Abstract
Human immunodeficiency virus (HIV) infection is associated with mood disorders and behavioral disinhibition. Impairments in sensorimotor gating and associated neurocognitive disorders are reported, but the HIV-proteins and mechanisms involved are not known. The regulatory HIV-1 protein, Tat, is neurotoxic and its expression in animal models increases anxiety-like behavior concurrent with neuroinflammation and structural changes in limbic and extra-limbic brain regions. We hypothesized that conditional expression of HIV-1 Tat1–86 in the GT-tg bigenic mouse model would impair sensorimotor gating and increase microglial reactivity in limbic and extralimbic brain regions. Conditional Tat induction via doxycycline (Dox) treatment (0–125 mg/kg, i.p., for 1–14 days) significantly potentiated the acoustic startle reflex (ASR) of GT-tg mice and impaired prepulse inhibition (PPI) of this response in a dose-dependent manner when Dox (100 mg/kg) was administered for brief (1 day) or prolonged (daily for 7 days) intervals. A greater proportion of active/reactive Iba1-labeled microglia was seen in the anterior cingulate cortex (ACC), dentate gyrus, and nucleus accumbens core when Tat protein was induced under either brief or prolonged expression conditions. Other subregions of the medial prefrontal cortex, amygdala, hippocampal formation, ventral tegmental area, and ventral pallidum also displayed Tat-induced microglial activation, but only the activation observed in the ACC recapitulated the pattern of ASR and PPI behaviors. Tat exposure also increased frontal cortex GFAP. Pretreatment with indomethacin attenuated the behavioral effects of brief (but not prolonged) Tat-exposure. Overall, exposure to HIV-1 Tat protein induced sensorimotor deficits associated with acute and persistent neuroinflammation in limbic/extralimbic brain regions.
Keywords: Acoustic Startle Reflex, Glial Fibrillary Acidic Protein, ionized calcium-binding adaptor protein 1, NeuroAIDS, Prepulse Inhibition, Trans-activating Transcriptor
1. Introduction
Human immunodeficiency virus (HIV) infection is associated with neurological dysfunction characterized by behavioral disinhibition, mood disorders (i.e. anxiety and depression), cognitive impairment, motor deficits, and central pathology, collectively termed “neuroAIDS” [1,2]. The advent of combination antiretroviral therapies has made it possible for HIV-positive individuals to slow the progression to acquired immunodeficiency syndrome (AIDS), but raises new challenges in maintaining quality-of-life for those living with HIV for extended periods.
Despite significant attenuation of viral load, antiretroviral therapies may not alleviate all symptoms of neuroAIDS, possibly due to the neurotoxic actions of HIV proteins from persistent viral reservoirs within the central nervous system (CNS) [3]. One such viral protein is the HIV-1 trans-activator of transcription (Tat). Tat can promote cell death via interacting mechanisms including direct extracellular actions at glutamatergic sites (including NMDA receptors [4]) and downstream activation of proinflammatory, NF-κB-regulated chemokines [5,6]. To begin to assess the behavioral sequelae associated with central actions of HIV-1 Tat, we have utilized the GT-tg bigenic mouse; a transgenic model that expresses GFAP-driven Tat protein under doxycycline (Dox) regulation in the CNS [7]. Consistent with clinical observations, inducing Tat expression in these mice is associated with elevated anxiety [8,9] and reductions of gray-matter density and fractional anisotropy abnormalities within mood-associated brain regions (including the hippocampus, amygdala, hypothalamus, and striatum [10,11]). Similar Tat-transgenic murine models also demonstrate anxiety-like behavior concurrent with neuronal cell death, astrocytosis, and microglial reactivity in striatal cells [12]. However, it is not known how executive function may be influenced by exposure to Tat in regions beyond the limbic and striatal areas.
To this end, we examined prepulse inhibition (PPI) of the acoustic startle reflex (ASR). This assay assesses sensorimotor gating in response to a startling acoustic stimulus, and the capacity to inhibit the startle response when a weak stimulus (a prepulse sound) is presented just prior to the startling stimulus [13]. Several brain regions influence PPI performance in rodents including the medial prefrontal cortex (mPFC), which plays an important inhibitory role alongside the ventral tegmental area [14,15] in modulating connections between the hippocampus, amygdala, and nucleus accumbens that culminate in the ventral pallidum to mediate the PPI response [15,16]. People with HIV-associated neurocognitive disorders demonstrate deficits in PPI responses not observed in HIV-positive participants without neurocognitive impairment or healthy non-afflicted individuals (which do not differ [17]). Previous findings in male rats demonstrate the capacity for postnatal Tat infusion into the hippocampus to disrupt ASR and PPI later in life [18], and for intra-hippocampal Tat to disrupt ASR and PPI in adulthood [19]. Among adult HIV-1 transgenic rats (that express 7 of the 9 clade B HIV-1 genes), temporal processing of visual and acoustic PPI is altered compared to controls, and appears dysregulated as a function of age [20–22]. Presently, we hypothesized that exposure to HIV-1 Tat in the CNS of age-matched adult mice would produce exposure-dependent impairment of ASR and PPI. Moreover, we anticipated that indirect, pro-inflammatory effects of Tat-induced toxicity throughout the ASR/PPI circuit would contribute to behavioral outcomes and expected microglial reactivity within related limbic/extralimbic subregions of the brain.
2. Materials and methods
All methods using animals were pre-approved by the Institutional Animal Care and Use Committees at Northeastern University (Boston, MA) and the Torrey Pines Institute for Molecular Studies (Port Saint Lucie, FL) and were conducted in accordance with ethical guidelines defined by the National Institutes of Health (NIH Publication No. 85–23).
2.1. Subjects
GT-tg bigenic male mice [7] used in behavioral and GFAP protein studies (n=204) were derived at the Torrey Pines Institute for Molecular Studies (Port Saint Lucie, FL) [9] from breeders originally provided by Dr. Johnny J. He. For immunohistochemical studies, GT-tg male mice (n=20) were obtained from a parallel colony at Northeastern University. The GT-tg breeder mice were back-crossed 7 generations onto a C57BL/6J strain. As such, C57BL/6J mice that lacked the HIV-1 Tat transgene were utilized as negative controls to rule out non-specific effects of Dox (n=25; The Jackson Laboratory, Bar Harbor, ME). All mice (~70 days old) were housed 4–5/cage and were maintained in a temperature- and humidity-controlled room on a 12:12h light/dark cycle (lights off at 19:00h) with ad libitum access to food and water.
2.2. Chemicals
To induce central HIV-1 Tat expression as previously described [7], mice were administered Dox at dosing regimens that have been established to optimize expression of central HIV-1 Tat protein in a manner dependent on Dox exposure (25–125 mg/kg, i.p., once daily for 1 to 14 days) [8,23]. In this animal model, Dox is demonstrated to induce expression of Tat mRNA [7] and protein [8,23] in an exposure-dependent manner where central Tat mRNA correlates with the transgene copy numbers expressed in brain [7]. Significant gross physiological changes (e.g., body weight) were not observed in response to Dox exposure with the exception of the two highest dosing regimens (Dox 125 mg/kg, i.p. for 7 days and Dox 100 mg/kg, i.p. for 14 days) which were associated with weight loss and attrition in GT-tg mice as previously reported [8], precluding characterization of these dosing regimens beyond what is reported herein. Additional mice were administered vehicle (saline, 0.9%) for minimal and maximal durations as negative control groups.
To attempt to counteract Tat-induced neuroinflammation, mice were pretreated with the cyclooxygenase-1 and -2 inhibitor, indomethacin (10 mg/kg/d, i.p., 20 h and again 30 min prior to Tat induction) or vehicle [sterile TRIS-HCL 0.2M (pH 8.2)] as a negative control. This dose of indomethacin was selected as it has been demonstrated previously to prevent the neuroinflammatory effects of methamphetamine in mice [24]. All chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
2.3. Acoustic startle and prepulse inhibition
Acoustic startle reflex (ASR) and prepulse inhibition (PPI) of ASR were assessed per established methods [13]. Briefly, ASR and PPI were conducted at the same time in sound-attenuating acoustic startle chambers from San Diego Instruments (San Diego, CA) with 70 dB background noise and a 5 min habituation prior to the first stimulus. A block of six 120 dB pulse-alone trials were presented first to stabilize startle responding. Fifty-two test trials followed, consisting of either a 120 dB (40 ms) pulse-alone stimulus, a stimulus preceded by a prepulse (4, 8, or 16 dB above background noise for 20 ms with a 100 ms delay), or no stimulus. Lastly, a final six pulse-alone trials were presented. All trials were presented in pseudorandom order with inter-trial intervals spanning 8–23 s. Acoustic startle response data are presented in an arbitrary metric of force that accounts for the whole-body flinch in response to stimuli. Prepulse inhibition was calculated for each prepulse using the formula: % PPI = 100 × [pulse alone − (prepulse + pulse)] / pulse alone.
2.4. Immunohistochemistry for microglia and assessment of activated microglia
Mice were anesthetized via 4% isoflurane and transcardially-perfused with saline, followed by 4% paraformaldehyde in PBS. Heads were fixed in paraformaldehyde and 15% sucrose for 24 h, after which brains were removed and stored in fresh paraformaldehyde. Brains were shipped to NeuroScience Associates (Knoxville, TN) for analysis via MultiBrain® Technology whereby samples were embedded together in a block, and freeze-sectioned at 30 µm in the coronal plane throughout the entire brain. Every 24th section (at 720 µm intervals) was stained for ionized calcium-binding adaptor protein 1 (Iba1) to reveal microglia [25] and visualized with 3,3’-diaminobenzidine as a chromogen. Iba1 is a protein specifically expressed in microglia and upregulated during the activation of these cells [25].
Microglial activation was classified by morphology [26,27] as determined an observer blind to pretreatment conditions. Briefly, subregions of the medial prefrontal cortex, amygdala, hippocampal formation, nucleus accumbens, ventral tegmental area, and ventral pallidum were examined (bilaterally as defined by the Allen mouse brain reference atlas [28]. Two consecutive bilateral sections per brain, with 2–3 brains per treatment group, were examined. Each hemisphere was analyzed separately; no differences in Iba1 expression were observed between left and right hemispheres. Microglia morphology was rated on a 1 to 4 scale, based on an established scoring system for microglia morphology and phenotypes [26,27,29]: 1 = Resting state microglia with ramified and long processes and a round cell body, 2 = Early activated microglia showing shortening of the processes, with minimal increases in immunoreactivity (i.e., darkened staining) in the cell body, 3 = Activated/reactive microglia displaying shortening of the distal and thickening of the proximal processes and increased immunoreactivity in the cell body, 4 = Reactive microglia showing a progression from the previous phenotype, but a cell body that is irregular and indistinct from the processes.
2.5. ELISA for frontal cortex GFAP content
Frontal cortex was grossly dissected from mouse brains and immediately flash frozen for storage at −80 °C. Tissue was homogenized in a 1× RIPA buffer (EMD Millipore, Darmstadt, Germany) with a cocktail of protease and phosphatase inhibitors (Product # 88668, Pierce, Rockford, IL) and centrifuged at 12,000 rpm for 10 min at 4 °C. Protein concentrations of supernatant were assessed via an assay for bicinchoninic acid (Pierce, Rockford, IL) and 10 µg of supernatant protein was loaded for each unknown sample. GFAP content was assessed using an enzyme-linked immunosorbant assay (ELISA) kit per manufacturer instructions (Product # NS830, EMD Millipore, Darmstadt, Germany). Samples, including standard curve (1.56 – 100 ng/ml) and unknowns, were run in duplicate. Intra- and inter-plate coefficients of variance were 2.9% and 5.6%, respectively.
2.6. Statistical analyses
All data are presented as mean ± S.E.M. ASR and immunohistochemistry labeling were assessed via one-way ANOVA with pharmacological manipulation as the between-subjects factor. PPI was assessed via repeated measures ANOVA with pharmacological manipulation as the between-subjects factor and prepulse dB as the within-subjects factor. Fisher’s Protected Least Significant Difference post-hoc tests determined group differences following main effects with a priori planned comparison of all groups to saline-administered controls. Direct statistical comparisons between strains were not conducted given that C57BL/6J mice lack the rtTA transcription factor present in the GT-tg mouse strain and would not serve as an appropriate direct control in behavioral assays. Rather, C57BL/6J mice were utilized as a control solely to ensure that non-specific effects of Dox administration itself did not account for observed changes in any dependent measure. No such interactions were detected. Analyses were considered significant when p ≤ 0.05.
3. Results
3.1. HIV-1 Tat potentiates acoustic startle and attenuates prepulse inhibition in an exposure-dependent manner
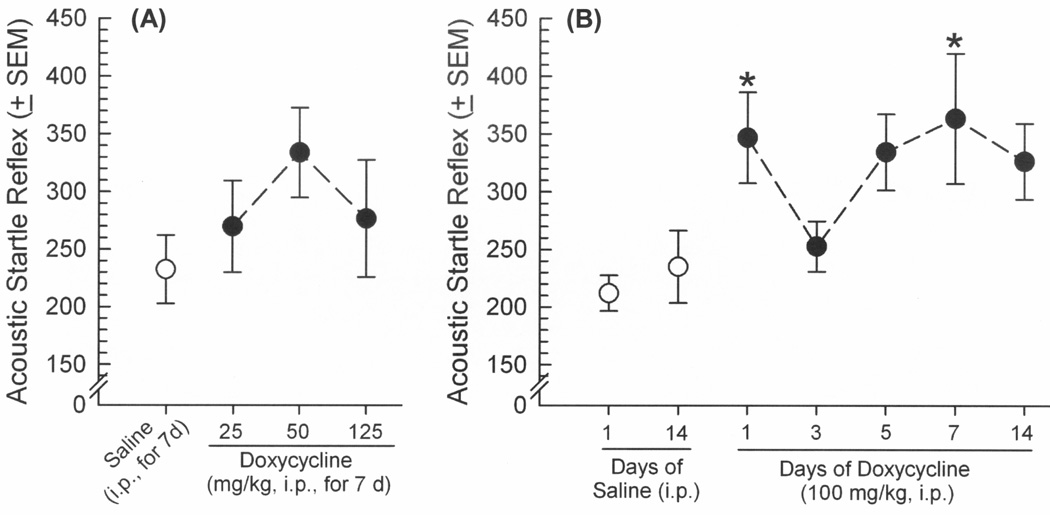
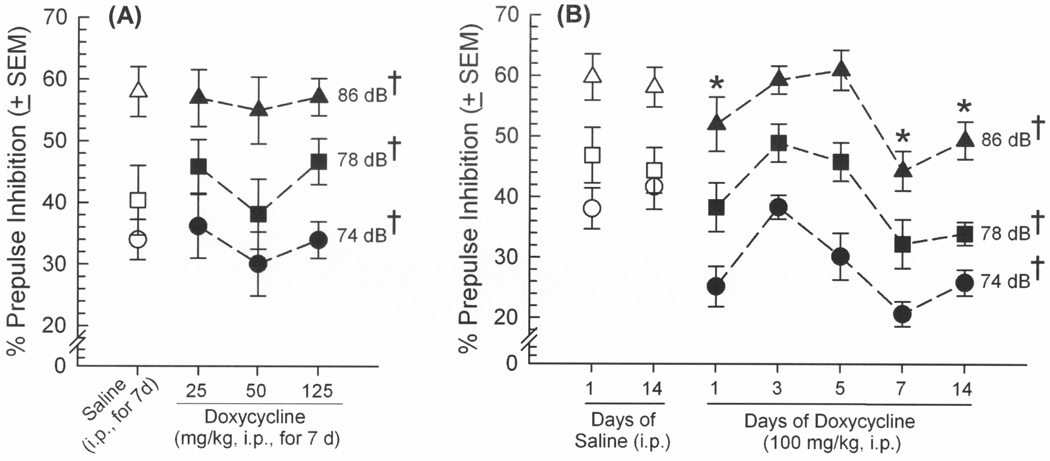
GT-tg mice were administered saline or Dox (25, 50, 100, or 125 mg/kg, i.p.) once daily for a chosen duration (1, 3, 5, 7, or 14 days) to induce expression of Tat protein. Exposure to the lowest (25 or 50 mg/kg) or highest (125 mg/kg) concentrations of Dox did not significantly influence ASR (Figure 1A) or PPI (Figure 2A). However, HIV-1 Tat induction via Dox (100 mg/kg/d) for a brief (1 day) or prolonged (7 days) exposure significantly potentiated ASR [F(6,61) = 2.76, p = 0.02] (Figure 1B) and impaired PPI [F(6,122) = 5.35, p = 0.0002] (Figure 2B) compared to uninduced, controls that received saline for 1 or 14 days (ASR: pDox1d = 0.01–0.03, pDox7d = 0.006–0.01; PPI: pDox1d = 0.02–0.03, pDox7d = 0.0003–0.0005). Prepulse inhibition (but not ASR) was also significantly impaired among those administered Dox 100 mg/kg/d for 14 days compared to uninduced controls (p = 0.005–0.008; Figure 2B). However, the Tat-exposure induced by this high dosing regimen (as well as the high 125 mg/kg Dox dose for 7 days) were previously observed to impair the locomotor behavior of GT-tg mice [8]. As this effect could be confounding in this assay, these Tat-induction conditions were thus not utilized further in the present report. Despite similar effects of treatment on ASR and PPI, these measures did not correlate at any dB (confirming prior reports [30]). Startle responses elicited by each prepulse amplitude also significantly differed from each other in PPI whether assessing the efficacy of Dox dose [F(2,72) = 74.95, p < 0.0001] (Figure 2A) or duration [F(2,122) = 235.22, p < 0.0001] (Figure 2B), validating the task (Figures 2A and 2B). No effects of saline or Dox on either ASR or PPI responses were observed among control C57BL/6J mice lacking the Tat transgene (Table 1).
Figure 1.
Acoustic startle reflex (arbitrary units ± SEM in response to 120 dB tone) among male GT-tg mice (n=10/group) administered saline (white circles) or doxycycline (black circles) in (A) increasing doses (25 – 125 mg/kg, i.p. for 7 days), or (B) increasing durations of exposure (100 mg/kg for 1 – 14 days) to induce HIV-1 Tat expression in brain. * indicates significant difference from saline-treated controls (one-way ANOVA, effect of treatment, p < 0.05).
Figure 2.
Prepulse inhibition of the acoustic startle reflex (% ± SEM) among male GT-tg mice (n=10/group) administered saline (open symbols), (A) increasing doses of doxycycline (25 – 125 mg/kg, i.p. for 7 days), or (B) increasing durations of exposure (1 – 14 days) to doxycycline (100 mg/kg) to induce HIV-1 Tat expression in brain. * indicates doxycycline treatment group significantly differs from either saline-treatment group, irrespective of dB (repeated measures ANOVA, main effect of treatment); † indicates each prepulse dB significantly differs from each other, irrespective of doxycycline treatment (repeated measures ANOVA, main effect of dB amplitude, p < 0.05).
Table 1.
Doxycycline has no influence on behavioral performance or Iba1-labeled microglia reactivity (mean ± SEM) among male C57BL/6J controls that do not carry the Tat transgene.
| Acoustic Startle and Prepulse Inhibition among C57BL/6J Mice | ||||
| Acoustic Startle Reflex (120dB) |
% Prepulse Inhibition (74dB) |
% Prepulse Inhibition (78dB) |
% Prepulse Inhibition (86dB) |
|
| Saline (0.9%, 14d) n = 12 | 163 ± 15 | 25 ± 4 | 41 ± 4 | 54 ± 4 |
| Doxycycline (100mg/kg, 14d) n = 12 | 156 ± 19 | 21 ± 4 | 36 ± 4 | 54 ± 4 |
| Microglial Reactivity via Iba1 Labeling among C57BL/6J Mice | ||||
| Agranular Insular Cortex |
Anterior Cingulate Cortex |
Prelimbic Cortex | Infralimbic Cortex | |
| Saline (0.9%, 7d) n = 8 | 1.7 ± 0.07 | 1.9 ± 0.04 | 1.4 ± 0.2 | 1.4 ± 0.2 |
| Doxycycline (100mg/kg, 7d) n = 12 | 1.7 ± 0.06 | 2.0 ± 0.05 | 1.5 ± 0.2 | 1.8 ± 0.2 |
3.2. Exposure to HIV-1 Tat that impairs sensorimotor gating promotes microglial reactivity in subregions of the medial prefrontal cortex and other extra/limbic regions
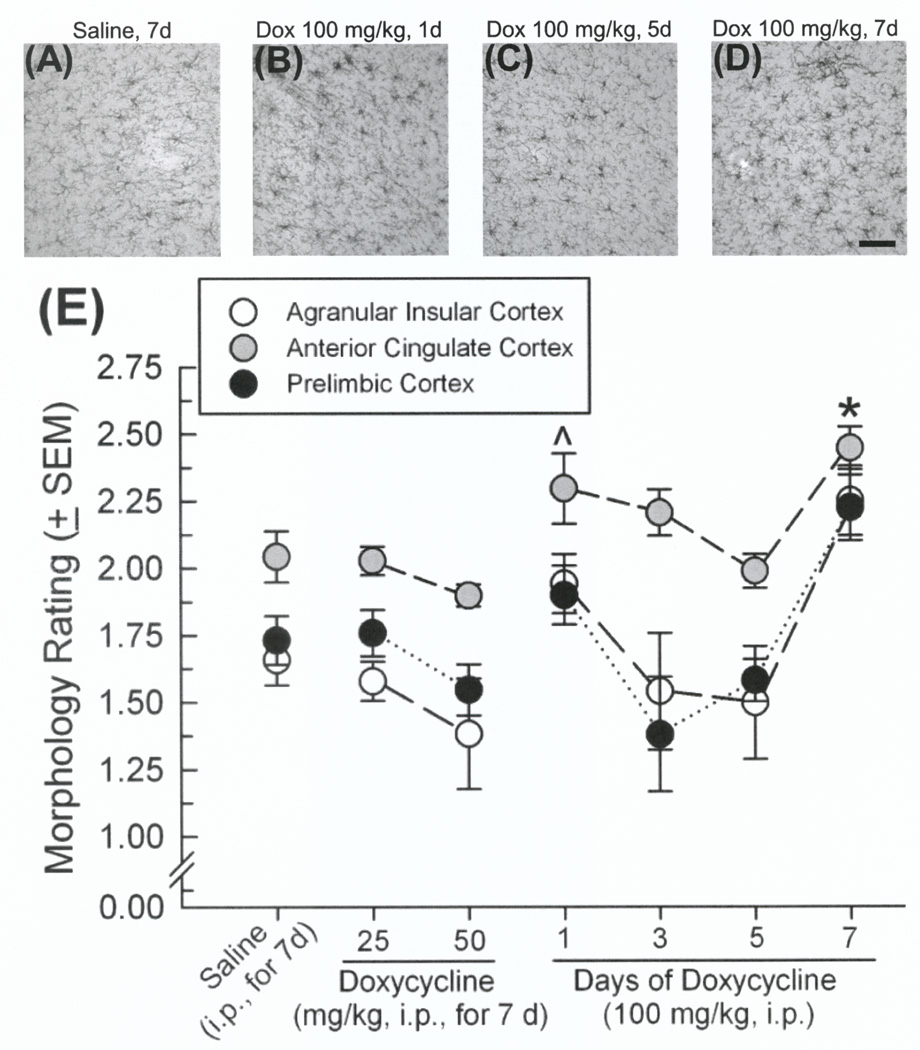
Microglial morphology was assessed for reactivity in subregions of the amygdala, hippocampal formation, mPFC, nucleus accumbens, ventral tegmental area, and ventral pallidum among GT-tg mice where Tat was induced via Dox (25, 50, or 100 mg/kg, i.p.) once daily for 1, 3, 5, or 7 days. Tat induction modulated microglial activation across subregions; however, mPFC was the only region to demonstrate a pattern entirely consistent with observed ASR and PPI behavior. In agranular insular [F(8,91) = 3.12, p = 0.003], anterior cingulate [F(8,91) = 6.79, p < 0.0001], and prelimbic [F(8,91) = 3.39, p = 0.002] mPFC cortices, the 100 mg/kg dose of Dox significantly enhanced microglial reactivity over saline administration when administered for 1 day (anterior cingulate cortex only: p = 0.03) or 7 days (agranular insular: p = 0.02, anterior cingulate: p = 0.0001, and prelimbic cortices: p = 0.02; Figure 3A–E). No significant differences were observed in the infralimbic mPFC cortex despite an apparent effect for mice receiving Dox (100 mg/kg, 7d) to demonstrate greater microglial reactivity (2.4 ± 0.01) than those receiving lesser dosing (saline, 7d: 1.7 ± 0.2, Dox25mg/kg,7d: 1.6 ± 0.2, Dox50mg/kg,7d: 1.6 ± 0.2, Dox100mg/kg,1d: 1.4 ± 0.4, Dox100mg/kg,3d: 1.7 ± 0.3, Dox100mg/kg,5d: 2.0 ± 0.1).
Figure 3.
(A–D) Representative photomicrographs of Iba1-labeled microglia in anterior cingulate cortex. (E) Morphology rating (mean ± SEM; 1 = resting, 2 = early activated, 3 = activated/reactive, 4 = reactive) of microglia in subregions of the medial prefrontal cortex (n=8–12 observations/group): agranular insular cortex (white circles), anterior cingulate cortex (grey circles), and prelimbic cortex (black circles). ^ indicates significant difference from saline-treated controls in the anterior cingulate cortex (one-way ANOVA, main effect of treatment). * indicates significant difference from saline-treated controls in all depicted subregions (one-way ANOVAs, effects of treatment, p < 0.05). Scale bar, 100 µm.
Within subregions of the amygdala, Tat induction via Dox significantly increased reactivity of microglia labeled with Iba-1 in all nuclei examined including the central amygdaloid [F(8,41) = 16.92, p < 0.05], anterior amygdaloid [F(8,39) = 3.13, p < 0.05], medial anterior [F(8,41) = 10.23, p < 0.05], medial posterior [F(8,41) = 7.80, p < 0.05], anterior cortical [F(8,41) = 3.19, p < 0.05], posterior cortical [F(8,41) = 7.43, p < 0.05], and amygdalo-hippocampal [F(8,41) = 4.52, p < 0.05] areas (Table 2). Some subregions appeared more sensitive to the neuroinflammatory effects of Tat induction with increased microglial reactivity at lower (25 or 50 mg/kg) Dox concentrations (central amygdaloid, medial anterior, or posterior cortical nuclei) compared to control saline-administration. Greater durations of Dox at a higher concentration (100 mg/kg) further bore out subregional differences with significant increases in microglial reactivity apparent after exposure for 3 or more days (central amygdaloid and anterior cortical nuclei), 5 or more days (anterior amygdaloid, medial anterior, or posterior cortical nuclei), or only apparent after 7 days (medial posterior nucleus and amygdalo-hippocampal area) of exposure compared to saline-administered controls.
Table 2.
Morphology rating (mean ± SEM; 1=resting, 2=early activated, 3=activated/reactive, 4=reactive) of microglia in subregions of the amygdala, hippocampal formation, nucleus accumbens, ventral tegmental area, and ventral pallidum (n=4–6 /group) among C57BL/6J or GT-tg mice administered saline or doxycycline (Dox; 25–100 mg/kg, i.p., for 1–7 days).
| Microglial Reactivity via Iba1 Labeling among C57BL/6J Mice and GT-tg mice | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain: | C57BL/6J | GT-tg | |||||||
| Doxycycline Treatment: |
Saline (7d) |
Dox (100 mg/kg, 7d) |
Saline (7d) |
Dox (25 mg/kg, 7d) |
Dox (50 mg/kg, 7d) |
Dox (100 mg/kg, 1d) |
Dox (100 mg/kg, 3d) |
Dox (100 mg/kg, 5d) |
Dox (100 mg/kg, 7d) |
| Amygdala | |||||||||
| Anterior amygdaloid nucleus | 1.68 ± 0.06 | 1.64 ± 0.05 | 1.64 ± 0.04 | 1.70 ± 0.04 | 1.78 ± 0.06 | 1.70 ± 0.06 | 1.77 ± 0.05 | 1.82 ± 0.07* (p=0.03) |
1.97 ± 0.09* (p=0.0003) |
| Central amygdaloid nucleus | 1.54 ± 0.05 | 1.67 ± 0.05 | 1.51 ± 0.04 | 1.64 ± 0.04* (p=0.04) |
1.94 ± 0.04* (p<0.0001) |
1.52 ± 0.05 | 1.76 ± 0.06* (p=0.0002) |
1.91 ± 0.04* (p<0.0001) |
1.97 ± 0.04* (p<0.0001) |
| Medial anterior nucleus | 1.78 ± 0.05 | 1.71 ± 0.06 | 1.66 ± 0.02 | 1.67 ± 0.04 | 1.93 ± 0.09* (p=0.004) |
1.67 ± 0.07 | 1.81 ± 0.05 | 1.89 ± 0.04* (p=0.01) |
2.31 ± 0.11* (p<0.0001) |
| Medial posterior nucleus | 1.89 ± 0.06 | 1.85 ± 0.06 | 1.71 ± 0.04 | 1.78 ± 0.04 | 1.78 ± 0.04 | 1.63 ± 0.02 | 1.80 ± 0.04 | 1.81 ± 0.04 | 2.11 ± 0.07* (p<0.0001) |
| Anterior cortical nucleus | 1.66 ± 0.06 | 1.68 ± 0.06 | 1.72 ± 0.05 | 1.77 ± 0.05 | 1.83 ± 0.05 | 1.76 ± 0.08 | 1.91 ± 0.09* (p=0.03) |
1.91 ± 0.07* (p=0.03) |
1.99 ± 0.05* (p=0.003) |
| Posterior cortical nucleus | 1.79 ± 0.04 | 1.81 ± 0.03 | 1.71 ± 0.05 | 1.78 ± 0.04 | 1.89 ± 0.04* (p=0.004) |
1.73 ± 0.02 | 1.83 ± 0.02 | 2.01 ± 0.06* (p<0.0001) |
2.06 ± 0.05* (p<0.0001) |
| Amygdalo-hippocampal area | 1.67 ± 0.12 | 1.63 ± 0.05 | 1.58 ± 0.03 | 1.58 ± 0.02 | 1.64 ± 0.07 | 1.62 ± 0.07 | 1.61 ± 0.06 | 1.68 ± 0.06 | 2.05 ± 0.12* (p<0.0001) |
| Hippocampal Formation | |||||||||
| CA1 | 1.81 ± 0.04 | 1.91 ± 0.02 | 1.91 ± 0.05 | 1.74 ± 0.04* (p=0.01) |
1.98 ± 0.03 | 1.88 ± 0.03 | 1.81 ± 0.06 | 1.97 ± 0.07 | 2.26 ± 0.06* (p<0.0001) |
| CA2 | 1.78 ± 0.14 | 2.06 ± 0.08 | 1.85 ± 0.13 | 1.90 ± 0.15 | 2.46 ± 0.10* (p=0.0001) |
1.85 ± 0.09 | 2.05 ± 0.06 | 2.36 ± 0.11* (p=0.0009) |
2.60 ± 0.06* (p<0.0001) |
| CA3 | 1.88 ± 0.06 | 1.90 ± 0.05 | 1.81 ± 0.09 | 1.80 ± 0.05 | 2.05 ± 0.08* (p=0.01) |
1.86 ± 0.06 | 1.94 ± 0.05 | 2.03 ± 0.06* (p=0.02) |
2.24 ± 0.09* (p<0.0001) |
| Dentate Gyrus | 1.81 ± 0.05 | 1.89 ± 0.08 | 1.93 ± 0.08 | 1.92 ± 0.06 | 2.50 ± 0.05* (p<0.0001) |
2.15 ± 0.10* (p=0.05) |
2.14 ± 0.10* (p=0.05) |
2.52 ± 0.05* (p<0.0001) |
2.46 ± 0.08* (p<0.0001) |
| Subiculum | 1.98 ± 0.04 | 1.85 ± 0.06 | 1.84 ± 0.07 | 1.82 ± 0.06 | 2.11 ± 0.06* (p=0.002) |
2.02 ± 0.08 | 1.99 ± 0.06 | 2.17 ± 0.06* (p=0.0004) |
2.48 ± 0.06* (p<0.0001) |
| Presubiculum | 1.09 ± 0.07 | 1.45 ± 0.15 | 1.50 ± 0.15 | 1.69 ± 0.08 | 1.87 ± 0.18 | 1.48 ± 0.21 | 1.83 ± 0.23 | 1.89 ± 0.10 | 2.17 ± 0.18* (p=0.004) |
| Parasubiculum | 1.79 ± 0.08 | 1.78 ± 0.09 | 1.87 ± 0.11 | 1.95 ± 0.04 | 2.18 ± 0.07* (p=0.007) |
1.93 ± 0.08 | 2.01 ± 0.08 | 2.11 ± 0.10* (p=0.03) |
1.84 ± 0.05 |
| Nucleus Accumbens | |||||||||
| Core | 1.86 ± 0.07 | 1.73 ± 0.05 | 1.54 ± 0.08 | 1.45 ± 0.06 | 1.81 ± 0.05* (p=0.01) |
1.91 ± 0.12* (p=0.001) |
1.65 ± 0.07 | 1.91 ± 0.06* (p=0.0003) |
2.16 ± 0.05* (p<0.0001) |
| Shell | 1.76 ± 0.08 | 1.92 ± 0.07 | 1.83 ± 0.15 | 1.78 ± 0.13 | 2.06 ± 0.18 | 1.84 ± 0.23 | 1.76 ± 0.13 | 1.89 ± 0.11 | 2.35 ± 0.21 |
| Lateral | 1.89 ± 0.04 | 1.84 ± 0.17 | 1.71 ± 0.08 | 1.93 ± 0.06 | 2.02 ± 0.14 | 1.79 ± 0.15 | 1.82 ± 0.06 | 2.13 ± 0.10 | 2.26 ± 0.21 |
| Ventral Tegmental Area | 1.44 ± 0.10 | 1.42 ± 0.03 | 1.54 ± 0.07 | 1.50 ± 0.07 | 1.83 ± 0.06* (p=0.005) |
1.48 ± 0.08 | 1.70 ± 0.09 | 1.86 ± 0.06* (p=0.002) |
1.92 ± 0.07* (p=0.0003) |
| Ventral Pallidum | 1.87 ± 0.04 | 1.87 ± 0.03 | 1.67 ± 0.03 | 1.76 ± 0.02 | 1.89 ± 0.06* (p<0.0001) |
1.70 ± 0.02 | 1.73 ± 0.02 | 2.04 ± 0.05* (p<0.0001) |
2.29 ± 0.03* (p<0.0001) |
Shaded cells indicate significant difference from respective saline-treated controls (one-way ANOVAs, effect of treatment, p < 0.05; post-hoc comparisons provided).
Within the hippocampal formation, regional differences in microglial reactivity were observed in CA1 [F(8,40) = 10.03, p < 0.0001], CA2 [F(8,40) = 7.85, p < 0.0001], CA3 [F(8,40) = 4.35, p < 0.0008], dentate gyrus [F(8,40) = 15.68, p < 0.0001], subiculum [F(8,41) = 11.73, p < 0.0001], presubiculum [F(8,41) = 3.59, p < 0.003], and parasubiculum [F(8,41) = 3.17, p < 0.007] (Table 2). Lower Dox exposure reduced reactivity in CA1 (25 mg/kg, 7d) but elevated it when administered at 50 mg/kg in CA2, CA3, dentate gyrus, subiculum, and parasubiculum. Greater durations of Dox (100 mg/kg) exposure produced significant microglial reactivity after 1 or more days in the dentate gyrus, 5 or more days in the CA2, CA3 and subiculum, at only 5 days in the parasubiculum, and after 7 days in CA1 or presubiculum as compared to controls.
The nucleus accumbens also demonstrated regional selectivity for Tat-induced neuroinflammation. The nucleus accumbens core [F(8,38) = 11.17, p < 0.0001], but not the shell or lateral accumbens, demonstrated Dox-dependent microglial activation with lower (50 mg/kg) or higher (100 mg/kg) Dox exposure for brief (1 day) or prolonged (5 – 7 days) durations (Table 2). Similar effects were observed in the ventral tegmental area [F(8,41) = 7.48, p < 0.0001] and ventral pallidum [F(8,40) = 28.16, p < 0.0001] with prolonged exposure (5 – 7 days) to lower (50 mg/kg) or higher (100 mg/kg) Dox dosing significantly increasing microglial activation (Table 2). Importantly, no differences were observed in any subregion examined among C57BL/6J controls that lacked the Tat transgene (Tables 1 and 2).
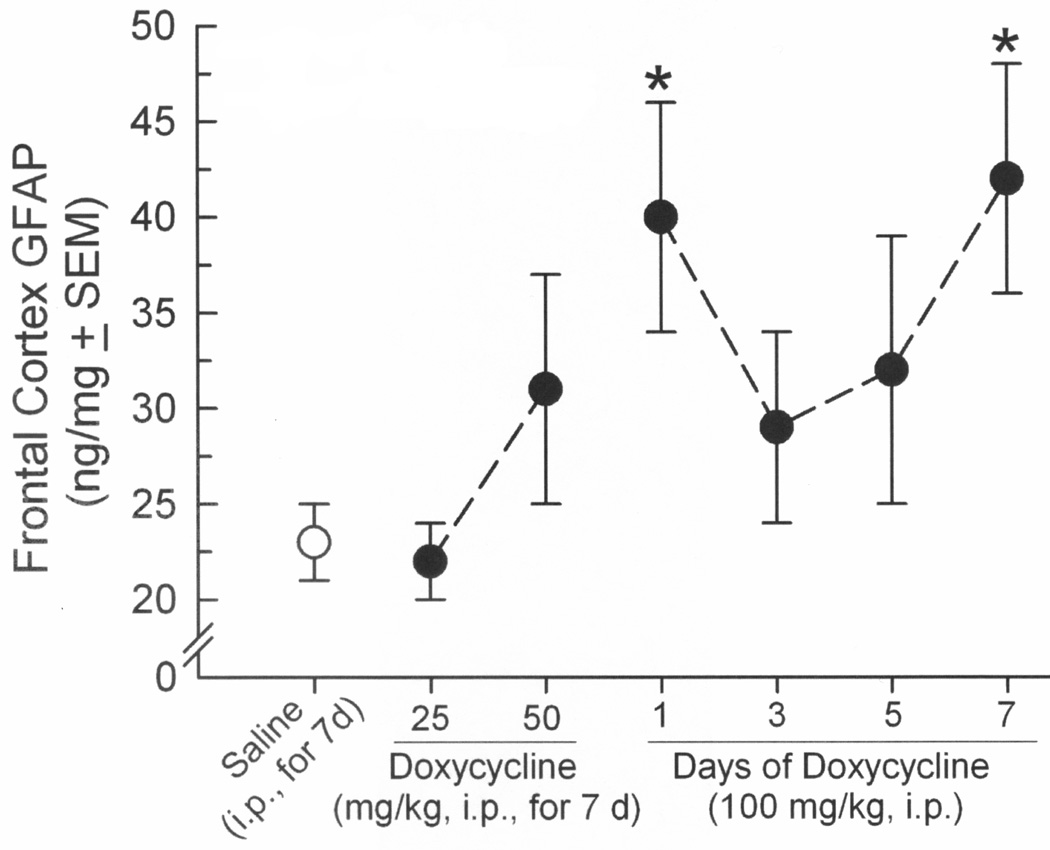
3.3. HIV-1 Tat dose-dependently elevated GFAP content in frontal cortex
The total content of GFAP was also assessed in frontal cortex, demonstrating a significant [F(6,47) = 2.22, p = 0.05] dose-dependent modulation by induction of Tat that recapitulated effects on microglial reactivity (Figure 4). Inducing Tat via Dox administration that was either acute (100 mg/kg, 1d; p = 0.03) or prolonged (100 mg/kg, 7d; p = 0.02) significantly increased GFAP protein in frontal cortex compared to saline-administered, uninduced controls (Figure 4). Significant differences from uninduced GT-tg mice were not observed at any other dosing regimen (Figure 4), nor were differences in GFAP detected among C57BL/6J controls that lacked Tat (C57BL/6JSaline,7d = 34 ± 6 ng/mg, C57BL/6JDox100mg/kg,7d = 29 ± 6 ng/mg).
Figure 4.
Frontal cortex GFAP content (ng/mg ± SEM) among male GT-tg mice that were administered saline (white circle) or had HIV-1 Tat dose-dependently induced by doxycycline (7–8 observations/group). * indicates significant difference from saline-treated controls (one-way ANOVA, effect of treatment, p = 0.05).
3.4. Inhibiting neuroinflammation via indomethacin attenuates brief, but not prolonged, induction of HIV-1 Tat effects on acoustic startle and prepulse inhibition
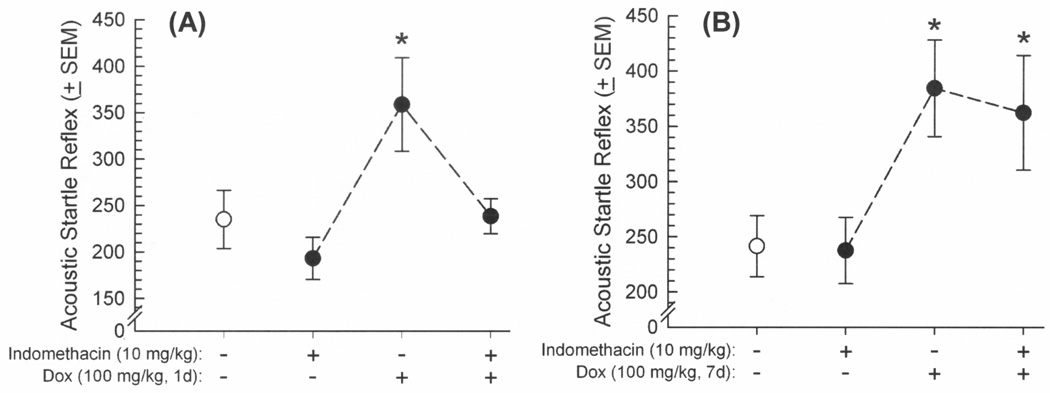
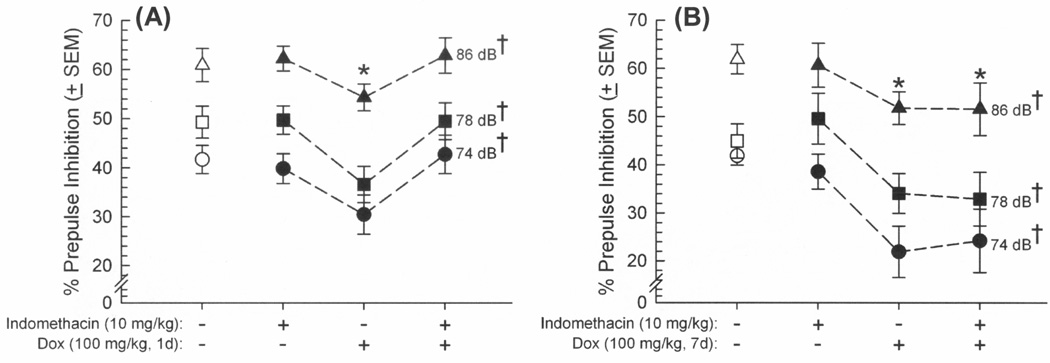
To assess the influence of Tat-induced neuroinflammation on ASR and PPI responding, GT-tg mice were administered two injections of vehicle or the cyclooxygenase-1 and -2 inhibitor, indomethacin (10 mg/kg/d, i.p.), prior to a either a brief 1-day Tat induction (Dox 100 mg/kg, i.p., for 1 day) or each day prior to a prolonged 7-day Tat induction (Dox 100 mg/kg, i.p., for 7 days). Indomethacin significantly influenced the effects of brief Dox exposure on ASR [F(3,44) = 4.65, p = 0.007] (Figure 5A) and PPI [F(3,88) = 2.93, p = 0.04] (Figure 6A). As before, induction of HIV-1 Tat significantly potentiated the ASR of GT-tg mice (p = 0.01; Figure 5A) and impaired PPI (p = 0.02; Figure 6A) compared to vehicle-treated, uninduced controls. Pretreatment with indomethacin also significantly attenuated the effect of Tat induction on ASR (p = 0.01; Figure 5A) and PPI (p = 0.01; Figure 6A) compared to Tat-induced mice that were pretreated with vehicle. However, although ASR was again elevated [F(3,35) = 3.72, p = 0.02] (Figure 5B) and PPI impaired [F(3,70) = 3.35, p = 0.03] (Figure 6B) following Tat induction through prolonged Dox treatment (7 days), the present indomethacin regimen was not able to mitigate these deficits (Figure 5B). As before, startle responses elicited by each prepulse amplitude significantly differed from each other in PPI whether assessing brief [F(2,88) = 189.92, p < 0.0001] (Figure 6A) or prolonged [F(2,70) = 102.43, p < 0.0001] (Figure 6B) Dox exposure, validating the PPI paradigm.
Figure 5.
Acoustic startle reflex (arbitrary units ± SEM in response to 120 dB tone) among male GT-tg mice (n=8–12/group) that received pretreatment with vehicle or indomethacin (10 mg/kg, i.p.) 20 h and again 30 min prior to inducing HIV-1 Tat (or not) via (A) a brief doxycycline exposure (100 mg/kg, 1d) or (B) a prolonged doxycycline exposure (100 mg/kg, 7d). * indicates significant difference from saline-treated controls (white circles; one-way ANOVA, effect of treatment, p < 0.05).
Figure 6.
Prepulse inhibition of the acoustic startle reflex (% ± SEM) among male GT-tg mice (n=8–12/group) that received pretreatment with vehicle or indomethacin (10 mg/kg, i.p.) 20 h and again 30 min prior to having HIV-1 Tat induced (or not) via (A) a brief doxycycline exposure (100 mg/kg, 1d) or (B) a prolonged doxycycline exposure (100 mg/kg, 7d). * indicates treatment group significantly differs from saline-treatment (white symbols) group, irrespective of dB (repeated measures ANOVA, main effect of treatment); † indicates each prepulse dB significantly differs from each other, irrespective of treatment (repeated measures ANOVA, main effect of dB amplitude, p < 0.05).
4. Discussion
The hypothesis that exposure to HIV-1 Tat protein in the CNS would impair sensorimotor responding was upheld. Inducing Tat via Dox dose-dependently potentiated the ASR of GT-tg bigenic mice and attenuated PPI of this reflex; effects that were absent among C57BL/6J controls that lacked the Tat transgene. The concentration of Dox that was previously demonstrated to optimally express Tat protein in the GT-tg brain (100 mg/kg, i.p. [8,23]), concurrent with impaired learning [23], enhanced anxiety-like behavior [8,9], and potentiated drug reward [31–33] was also the most efficacious concentration observed herein. Notably, effects on ASR and PPI were not linear; rather, acute Tat induction (after 1 day of Dox) or prolonged exposure (after 7 days of Dox) equally potentiated startle and impaired PPI. The results support the hypothesis that Tat effects may involve indirect neuroinflammatory processes in extra/limbic brain regions, as the reactivity of Iba1-labeled microglia was potentiated after 1 day of induction in the anterior cingulate cortex, and after 7 days of induction in the agranular insular, anterior cingulate, and prelimbic cortices of the mPFC. Two other brain regions also demonstrated microglial activation in response to brief or prolonged Tat induction (dentate gyrus and nucleus accumbens core); however, neither recapitulated the pattern of behavioral perturbation as well as the anterior cingulate cortex. Additionally, the astroglial protein, GFAP, was significantly increased in frontal cortex after 1 or 7 days of Tat induction. Pre-treatment with an anti-inflammatory cyclooxygenase-1 and -2 inhibitor attenuated the behavioral effects of acute, but not prolonged, Tat induction, suggesting the importance of neuroinflammatory factors in mediating the early effects of Tat exposure.
These data support prior findings in rats that indicate HIV-1- [20–22] or Tat- [18–19] induced disruption of ASR or PPI, but extend them to elucidate associated neuroinflammatory pathology within extra/limbic brain regions of a transgenic mouse model. Findings are broadly consistent with demonstrations of Tat-induced neuroinflammation in vivo, including biomarkers of neuroinflammation in the cortex of GT-tg bigenic mice [34], activation of astrocytes and elevated immunoreactive microglial markers in the striatum of Tat-induced mice that are co-administered morphine [35], and demonstrated microgliosis in the cortex of CX3CR1GFP/+ mice [36]. Moreover, a single exogenous administration of Tat protein to the hippocampus produced microgliosis within 24 h that lasted for 28 days in chimeric C57BL/6 mice genetically marked to distinguish infiltrating- from resident-immune cells [37]. These results are similar to findings with another Tat-transgenic mouse model, wherein Tat induced an increase in Iba1-labeled cells within striatum concurrent with co-localized markers of nitrosative cellular stress, increased neuronal cell death, astrogliosis, and decreased dendritic spine density [12].
In the present experiments, acute vs. prolonged Tat exposure elicited commensurate impairment in sensorimotor gating; however, cyclooxygenase inhibition was observed only to mitigate brief Tat exposure. These data suggest that different potential mechanisms may underlie the observed disruptions of ASR/PPI, involving initial acute and sublethal neuroinflammatory processes compared to a later onset of inflammation-associated neurotoxic events. In support of this suggestion, acute intra-hippocampal injections of Tat1–72 were observed to significantly elevate cyclooxygenase-2 mRNA and protein in the hippocampus of C57BL/6J mice [38]. Microglial activation in this model could be attenuated via pretreatment with the cyclooxygenase-2 inhibitor, NS-398 [38]. However, among HIV-transgenic rats, which is a chronic whole-animal model of constitutive viral protein exposure, mRNA elevations in neuroinflammatory markers, including cyclooxygenase-1 and -2 or membrane and cytosolic prostaglandin E2 synthase, were not detected [39]. Despite this, we have observed structural gray-matter reductions in some brain regions comprising the ASR/PPI circuit using MRI (including cortical, amygdalar, and hippocampal brain regions) [10] and other researchers have demonstrated profound neuronal death [12] following prolonged Tat exposure. As such, acute Tat exposure may elicit potentially-reversible neuroinflammatory effects as prologue to later toxic events. Conceivably, novel therapeutics that target Tat's neuroinflammatory actions may provide protection from subsequent cellular toxicity. One connatural compound, didehydro-Cortistatin A, targets Tat transactivation directly and has been recently shown to attenuate Tatmediated increases in pro-inflammatory cytokines including IL-1β and IL-6 in vitro [40]. The present results suggest that mitigation of Tat's neuroinflammatory processes that disrupt sensorimotor gating via actions in localized brain regions (i.e. anterior cingulate and associated extra/limbic nuclei) may prove viable priorities for adjunctive HIV drug development. It must be noted that indomethacin may attenuate affective dysfunction associated with acute onset of sickness behavior in rodents, which could ameliorate some behavioral observations [41]. However, this caveat is less likely to have influenced the present behavioral outcomes given that we and others [42] have not observed indomethacin to influence ASR or PPI on its own.
The present findings were limited to the examination of Iba1 immunohistochemistry, but were evaluated in a blind manner by an independent observer who used established methodology and scoring parameters [25–27,29]. Although additional in-depth studies of Tat-induced markers of neuroinflammation are needed, the results after 7 days of induction suggest that Tat exposure promotes microglial (and potential astroglial) pathology and inflammation. Moreover, our prior MRI findings support the idea that multiple brain areas are affected by conditional Tat expression [10–11], supporting the evaluation of additional brain regions for future histochemical studies aimed at assessing the magnitude of neuroinflammation over time following Tat induction. Notably, hippocampal inputs to mPFC appear critical for output to PPI-relevant brain regions (including nucleus accumbens) [43]. Hippocampus is also noted to be a region of high Tat mRNA expression in the GT-tg bigenic mouse model [7], potentially contributing to the present behavioral observation. Given recent demonstrations of Tat's sublethal effects to reduce spine density on medium spiny neurons in hippocampus [44] and similar HIV-1-related effects in nucleus accumbens [45], the involvement of the greater limbic/extralimbic circuit for other tasks that involve attentional processing presents as an important target for future investigations.
In addition to indirect neuroinflammatory actions, Tat may also utilize direct mechanisms to influence sensorimotor gating. Prepulse inhibition is known to be attenuated by dopamine agonists in mice, particularly those with preference for D1-like receptors [46]. Tat is known to allosterically modulate the dopamine transporter [47], elevating synaptic dopamine concentrations [48] and increasing the efficacy of binding ligands [49]. Tat-induced enhancement of dopamine levels could further promote the deficits observed in PPI presently. However, although additional mechanistic studies are needed, these results still emphasize the importance of HIV-1 Tat as a therapeutic target, given the multiple mechanisms for cellular and behavioral dysfunction that lie downstream of its actions.
It is important to note that Tat is not the only HIV-1 protein that may contribute to HIV-1 effects on attentional dysfunction or impaired ASR/PPI. Perinatal infusion of intrahippocampal glycoprotein 120 (gp120) produces limited deficits in geotaxis of rats and ASR sensitivity in early development [50], disrupting PPI transiently later in life [51]. Like Tat, effects of gp120 may be related to dopaminergic dysfunction, given that the dopamine enhancer, methamphetamine, attenuates differences in ASR or PPI of male and female transgenic mice [52] and the non-selective dopamine receptor agonist, apomorphine, attenuates peak amplitude shifts in ASR among rats receiving perinatal gp120 infusions [51]. As such, additional viral proteins are likely to contribute to HIV dysfunction of attentional and sensorimotor processes.
Some additional caveats for the present work must be reviewed. Foremost, the conditionally-inducible model of Tat expression requires induction with acute-to-prolonged Dox administration. It is possible that some inflammatory effects of Tat protein could be offset by Dox itself, which is reported to have modest anti-inflammatory effects at high doses [53,54]. However, this seems unlikely given that minocycline has been shown to attenuate inflammatory PPI deficits (not exacerbate them as was observed with Tat exposure herein) [55]. Strain differences in ASR were also observed, with control C57BL/6J mice demonstrating a lower ASR than control (saline-treated) GT-tg bigenic mice. These findings are consistent with prior reports wherein C57BL/6J mice demonstrated an ASR that was intermediate to several inbred strains [30]. Similarly, locomotor differences between C57BL/6J and GT-tg mice have been reported, but do not account for differences in affect [8]. It must be noted that motor deficits were previously associated with maximal Dox treatment conditions (125 mg/kg for 7d, or 100 mg/kg for 14d) [8]. As such, these manipulations were not followed up for morphological/biochemical analyses, given that the influence of potential motor confounds at these doses could not be ruled out from the present data. Lastly, the enhancement of GFAP may indicate astrogliosis (as has been observed in a similar transgenic Tat mouse model [12]); albeit, it cannot be ruled out that GFAP was enhanced via proinflammatory activation of astroglia independent of death in this cell type.
5. Conclusions
Together, these findings demonstrate that exposure to the HIV-1 regulatory protein, Tat, is sufficient to impair ASR and PPI responding, potentially due to neuroinflammatory effects that involve subregions of the mPFC, hippocampal formation, amygdala, nucleus accumbens, ventral tegmental area, and ventral pallidum. The implications of the present work extend beyond reflexive responding, and elucidate a central pathology within a hippocampus/mPFC/midbrain circuit that is essential not only for PPI, but also for anxiety- and reward-responding that may also be perturbed in neuroAIDS. As such, neuroinflammatory factors within these regions are likely to be important therapeutic targets for HIV-related behaviors beyond sensorimotor responding.
Highlights.
Low or high exposure to Tat amplified startle and impaired prepulse inhibition
Low or high Tat activated microglia in mPFC and extra/limbic brain regions
GFAP increased in mPFC concurrent with low or high Tat
Indomethacin pretreatment mitigated effects of brief (not prolonged) Tat exposure
Acknowledgements
This research was supported by F31 NS064872-01 from NINDS to ANC, R01MH085607 from NIMH to JPM, and funds from the State of Florida, Executive Office of the Governor’s Department of Economic Opportunity. We thank Dr. Johnny J. He for the gift of the GT-tg breeder mice and Elizabeth Sypek for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burdo TH, Katner SN, Taffe MA, Fox HS. Neuroimmunity, drugs of abuse, and neuroAIDS. J Neuroimmune Pharmacol. 2006;1:41–49. doi: 10.1007/s11481-005-9001-3. [DOI] [PubMed] [Google Scholar]
- 2.Kopnisky KL, Bao J, Lin YW. Neurobiology of HIV, psychiatric and substance abuse comorbidity research: workshop report. Brain Behav Immun. 2007;21:428–441. doi: 10.1016/j.bbi.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Kraft-Terry SD, Stothert AR, Buch S, Gendelman HE. HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiol Dis. 2010;37:542–548. doi: 10.1016/j.nbd.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potter MC, Figuera-Losada M, Rojas C, Slusher BS. Targeting the glutamatergic system for the treatment of HIV-associated neurocognitive disorders. J Neuroimmune Pharmacol. 2013;8:594–607. doi: 10.1007/s11481-013-9442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Copeland KF. Modulation of HIV-1 transcription by cytokines and chemokines. Mini Rev Med Chem. 2005;5:1093–1101. doi: 10.2174/138955705774933383. [DOI] [PubMed] [Google Scholar]
- 6.Royal W, 3rd, Zhang L, Guo M, Jones O, Davis H, Bryant JL. Immune activation, viral gene product expression and neurotoxicity in the HIV-1 transgenic rat. J Neuroimmunol. 2012;247:16–24. doi: 10.1016/j.jneuroim.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paris JJ, Singh HD, Ganno ML, Jackson P, McLaughlin JP. Anxiety-like behavior of mice produced by conditional central expression of the HIV-1 regulatory protein, Tat. Psychopharmacology (Berl.) 2013;231:2349–2360. doi: 10.1007/s00213-013-3385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paris JJ, Fenwick J, McLaughlin JP. Progesterone protects normative anxiety-like responding among overiectomized female mice that conditionally express the HIV-1 regulatory protein, Tat, in the CNS. Horm Behav. 2014;65:445–453. doi: 10.1016/j.yhbeh.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey AN, Liu X, Mintzopoulos D, Paris JJ, Muschamp JW, McLaughlin JP, Kaufman MJ. Conditional Tat protein expression in the GT-tg bigenic mouse brain induces gray matter density reductions. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:49–54. doi: 10.1016/j.pnpbp.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey AN, Liu X, Mintzopoulos D, Paris JJ, McLaughlin JP, Kaufman MJ. Conditional Tat protein brain expression in the GT-tg Bigenic Mouse Induces Cerebral Fractional Anisotropy Abnormalities. Curr HIV Res. 2015 doi: 10.2174/1570162x13666150126125244. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn YK, Podhaizer EM, Farris SP, Miles MF, Hauser KF, Knapp PE. Effects of chronic HIV-1 Tat exposure in the CNS: heightened vulnerability of males versus females to changes in cell numbers, synaptic integrity, and behavior. Brain Struct Funct. 2015;220:605–623. doi: 10.1007/s00429-013-0676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geyer MA, Dulawa SC. Assessment of murine startle reactivity, prepulse inhibition, and habituation. Curr Protoc Neurosci. 2003;8:8.17. doi: 10.1002/0471142301.ns0817s24. [DOI] [PubMed] [Google Scholar]
- 14.Bubser M, Koch M. Prepulse inhibition of the acoustic startle response of rats is reduced by 6-hydroxydopamine lesions of the medial prefrontal cortex. Psychopharmacology (Berl.) 1994;113:487–492. doi: 10.1007/BF02245228. [DOI] [PubMed] [Google Scholar]
- 15.Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- 16.Swerdlow NR, Geyer MA. Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr Bull. 1998;24:285–301. doi: 10.1093/oxfordjournals.schbul.a033326. [DOI] [PubMed] [Google Scholar]
- 17.Minassian A, Henry BL, Woods SP, Vaida F, Grant I, Geyer MA, Perry W Translational Methamphetamine AIDS Research Center (TMARC) Group. Prepulse inhibition in HIV-associated neurocognitive disorders. J Int Neuropsychol Soc. 2013;19:709–717. doi: 10.1017/S1355617713000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitting S, Booze RM, Mactutus CF. Neonatal hippocampal Tat injections: developmental effects on prepulse inhibition (PPI) of the auditory startle response. Int J Dev Neurosci. 2006;24:275–283. doi: 10.1016/j.ijdevneu.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitting S, Booze RM, Hasselrot U, Mactutus CF. Intrahippocampal injections of Tat: effects on prepulse inhibition of the auditory startle response in adult male rats. Pharmacol Biochem Behav. 2006;84:189–196. doi: 10.1016/j.pbb.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Moran LM, Aksenov MY, Booze RM, Webb KM, Mactutus CF. Adolescent HIV-1 transgenic rats: evidence for dopaminergic alterations in behavior and neurochemistry revealed by methamphetamine challenge. Curr HIV Res. 2012;10:415–424. doi: 10.2174/157016212802138788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moran LM, Booze RM, Mactutus CF. Time and time again: temporal processing demands implicate perceptual and gating deficits in the HIV-1 transgenic rat. J Neuroimmune Pharmacol. 2013;8:988–997. doi: 10.1007/s11481-013-9472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moran LM, Booze RM, Webb KM, Mactutus CF. Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Exp Neurol. 2013;239:139–147. doi: 10.1016/j.expneurol.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res. 2012;229:48–56. doi: 10.1016/j.bbr.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonçalves J, Baptista S, Martins T, Milhazes N, Borges F, Ribeiro CF, Malva JO, Silva AP. Methamphetamine-induced neuroinflammation and neuronal dysfunction in the mice hippocampus: preventive effect of indomethacin. Eur J Neurosci. 2010;31:315–326. doi: 10.1111/j.1460-9568.2009.07059.x. [DOI] [PubMed] [Google Scholar]
- 25.Schluesener HJ, Seid K, Kretzschmar J, Meyermann R. Allograft-inflammatory factor-1 in rat experimental autoimmune encephalomyelitis, neuritis, and uveitis: expression by activated macrophages and microglial cells. Glia. 1998;24:244–251. doi: 10.1002/(sici)1098-1136(199810)24:2<244::aid-glia9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Davis EJ, Foster TD, Thomas WE. Cellular forms and functions of brain microglia. Brain Res Bull. 1994;34:73–78. doi: 10.1016/0361-9230(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 27.Yoichi K. Activated and phagocytic microglia. In: Walz W, editor. Cerebral ischemia: molecular and cellular pathophysiology. New Jersey: Humana Press; 1999. pp. 251–271. [Google Scholar]
- 28.Dong HW. The Allen reference atlas: a digital color brain atlas of the C57BL/6J male mouse. New York: Wiley; 2008. [Google Scholar]
- 29.Ladeby R, Wirenfeldta M, Garcia-Ovejerob D, Fengera C, Dissing-Olesena L, Dalmaua I, Finsena B. Microglial cell population dynamics in the injured adult central nervous system. Brain Res Rev. 2005;48:196–206. doi: 10.1016/j.brainresrev.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl.) 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- 31.McLaughlin JP, Ganno ML, Eans SO, Mizrachi E, Paris JJ. HIV-1 Tat protein exposure potentiates ethanol reward and reinstates extinguished ethanol-conditioned place preference. Curr HIV Res. 2015;12(6):415–423. doi: 10.2174/1570162x1206150311160133. [DOI] [PubMed] [Google Scholar]
- 32.Paris JJ, Carey AN, Shay CF, Gomes SM, He JJ, McLaughlin JP. Effects of conditional central expression of HIV-1 Tat protein to potentiate cocaine-mediated psychostimulation and reward among male mice. Neuropsychopharmacology. 2014;39:380–388. doi: 10.1038/npp.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paris JJ, Fenwick J, McLaughlin JP. Estrous Cycle and HIV-1 Tat Protein Influence Cocaine-Conditioned Place Preference and Induced Locomotion of Female Mice. Curr HIV Res. 2015;12(6):388–396. doi: 10.2174/1570162x13666150121105221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou W, Kim BO, Zhou BY, Liu Y, Messing A, He JJ. Protection against human immunodeficiency virus type 1 Tat neurotoxicity by ginkgo biloba extract EGb 761 involving glial fibrillary acidic protein. Am J Pathol. 2007;171:1923–1935. doi: 10.2353/ajpath.2007.070333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Geurin T, Chauhan A, Reid R, Xu R, Nath A, Knapp PE, Hauser KF. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia. 2008;56:1414–1427. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marker DF, Tremblay ME, Lu SM, Majewska AK, Gelbard HA. A thin-skull window technique for chronic two-photon in vivo imaging of murine microglia in models of neuroinflammation. J Vis Exp. 2010;43:pii, 2059. doi: 10.3791/2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu SM, Tremblay MÈ, King IL, Qi J, Reynolds HM, Marker DF, Varrone JJ, Majewska AK, Dewhurst S, Gelbard HA. HIV-1 Tat-induced microgliosis and synaptic damage via interactions between peripheral and central myeloid cells. PLoS One. 2011;6:e23915. doi: 10.1371/journal.pone.0023915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flora G, Pu H, Hennig B, Toborek M. Cyclooxygenase-2 is involved in HIV-1 Tat-induced inflammatory responses in the brain. Neuromolecular Med. 2006;8:337–352. doi: 10.1385/NMM:8:3:337. [DOI] [PubMed] [Google Scholar]
- 39.Rao JS, Kim HW, Kellom M, Greenstein D, Chen M, Kraft AD, Harry GJ, Rapoport SI, Basselin M. Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in brain of HIV-1 transgenic rats. J Neuroinflammation. 2011;8:101. doi: 10.1186/1742-2094-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Mediouni S, Jablonski J, Paris JJ, Clementz MA, Thenin-Houssier S, McLaughlin JP, Valente ST. Didehydro-Cortistatin A Inhibits HIV-1 Tat Mediated Neuroinflammation and Prevents Potentiation of Cocaine Reward in Tat Transgenic Mice. Curr HIV Res. 2015;13(1):64–79. doi: 10.2174/1570162x13666150121111548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Paiva VN, Lima SN, Fernandes MM, Soncini R, Andrade CA, Giusti-Paiva A. Prostaglandins mediate depressive-like behaviour induced by endotoxin in mice. Behav Brain Res. 2010;215:146–151. doi: 10.1016/j.bbr.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 42.Frau R, Bini V, Pes R, Pillolla G, Saba P, Devoto P, Bortolato M. Inhibition of 17α-hydroxylase/C17,20 lyase reduces gating deficits consequent to dopaminergic activation. Psychoneuroendocrinology. 2014;39:204–213. doi: 10.1016/j.psyneuen.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yee BK. Cytotoxic lesion of the medial prefrontal cortex abolishes the partial reinforcement extinction effect, attenuates prepulse inhibition of the acoustic startle reflex and induces transient hyperlocomotion, while sparing spontaneous object recognition memory in the rat. Neuroscience. 2000;95:675–689. doi: 10.1016/s0306-4522(99)00441-8. [DOI] [PubMed] [Google Scholar]
- 44.Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, Fox MA, Su J, Medina AE, Krahe TE, Knapp PE, Guido W, Hauser KF. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry. 2013;73:443–453. doi: 10.1016/j.biopsych.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roscoe RF, Jr, Mactutus CF, Booze RM. HIV-1 Transgenic Female Rat: Synaptodendritic Alterations of Medium Spiny Neurons in the Nucleus Accumbens. J Neuroimmune Pharmacol. 2014;9:642–653. doi: 10.1007/s11481-014-9555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ralph-Williams RJ, Lehmann-Masten V, Otero-Corchon V, Low MJ, Geyer MA. Differential effects of direct and indirect dopamine agonists on prepulse inhibition: a study in D1 and D2 receptor knock-out mice. J Neurosci. 2002;22:9604–9611. doi: 10.1523/JNEUROSCI.22-21-09604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu J, Mactutus CF, Wallace DR, Booze RM. HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: Dissociation of [3H]dopamine uptake and [3H]2β-carbomethoxy-3-β-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J Pharmacol Exp Ther. 2009;329:1071–1083. doi: 10.1124/jpet.108.150144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. Hyperdopaminergic tone in HIV-1 protein treated rats and cocaine sensitization. J Neurochem. 2010;115:885–896. doi: 10.1111/j.1471-4159.2010.06968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Midde NM, Huang X, Gomez AM, Booze RM, Zhan CG, Zhu J. Mutation of tyrosine 470 of human dopamine transporter is critical for HIV-1 Tat-induced inhibition of dopamine transport and transporter conformational transitions. J Neuroimmune Pharmacol. 2013;8:975–987. doi: 10.1007/s11481-013-9464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal gp120 injection: an examination early in development. Neurotoxicology. 2007;28:101–107. doi: 10.1016/j.neuro.2006.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal glycoprotein 120 injection: the role of dopaminergic alterations in prepulse inhibition in adult rats. J Pharmacol Exp Ther. 2006;318:1352–1358. doi: 10.1124/jpet.106.105742. [DOI] [PubMed] [Google Scholar]
- 52.Henry BL, Geyer MA, Buell MR, Perry W, Young JW, Minassian A Translational Methamphetamine AIDS Research Center (TMARC) Group. Prepulse inhibition in HIV-1 gp120 transgenic mice after withdrawal from chronic methamphetamine. Behav Pharmacol. 2014;25:12–22. doi: 10.1097/FBP.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaudhry K, Rogers R, Guo M, Lai Q, Goel G, Liebelt B, Ji X, Curry A, Carranza A, Jimenez DF, Ding Y. Matrix metalloproteinase-9 (MMP-9) expression and extracellular signal-regulated kinase 1 and 2 (ERK1/2) activation in exercise-reduced neuronal apoptosis after stroke. Neurosci Lett. 2010;474:109–114. doi: 10.1016/j.neulet.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 54.Chen W, Hartman R, Ayer R, Marcantonio S, Kamper J, Tang J, Zhang JH. Matrix metalloproteinases inhibition provides neuroprotection against hypoxia-ischemia in the developing brain. J Neurochem. 2009;111:726–736. doi: 10.1111/j.1471-4159.2009.06362.x. [DOI] [PubMed] [Google Scholar]
- 55.Zhu F, Liu Y, Zheng Y, Zhao J. Minocycline alleviates behavioral deficits and inhibits microglial activation induced by intrahippocampal administration of granulocyte-macrophage colony-stimulating factor in adult rats. Neuroscience. 2014;266:275–281. doi: 10.1016/j.neuroscience.2014.01.021. [DOI] [PubMed] [Google Scholar]