Abstract
Objective
To test whether genotype of the serotonin transporter-linked polymorphic region (5HTTLPR) and atypical attachment interact to predict externalizing psychopathology prospectively in a sample of children with a history of early institutional care.
Methods
Caregiver report of externalizing behavior at 54 months was examined in 105 children initially reared in institutional care and enrolled in the Bucharest Early Intervention Project, a randomized controlled trial of high quality foster care. 5HTTLPR genotype, attachment status at 42 months of age (typical [secure, avoidant, or ambivalent] or atypical [disorganized-controlling, insecure-other]), as well as their interaction, were examined as predictors of externalizing behavior at age 54 months.
Results
5HTTLPR genotype and atypical attachment at age 42 months interacted to predict externalizing behavior at age 54 months. Specifically, children with the s/s genotype with an atypical attachment had the highest externalizing scores. However, s/s children with a typical attachment demonstrated the lowest externalizing scores, even after controlling for intervention group status. There was no association between attachment status and externalizing behavior among children carrying at least one copy of the l allele.
Discussion
These findings indicate that genetic variation in the serotonergic system moderates the association between atypical attachment status and externalizing in young children. Our findings suggest that children, as a result of genetic variability in the serotonergic system, demonstrate differential sensitivity to the attachment relationship.
Keywords: Differential sensitivity, institutionalization, 5HTTLPR, externalizing
The serotonin transporter (5HTT, SLC6A4) is a key regulator of serotonin signaling1. Within the promoter of this gene is one of the most studied polymorphic variants in psychiatric genetics, the serotonin transporter-linked polymorphic region (5HTTLPR), beginning with a report of gene × environment interaction in which individuals with one or two copies of the short (s) allele had greater symptoms of depression following life stress than their counterparts with two long (l) alleles2. Although these findings have been replicated3, a number of failed replication studies involving the 5HTTLPR gene exist4 and the diathesis-stress model for the 5HTTLPR genotype has been challenged5. A recent meta-analysis of 77 gene × environment studies of the 5HTTLPR genotype indicated that the short (s) allele was associated with differential responsiveness to the environmental context6. Specifically, allelic variability across individuals for the 5HTTLPR have been found to result in both increased vulnerability to negative environments and increased resilience or benefit in positive environments.
Three previous studies have examined the association between the 5HTTLPR genotype and early care in institutional settings with child outcomes7–9. In all three studies children with the s/s genotype appeared to demonstrate greater sensitivity to the environment. Kumsta et al.9 found that s/s children adopted from Romanian institutions who experienced no adverse life events between ages 11 and 15 appeared to preferentially benefit from the high quality caregiving received in their adoptive homes. These children had lower levels of attention-deficit/hyperactivity disorder than their counterparts with the same early caregiving experiences and genotype who experienced adverse life events between ages 11 and 15. In two studies from the Bucharest Early Intervention Project (BEIP), a randomized controlled trial of foster care for institutionalized youth, children with the s/s genotype appeared to have the greatest sensitivity to the environment. In the first study from the BEIP cohort, the s/s genotype, in combination with the met allele of Brain Derived Neurotrophic Factor (BDNF), predicted differential sensitivity in longitudinal changes in indiscriminate behavior depending on randomization to the high quality foster care8. In the second study, Brett et al.7 found that the effect of the intervention on subsequent externalizing psychopathology was moderated by the 5HTTLPR genotype. Genotype was not associated with externalizing behavior at baseline (mean age 22 months). Among the l carriers, the intervention did not differentially predict later externalizing from 30 to 54 months. Among children with the s/s genotype, a trajectory of increased externalizing scores was found for those children randomized to the care as usual group. However, over time decreased externalizing scores were found for those s/s individuals randomized to the foster care group. These results suggest that the s/s genotype confers increased sensitivity, to environmental experiences, particularly caregiving. In addition to caregiving being linked to externalizing, variations in caregiving were linked to caregiver—child attachment10.
Considerable evidence exists linking externalizing psychopathology and attachment, frequently characterized by either dimensional measures of attachment security or using categorical approaches that typically compare disorganized attachment to secure attachment11,12. Meta-analytic work of 34 studies, comprising of a total of 3,778 participants, found a significant moderate association (Cohen’s d = .34) between disorganized attachment and externalizing behavior. Among risk groups, including preschool age children recruited following disclosure of sexual abuse, disorganized attachment was associated with 4.61 increased odds of clinically significant externalizing problems13. In another risk group, boys with disorganized attachments demonstrated increases in externalizing behavior longitudinally14. Providing further support for the link between attachment and externalizing psychopathology, an attachment-based intervention study resulted in decreases in child externalizing behavior among families with high levels of stress15. In the BEIP, improvements in attachment were a key outcome of the high quality foster care intervention16, and security of attachment mediated the reduction in psychiatric symptomatology17.
Previously several studies in high-risk children have examined the interaction of the 5HTTLPR and attachment18. In a small study of 37 Ukrainian preschoolers, children carrying the s allele with a history of early institutional care were more likely to exhibit disorganized attachment, while those with the l/l genotype appeared to be less impacted by the negative early caregiving experience. In a study of 91 adolescent youth, Zimmerman et al.19 found that youth with the s/s genotype with insecure parental attachment demonstrated increased aggression, while youth with the s/s genotype and secure parental attachment demonstrated more agreeable autonomy. Lastly, in a longitudinal study of 89 young children, attachment security interacted with 5HTTLPR genotype to predict self-regulatory abilities20, a critical factor contributing to externalizing behavior. Within the BEIP, attachment has not previously been considered in the association between 5HTTLPR genotype and externalizing psychopathology.
Data from the BEIP revealed that foster care intervention was not predictive of externalizing outcomes at 54 months, despite intervention effects for the internalizing symptom domain. It may be that differential sensitivity associated with allelic variation in 5HTTLPR may mask the direct effects of both intervention and candidate genes21. Hence, we sought to examine the interaction of 5HTTLPR genotype and attachment with the prospective prediction of externalizing behavior within the BEIP study22. Attachment was categorized as typical (i.e., secure, avoidant, and ambivalent) versus atypical (i.e., disorganized-controlling and insecure-other), following the Smyke et al.16 approach. This common approach was utilized given the research linking disorganized and other atypical attachment classifications to psychopathology16,23,24.
We hypothesized that children with the s/s genotype and a typical attachment would demonstrate the lowest externalizing symptoms, whereas those with the same genotype and an atypical attachment would have the highest symptoms among the entire sample. Additionally, given previous findings of genetic differential sensitivity to the BEIP foster care intervention7 we sought to examine whether attachment status interacted with the intervention, as well as isolate the relative predictive power of both attachment and the randomized group status on externalizing symptoms at age 54 months.
Methods
Participants
Participants were enrolled in the BEIP22, with inclusion and exclusion criteria described elsewhere21,25. Participants included 136 abandoned children living in institutions in Bucharest Romania and at the time of initial assessment between 6 and 30 months of age. Following baseline assessments, 68 of the children (33 males and 35 females) were randomly assigned to the care as usual group (CAUG) and 68 (34 males and 34 females) to the foster care group (FCG). Children were excluded from the study for medical reasons including diagnosed genetic syndromes, significant evidence of fetal alcohol syndrome or microcephaly. The foster care network was created and supported by the project as an intentional alternative to institutional care26. Detail about the ethical issues and study design are described elsewhere22,27–29.
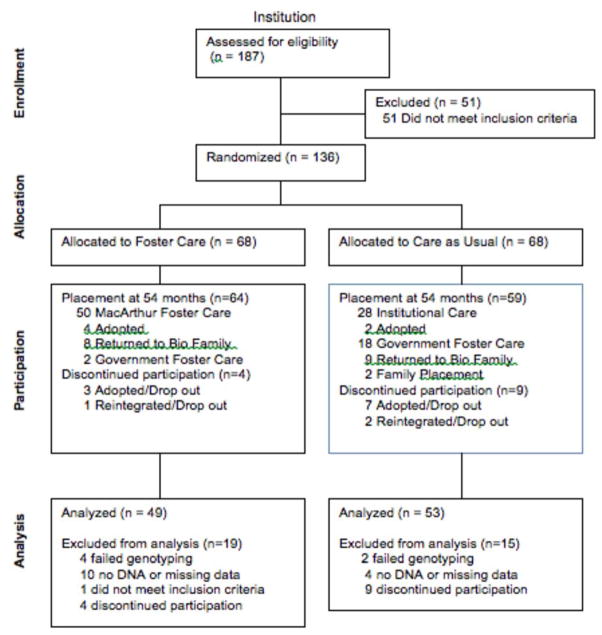
Following randomization, all subsequent decisions regarding placement were made by local child protection authorities in accordance with Romanian law. Figure 1 depicts placement at 54 months and flow of participants, including reasons participants were not included in final sample. All analyses that include intervention group follow an intent-to-treat approach, so that children are analyzed within their originally assigned group regardless of placement at 54 months of age as this is expected to provide the most stringent statistical test of the intervention. Psychopathology data and genotype data were available for 102 children, as described elsewhere7.
Figure 1.
Consort Diagram
Measures
Externalizing Behavior
Baseline
The Infant Toddler Social Emotional Assessment (ITSEA)30 is a 195-item caregiver report questionnaire used to assess problem behaviors and competencies in children greater than 12 months of age. The ITSEA was administered to caregivers/parents at the baseline assessment. For the present study, we used the externalizing domain score, which comprises activity/impulsivity, aggression/defiance, and peer aggression. ITSEA responses were obtained from foster mothers for children in foster care. For children in institutions, an institutional caregiver, who was considered the child’s favorite caregiver by staff consensus, or, if the child had no favorite, a caregiver who worked with the child regularly, reported on the child’s behavior at each time point.
54 months of age
Psychiatric morbidity at 54 months of age was assessed using the Preschool Age Psychiatric Assessment (PAPA)31. The PAPA is a semi-structured psychiatric interview assessing caregiver report of child behavior and emotional scores. Test–retest reliability of the PAPA is comparable to structured psychiatric interviews used to assess older children and adults. The PAPA 1.3 was translated into Romanian, back-translated into English, and assessed for meaning at each step by bilingual research staff. The BEIP lead interviewer was trained in administration of the PAPA by the group who developed the measure, and he subsequently trained other Romanian interviewers. Selection of the reporter was as described for the ITSEA. The reliability of the PAPA in this population is described in detail elsewhere21. For this study, total externalizing score, a composite measure of symptoms of conduct disorder, oppositional defiant disorder, and attention-deficit/hyperactivity disorder, was utilized.
Attachment
Attachment quality was assessed at 42 months of age16 using the Strange Situation Procedure32 and coded using the MacArthur Preschool system33. Coders, blind to group status, were trained to reliability in the MacArthur Preschool System to determine presence or absence of a typical attachment, a classification used by our group in previous work16. Typical attachment (lower risk) included those with a secure, avoidant, or ambivalent attachment classification. Atypical attachment (higher risk) included those with a disorganized-controlling or insecure-other attachment classification. Institutionalized children were assessed with their “favorite” caregiver who worked regularly with the child and knew the child well.
Genotyping
DNA was extracted from MasterAmp buccal swabs using Epicentre Biotechnologies MasterAmp DNA extraction solution following manufacturer’s recommendations. 5HTTLPR (rs4795541) allele status was determined using standard PCR methods and gel electrophoresis with careful attention to magnesium concentrations. Variation of Mg levels from 1mM to 2mM did not result in genotype differences as has been previously demonstrated in other studies34. All samples were run in triplicate, with known controls. Genotype was tested to confirm Hardy-Weinberg Equilibrium (p = 0.96, N=102). 5HTTLPR genotype frequencies were .47 for the s allele and .53 for the l allele. Genotype was not determined on 6 individuals for whom DNA was obtained (3 CAUG, 3 FCG). No significant differences in group, ethnicity, or externalizing scores were found between those with and without genotype or DNA.
Human Subjects
The study was approved by Institutional Review Boards at Tulane University, Boston Children’s Hospital/Harvard Medical School, University of Maryland, and by the local commissions on child protection in each sector of Bucharest. The study also was reviewed in 2002 by an ad hoc ethics commission appointed by the Romanian government22,27–29.
Data Analysis
Bivariate associations examined associations between genotype and group, demographic characteristics (i.e., sex and ethnicity), and attachment status at 42 months.
Univariate ANCOVAs were used to examine the effect of 5HTTLPR genotype, atypical attachment, and their interaction on externalizing at 54 months, statistically controlling for sex, ethnicity, and baseline externalizing behaviors.
Results
A total of 102 individuals provided both genetic data and externalizing scores for at least one time point (i.e., baseline or 54 months). 5HTTLPR genotype was unrelated to intervention group, demographic variables (including sex and ethnicity), and baseline externalizing (Table 1). Though not statistically significant, those with the s/s genotype were most likely to have a typical attachment, followed by those with the s/l genotype, while those with the l/l genotype had the lowest rates of typical attachment.
Table 1.
Demographics by 5HTTLPR genotype.
| s/s (n = 22) | s/l (n = 51) | l/l (n = 29) | χ2 or F | p | |
|---|---|---|---|---|---|
| Sex (% Male) | 55% | 63% | 38% | 4.59 | .10 |
| Ethnicity (% Romanian) | 46% | 51% | 55% | 1.66 | .80 |
| Attachment 42 months (% Typical) | 82% | 69% | 61% | 2.60 | .27 |
| Externalizing scores at baseline, M (SD) | 0.51 (0.49) | 0.60 (0.39) | 0.61 (0.36) | 0.37 | .69 |
| Externalizing scores at 54 months, M (SD) | 7.09 (7.76) | 8.48 (7.15) | 7.61 (5.59) | 0.35 | .70 |
Intervention group was associated with attachment status at 42 months (χ2(101) = 5.18, p = .02), where children randomly assigned to the FCG intervention at baseline were significantly more likely to have a typical attachment (79%) than those in the CAUG (58%)16.
5HTTLPR individual genotype analysis (s/s vs. s/l vs. l/l)
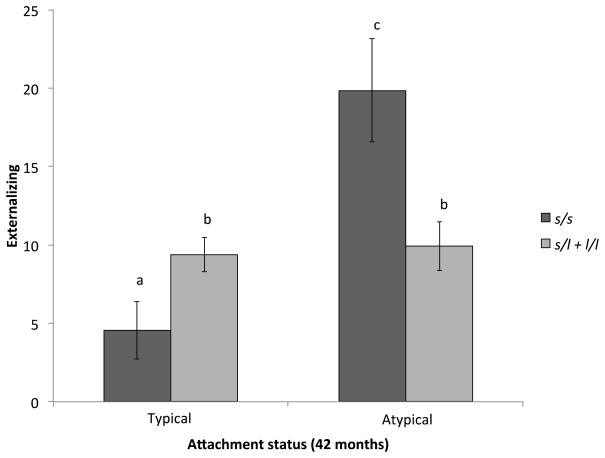
There was no main effect of 5HTTLPR genotype on externalizing scores at 54 months (s/s 7.50 [1.69], s/l 8.53 [1.08], and l/l 8.24 [1.41], p = .87). The main effect of attachment at 42 months on externalizing at 54 months was also not statistically significant, as children with typical and atypical attachment classifications had comparable levels of externalizing behaviors (7.71 [0.94] vs. 9.41 [1.40], p = .33). However, a significant genotype (s/s vs. s/l vs. l/l) × attachment interaction was found (F(2,73) = 6.28, p = .003) (Figure 2). Post hoc tests conducted within genotype indicated that there was no effect of attachment status within individuals with the l/l (F(1,18) = 1.02, p = .33) or s/l group (F(1,37) = 0.00, p = .99). The effect was driven by s/s individuals, where attachment status strongly predicted externalizing scores at 54 months (F(1,12) = 17.27, p = .001). Individuals with the s/s genotype with a typical attachment strategy had significantly lower externalizing symptoms (3.99 [1.72]) than s/s individuals with an atypical attachment (20.49 [3.46]), which produced a very large effect (Cohen’s d = 2.92).
Figure 2.
Externalizing scores at 54 months by attachment status and 5HTTLPR genotype (s/s vs. s/l and l/l). Columns with the same letter do not significantly differ from one another using Least Significant Difference post hoc test (p > .05).
Post hoc analyses were conducted to examine the effect of 5HTTLPR genotype on externalizing scores at 54 months based on children’s attachment status at 42 months. Genotype was not a significant predictor of externalizing among child with a typical attachment (F(2,49) = 2.03, p = .14). However, a significant effect of genotype was found among children with an atypical attachment (F(2,21) = 4.20, p = .03). Those with the s/s genotype had significantly higher externalizing scores at 54 months (18.04 [3.39] than both the s/l (9.21 [2.04]) and l/l (6.70 [1.96]) groups (ps < .02). These findings remained significant with baseline externalizing omitted as a covariate. The findings also remained statistically significant after controlling for intervention group status (i.e., CAUG vs. FCG).
5HTTLPR grouped genotype analysis (s/s vs. s/l and l/l)
Our initial findings indicate the effects are driven by individuals with the s/s genotype, thus, we examined externalizing scores collapsing across genotypes with any l alleles to test directly whether s/s individuals significantly differed from those with either the s/l or l/l genotype. There was a significant genotype (s/s vs. s/l and l/l) × attachment interaction was found (F(1,75) = 12.01, p < .001) (Figure 2). Again, the effect was driven by differences in the s/s genotype based on typical attachment status. Thus, the findings were essentially unchanged using the grouped genotype approach.
Intervention Group, Attachment Status, and 5HTTLPR Genotype on Externalizing
Next, hierarchical multiple regression models were used to test the relative predictive value of intervention group and attachment status in their interaction with genotype (see Table 2). In Model 1, sex, ethnicity, and baseline externalizing scores were entered in Step 1, intervention group, attachment status, and grouped genotype (s/s vs. s/l and l/l) were entered in Step 2, the genotype × attachment status interaction was entered in Step 3, and the genotype × intervention group interaction was entered in Step 4. Steps 1, 3, and 4 all resulted in significant increases in the variance explained in externalizing scores at 54 months. The final step, which included the intervention × genotype interaction, predicted additional variance in externalizing at 54 months above the effects in the prior steps (ΔR2 = .05, p = .035), indicating that the addition of the intervention × genotype interaction provides additional predictive power.
Table 2.
Summary table of the hierarchical multiple regression model for intervention group, attachment status, and 5HTTLPR genotype predicting externalizing at 54 months.
| Model 1 | Model 2 | ||||
|---|---|---|---|---|---|
| Predictor | ΔR2 | β | Predictor | ΔR2 | β |
| Step 1 | .10* | Step 1 | .10* | ||
| Covariatesa | Covariatesa | ||||
| Step 2 | .01 | Step 2 | .01 | ||
| Intervention group | .03 | Intervention group | .03 | ||
| Attachment status | .12 | Attachment status | .12 | ||
| 5HTTLPR genotype | −.05 | 5HTTLPR genotype | −.05 | ||
| Step 3 | .13*** | Step 3 | .14*** | ||
| Attachment status × | .46*** | Intervention group × | - | ||
| Genotype | Genotype | .73*** | |||
| Step 4 | .05* | Step 4 | .04 | ||
| Intervention group × | −.49* | Attachment status × | .30* | ||
| Genotype | Genotype | ||||
| Total R2 | .29*** | Total R2 | .29*** | ||
Note.
Covariates included age, ethnicity, and baseline externalizing.
p = .051.
p < .05.
p < .001.
In Model 2, the analysis was repeated but steps 3 and 4 were reversed to examine the effect of the 5HTTLPR genotype × attachment interaction over and above the effects of prior steps in the model. Again, steps 1, 3, and 4 were significant predictors in the model. In Step 4, the attachment status × genotype interaction was a significant predictor of the variance in externalizing at 54 months over and above the effects of the prior steps (ΔR2 = .04, p = .049).
Discussion
The present study demonstrated an interaction between 5HTTLPR genotype and atypical attachment in the prediction of externalizing behavior at age 54 months in young children with a history of early care in institutional settings. Specifically, an atypical attachment at 42 months, in combination with the s/s genotype, predicted the highest levels of externalizing symptoms, while those with this same genotype, with a typical attachment, had the lowest externalizing scores over and above any effect of intervention group status. Individuals with the s/l and l/l genotypes had externalizing symptoms that fell at the intermediate level and did not significantly vary based on attachment status nor intervention group status, consistent with our previous findings of differential sensitivity in s/s individuals7. These findings extend our understanding of one pathway by which attachment predicts externalizing psychopathology following early adversity.
Prior research has indicated that both genetic and environmental factors, but particularly their interaction, play a role in the etiology of externalizing disorders35. At the baseline assessment, when children were on average age 22 months, there were no significant intervention group or genotype differences in externalizing. The absence of a direct genetic on externalizing symptoms is consistent with differential susceptibility theory5. The failure to detect a main effect of genotype on attachment, while at the surface appearing inconsistent with previous work, offers perhaps some insight into conflicting findings as to which allele predisposes a child toward secure attachment, the l or the s18,36,37. Importantly, the prospective association of typical attachment and later externalizing symptoms in the s/s group was still detected even after controlling for intervention group status. These results indicate that both the effects of intervention group and attachment status are moderated by genotype, and that both interactions independently predict meaningful differences in externalizing scores at 54 months.
These results complement a recent study of the 5HTTLPR exploring the moderating effect of attachment security on adolescent aggressiveness19. Specifically, adolescents with the s/s genotype with secure attachment demonstrated low levels of aggression, however children with the s/s who were insecurely attached were reported as being more aggressive and demonstrated more hostile behavior. Thus, rather than finding simple main effects of 5HTTLPR genotype on externalizing outcomes, there is converging evidence that attachment interacts with genetic variation to predict this form of psychopathology.
As a result of the mixed literature regarding the association between attachment and later externalizing there is increasing interest in studies exploring the moderation of attachment by genetic variants on a range of other outcomes18,19,36. Specifically, although no association was found in a large study between disorganized attachment in infancy with externalizing measured in preschool and high school38, other work has found prospective associations between infant attachment and childhood externalizing problems39. The present finding of differential sensitivity provides novel insight into these previous discrepant results. Significant differences in externalizing in children with the s/s genotype indicate genetic differential sensitivity to attachment, such that for the s/s children, attachment is likely important in the development of externalizing symptomatology, while for those children with the l allele attachment may have less influence. Further, these findings extend our previous study linking intervention group, 5HTTLPR, and externalizing. We have now shown that in s/s children, intervention group status moderated externalizing, above and beyond the effect of attachment × genotype interaction. Attachment status was unrelated to externalizing outcomes for individuals without the s/s genotype. This absence of significant differences in externalizing in children with the l allele (e.g., s/l and l/l) provides further support of the differential susceptibility model40, and is in concert with recent non-human animal work indicating that the low expressing version of the 5-HTT gene may promote phenotypic plasticity based on environmental experience41.
These findings should be considered in light of a number of limitations. We were limited in available sample size. Additionally, the consistency with other work19 further bolsters the present studies findings. Nevertheless, replication is warranted, particularly in other samples of children who have and have not experienced early environmental adversity, in order to determine the generalizability of the findings. We purposely selected one genetic variant, the well-studied 5HTTLPR genotype, for our candidate gene approach in studying differential susceptibility in the prediction of externalizing symptoms. However, the etiology of any domain of psychopathology is complex and likely involves many different genes as well as gene × gene interactions. Indeed, the dopaminergic system has relevance to externalizing psychopathology and has also been found to interact with caregiving the prediction of this construct42–44. Thus, in elucidating the complex etiological components to the development of externalizing problems, multiple genes will need to be considered.
In conclusion, this study provides evidence that attachment interacts with 5HTTLPR genotype consistent with differential sensitivity. Those individuals with the s/s genotype were most likely to benefit from the development of a typical attachment at 42 months, as evidence by lower externalizing symptoms at age 54 months. Conversely, individuals with the s/s genotype were also most sensitive to the negative consequences of an atypical attachment, given the higher levels of externalizing found in this population. These findings underscore the importance of developing typical attachments in early life, the complex nature of early social emotional development in children that encompasses both genetic and environmental components, and the interaction between these factors, in the development of psychopathology in young children.
Acknowledgments
Funding for this study was provided by the National Institutes of Health (MH091363 to CAN), the John D. and Catherine T. MacArthur Foundation (to CAN), and a NARSAD Young Investigator Award (to SSD) and R21 MH094688 (to SSD).
Footnotes
There are no author conflicts of interest.
References
- 1.Heils A, Teufel A, Petri S, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 2.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 3.Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–454. doi: 10.1016/j.ypsy.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastiaansen JA, Servaas MN, Marsman JBC, et al. Filling the Gap: Relationship Between the Serotonin-Transporter-Linked Polymorphic Region and Amygdala Activation. Psychol Sci. 2014 doi: 10.1177/0956797614548877. [DOI] [PubMed] [Google Scholar]
- 5.Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- 6.van IJzendoorn MH, Belsky J, Bakermans-Kranenburg MJ. Serotonin transporter genotype 5HTTLPR as a marker of differential susceptibility? A meta-analysis of child and adolescent gene-by-environment studies. Transl Psychiatry. 2012;2:e147. doi: 10.1038/tp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brett ZH, Humphreys KL, Smyke AT, et al. 5HTTLPR genotype moderates the longitudinal impact of early caregiving on externalizing behavior. Dev Psychopathol. 2014 doi: 10.1017/S0954579414001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drury SS, Gleason MM, Theall KP, et al. Genetic sensitivity to the caregiving context: The influence of 5httlpr and BDNF val66met on indiscriminate social behavior. Physiol Behav. 2012;106:728–735. doi: 10.1016/j.physbeh.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumsta R, Stevens S, Brookes K, et al. 5HTT genotype moderates the influence of early institutional deprivation on emotional problems in adolescence: Evidence from the English and Romanian Adoptee (ERA) study. J Child Psychol Psychiatry Allied Discip. 2010;51:755–762. doi: 10.1111/j.1469-7610.2010.02249.x. [DOI] [PubMed] [Google Scholar]
- 10.NICHD Early Care Research Network. Infant-mother attachment classification: risk and protection in relation to changing maternal caregiving quality. Dev Psychol. 2006;42(1):38–58. doi: 10.1037/0012-1649.42.1.38. [DOI] [PubMed] [Google Scholar]
- 11.Lyons-Ruth K, Easterbrooks MA, Cibelli CD. Infant attachment strategies, infant mental lag, and maternal depressive symptoms: predictors of internalizing and externalizing problems at age 7. Dev Psychol. 1997;33:681–692. doi: 10.1037/0012-1649.33.4.681. [DOI] [PubMed] [Google Scholar]
- 12.Bohlin G, Eninger L, Brocki KC, Thorell LB. Disorganized attachment and inhibitory capacity: Predicting externalizing problem behaviors. J Abnorm Child Psychol. 2012;40:449–458. doi: 10.1007/s10802-011-9574-7. [DOI] [PubMed] [Google Scholar]
- 13.Beaudoin G, Hébert M, Bernier A. Contribution of attachment security to the prediction of internalizing and externalizing behavior problems in preschoolers victims of sexual abuse. Rev Eur Psychol Appliquée/European Rev Appl Psychol. 2013;63:147–157. doi: 10.1016/j.erap.2012.12.001. [DOI] [Google Scholar]
- 14.Pasco Fearon RM, Belsky J. Infant-mother attachment and the growth of externalizing problems across the primary-school years. J Child Psychol Psychiatry Allied Discip. 2011;52:782–791. doi: 10.1111/j.1469-7610.2010.02350.x. [DOI] [PubMed] [Google Scholar]
- 15.Van Zeijl J, Mesman J, Van IJzendoorn MH, et al. Attachment-based intervention for enhancing sensitive discipline in mothers of 1- to 3-year-old children at risk for externalizing behavior problems: a randomized controlled trial. J Consult Clin Psychol. 2006;74:994–1005. doi: 10.1037/0022-006X.74.6.994. [DOI] [PubMed] [Google Scholar]
- 16.Smyke AT, Zeanah CH, Fox NA, Nelson CA, Guthrie D. Placement in foster care enhances quality of attachment among young institutionalized children. Child Dev. 2010;81(1):212–23. doi: 10.1111/j.1467-8624.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGoron L, Gleason MM, Smyke AT, et al. Recovering from early deprivation: Attachment mediates effects of caregiving on psychopathology. J Am Acad Child Adolesc Psychiatry. 2012;51:683–693. doi: 10.1016/j.jaac.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakermans-Kranenburg MJ, Dobrova-Krol N, van IJzendoorn M. Impact of institutional care on attachment disorganization and insecurity of Ukrainian preschoolers: Protective effect of the long variant of the serotonin transporter gene (5HTT) Int J Behav Dev. 2012;36:11–18. doi: 10.1177/0165025411406858. [DOI] [Google Scholar]
- 19.Zimmermann P, Mohr C, Spangler G. Genetic and attachment influences on adolescents’ regulation of autonomy and aggressiveness. J Child Psychol Psychiatry Allied Discip. 2009;50:1339–1347. doi: 10.1111/j.1469-7610.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 20.Kochanska G, Philibert RA, Barry RA. Interplay of genes and early mother-child relationship in the development of self-regulation from toddler to preschool age. J Child Psychol Psychiatry Allied Discip. 2009;50:1331–1338. doi: 10.1111/j.1469-7610.2008.02050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeanah CH, Egger HL, Smyke AT, et al. Institutional rearing and psychiatric disorders in Romanian preschool children. Am J Psychiatry. 2009;166(7):777–85. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- 22.Zeanah CH, Nelson CA, Fox NA, et al. Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Dev Psychopathol. 2003;15:885–907. doi: 10.1017/S0954579403000452. [DOI] [PubMed] [Google Scholar]
- 23.Green J, Goldwyn R. Annotation: Attachment disorganisation and psychotherapy: New findings in attachment research and their potential implications for developmental psychopathology in childhood. J Child Psychol Psychiatry Allied Discip. 2002;43:835–846. doi: 10.1111/1469-7610.00102. [DOI] [PubMed] [Google Scholar]
- 24.van Ijzendoorn MH, Schuengel C, Bakermans-Kranenburg MJ. Disorganized attachment in early childhood: meta-analysis of precursors, concomitants, and sequelae. Dev Psychopathol. 1999;11:225–249. doi: 10.1017/S0954579499002035. [DOI] [PubMed] [Google Scholar]
- 25.Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science (80–) 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- 26.Smyke AT, Koga SF, Johnson DE, et al. The caregiving context in institution-reared and family-reared infants and toddlers in Romania. J Child Psychol Psychiatry. 2007;48:210–218. doi: 10.1111/j.1469-7610.2006.01694.x. [DOI] [PubMed] [Google Scholar]
- 27.Millum J, Emanuel EJ. Ethics. The ethics of international research with abandoned children. Science. 2007;318:1874–1875. doi: 10.1126/science.1153822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson CA, Fox NA, Zeanah CH. Romania’s Abandoned Children. Cambridge, MA: Harvard University Press; 2014. [Google Scholar]
- 29.Zeanah CH, Fox NA, Nelson CA. The Bucharest Early Intervention Project: case study in the ethics of mental health research. J Nerv Ment Dis. 2012;200:243–7. doi: 10.1097/NMD.0b013e318247d275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter AS, Briggs-Gowan MJ, Jones SM, Little TD. The Infant-Toddler Social and Emotional Assessment (ITSEA): Factor structure, reliability, and validity. J Abnorm Child Psychol. 2003;31:495–514. doi: 10.1023/A:1025449031360. [DOI] [PubMed] [Google Scholar]
- 31.Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A. Test-Retest Reliability of the Preschool Age Psychiatric Assessment (PAPA) J Am Acad Child Adolesc Psychiatry. 2006;45:538–549. doi: 10.1016/S0084-3970(08)70314-1. [DOI] [PubMed] [Google Scholar]
- 32.Ainsworth MDS, Blehar MC, Waters E, Wall S. Patterns of Attachment: A Psychological Study of the Strange Situation. 1978:xviii + 391. doi: 10.1093/jaarel/52.2.411-a. [DOI] [Google Scholar]
- 33.Cassidy J, Marvin RS the MacArthur Working Group. . Attachment organization in preschool children: Procedures and coding manual. 1992 [Google Scholar]
- 34.Yonan AL, Palmer AA, Gilliam TC. Hardy-Weinberg disequilibrium identified genotyping error of the serotonin transporter (SLC6A4) promoter polymorphism. Psychiatr Genet. 2006;16:31–34. doi: 10.1097/01.ypg.0000174393.79883.05. [DOI] [PubMed] [Google Scholar]
- 35.Hicks BM, South SC, Dirago AC, Iacono WG, McGue M. Environmental adversity and increasing genetic risk for externalizing disorders. Arch Gen Psychiatry. 2009;66:640–648. doi: 10.1001/archgenpsychiatry.2008.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spangler G, Johann M, Ronai Z, Zimmermann P. Genetic and environmental influence on attachment disorganization. J Child Psychol Psychiatry. 2009;50:952–961. doi: 10.1111/j.1469-7610.2008.02054.x. [DOI] [PubMed] [Google Scholar]
- 37.Luijk MPCM, Roisman GI, Haltigan JD, et al. Dopaminergic, serotonergic, and oxytonergic candidate genes associated with infant attachment security and disorganization? in search of main and interaction effects. J Child Psychol Psychiatry Allied Discip. 2011;52:1295–1307. doi: 10.1111/j.1469-7610.2011.02440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlson EA. A prospective longitudinal study of attachment disorganization/disorientation. Child Dev. 1998;69:1107–1128. doi: 10.2307/1132365. [DOI] [PubMed] [Google Scholar]
- 39.Munson JA, McMahon RJ, Spieker SJ. Structure and variability in the developmental trajectory of children’s externalizing problems: impact of infant attachment, maternal depressive symptomatology, and child sex. Dev Psychopathol. 2001;13:277–296. doi: 10.1017/S095457940100205X. [DOI] [PubMed] [Google Scholar]
- 40.Belsky J, Pluess M, Widaman KF. Confirmatory and competitive evaluation of alternative gene-environment interaction hypotheses. J Child Psychol Psychiatry Allied Discip. 2013;54:1135–1143. doi: 10.1111/jcpp.12075. [DOI] [PubMed] [Google Scholar]
- 41.Kästner N, Richter SH, Lesch KP, Schreiber RS, Kaiser S, Sachser N. Benefits of a “vulnerability gene”? A study in serotonin transporter knockout mice. Behav Brain Res. 2015;283:116–120. doi: 10.1016/j.bbr.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 42.Bakermans-Kranenburg MJ, van Ijzendoorn MH. Gene-environment interaction of the dopamine D4 receptor (DRD4) and observed maternal insensitivity predicting externalizing behavior in preschoolers. Dev Psychobiol. 2006;48:406–409. doi: 10.1002/dev.20152. [DOI] [PubMed] [Google Scholar]
- 43.Bakermans-Kranenburg MJ, Van IJzendoorn MH, Pijlman FTA, Mesman J, Juffer F. Experimental evidence for differential susceptibility: dopamine D4 receptor polymorphism (DRD4 VNTR) moderates intervention effects on toddlers’ externalizing behavior in a randomized controlled trial. Dev Psychol. 2008;44:293–300. doi: 10.1037/0012-1649.44.1.293. [DOI] [PubMed] [Google Scholar]
- 44.Marsman R, Oldehinkel AJ, Ormel J, Buitelaar JK. The dopamine receptor D4 gene and familial loading interact with perceived parenting in predicting externalizing behavior problems in early adolescence: The TRacking Adolescents’ Individual Lives Survey (TRAILS) Psychiatry Res. 2013;209:66–73. doi: 10.1016/j.psychres.2012.10.022. [DOI] [PubMed] [Google Scholar]