/hyperactivity disorder and smoking treatment failure. Nicotine Tob Res. 2005;7:453–460. doi: 10.1080/14622200500125310. [DOI] [PubMed] [Google Scholar]
- 10.Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse. 1995;7:373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell JT, McIntyre EM, McClernon FJ, Kollins SH. Smoking motivation in adults with attention-deficit/hyperactivity disorder using the wisconsin inventory of smoking dependence motives. Nicotine Tob Res. 2014;16:120–125. doi: 10.1093/ntr/ntt144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Voorhees E, McClernon FJ, Fuemmeler B, English J, Holdaway A, Hallyburton M, Dew R, Kollins S. An examination of differences in variables maintaining smoking behavior in adult smokers with and without attention-deficit/hyperactivity disorder. Addict Res Theory. 2012;20:72–81. [Google Scholar]
- 13.Jentsch JD. Impaired visuospatial divided attention in the spontaneously hypertensive rat. Behav Brain Res. 2005;157:323–330. doi: 10.1016/j.bbr.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Sanabria F, Killeen PR. Evidence for impulsivity in the Spontaneously Hypertensive Rat drawn from complementary response-withholding tasks. Behav Brain Funct. 2008;4:7. doi: 10.1186/1744-9081-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh YL, Yang CC. Age-series characteristics of locomotor activities in spontaneously hypertensive rats: a comparison with the Wistar-Kyoto strain. Physiol Behav. 2008;93:777–782. doi: 10.1016/j.physbeh.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 16.Hill JC, Herbst K, Sanabria F. Characterizing operant hyperactivity in the Spontaneously Hypertensive Rat. Behav Brain Funct. 2012;8:5. doi: 10.1186/1744-9081-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sagvolden T, Johansen EB, Wøien G, Walaas SI, Storm-Mathisen J, Bergersen LH, Hvalby Ø, Jensen V, Aase H, Russell VA, Killeen PR, DasBanerjee T, Middleton FA, Faraone SV. The spontaneously hypertensive rat model of ADHD – The importance of selecting the appropriate reference strain. Neuropharmacology. 2009;57:619–626. doi: 10.1016/j.neuropharm.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Hiler KA, Tolley EA, Matta SG, Sharp BM. Genetic factors control nicotine self-administration in isogenic adolescent rat strains. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology. 2009;204:391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol. 1992;105:849–856. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 22.Dunnett C. A multiple comparison procedure for comparing several treatments with a control. J A Stat Assoc. 1955;50:1096–1121. [Google Scholar]
- 23.Rauhut AS, Zentner IJ, Mardekian SK, Tanenbaum JB. Wistar Kyoto and Wistar rats differ in the affective and locomotor effects of nicotine. Physiol Behav. 2008;93:177–188. doi: 10.1016/j.physbeh.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Huston JP, de Souza Silva MA, Topic B, Müller CP. What’s conditioned in conditioned place preference? Trends Pharmacol Sci. 2013;34:162–166. doi: 10.1016/j.tips.2013.01.004. [DOI] [PubMed] [Google Scholar]