Abstract
Aims
Oral anticoagulation therapy prevents stroke and improves survival in patients with atrial fibrillation, but the therapy is underutilized. We sought to identify the prevalence of contraindications for oral anticoagulation and the proportion of patients potentially eligible for different agents.
Methods
We identified patients with nonacute atrial fibrillation in a nationally representative 5% sample of 2009 Medicare data. We divided the population into patients ineligible for any oral anticoagulant, patients eligible for warfarin only, and patients eligible for any anticoagulant. We compared patient characteristics and the use of anticoagulation among the subgroups.
Results
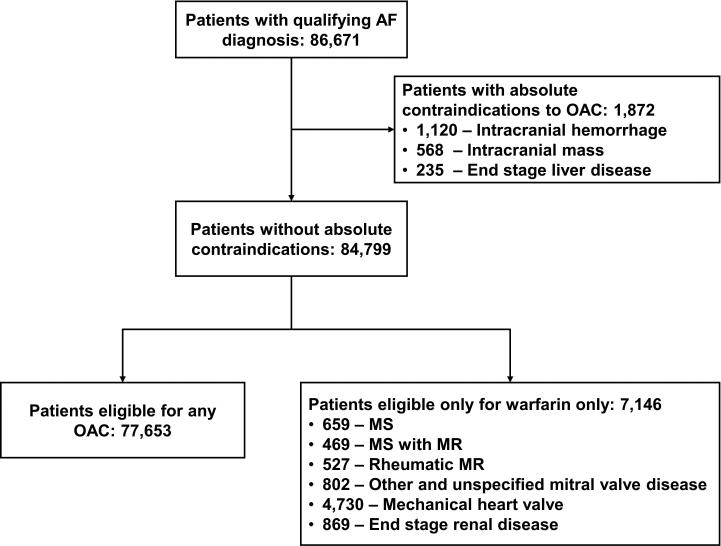
Among 86,671 patients with atrial fibrillation, 1872 (2.2%) were ineligible for anticoagulation because of an absolute contraindication, most frequently a history of intracranial hemorrhage (60%). Patients ineligible for any anticoagulant were the same age as the overall group (mean age, 80.5 vs 80.4 years). However, they had higher rates of dementia (19% vs 8.6%) and heart failure (59% vs 43%) and higher mean CHADS2 scores (3.8 vs 2.8). Of the remaining 84,799 patients eligible for anticoagulation, 7146 (8.4%) had were eligible for warfarin only (most commonly because of mechanical heart valves [66%] and end-stage renal disease [12%]). Sixty-five percent of patients eligible for anticoagulation received warfarin, and the proportion was similar for patients with a relatively high risk of bleeding.
Conclusions
Older adults with atrial fibrillation rarely have absolute contraindications to oral anticoagulation therapy. Among patients without contraindications, most appeared to be eligible for any anticoagulant, and relatively high-risk features appeared not to influence warfarin use.
Introduction
Treatment with oral anticoagulation has been demonstrated to significantly reduce stroke in patients with atrial fibrillation [1]. Guidelines recommend oral anticoagulation for patients with atrial fibrillation and additional risk factors for stroke [2,3]. However, historical data have shown underutilization of anticoagulation therapy, with approximately half of all patients with atrial fibrillation not receiving treatment [4-8]. Several reasons have been cited for undertreatment, including the prevalence of contraindications. However, contraindications to oral anticoagulation therapy are often relative and subject to provider interpretation. There remain few consistent, absolute contraindications to the use of anticoagulation therapy. In addition, oral vitamin K antagonist therapy, the historical mainstay of therapy, is a challenging treatment strategy, because the drugs require regular blood draws for monitoring and are associated with numerous drug and food interactions. Management challenges may be a factor in undertreatment. Several alternative agents have become available, yet it is not clear what proportion of patients is eligible for these drugs, which are approved for only a subset of patients with atrial fibrillation.
The objectives of the current study were to identify the proportion of older patients with atrial fibrillation who have absolute contraindications to oral anticoagulation; to identify the proportion of patients with atrial fibrillation who can only receive warfarin for anticoagulation; and to assess the use of anticoagulation therapy in eligible patients who have a relatively higher risk of bleeding.
Methods
Data Source
We obtained a nationally representative 5% sample of Medicare standard analytic files and corresponding denominator files from the US Centers for Medicare & Medicaid Services for 2009 through 2010. Inpatient files contain institutional claims for facility costs covered under Medicare Part A, and outpatient files contain claims from institutional outpatient providers. Carrier files contain noninstitutional provider claims for services covered under Medicare Part B. Denominator files contain beneficiary demographic characteristics and information about program eligibility and enrollment.
Study Populations
We defined a 2010 cohort of beneficiaries with prevalent atrial fibrillation based on claims diagnoses in 2009. To establish a diagnosis of nonacute atrial fibrillation that was unlikely to be due to a reversible cause, we required at least 2 diagnoses of atrial fibrillation (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 427.31) in any position on separate inpatient or outpatient claims at least 6 months apart. We required at least 1 outpatient diagnosis to establish that beneficiaries were treated as outpatients. We required that beneficiaires were 65 years or older, were living in the United States on January 1, 2010, and had continuous enrollment in fee-for-service Medicare in the prior calander year.
We defined 3 subpopulations of Medicare beneficiaries with prevalent atrial fibrillation: patients who were unlikely to be eligible for anticoagulation therapy because of absolute contraindications (“ineligible”); patients who were eligible for warfarin only because of absolute contraindications to novel anticoagulant use (“warfarin only”); and patients who were eligible for any oral anticoagulant. We searched Medicare inpatient, outpatient, and carrier claims files from 2009 for evidence of contraindications to anticoagulation therapy. We identified beneficiaries with absolute contraindications to any anticoagulation therapy based on diagnoses of intracranial hemorrhage (ICD-9-CM 430, 431, 432.x), intracranial mass (ICD-9-CM 191.x, 225.x, 239.6, 198.3), or end-stage liver disease using the algorithm from Goldberg et al [9] (see Supplemental Material). Patients with any contraindication to novel anticoagulant use were considered eligible for warfarin only. We defined these contraindications as valvular atrial fibrillation (ie, mitral stenosis [ICD-9-CM 394.0]; rheumatic mitral insufficiency [ICD-9-CM 394.1]; mitral stenosis with insufficiency [ICD-9-CM 394.2] or other and unspecified mitral valve diseases [ICD-9-CM 394.9]); presence of a mechanical valve (ICD-9-CM procedure codes 35.22 [10] or 35.24 or in situ diagnosis code V43.3); or end-stage renal disease (Medicare denominator file ESRD indicator, “Y”). Finally, the remaining patients not identified with contraindications were considered eligible for any anticoagulation therapy.
Patient Characteristics
Demographic characteristics included age, sex, race, and state of residence. We used self-reported race categories “black” and “white” and combined all other categories as “other” [11]. On the basis of state of residence, we grouped beneficiaries into 4 geographic regions. We identified comorbid conditions using well-validated coding algorithms [12,13] and searched all claims in 2009 for dementia, diabetes mellitus, ischemic heart disease, peripheral vascular disease, heart failure, cerebrovascular disease, hypertension, chronic obstructive pulmonary disease, renal disease, stroke or transient ischemic attack, cancer, and valvular heart disease. We also searched for evidence of atrial flutter (ICD-9-CM code 427.32). We used the approach of Gage et al [14] to define the CHADS2 score and Lip et al [15] to define the CHA2DS2-VASc score. While these scores were not developed for patients with Rheumatic disease or mechanical valve replacements, they are used to risk-stratify patients with non-valvular AF for anticoagulation and can be useful markers of stroke risk in an overall AF population. We identified the existence of a pacemaker or implantable cardioverter-defibrillator on the basis of Current Procedural Terminology (CPT) and ICD-9-CM codes for device in situ, implantation, revision, or monitoring.
In addition to absolute contraindications for anticoagulation therapies, we examined the prevalence of high-risk characteristics or relative contraindications that may influence anticoagulation patterns (hereafter, “relative high risk”). We considered advanced age (85 years or older) [16-18], and also searched the prior year Medicare claims for evidence of dementia, gastrointestinal hemorrhage, thrombocytopenia, anemia, hematological malignancy, and traumatic intracranial hemorrhage [12,13,19].
Medications
We examined medication use in the 90 days prior to January 1, 2010. We searched for evidence of international normalized ratio (INR) testing as a proxy for warfarin therapy. We searched carrier and outpatient facility claims for prothrombin time laboratory test (CPT 85610) or home INR monitoring instruction, equipment, or interpretation of results (Healthcare Common Procedure Coding System [HCPCS] codes G0248, G0249, G0250) [20]. We required 2 separate INR tests within 90 days to avoid misclassification related to routine INR testing in patients not on warfarin therapy. As a sensitivity analysis in the subset of beneficiaries enrolled in Medicare Part D, we searched Part D claims for a warfarin prescription claim (generic name “WARFARIN SODIUM”).
Statistical Analysis
We describe the prevalence of contraindications using frequencies with percentages. To describe baseline characteristics of each study subpopulation, we present categorical variables as frequencies and continuous variables as means with SDs. We tested for differences between subgroups using χ2 tests for categorical variables and Kruskal-Wallis tests for continuous variables.
Among all patients eligible for any anticoagulation therapy, we describe rates of relative contraindications overall and by use of warfarin ascertained by prior INR testing. We tested for differences in relative contraindication rates between subgroups using χ2 tests. As a sensitivity analysis, we compared rates of contraindications by warfarin use on the basis of prescription drug claims in the subgroup of beneficiaries enrolled in Medicare Part D.
We used SAS software version 9.3 (SAS Institute Inc, Cary, North Carolina) for all analyses. We chose a 2-tailed α threshold of 0.05 for statistical significance. The institutional review board of the Duke University Health System approved the study.
Results
The study population consisted of 86,671 patients with atrial fibrillation. Of these patients, 1872 (2.2%) were ineligible for oral anticoagulation therapy; the most common reason for ineligibility was prior intracranial hemorrhage (n = 1120; 60%; Figure 1). We subsequently identified 7146 patients (8.2% overall) as candidates for warfarin only, most commonly due to the presence of a mechanical heart valve (n = 4730 [66%]) or end-stage renal disease (n = 869 [12%]). The remaining patients (n = 77,653 [90% overall]) were deemed potentially eligible for any oral anticoagulation therapy. A total of 43,818 patients (51% of the overall study population) were enrolled in Medicare Part D.
Figure 1.
Derivation of the Study Population
Abbreviations: AF, atrial fibrillation; MS, mitral stenosis; MR, mitral regurgitation; OAC, oral anticoagulation therapy.
Note: Subgroup numbers may not sum to totals due to overlap in diagnoses.
Demographic characteristics and medical history for the 3 subgroups are shown in Table 1. Differences in age and sex were modest. Compared with the overall population, patients who were ineligible for any anticoagulation therapy had the highest rates of dementia (19% vs 9%), diabetes mellitus (46% vs 36%), ischemic heart disease (67% vs 55%), peripheral vascular disease (42% vs 30%), heart failure (59% vs 43%), and prior stroke or transient ischemic attack (54% vs 20%). Mean CHADS2 and CHA2DS2-VASc scores were also highest in the ineligible group (mean CHADS2 score, 3.8 vs 3.1 for warfarin only vs 2.8 for any anticoagulation; P < .001).
Table 1.
Characteristics of the Study Population by Eligibility for Oral Anticoagulation Therapy
| Characteristic | Eligibility | P Value | |||
|---|---|---|---|---|---|
| Overall Population (N = 86,671) | Ineligible (n = 1872) | Warfarin Only (n = 7146) | Any Oral Anticoagulant (n = 77,653) | ||
| Age, mean (SD), y | 80.4 (7.4) | 80.5 (7.3) | 79.1 (6.9) | 80.5 (7.4) | < .001 |
| Age group, No. (%) | < .001 | ||||
| 65-69 y | 7607 (8.8) | 156 (8.3) | 777 (10.9) | 6674 (8.6) | |
| 70-74 y | 12,912 (14.9) | 285 (15.2) | 1192 (16.7) | 11,435 (14.7) | |
| 75-79 y | 17,978 (20.7) | 378 (20.2) | 1660 (23.2) | 15,940 (20.5) | |
| ≥ 80 y | 48,174 (55.6) | 1053 (56.3) | 3517 (49.2) | 43,604 (56.2) | |
| Women | 49,764 (57.4) | 1067 (57.0) | 4067 (56.9) | 44,630 (57.5) | .61 |
| Race, No. (%) | < .001 | ||||
| Black | 2741 (3.2) | 77 (4.1) | 246 (3.4) | 2418 (3.1) | |
| White | 81,847 (94.4) | 1726 (92.2) | 6686 (93.6) | 73,435 (94.6) | |
| Other | 2083 (2.4) | 69 (3.7) | 214 (3.0) | 1800 (2.3) | |
| US geographic region, No. (%) | < .001 | ||||
| Midwest | 21,671 (25.0) | 412 (22.0) | 1704 (23.8) | 19,555 (25.2) | |
| Northeast | 19,518 (22.5) | 487 (26.0) | 1966 (27.5) | 17,065 (22.0) | |
| South | 32,454 (37.4) | 660 (35.3) | 2378 (33.3) | 29,416 (37.9) | |
| West | 13,028 (15.0) | 313 (16.7) | 1098 (15.4) | 11,617 (15.0) | |
| Comorbid conditions, No. (%) | |||||
| Atrial flutter | 8463 (9.8) | 274 (14.6) | 1039 (14.5) | 7150 (9.2) | < .001 |
| Cancer | 14,823 (17.1) | 548 (29.3) | 1248 (17.5) | 13,027 (16.8) | < .001 |
| Cerebrovascular disease | 24,575 (28.4) | 1447 (77.3) | 2359 (33.0) | 20,769 (26.7) | < .001 |
| Stroke or TIA | 16,953 (19.6) | 1015 (54.2) | 1524 (21.3) | 14,414 (18.6) | < .001 |
| COPD | 30,168 (34.8) | 863 (46.1) | 3225 (45.1) | 26,080 (33.6) | < .001 |
| Dementia | 7462 (8.6) | 360 (19.2) | 468 (6.5) | 6634 (8.5) | < .001 |
| Diabetes mellitus | 30,862 (35.6) | 865 (46.2) | 2806 (39.3) | 27,191 (35.0) | < .001 |
| Heart failure | 37,064 (42.8) | 1095 (58.5) | 4326 (60.5) | 31,643 (40.7) | < .001 |
| Hypertension | 77,160 (89.0) | 1771 (94.6) | 6456 (90.3) | 68,933 (88.8) | < .001 |
| Ischemic heart disease | 47,935 (55.3) | 1253 (66.9) | 5041 (70.5) | 41,641 (53.6) | < .001 |
| Peripheral vascular disease | 26,118 (30.1) | 785 (41.9) | 2598 (36.4) | 22,735 (29.3) | < .001 |
| Renal disease | 15,922 (18.4) | 540 (28.8) | 2268 (31.7) | 13,114 (16.9) | < .001 |
| Valvular heart disease | 33,522 (38.7) | 921 (49.2) | 6722 (94.1) | 25,879 (33.3) | < .001 |
| CHADS2 score, mean (SD) | 2.8 (1.3) | 3.8 (1.4) | 3.1 (1.3) | 2.8 (1.3) | < .001 |
| 0 | 1605 (1.9) | — a | 129 (1.8) | 1466 (1.9) | < .001 |
| 1 | 10,525 (12.1) | 86 (4.6) | 648 (9.1) | 9791 (12.6) | < .001 |
| ≥ 2 | 74,541 (86.0) | 1776 (94.9) | 6369 (89.1) | 66,396 (85.5) | < .001 |
| CHA2DS2-VASc, mean (SD) | 5.1 (1.6) | 6.2 (1.6) | 5.4 (1.6) | 5.0 (1.6) | < .001 |
| 1 | 587 (0.7) | — a | 27 (0.4) | 556 (0.7) | < .001 |
| ≥ 2 | 86,084 (99.3) | 1868 (99.8) | 7119 (99.6) | 77,097 (99.3) | < .001 |
| Implantable device, No. (%) | |||||
| ICD | 5705 (6.6) | 169 (9.0) | 769 (10.8) | 4767 (6.1) | < .001 |
| Pacemaker | 21,366 (24.7) | 519 (27.7) | 2433 (34.0) | 18,414 (23.7) | < .001 |
Abbreviations: COPD, chronic obstructive pulmonary disease; ICD, implantable cardioverter-defibrillator; TIA, transient ischemic attack.
In accordance with the privacy policy of the Centers for Medicare & Medicaid Services, data for cells containing 10 or fewer observations are not reported.
Of the 84,799 patients without absolute contraindications, 39,592 (47%) represented groups that may be considered at higher risk for anticoagulation therapy or have relative contraindications. The details of these conditions are shown in Table 2. Age greater than 85 years (n = 22,451 [27%]) and dementia (n = 7102 [8.4%]) were the most common potentially high-risk features.
Table 2.
Among Patients Without Absolute Contraindications, Proportion With Conditions That Place Them at Relatively High Risk of Adverse Events With Anticoagulation
| Conditiona | Patients, No. (%) (N = 84,799) |
|---|---|
| High risk for anticoagulation | 39,592 (47.0) |
| Age > 85 years | 22,451 (26.5) |
| Anemia | 13,527 (16.0) |
| Prior gastrointestinal bleed | 7973 (9.4) |
| Dementia | 7102 (8.4) |
| Thrombocytopenia | 2568 (3.0) |
| Hematological malignancy | 1791 (2.1) |
| Traumatic intracranial hemorrhage | 170 (0.2) |
Conditions are not mutually exclusive, and patients may be represented in more than 1 group.
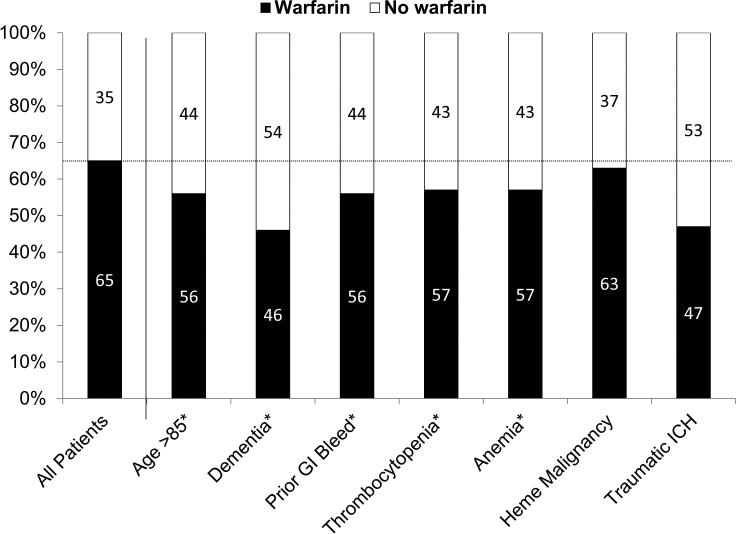
Among patients without absolute contraindications, 65% (n = 54,768/84,799) were treated with warfarin according to INR testing. Rates of warfarin use among high-risk subgroups are shown in Figure 2. Warfarin use was lowest in patients with dementia (46%) and traumatic intracranial hemorrhage (47%), but ranged from 56% to 63% in the remaining groups (including advanced age, prior gastrointestinal bleeding, thrombocytopenia, anemia, and hematologic malignancy). Among patients at high risk of thromboembolism without absolute contraindications, 79% (n = 3,725/4,730) of those with mechanical heart valves and 74% (n = 1,126/1,452) of those with mitral valve disease were treated with warfarin. In sensitivity analyses in the Medicare Part D sample, 62% (n = 27,312/43,818) were treated with warfarin based on prescription claims and rates of warfarin use among high-risk subgroups were similar (see Supplemental Material).
Figure 2.
Among Patients Without Absolute Contraindications, Rates of Warfarin Use (Defined by INR Testing) Overall and Within Relatively High-Risk Patients
Abbreviations: GI; gastrointestinal; ICH: intracranial hemorrhage; INR: international normalized ratio; OAC oral anticoagulation.
Discussion
In our analysis of older patients with atrial fibrillation, we found that a minority had absolute contraindications to oral anticoagulation therapy, and as a group, they had a high risk of thromboembolic events. Of the remaining patients who were eligible for anticoagulation therapy, most appeared to be eligible for either warfarin or novel oral anticoagulants. However, whereas nearly half of eligible patients may have had characteristics placing them at higher risk of bleeding anticoagulation therapy, the majority of patients in these subgroups were prescribed warfarin and rates of warfarin use were only slightly lower than in the overall eligible study population. Our findings may have implications for improving the care of patients with atrial fibrillation.
Several prior studies have reported rates of anticoagulation contraindications from less than 20% to more than 50% [21-25]. There are many reasons for this variability. The identification of contraindications to anticoagulation therapy may be subjective, and often there is significant local practice variation [26]. In addition, many such conditions vary greatly in severity. For example, mild hepatic impairment may be a subjective contraindication, whereas decompensated hepatic failure with coagulopathy is a more consistent reason to withhold therapy. Finally, the severity of the contraindication must be weighed against the underlying thromboembolic risk of the patient. Moderate relative contraindications may not be of sufficient risk to justify withholding anticoagulation from patients at substantial risk of stroke. Therefore, it is difficult to attribute undertreatment of a population to prevalence of significant contraindications.
Thus, we sought first to identify rates of comorbid conditions that are consistently considered absolute contraindications—major intracranial pathology (prior intracranial hemorrhage and/or intracranial masses) or decompensated liver disease. We found that a small minority of older patients meets these criteria. Importantly, patients at high risk for catastrophic bleeding on anticoagulation therapy also represent patients at high thromboembolic risk; on average, they had high CHADS2 and CHA2DS2-VASc scores and high rates of other cardiovascular comorbid conditions, compared with patients who did not have absolute contraindications to anticoagulation therapy. Therefore, it is imperative that providers carefully assess each of these risks to balance them and select the most beneficial treatment strategy overall.
The use of oral anticoagulation therapy is often based on a patient's stroke risk, relative to any comorbid conditions that might convey substantial risk for bleeding. Such features often are cited as relative contraindications, but they largely represent patient characteristics that put them at higher risk for bleeding and may lead to withholding of anticoagulation therapy, depending on its perceived benefit. Our data showed that among such subgroups, patients with dementia and traumatic intracranial hemorrhage had the lowest rates of warfarin use (46% and 47%, respectively). In contrast, the remaining groups (age 85 years or older, prior gastrointestinal bleeding, blood dyscrasias) had subtle variation in use anticoagulation therapy from the overall cohort (56%-63% vs 65%). Each represents a potential risk factor either for bleeding or for increased risk of adverse events in the setting of bleeding, yet such conditions appeared to have had little impact on warfarin use in the study population. Nevertheless, these data may also reflect the highly variable nature of such treatment patterns, without consistent criteria. Physicians are left to make highly subjective judgments of risk versus benefit [27], and likely benefit from shared decision-making models and aids [28].
The vast majority of patients eligible for anticoagulation therapy in our cohort did not have an identifiable condition that limited them to warfarin (ie, mechanical valve, severe renal disease, or valvular atrial fibrillation). These findings have important implications. Several newer oral anticoagulants have been approved, or are in development, for stroke prevention in patients with atrial fibrillation. However, these agents have not overtaken warfarin as the dominant strategy [29], and it remains unclear what proportion of patients should receive such agents. They have been demonstrated to be at least as effective as warfarin at preventing thromboembolism and have also demonstrated lower rates of bleeding [30-34]. Our data show a large proportion of patients with atrial fibrillation (90%) could be eligible and may benefit. Nevertheless, there may remain barriers including access, dosing, cost, reversibility, and interactions with concomitant medications.
Rates of oral anticoagulation therapy in the Medicare population have been previously reported to be approximately 56% and 59% using Medicare Part D data from 2006 and 2007, respectively [35]. We found a rate of 65% using INR data (62% using Part D data from 2010). There are likely several explanations for these differences. The prior data did not exclude patients with absolute contraindications, as ours did. Also, 2006-2007 was a period of still increasing enrollment in Medicare Part D, compared to 2010. Lastly, the higher rate in our study may reflect an overall increase in treatment rates in this population, as has been previously suggested [36].
Our study has limitations. The definitions of contraindications are subjective, perhaps even those we deemed “absolute.” Nevertheless, we used commonly accepted conditions in which most providers would not consider the use of anticoagulation therapy. In addition, our study was based on administrative claims data and thus was subject to the limitations inherent in such methods. These include diagnosis biases related to billing and the limitations of the coding schema, ascertainment bias of limited look-back, and exclusion of over-the-counter therapies such as aspirin. Furthermore, the severity of coded conditions could not be ascertained, which may be particularly relevant for renal disease in which medication dosing can vary by severity. Similarly, patient and provider preferences were not captured; we cannot attribute treatment decisions directly to the presence or absence of measured contraindications. Lastly, we did not have data on concomitant medications, which may also influence both rates of anticoagulation therapy (by using INR measurement as a surrogate) and the choice of whether to use anticoagulation therapy. However, the timing of the study predates the approval of any novel anticoagulant.
In conclusion, older patients with atrial fibrillation rarely have absolute contraindications to oral anticoagulation therapy, and those who do are also at high risk for thromboembolic events. Despite the infrequency of absolute contraindications, anticoagulation therapy is underutilized. Among eligible patients, the majority appeared to be medically eligible for any anticoagulant. These findings could provide an opportunity for improvement in the care of patients with atrial fibrillation, and additional studies are needed to identify the appropriate indications, if any, for withholding anticoagulation therapy.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by grants R01HL092577, RC1HL101056, and R01HL102214 from the National Heart, Lung, and Blood Institute; and by grant R01AG028321 from the National Institute on Aging. Dr Steinberg was supported by grant T32HL007101.
Footnotes
Author Contributions: Drs Steinberg, Curtis, and Piccini conceived and designed the study. All authors analyzed and interpreted the data. Ms Greiner and Mr Hammill conducted the statistical analysis. Dr Steinberg and Ms Greiner drafted the manuscript. All authors critically revised the manuscript for important intellectual content. Drs Steinberg, Curtis, and Benjamin obtained funding. Drs Curtis and Piccini supervised the study.
Additional Contributions: Damon M. Seils, MA, Duke University, provided editorial assistance and prepared the manuscript. Mr Seils did not receive compensation for his assistance apart from his employment at the institution where the study was conducted.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Institute on Aging, or the National Institutes of Health.
Disclosures: None.
References
- 1.Hart RG, Benavente O, McBride R, et al. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131:492–501. doi: 10.7326/0003-4819-131-7-199910050-00003. [DOI] [PubMed] [Google Scholar]
- 2.Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 3.Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:104–123. doi: 10.1161/CIR.0b013e3181fa3cf4. [DOI] [PubMed] [Google Scholar]
- 4.Fang MC, Stafford RS, Ruskin JN, et al. National trends in antiarrhythmic and antithrombotic medication use in atrial fibrillation. Arch Intern Med. 2004;164:55–60. doi: 10.1001/archinte.164.1.55. [DOI] [PubMed] [Google Scholar]
- 5.Gage BF, Boechler M, Doggette AL, et al. Adverse outcomes and predictors of underuse of antithrombotic therapy in medicare beneficiaries with chronic atrial fibrillation. Stroke. 2000;31:822–827. doi: 10.1161/01.str.31.4.822. [DOI] [PubMed] [Google Scholar]
- 6.Bungard TJ, Ghali WA, Teo KK, et al. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160:41–46. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- 7.Go AS, Hylek EM, Borowsky LH, et al. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131:927–934. doi: 10.7326/0003-4819-131-12-199912210-00004. [DOI] [PubMed] [Google Scholar]
- 8.Ogilvie IM, Newton N, Welner SA, et al. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638–645.e4.. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg D, Lewis J, Halpern S, et al. Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol Drug Saf. 2012;21:765–769. doi: 10.1002/pds.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schelbert EB, Vaughan-Sarrazin MS, Welke KF, et al. Hospital volume and selection of valve type in older patients undergoing aortic valve replacement surgery in the United States. Circulation. 2005;111:2178–2182. doi: 10.1161/01.CIR.0000163567.03454.EB. [DOI] [PubMed] [Google Scholar]
- 11.Arday SL, Arday DR, Monroe S, et al. HCFA’s racial and ethnic data: current accuracy and recent improvements. Health Care Financ Rev. 2000;21:107–116. [PMC free article] [PubMed] [Google Scholar]
- 12.Birman-Deych E, Waterman AD, Yan Y, et al. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 13.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 14.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 15.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 16.Lip GY, Banerjee A, Lagrenade I, et al. Assessing the risk of bleeding in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation project. Circ Arrhythm Electrophysiol. 2012;5:941–948. doi: 10.1161/CIRCEP.112.972869. [DOI] [PubMed] [Google Scholar]
- 17.Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORR(2)HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation: the AMADEUS (Evaluating the Use of SR34006 Compared to Warfarin or Acenocoumarol in Patients With Atrial Fibrillation) Study. J Am Coll Cardiol. 2012;60:861–867. doi: 10.1016/j.jacc.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 18.Scowcroft AC, Lee S, Mant J. Thromboprophylaxis of elderly patients with AF in the UK: an analysis using the General Practice Research Database (GPRD) 2000-2009. Heart. 2013;99:127–132. doi: 10.1136/heartjnl-2012-302843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kociol RD, Greiner MA, Hammill BG, et al. B-type natriuretic peptide level and postdischarge thrombotic events in older patients hospitalized with heart failure: insights from the Acute Decompensated Heart Failure National Registry. Am Heart J. 2012;163:994–1001. doi: 10.1016/j.ahj.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Qualls LG, Greiner MA, Eapen ZJ, et al. Postdischarge international normalized ratio testing and long-term clinical outcomes of patients with heart failure receiving warfarin: findings from the ADHERE registry linked to Medicare claims. Clin Cardiol. 2013;36:757–765. doi: 10.1002/clc.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flaker GC, McGowan DJ, Boechler M, et al. Underutilization of antithrombotic therapy in elderly rural patients with atrial fibrillation. Am Heart J. 1999;137:307–312. doi: 10.1053/hj.1999.v137.91403. [DOI] [PubMed] [Google Scholar]
- 22.Smith NL, Psaty BM, Furberg CD, et al. Temporal trends in the use of anticoagulants among older adults with atrial fibrillation. Arch Intern Med. 1999;159:1574–1578. doi: 10.1001/archinte.159.14.1574. [DOI] [PubMed] [Google Scholar]
- 23.Bradley BC, Perdue KS, Tisdel KA, et al. Frequency of anticoagulation for atrial fibrillation and reasons for its non-use at a Veterans Affairs medical center. Am J Cardiol. 2000;85:568–572. doi: 10.1016/s0002-9149(99)00813-9. [DOI] [PubMed] [Google Scholar]
- 24.Kalra L, Yu G, Perez I, et al. Prospective cohort study to determine if trial efficacy of anticoagulation for stroke prevention in atrial fibrillation translates into clinical effectiveness. BMJ. 2000;320:1236–1239. doi: 10.1136/bmj.320.7244.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kakkar AK, Mueller I, Bassand JP, et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS One. 2013;8:e63479. doi: 10.1371/journal.pone.0063479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenman MB, Simon TA, Teal E, et al. Perceived or actual barriers to warfarin use in atrial fibrillation based on electronic medical records. Am J Ther. 2012;19:330–337. doi: 10.1097/MJT.0b013e3182546840. [DOI] [PubMed] [Google Scholar]
- 27.Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298–2307. doi: 10.1161/CIRCULATIONAHA.111.055079. [DOI] [PubMed] [Google Scholar]
- 28.Seaburg L, Hess EP, Coylewright M, et al. Shared decision making in atrial fibrillation: where we are and where we should be going. Circulation. 2014;129:704–710. doi: 10.1161/CIRCULATIONAHA.113.004498. [DOI] [PubMed] [Google Scholar]
- 29.Steinberg BA, Holmes DN, Piccini JP, et al. Early adoption of dabigatran and its dosing in US patients with atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation. J Am Heart Assoc. 2013;2:e000535. doi: 10.1161/JAHA.113.000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 31.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 32.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 33.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 34.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 35.Piccini JP, Mi X, Dewald TA, et al. Pharmacotherapy in Medicare beneficiaries with atrial fibrillation. Heart Rhythm. 2012;9:1403–1408. doi: 10.1016/j.hrthm.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shroff GR, Solid CA, Herzog CA. Temporal trends in ischemic stroke and anticoagulation therapy among Medicare patients with atrial fibrillation: a 15-year perspective (1992-2007). JAMA Intern Med. 2013;173:159–160. doi: 10.1001/jamainternmed.2013.1579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.