Abstract
Micro Abstract
The incorporation of bevacizumab with concurrent chemoradiation (CRT) in the treatment of locally advanced non-small cell lung cancer (NSCLC) could improve efficacy in this disease stage. This trial accrued patients in 2 strata (High and Low risk for hemoptysis) and in 3 separate cohorts depending on the timing of the bevacizumab. Bevacizumab could not be safely integrated or effectively combined with CRT in inoperable NSCLC patients. Future trials combining bevacizumab and CRT are not warranted.
Purpose
The aim of this trial was to determine feasibility of incorporating bevacizumab (B) into concurrent chemoradiation (CRT) for locally advanced non-small cell lung cancer (NSCLC).
Patients and Methods
Patients with unresectable stage III NSCLC, performance status 0-1, and adequate organ function were accrued in 2 strata, Low and High Risk (squamous histology, hemoptysis, tumor with cavitation and/or adjacent to a major vessel). Cohort 1 patients received cisplatin 50mg/m2 days (d) 1 and 8, etoposide 50mg/m2 (d 1-5) for 2 cycles concurrent with RT (64.8 Gy) followed by docetaxel (D) 75mg/m2 and bevacizumab (B) 15 mg/kg for 3 cycles. If safety was established, then accrual would continue to Cohort 2 (B, d15, 36, 57) and then subsequently to Cohort 3 (B, d1, 22, 43).
Results
Twenty-nine patients (17 Low and 12 High Risk) registered to Cohort 1. Twenty-six patients (including 4 squamous, 1 adenosquamous) were assessable. Twenty-five completed CRT. Grade 3/4 toxicities during CRT included acceptable rates of hematologic toxicity, esophagitis, and pneumonitis. Of 21 assessable for safety with D/B consolidation, major adverse events (AEs) were pneumonitis (2 grade 3) and 2 episodes of fatal hemoptysis in the High Risk group, resulting in closure of this stratum. The Low Risk stratum subsequently closed because of slow accrual. Median overall survival was 46 months for Low Risk and 17 months for High Risk strata.
Conclusion
Bevacizumab was not safely integrated into CRT for stage III NSCLC in patients considered at high risk for hemoptysis. In lower risk patients, data are insufficient to determine safety or efficacy.
Keywords: Lung cancer, concurrent chemoradiotherapy, bevacizumab
INTRODUCTION
The standard treatment for good performance status patients with inoperable Stage III non-small cell lung cancer (NSCLC) is concurrent chemoradiotherapy (CRT).1,2 Despite the advances made with concurrent therapy, a great proportion of patients continue to die from recurrent disease, indicating that new treatment strategies are necessary. Optimal chemotherapeutic regimens and radiotherapy dose and schedules remain to be defined. Ongoing trials are incorporating newer chemotherapeutic and molecularly targeted agents into combined modality therapy with thoracic radiotherapy in an attempt to improve therapeutic outcomes.
The underlying hypothesis for SWOG-coordinated studies of CRT in unresectable Stage III NSCLC include full dose chemotherapy given during concurrent thoracic radiation to optimize efficacy by addressing both loco-regional disease and distant micrometastases early on. Based on these concepts, SWOG has pursued a strategy of combining cisplatin and etoposide with radiation in a series of sequential studies: S9019 S9504 and S0023.3-6 Both drugs can be safely delivered with concurrent thoracic radiation at systemic doses. Pertinent to this study design was, S9504, in which patients were treated with concurrent therapy followed by consolidation docetaxel.4 Docetaxel was selected for this consolidation approach based on its clinical activity in second-line therapy (after failure of platinum-based treatment).7,8 The long term results of this phase II study demonstrated tolerability and efficacy with a median survival of 26 months and an overall survival of 29% at 5 years.5 In a follow-up intergroup study, S0023, patients were treated with the S9504 regimen and then randomized to receive gefitinib or placebo as maintenance therapy.6 Although this study did not demonstrate efficacy of gefitinib, results confirmed favorable outcomes with the S9504 approach. A subsequent HOG Phase III trial evaluating consolidation docetaxel was ongoing at the time S0533 was designed. Hence, the S9504 platform was utilized as the basis for the S0533 trial described here.
Many research efforts have been focused on developing treatments based on the inhibition of tumor angiogenesis. Vascular endothelial growth factor (VEGF) is an ideal target because it is the most potent and specific of the endothelial mitogens.9 Its presence has been correlated with a poor prognosis, and many human tumors, including NSCLC, have upregulated VEGF mRNA.10,11 Bevacizumab is a humanized monoclonal antibody directed against VEGF.12 In a randomized Phase II trial, bevacizumab was combined with carboplatin and paclitaxel in chemo-naïve patients with advanced NSCLC.13 The study suggested that bevacizumab at 15 mg/kg given every three weeks with chemotherapy might increase response and prolong time to progression. However, an increased incidence of pulmonary hemorrhage occurred that was associated with centrally located masses and/or cavitary lesions and 4/6 of these patients had squamous cell carcinoma histology. The risk of pulmonary hemorrhage in the squamous subset was 31% compared to 4% for the other pathologic subtypes. As a result, in the subsequent phase III trial, ECOG E4599, patients who had lung cancer of squamous histology were excluded.14 Results of this study indicated that patients who received carboplatin/paclitaxel with bevacizumab had a significantly better survival when compared to those who received chemotherapy alone resulting in subsequent FDA approval of this regimen for the treatment of metastatic, non-squamous NSCLC.
In addition to synergy with chemotherapeutic agents, there are data in preclinical models suggesting that inhibiting angiogenesis may potentiate radiation.15,16 VEGFR inhibitors have been evaluated in preclinical lung cancer models and have shown tumor growth delay when given with radiation especially on a concurrent treatment schedule.17 Building on emerging data with bevacizumab in the metastatic setting, it would seem logical to try and incorporate bevacizimab into treatment for locally advanced NSCLC in an effort to increase the therapeutic ratio. This pilot study was designed to define the appropriate timing for the addition of bevacizumab onto our standard platform of full dose concurrent CRT, as well as to delineate the tolerability of this approach in patients with NSCLC of all histologies. It was postulated that by radiating the primary tumor there would be a reduced occurrence of the pulmonary hemorrhage associated with bevcizumab treatment. The safe, successful incorporation of bevacizumab into this treatment paradigm could ultimately result in more efficacious management of unresectable NSCLC.
PATIENTS AND METHODS
Patient Eligibility
Patients with histologic or cytologic proof of unresectable Stage IIIA (N2) or Stage IIIB NSCLC cancer with measurable or non-measurable disease were eligible. Lymph nodes were considered positive if histologically proven, >1 cm and positron emission tomography positive, and/or >3 cm on CT scan. Mediastinoscopy was not required for confirmation of N2 status. Additional eligibility criteria included a Zubrod PS of 0-1; no prior chemotherapy, radiotherapy, or surgical resection for lung cancer; and adequate organ function. Patients had to have a forced expiratory volume in 1 second (FEV1) ≥ 2L or a predicted FEV1 in the contralateral lung of ≥ 0.8L. No full dose anticoagulation or pathologic condition that carried a high bleeding risk, ulcer, non-healing wound, or bone fracture was allowed. Patients could not have had a fine needle/core biopsy, or mediastinoscopy within 7 days of registration.
The protocol was approved by institutional review boards at each site, and was registered with ClinicalTrials.gov (ClinicalTrials.gov Identifier: NCT00334815) prior to enrolling patients. All patients were informed of the investigational nature of the trial and provided written informed consent. Patients were offered optional participation in SWOG S9925 (Lung Cancer Specimen Repository).
Study Treatment
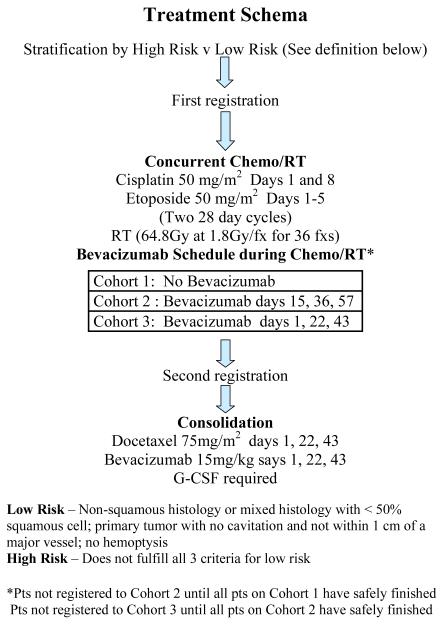
The treatment plan is depicted in Figure 1. All patients received concurrent chemotherapy and radiotherapy. Chemotherapy consisted of cisplatin 50 mg/m2 on days 1, 8, 29, and 36 with etoposide 50 mg/m2 on days 1 to 5 and 29 to 33. Thoracic radiotherapy commenced within 24 hours of chemotherapy. Radiation field arrangements were determined by 3D planning to the primary lesion and involved lymph nodes. The radiation prescription was 6480 cGy, given in 36 fx at 180 cGy/day, 5 days a week without interruption.
1).
Treatment schema of SWOG 0533
Four to seven weeks after completion of radiation, patients without progressive disease by Response Evaluation Criteria in Solid Tumors (RECIST) were registered to receive three cycles of docetaxel 75 mg/m2 on day one of every 21 days. Prophylactic use of pegfilgrastim or G-CSF was required with all three cycles of docetaxel consolidation.
Patient accrual proceeded in 3 sequential cohorts stratified by High Risk versus Low Risk patients. Patients were classified as Low Risk if all of the following criteria were met: 1) non-squamous histology or mixed histology with < 50% squamous cell carcinoma; 2) a primary tumor with no cavitation and not within 1 cm of a major blood vessel; 3) no history of hemoptysis (bright red blood of ½ teaspoon or more) within 28 days prior to registration. Patients who did not fulfill all three of the Low Risk categories were classified as High Risk. The treatment plan for the 3 cohorts differed only with regard to the timing of the bevacizumab. The first patient cohort would receive bevacizumab 15 mg/kg only during consolidation docetaxel following completion of concurrent CRT. If safe, based on predefined protocol-specific criteria, the second cohort would receive bevacizumab starting on day 15 of induction CRT. The last cohort of patients would start bevacizumab on day 1 of CRT. Accrual to any subsequent cohort of either stratum did not occur until safety data from the previous cohorts in both strata and other trials had been reviewed. The intent of this approach was to provide a more controlled setting for the addition of bevacizumab to combined modality treatment of all NSCLC histologies.
Toxicity Evaluation and Treatment Modifications
This study used the National Cancer Institute Common Toxicity Criteria version 3.0 for toxicity and adverse event reporting. During concurrent CRT, cisplatin was omitted on day 8 or 36 for grade 4 or febrile neutropenia, grade 4 esophagitis, or grade ≥ 2 renal toxicity. On day 29, chemotherapy was delayed 1 week for an absolute neutrophil count < 1,500/μl, a platelet count < 100,000/μl, or ≥ grade 3 non-hematologic toxicity. If febrile neutropenia occurred during the previous cycle, etoposide was reduced to 4 days . Cisplatin dose was reduced or omitted if the creatinine clearance was ≤ 45 cc/min. A break in radiation was allowed only for grade 4 neutropenia or esophagitis requiring parenteral alimentation.
Patients could not have persistent or new hemoptysis or > Grade 2 esophagitis before going on to docetaxel consolidation. Patients who developed febrile neutropenia, or grade 4 neutropenia or thrombocytopenia during consolidation required a dose reduction to 55 mg/m2.
Once the patient received the first dose of bevacizumab, toxicities were assessed weekly. These toxicities were reported using a web-based dose-limiting toxicity reporting form. Prior to each treatment, there was special attention given to blood pressure, proteinuria, bleeding, cardiovascular events, reversible posterior leukoencephalopathy syndrome, esophagitis, esophageal stricture and tracheoesophageal/bronchial fistulae.
Study Evaluation and Follow-Up
Prestudy evaluation included a medical history and physical exam, performance status determination, laboratory analysis, pulmonary function tests, ECG, an MRI or CT scan of the brain, and a CT scan of the chest including the liver and adrenal glands. During CRT, CBCs were obtained weekly and, during consolidation, before each cycle and during week 2. A history and physical, and chemistries were obtained prior to each treatment cycle. Once bevacizumab was started, weekly toxicity assessments were required and continued until 60 days after the bevacizumab was discontinued or until all adverse events had resolved. Response assessment occurred at the end of CRT and docetaxel/bevacizumab and then every 2-3 months for 2 years, and then every 6 months until 4 years after the initial registration.
Statistical Methods
The primary objective was to assess toxicities, especially the risk of hemorrhage, associated with the addition of bevacizumab to combined modality therapy. This would be assessed in up to 3 different cohorts to determine the earliest timepoint tolerable to incorporate bevacizumab. Initially, 35 Low Risk and 35 High Risk patients would be accrued to the first cohort assuming that 80% would be registered for consolidation therapy and be evaluable for bevacizumab-associated toxicities. If the first cohort was deemed safe, than an additional 28 evaluable patients per stratum would be accrued to the second cohort. If the second cohort was determined to be tolerable, than another 28 patients per stratum would be accrued to the third cohort. The design was sufficient to distinguish between the null hypothesis of an unacceptable rate (≥ 20%) of grade 4 or higher hemorrhage versus the alternative hypothesis of an acceptable rate (≤ 5%) of these toxicities with 84% exact power, using a one-sided test based on binomial distribution with a significance of 5%. Progression-free survival (PFS) and overall survival (OS) were measured from the date of initial enrollment. Estimates were calculated using the method of Kaplan-Meier.18 Confidence intervals for the median PFS and OS estimates were constructed using the method of Brookmeyer-Crowley.19 The response rate (RR) was defined as the number of both confirmed and unconfirmed complete and partial responses among the subset of patients with measurable disease (per RECIST) at baseline. The disease control rate (DCR) was defined as the number of patients with a best response of stable disease or better among the patients with measurable disease at baseline. Clopper-Pearson (exact) 95% confidence intervals were calculated for binary outcomes, such as RR and DCR and individual toxicity rates.
RESULTS
Trial History
SWOG 0533 was activated in June 2006. Because of concerns regarding toxicity monitoring, the trial was initially opened at a limited number of SWOG institutions. The study was temporarily closed April 2007 secondary to a CTEP action letter regarding reports of tracheoesophageal (TE) fistulae in patients with small cell lung cancer being treated with concurrent CRT and bevacizumab. Additionally, there were multiple amendments throughout the study course due to new reports of bevacizumab-related toxicity. When it was deemed that the trial could be conducted safely and toxicities could rapidly be communicated, it was opened group-wide in June 2008. The High Risk stratum closed secondary to toxicity in March 2009 and the Low Risk stratum closed in March 2010 because of slow accrual. Patients were only enrolled to Cohort 1.
Patient Characteristics
Seventeen patients were accrued to the low-risk stratum and 15 were eligible. In the high-risk stratum there were 12 patients, 11 eligible. Of the 3 ineligible patients, 2 had incorrect stage and one had inadequate pulmonary function. One High Risk patient never received any treatment. The characteristics of the 26 assessable patients are presented in Table 1. In the High Risk stratum, there were a higher proportion of males and patients with stage IIIB. There were 4 patients with squamous cell carcinoma in this stratum.
Table 1.
PATIENT CHARACTERISTICS
|
LOW RISK (n = 15) |
HIGH RISK (n = 11) |
|
|---|---|---|
|
| ||
| Age (yr) | 54.5 (32-70) | 63 (51-77) |
| Sex (Male) | 6 (40%) | 7 (64%) |
| Race (White) | 14 (93%) | 9 (82%) |
| PS = 0 | 9 (60%) | 5 (45%) |
|
| ||
| HISTOLOGY | ||
| Adenocarcinoma | 10 | 3 |
| Squamous | 0 | 4 |
| Large Cell | 1 | 1 |
| Mixed | 1 | 0 |
| Other | 3 | 3 |
|
| ||
| STAGE | ||
| IIIA | 6 | 1 |
| IIIB | 9 | 9 |
| Unknown | - | 1 |
Twenty-five patients completed the CRT as planned. There was one major protocol deviation: a patient in the initial cohort received one dose of bevacizumab concurrent with CRT due to a treatment assignment error.
Fourteen patients from the Low Risk stratum and 7 High Risk patients were treated with consolidation docetaxel and bevacizumab. Nine and 3 patients, respectively, completed the consolidation treatment as planned. Reasons for incompletion of treatment included 5 for adverse events, 2 deaths, one patient refusal, and one patient who developed a cavitating lesion.
Toxicity
Grades 3 and 4 toxicities that occurred during concurrent CRT and the consolidation phase of treatment are represented in Table 2. Overall, the concurrent CRT was well tolerated. Grades 3 or 4 neutropenia or leukopenia were the most common toxicities and occurred in 38% and 23% of the patients, respectively. There was an 11.5% incidence of Grade 3-4 febrile neutropenia. Grade 3 esophagitis occurred in only 2 patients (8%) and there was one episode of grade 3 pneumonitis.
Table 2.
TOXICITIES
| Treatment | Chemo/RT | Consolidation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stratum | Low Risk (n = 15) |
High Risk (n = 11) |
Low Risk (n = 14) |
High Risk (n = 7) |
||||||||
| Toxcity Grade | 3 | 4 | 5 | 3 | 4 | 5 | 3 | 4 | 5 | 3 | 4 | 5 |
| Neutropenia | 3 | 1 | - | 3 | 3 | - | - | - | - | - | - | - |
| Leukopenia | 1 | 2 | - | 2 | 1 | - | - | - | - | - | - | - |
| Thrombocytopenia | 2 | - | - | - | - | - | - | - | - | - | - | - |
| Anemia | 1 | - | - | 1 | - | - | - | - | - | 1 | 1 | - |
| Febrile Neutropenia | - | - | - | 2 | 1 | - | - | - | - | - | - | - |
| Electrolytes | 2 | - | - | 2 | - | - | - | - | - | 1 | - | - |
| Renal | - | - | - | 1 | - | - | - | - | - | - | - | - |
| Nausea | 1 | - | - | 1 | - | - | - | - | - | - | - | - |
| Esophagitis | 1 | - | - | 1 | - | - | 1 | - | - | - | - | - |
| Pneumonitis | - | - | - | 1 | - | - | - | - | - | 1 | - | - |
| Hemorrhage | - | - | - | - | - | - | - | - | - | 1 | - | 2 * |
1) Adenocarcinoma, central tumor, Grade 5 hemoptysis 29 days after cycle 2
2) Squamous Ca, cavitation, Grade 5 hemoptysis 13 days after cycle 2
During the consolidation docetaxel and bevacizumab there was no significant neutropenia or leukopenia, likely as a result of the mandated use of pegfilgrastim or GCSF. There were 2 episodes of grades 3 and 4 anemia. No further esophagitis occurred during the consolidation treatment. There were an additional 2 instances of grade 3 pneumonitis for a total incidence of 11.5% over both treatment phases.
There were 2 episodes of grade 5 pulmonary hemorrhage. Both occurred in High Risk patients and resulted in the closure of this stratum. One patient had squamous cell carcinoma with cavitation, with the event occurring 13 days after the second cycle of consolidation treatment. The second patient had adenocarcinoma and a centrally located tumor. Fatal hemoptysis developed 29 days after the second consolidation cycle. The third cycle of consolidation had already been delayed because of a hospitalization for pneumonia. During consolidation therapy there was 1 episode of grade 3 gastrointestinal hemorrhage in a High Risk patient. There were an additional 3 occurrences of grade 1 upper/lower respiratory tract bleeding among the Low Risk patients. There was only one incidence of grade 1 proteinuria and no vascular events.
Efficacy
Twenty-four patients had measurable disease at baseline and were included in the analysis of response. Nine out of 14 patients on the Low Risk stratum had either a confirmed or unconfirmed partial response (PR) for a response rate of 64% (95% CI: 35% - 87%). Seven out of 10 High Risk patients had a PR for a response rate of 70% (95% CI: 35% - 93%). The disease control rates were 93% (95% CI: 66% - 100%) and 90% (95% CI: 56% - 100%), respectively
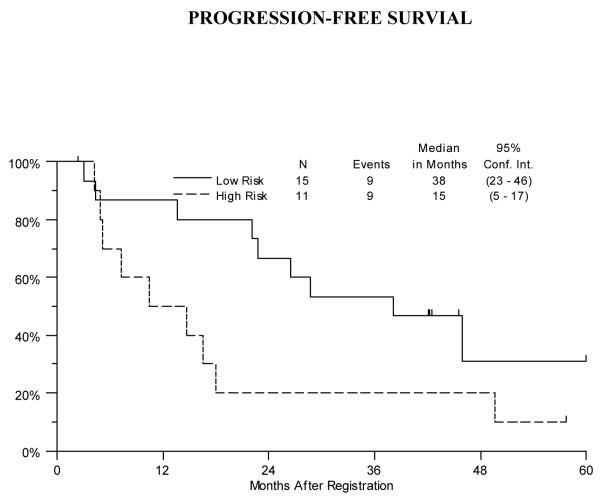
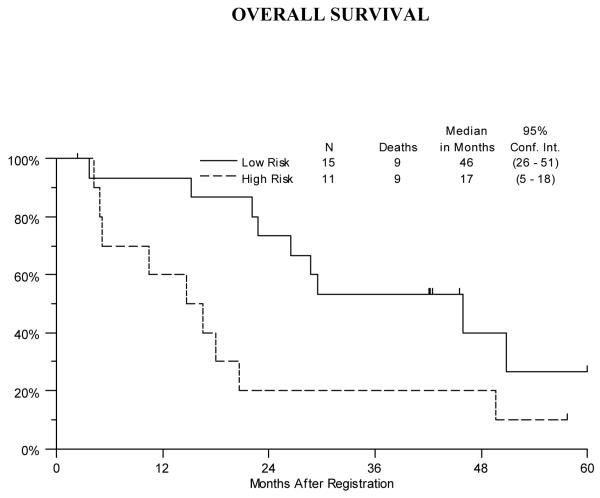
The median progression-free survival was 38 months (95% CI: 23-46 months) for the Low Risk patients and 15 months (95% CI: 5-17-months) for the High Risk patients. (Figure 2) Median overall survival was 46 months (95% CI: 26-51 months) for the Low Risk stratum and 17 months (95% CI: 5-18 months) for the High Risk stratum. (Figure 3)
2).
Progression-free survival for Low and High Risk Strata
3).
Overall survival for the Low and High Risk Strata
DISCUSSION
SWOG has had success with incorporating full dose cisplatin and etoposide chemotherapy with radiation as treatment for locally advanced NSCLC with acceptable rates of hematologic toxicity, esophagitis, and pneumonitis A randomized phase III Hoosier Oncology Group (HOG) study designed to validate the concept of docetaxel consolidation, ongoing at the time S0533 was activated, did not support the use of docetaxel consolidation treatment as there was no significant difference in survival between the treatment arm receiving the docetaxel compared to the study arm that did not receive additional chemotherapy after CRT.20 It is important to note that despite the results of the HOG study, consolidation therapy after concurrent CRT is consistently utilized in trial designs for the treatment of locally advanced NSCLC. Interestingly, patients on the HOG trial who did not receive consolidation docetaxel still had a 23.2 month median survival which further supports the CRT cisplatin-etoposide platform used in these trials.
Some of the most recent advances in the treatment of NSCLC have centered on the development of a number of molecularly-targeted agents such as inhibitors of VEGF and EGFR. Thus, it was rational to attempt to include these novel drugs in the treatment of locally advanced disease in an attempt to improve cure rates. Bevacizumab carries with it some unique and disturbing toxicities, particularly pulmonary hemorrhage that prevents its use in many patients with NSCLC.13,21 There was very little information regarding the use of bevacizumab in conjunction with radiation and chemotherapy in the treatment of locally advanced disease at the time this protocol was initiated. We elected to take a cautious approach by initially administering bevacizumab with consolidation chemotherapy after the conclusion of the concurrent CRT. Our rationale was based on the evidence that palliative radiotherapy is effective in controlling hemoptysis. A metaananlysis of 9 trials showed symptom improvement in 80% of patients and 5 trials reported complete resolution of bleeding in 74% of patients receiving low dose radiation (10-30 Gy) and 69% of patients receiving higher dose.22 Death from pulmonary hemorrhage after definitive CRT is rare, albeit data on this subject is lacking. On the HOG trial and S0023 where all patients received CRT, no toxic deaths were reported from pulmonary hemorrhage.6,20 A single institution retrospective analysis from Korea attempted to identify risk factors for fatal hemoptysis associated with CRT.23 There was an 8% incidence of fatal pulmonary hemorrhage. A multivariate analysis identified central tumor location and poor performance status as risk factors for fatal hemoptysis. Although histology was not a significant variable, 10 of the 12 affected patients had squamous cell histology and most of the patients had radiographic evidence of radiation pneumonitis/fibrosis. The successful conduct of a trial with significant safety concerns in a cooperative group setting is a daunting task. To ensure careful monitoring of all toxicities especially during bevacizumab treatment, a Rapid Toxicity Reporting Form had to be submitted weekly on all patients once bevacizumab treatment commenced. A summary report of all significant toxicities was generated and sent to the study coordinators on a weekly basis. The trial was also initially opened in a limited number of institutions in order to ensure that the reporting system that was developed was feasible and would result in the timely collection of toxicity data. Despite this cautious stepwise design, 2 episodes of fatal hemoptysis occurred among the High Risk patients in the first study cohort, resulting in immediate closure of this stratum. Both events occurred at the same time during consolidation after 2 cycles of chemotherapy and bevacizumab. Treating the primary tumor with CRT did not prevent the subsequent occurrence of fatal pulmonary hemorrhage.
Other investigators have also been unable to integrate bevacizumab into a CRT regimen for locally advanced disease in a fashion enabling transition to clinical practice. Socinski et al. recently reported the results of a 45 patient trial in locally advanced NSCLC.24 Patients on this study received induction carboplatin/paclitaxel/bevacizumab followed by weekly carboplatin/paclitaxel and bevacizumab (every other week) ± erlotinib with 74 Gy thoracic conformal radiation and then consolidation bevacizumab and erlotinib. The study was closed for patients with squamous cell histology after 2 grade 5 hemorrhagic events both of which occurred after CRT, 78 and 69 days after the last dose of bevacizumab. There was also an additional episode during concurrent treatment.
Another toxicity of concern during the course of the trial was the development of TE fistulae, which is generally an uncommon event as a result of CRT for lung cancer treatment. In a simultaneous, ongoing study in limited stage small cell lung cancer (SCLC), among 29 patients there were 2 confirmed and one suspected episode of TE fistulae.25,26 All 3 patients had grade 3 esophagitis during CRT and bevacizumab induction therapy. Subsequently, an additional patient developed a fatal TE fistula during maintenance treatment. There was some speculation that this toxicity might be histology related. However, in an independent study in NSCLC, 2 of 5 patients developed TE fistulae during maintenance treatment with chemotherapy and bevacizumab.26 Both patients also had severe esophageal toxicity after CRT and bevacizumab. The insinuation is that severe esophageal toxicity as a result of this treatment may predispose patients to the development of TE fistulas. In the Socinski trial the grade 3/4 esophagitis rate was 29% and there was one TE fistula 3½ months after CRT. No patients on our study developed TE fistulae most likely because the trial was amended to exclude patients with > grade 2 esophagitis from receiving bevacizumab.
It is difficult to ascertain if the addition of bevacizumab was efficacious in the treatment of the patients in the Low Risk stratum. The median progression-free and over all survival estimates for the Low Risk patients were 38 and 46 months which are much better than previous trials but may be a product of the small number of patients accrued. This study still supports the use of cisplatin/etoposide and concurrent radiotherapy as an effective treatment. The non-squamous group in the previously referenced Socinski trial had an 18.7 month median overall survival and a 21% 5-year survival which was no better than results from previous trials.24,27
There are several important lessons learned from the conduct of S0533. First, we were unable to successfully integrate bevacizumab into concurrent CRT for Stage III NSCLC, particularly in patients considered at high risk for pulmonary hemorrhage, i.e., squamous cell carcinoma histology, central tumors, tumor cavitation. For patients considered at lower risk, the data are insufficient to determine efficacy or safety.
Caution should be taken when using bevacizumab for recurrent disease after previous treatment with concurrent CRT, assuming the primary endobronchial tumor remains. This may be especially true in patients who experienced severe esophagitis and/or pneumonitis during or after concurrent therapy considering several of the reported events of pulmonary hemorrhage and TE fistulae occurred long after initial treatment was complete.
There are numerous new molecularly targeted agents that are of interest in the treatment of NSCLC. With many of them, especially those with antiangiogenic activity, careful study design and close toxicity monitoring is imperative to properly integrate their use in multimodality therapy. Our experience with SWOG S0533 indicates that, with very careful planning, a trial of this nature can be safely conducted within a cooperative group setting.
Clinical Practice Points.
Concurrent CRT is the standard of care treatment for good performance status patients with locally advanced, inoperable NSCLC. Bevacizumab combined with chemotherapy has resulted in an improvement in survival for patients with metastatic NSCLC.
Our trial was conducted within the SWOG. Patients were identified as High and Low risk for hemoptysis and were accrued separately to these strata. The study was designed to define the appropriate timing and safety of the addition of bevacizumab to the CRT platform.
Bevacizumab could not be safely integrated with CRT for patients at High risk for hemoptysis. Among Low risk patients the data were insufficient to determine efficacy. Other investigators have come the same conclusion that the incorporation of bevacizumab is not recommended in the treatment paradigm for locally advanced NSCLC
Trials incorporating new agents that require close monitoring can be conducted within a cooperative group setting with the proper study design.
Acknowledgement
Supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA11083, CA42777, CA45808, CA12644, CA52654, CA46282, CA46113, CA58658, CA67575, CA63850, CA37981, CA46441, CA14028, and in part by Genentech (Roche)
Footnotes
Results presented in part at the 48th Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2012, Chicago, Illinois; American Society for Radiation Oncology Annual Meeting (October 28-31, 2012, Boston, MA); and Chicago Multidisciplinary Symposium in Thoracic Oncology (September 6-8, 2012, Chicago, IL)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small cell lung cancer. J Clin Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 2.Curran WJ, Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albain KS, Crowley JJ, Turrisi AT, et al. Concurrent cisplatin, etoposide plus radiotherapy for pathologic stage IIIB non-small cell lung cancer: a Southwest Oncology Group phase II study (S9019) J Clin Oncol. 2002;20:3454–3460. doi: 10.1200/JCO.2002.03.055. [DOI] [PubMed] [Google Scholar]
- 4.Gandara DR, Chansky K, Albain KS, et al. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small cell lung cancer: phase II Southwest Oncology Group study S9504. J Clin Oncol. 2003;21:2004–2010. doi: 10.1200/JCO.2003.04.197. [DOI] [PubMed] [Google Scholar]
- 5.Gandara DR, Chansky K, Albain KS, et al. Long-term survival with concurrent chemoradiation therapy followed by consolidation docetaxel in stage IIIB non-small cell lung cancer: a phase II Southwest Oncology Group study (S9504) Clin Lung Cancer. 2006;8:116–121. doi: 10.3816/CLC.2006.n.039. [DOI] [PubMed] [Google Scholar]
- 6.Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008;26:2450–2456. doi: 10.1200/JCO.2007.14.4824. [DOI] [PubMed] [Google Scholar]
- 7.Gandara DR, Vokes E, Green M, et al. Activity of docetaxel in platinum-treated non-small cell lung cancer: Results of a phase II multicenter trial. J Clin Oncol. 2000;18:131–135. doi: 10.1200/JCO.2000.18.1.131. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 9.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10934–10934. [PubMed] [Google Scholar]
- 10.Toi M, Matsumoto T, Bando H. Vascular endothelial growth factor: its prognostic, predictive, and therapeutic implications. Lancet Oncol. 2001;2:667–673. doi: 10.1016/S1470-2045(01)00556-3. [DOI] [PubMed] [Google Scholar]
- 11.Yuan A, Yu C-J, Kuo S-H, et al. Vascular endothelial growth factor 189 mRNA isoform expression specifically correlates with tumor angiogenesis, patient survival, and postoperative relapse in non-small cell lung cancer. J Clin Oncol. 2001;19:432–441. doi: 10.1200/JCO.2001.19.2.432. [DOI] [PubMed] [Google Scholar]
- 12.Cababe E, Wakelee H. Role of anti-angiogenesis agents in treating NSCLC: focus on bevacizumab and VEGFR tyrosine kinase inhibitors. Curr Treat Options Oncol. 2007;8:15–27. doi: 10.1007/s11864-007-0022-4. [DOI] [PubMed] [Google Scholar]
- 13.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2189. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 14.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 15.Geng L, Donnelly E, McMahon G, et al. Inhibition of vascular endothelial growth factor receptor signaling leads to reversal of tumor resistance to radiotherapy. Cancer Res. 2001;61:2413–2419. [PubMed] [Google Scholar]
- 16.Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 17.Williams KJ, Telfer BA, Brave S, et al. ZD6474, a potent inhibitor of vascular endothelial growth factor signaling, combined with radiotherapy: schedule-dependent enhancement of antitumor activity. Clin Cancer Res. 2004;10:8587–8593. doi: 10.1158/1078-0432.CCR-04-1147. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 20.Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol. 2008;26:5755–5760. doi: 10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 21.Sandler AB, Schiller JH, Gray R, et al. Retrospective evaluation of the clinical and radiographic risk factors associated with severe pulmonary hemorrhage in first-line advanced, unresectable non-small-cell lung cancer treated with carboplatin and paclitaxel plus bevacizumab. J Clin Oncol. 2009;27:1405–1412. doi: 10.1200/JCO.2008.16.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systemic review. J Clin Oncol. 2008;26:4001–4011. doi: 10.1200/JCO.2007.15.3312. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y-H, Kim E-Y, Ban H-J, et al. Risk factors for fatal hemoptysis after concurrent chemoradiation therapy in patients with non-small cell lung carcinoma. Chonnam Med J. 2010;46:19–24. [Google Scholar]
- 24.Socinski MA, Stinchcombe TE, Moore DT, et al. Incorporating bevacizumab and erlotinib in the combined-modality treatment of stage III non-small-cell lung cancer: results of a phase I/II trial. J Clin Oncol. 2012;30:3953–3959. doi: 10.1200/JCO.2012.41.9820. [DOI] [PubMed] [Google Scholar]
- 25.Patton JF, Spigel DR, Greco FA, et al. Irinotecan (I), carboplatin (C), and radiotherapy (RT) followed by maintenance bevacizumab (B) in the treatment (tx) of limited-stage small cell lung cancer (LS-SCLC): Update of a phase II trial of the Minnie Pearl Cancer Res Network. J Clin Oncol. 2006;24:385a. (suppl; abstr 7085) [Google Scholar]
- 26.Spigel DR, Hainsworth JD, Yardley DA, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol. 2009;28:43–48. doi: 10.1200/JCO.2009.24.7353. [DOI] [PubMed] [Google Scholar]
- 27.Socinski MA, Morris DE, Halle JS, et al. Induction and concurrent chemotherapy with high dose conformal radiation in unresectable stage IIIA and IIIB non-small-cell lung cancer: a dose escalation phase I/II trial. J Clin Oncol. 2004;22:4341–4350. doi: 10.1200/JCO.2004.03.022. [DOI] [PubMed] [Google Scholar]