Abstract
Mammalian skilled forelimb movements are remarkable in their precision, a feature that emerges from the continuous adjustment of motor output. Here we discuss recent progress in bridging the gap between theory and neural implementation in understanding the basis of forelimb motor refinement. One influential theory is that feedback from internal copy motor pathways enables fast prediction, through a forward model of the limb, an idea supported by behavioral studies that have explored how forelimb movements are corrected online and can adapt to changing conditions. In parallel, neural substrates of forelimb internal copy pathways are coming into clearer focus, in part through the use of genetically tractable animal models to isolate spinal and cerebellar circuits and explore their contributions to movement.
Introduction
One of the more impressive accomplishments of mammalian motor behavior is the ability to move the forelimb rapidly and precisely toward a target. A striking feature of these goal-directed reaching movements is a consistency in execution – for example, the trajectory and speed of the limb are remarkably regular trial after trial [1]. Moreover, this precision is adaptable – if the environment is altered, subsequent reaches are quickly recalibrated [2–4].
In principle, the motor system might accomplish such regularity through the use of feed-forward command pathways that translate motor plans into desired patterns of muscle contraction. However, the consistency of reaching movements is difficult to explain purely through feed-forward mechanisms, in part because motor commands are noisy and variable [5–8]. One way to compensate for this variability is to make use of feedback pathways that report the effects of motor commands, enabling motor output to be corrected (Figure 1). Indeed, corrective movements are evident throughout the course of a reach [9–11], implying the existence of neural feedback strategies that guide the continuous updating of limb movement.
Figure 1. Internal copy and sensory feedback pathways for forelimb motor control.
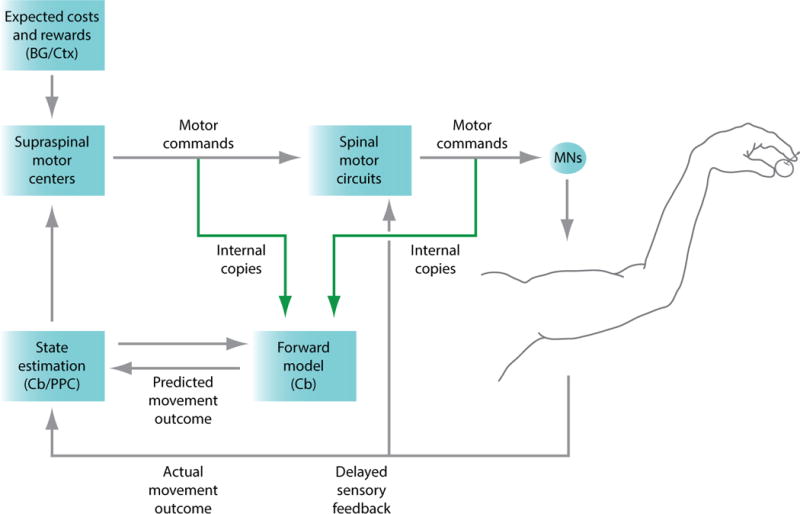
One putative model for the control of forelimb movements is an optimal feedback control scheme that incorporates internal copy feedback for forward model implementation. In this scenario, cortical and subcortical supraspinal motor centers convey feed-forward forelimb motor commands to spinal motor circuits, where these commands are further processed before modulating motor neuron (MN) activity and eliciting forelimb muscle contraction; these motor commands are subject to noise and variability. Sensory feedback from the limb conveys information about the effects of motor commands to local spinal circuits and to supraspinal sensory pathways, but is subject to peripheral and central temporal delays. To compensate for these delays, multiple motor command pathways give rise to collaterals that transmit internal copies (green arrows) to cerebellar (Cb) circuits, where they are used to generate predictions of the sensory consequences of motor commands (forward model). These forward model predictions can be combined with delayed sensory feedback to extrapolate an estimate of limb state (state estimation), a process thought to occur at least in part through communication between the cerebellum and posterior parietal cortex (PPC). State estimates are combined with an ongoing evaluation of movement cost and reward, potentially derived from basal ganglia (BG)–cortical (Ctx) pathways, to modulate motor cortex and generate motor command updates that are relevant to the current objectives of the task. In addition, by reducing the mismatch between predicted and actual movement outcome, forward models can be adapted to novel environmental conditions to refine subsequent movements [8,14,16,19].
Where do these feedback signals arise? Proprioception and vision provide the most conspicuous sources of feedback during movement, yet they carry inherent temporal delays that limit their use during very rapid corrections [12]. Faced with these temporal limitations, there has been much speculation about sources of more rapid feedback that can be deployed to update movement. One persuasive idea, called a forward model, is that motor commands are copied and conveyed internally to the cerebellum, where they are used to generate rapid predictions of the sensory consequences of motor actions (Figure 1). These predictions could, in theory, be used to update motor commands before sensory feedback arrives. Moreover, during longer movements, they could be merged with delayed sensory feedback to improve the estimate of current limb state, enabling more effective modification of motor output (for review see [12–15]).
Here we highlight recent behavioral studies that test and clarify potential contributions of forward models and internal feedback to forelimb motor control. We then describe experimental progress in defining their underlying neural substrates.
Forward models for forelimb movement
Forward models offer many theoretical benefits for motor and sensory systems [12–15], of which we focus on two: rapid online corrections within a movement, and flexible adaptation of future movements.
Rapid online correction
Several experimental systems have been used to evaluate the potential contribution of forward models to online motor correction. In principle, if an erroneous motor command is generated, a forward model can be used to predict the consequences and correct motor output during a movement without waiting for delayed sensory information. The study of saccades, rapid eye movements that are completed before visual feedback arrives, has provided substantial experimental support for this idea, revealing that corrective movements arise in the absence of sensory feedback [15]. In contrast, reaching movements typically last hundreds of milliseconds, providing ample time for sensory feedback to impact reach trajectory, and making it harder to disentangle the influence of internal and sensory feedback experimentally.
How can the contributions of internal copies and sensory feedback be reconciled to explain online reaching corrections? One way to view a role for all types of feedback, sensory and internal, is that they provide information necessary for estimating the state of the limb. An advantage of internal feedback in particular is that implementing a forward model can help overcome the temporal delays of sensory feedback. In this framework, delayed sensory information is integrated continuously with forward model output to extrapolate an instantaneous estimate of limb state [12,14,16] (Figure 1). Recent studies have provided experimental support for the hypothesis that state estimates are augmented by internal copy information, and therefore estimates made from sensory feedback alone will be less accurate. Bastian and colleagues compared the accuracy of subjects’ proprioceptive assessment of limb location during active movements, when the subject initiates motor commands and presumably generates internal copies, and passive movements, when the arm is moved externally and no commands or copies are generated. Subjects provide more accurate proprioceptive reports during active than passive movements [17], a phenomenon that is disrupted in cerebellar patients [18••], implying that internal copies conveyed to the cerebellum during self-initiated movements enhance the accuracy of limb state estimation.
A question that naturally follows is how do these state estimates contribute to the online updating of forelimb movement? An advance in the field has been the development of optimal feedback control theory, which provides a formal description of the relationship between motor commands, feedback, cost and reward during movement execution [16,19] (Figure 1). In this view, limb state estimates provide information used by motor command pathways to generate appropriate corrections, enabling a level of motor refinement beyond the capability of simple sensorimotor reflex arcs. Moreover, the ongoing evaluation of estimated costs and rewards of movement ensures that the corrections that are implemented are relevant and optimal given the current state of the limb [14–16,19] (Figure 1).
One major prediction of optimal feedback control is that environmental perturbations will trigger online corrections that depend not only on estimates of limb state, but also on the objectives of the task. Indeed, Nashed and colleagues found that corrective movements that arise ~50–100 ms after mid-reach perturbation of the limb differ depending on the spatial properties of the goal [20], and are flexible enough to redirect to an entirely new goal based on the estimated state of the limb [21•]. Similarly, by visually perturbing the location of the hand and target, Dimitriou and colleagues found that the strength of corrective responses is continually tuned to changing task demands [22•]. These studies provide evidence that feedback is used to generate rapid online corrections that account for the dynamics of the limb and help attain a goal with minimal effort. In principle, feedback control theory does not require that forward models be implemented for limb state estimation [8]. The temporal benefits of forward models and the evidence that internal copies can improve state estimation, however, make a compelling case for a motor control system that merges internal and external feedback for online movement correction [14,16] (Figure 1).
Adaptation of future movements
In addition to within-reach corrections, the forelimb motor system also exhibits considerable trial-to-trial adaptability [2–4], reshaping motor output to accommodate to changes like muscle fatigue and tool use. One way to achieve this motor adaptation would be to remap an existing forward model by comparing its output with sensory reports; by reducing mismatch between forward model predictions and actual sensory outcome, subsequent movements can progressively accommodate to changing conditions [2,15,23] (Figure 1).
How might forward model and sensory signals be represented and compared to drive adaptation? Recent studies from Smith and colleagues revealed that adaptation emerges from the actual movement experienced rather than the original motor plan [24], and that even when limb perturbation is not well approximated by spatial variables (e.g. position and velocity), internal models nevertheless represent limb dynamics in a spatial framework [25]. Exploring the role of sensory error signals, Izawa and Shadmehr provide evidence that while both sensory and reward prediction errors drive movement correction, only sensory errors elicit remapping of the forward model of the limb [26]. Moreover, using proprioceptive and visual perturbations, Marko and colleagues found that adaptation sensitivity decreases as the magnitude of sensory errors increases [27]. Together these findings shed light on the structure of forward model representations, and reveal that the type and degree of error have a considerable effect on the adaptive response.
The studies described above provide evidence that online corrections and across-trial adaptation exhibit features consistent with the use of forward models, raising the question of whether these two corrective motor strategies arise from the same or different processes. In separate studies, Magescas and colleagues and Yousif and Diedrichsen found that online corrections can arise with or without adaptation depending on the nature of perturbation, implying that these phenomena, and thus their underlying mechanisms, are dissociable [28,29]. Moreover, motor learning need not result from a forward model-based process at all; Huang and colleagues have shown that task success, independent of sensory error driven adaptation, can reshape motor output [30], supporting the existence of model-free approaches to motor learning [23,26]. Thus the implementation of forward models for online correction, the remapping of forward models for adaptation, and non-forward-model-based learning all appear to be important but separable phenomena that contribute to forelimb motor control.
Neural substrates for forward models and internal copies
Defining the neural basis of forelimb online correction and adaptation requires understanding how underlying neural circuits might convey internal copies and implement forward models. Substantial progress has been made using behavioral and electrophysiological approaches, complemented recently by the use of genetically tractable model systems to access and manipulate discrete motor circuits and examine the behavioral consequences. While we limit our discussion here to cerebellar and spinal circuits, we note that there is substantial evidence implicating multiple regions of the cerebral cortex in the integration of sensory and forward model cues for forelimb movement updating, as discussed elsewhere [31–34].
Cerebellum
The cerebellum represents one putative location for forelimb forward models; cerebellar cortical circuits have long been ascribed pattern recognition and adaptive properties [35–37], and damage or disruption of these pathways provides compelling evidence that they generate predictions of limb motor output [38–41]. How might neurons in the cerebellar cortex process internal copy and sensory information?
Cerebellar granule cells are presumptive recipients of internal copy signals, via mossy fiber inputs, providing one potential location for the convergence of sensory and copy information. Ekerot and Jörntell used electrophysiological recordings in the cat to identify granule cells that receive input specific for forelimb sensory modality and receptive field [42], suggesting that at least a subset of granule cells are specialized for discrete features of forelimb control. Moreover, Huang and colleagues used cell-type-specific axonal tracing in mice to identify sensory and brainstem motor pathways converging onto individual granule cells [43•], supporting the idea that these cells are equipped to integrate forelimb sensory feedback with internal copy information during movement.
Purkinje cells represent the output neurons of the cerebellar cortex, implying that their activity carries information central for motor refinement. Using a forelimb tracking task in monkeys, Popa and colleagues found that Purkinje simple spike firing, driven by granule cell input, correlates with both prediction of movement error and error feedback after movement [44], suggesting that Purkinje cells respond to multiple features of internal and external feedback [45]. Purkinje cell complex spikes, driven by climbing fiber input, are thought to encode error signals that modify internal models, and Yang and Lisberger provide evidence that the duration of complex spikes correlates with the degree of learning during monkey smooth pursuit eye movements [46•]. Supporting these findings, Najafi and colleagues used two-photon calcium imaging in mice during eyeblink conditioning to reveal that Purkinje cell dendritic signaling encodes instructive sensory stimuli probabilistically [47,48], providing further support that teaching signals are encoded in a graded manner by Purkinje cells. These graded responses might influence the type of motor learning that can occur; using an optogenetic approach in mice to activate climbing fibers during vestibulo-ocular reflex training, Raymond and colleagues showed that equivalent climbing fiber error signals can have distinct effects on motor adaptation [49,50••], likely depending on the state of Purkinje cells and their inputs. Together these studies clarify how Purkinje cell predictive and sensory signaling might reflect the substrate of a forward model, which can be adapted by error in a graded and context dependent manner.
If forward model signals are encoded in cerebellar cortex, this should be evident in cerebellar output. Studies of vestibular processing provide substantial support for the idea that deep cerebellar nuclei, which receive Purkinje cell input and contain the output neurons of the cerebellum, use internal models during movement to interpret sensory information [51]. Laurens and colleagues used electrophysiological and modeling approaches to show that Purkinje cell and deep cerebellar nuclei circuits in macaque encode erroneous acceleration during unnatural movement stimulation, suggesting that an internal model of gravity is implemented to avoid ambiguities inherent in vestibular signaling [52]. Moreover, Brooks and Cullen showed that sensory signals are cancelled in deep cerebellar nuclei during active but not passive movement in monkeys, implying that internal copy information is used to anticipate and distinguish self-initiated motion [53••]. Thus salient features of cerebellar output conform to the assignment of the cerebellum as a locus for forward model processing.
Spinal internal copy circuits
If cerebellar circuits implement a forward model of the limb, then cerebellar input pathways must supply the requisite internal copy information. We focus here on spinal motor circuits as a tractable location to look for forelimb internal copy pathways, given their direct proximity to motor neurons and close functional correspondence to motor output [54].
Spinal neurons whose axons convey copy signals have been identified in many species [55], suggesting a phylogenetically old strategy for generating internal feedback. Indeed, recent studies clarify roles for spinal copy pathways in filtering the sensory consequences of swimming movements in teleosts [56], and stabilizing ocular movements during locomotion in Xenopus [57]. From evolutionarily old origins, spinal internal copy pathways have presumably emerged and expanded in concert with the elaboration of mammalian limb movements. The ventral spinocerebellar tract has long been thought to convey internal copy information about rhythmic spinal activity to the cerebellum [58–60], whereas the dorsal spinocerebellar tract has been thought to mainly transmit sensory feedback [61,62]. Fedirchuk and colleagues, however, recently found that dorsal spinocerebellar tract neurons can also convey signals related to central rhythmic activity during fictive locomotion and scratching in the absence of sensory input [63], suggesting that these neurons may also be involved in conveying internal copy information. Moreover, recent work by Hantman and Jessell suggests that innervation of dorsal spinocerebellar tract neurons by descending motor copy pathways might provide a pre-cerebellar spinal locus for the anticipatory modulation of sensory feedback [64].
To date, cervical propriospinal neurons (PNs) represent the best characterized internal copy circuit involved in reaching movements. PNs receive input from descending motor command pathways and send bifurcated output; one axonal branch projects to forelimb-innervating motor neurons, and the other projects to the lateral reticular nucleus (LRN), a major pre-cerebellar relay [65,66] (Figure 2a). These dual projections provide an anatomically plausible substrate for conveying internal copies of pre-motor signals to the cerebellum [10].
Figure 2. Propriospinal neurons modulate forelimb movement through an internal copy feedback loop.
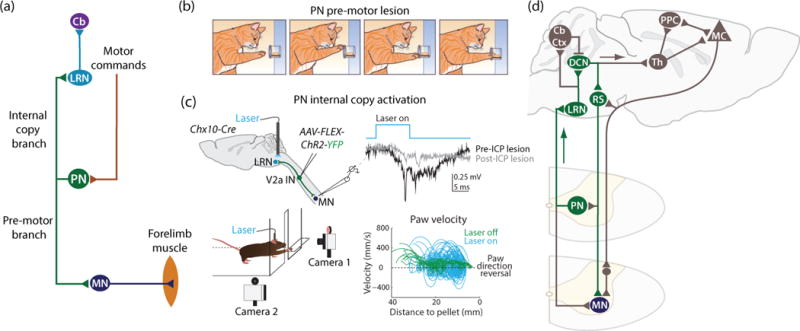
(a) PNs receive input from descending motor command pathways. Bifurcating PN axons innervate forelimb motor neurons (MN; pre-motor branch) and the lateral reticular nucleus (LRN; internal copy branch), which projects to the cerebellum (Cb). (b) After a mechanical lesion of the PN pre-motor axon branch, cats exhibit reaching ataxia as the paw approaches a food traget located inside of a tube [65]. (c) To activate the PN internal copy branch selectively, channelrhodopsin (ChR2) was expressed in PNs via injection of conditional virus (AAV-FLEX-ChR2-YFP) into cervical spinal cord of mice that selectively express Cre recombinase in Chx10+ V2a interneurons (IN). Photostimulation of ChR2+ PN terminals in the LRN activates the PN internal copy pathway without affecting the pre-motor PN branch [69]. Motor field potential recordings, as well as intracellular motor neuron recordings (not shown), during photostimulation reveal rapid forelimb motor neuron recruitment. These effects are substantially diminished by lesioning the inferior cerebellar peduncles (ICP), which contain projections from the LRN to the cerebellum, implicating a rapid cerebellar-motor feedback loop. Photoactivation of the PN-LRN pathway disrupts reaching movements (blue traces), quantified through highresolution 3D reconstruction of mouse reaching kinematics; movements exhibited a dramatic increase in the incidence of paw direction reversals and variation in velocity and acceleration during reaching without affecting digit abduction (not shown), suggesting a selective perturbation of circuits involved in reaching behavior. (d) Schematic of putative PN pathways as they might relate to feedback control (Figure 1). A rapid subcortical PN-LRN feedback loop (green) could modulate forelimb movement by recruiting deep cerebellar nuclei (DCN), which excite reticulospinal (RS) projections to motor neurons (MN). Additional loops (brown) might engage cerebellar cortex (Cb Ctx) and cerebral cortex via thalamus (Th), where posterior parietal cortex (PPC) and motor cortex (MC) communicate bidirectionally (see text). (b) modified from [65]; (c) modified from [69].
Lesion studies in cats have provided evidence that reaching movements are preferentially disrupted by severing PN pre-motor projections (Figure 2b), and suggest that one role of PNs is to correct reaching rapidly after a sudden change in target position [10,65]. In a recent study, Kinoshita and colleagues used a viral approach in primates to silence PN activity, revealing a disruption of both reaching and grasping [67••], suggesting that PN function may have expanded during evolution to control increasingly dexterous hand movements in primates [65]. An electrophysiological study in humans by Giboin and colleagues suggests that PN recruitment is enhanced during goal-directed reaching, and that PN inhibition by sensory feedback may help to update the descending command and terminate movement [68], supporting earlier findings in cats [10]. These studies provide substantial insight into evolutionarily conserved and divergent aspects of PN function, and raise the question of whether the PN internal copy pathway has any impact on forelimb movement (Figure 2a).
Addressing this question requires a means to access and manipulate the internal copy axonal branch, a task for which mouse genetics offers advantages over less selective electrical stimulation approaches. In a recent study, Azim and colleagues identified a population of excitatory mouse PNs within the genetically defined spinal V2a interneuron class [69••], a cardinal interneuron subtype involved in motor control. Employing this genetic access, they used optogenetic stimulation to activate the PN internal copy branch selectively, revealing that recruitment of the PN-LRN pathway modifies forelimb motor output through a rapid cerebellar feedback loop, eliciting severe disruption of reaching behavior (Figure 2c). The short latency of this pathway suggests a capacity for the PN internal copy circuit to update movement continuously during a reach. Intriguingly, Pivetta and colleagues found that in addition to V2a interneurons, a variety of genetically defined excitatory and inhibitory spinal interneuron subtypes in mice exhibit PN-like axonal bifurcation [70••], supporting the earlier identification of a separate inhibitory class of PNs in cats [65]. Moreover, these separate internal copy pathways organize into distinct target zones in the LRN [70], suggesting that LRN mossy fiber projection neurons are tasked with processing and integrating multiple channels of internal copy information [66]. These studies imply that the transmission of internal copy signals to cerebellar pathways is a common feature of spinal motor circuits, and the variety of cervical spinal cord neurons dedicated to this task could be a reflection of the diverse repertoire of mammalian forelimb behaviors.
Which pathways are recruited by PNs, and how might they correspond to putative neural substrates for internal and sensory feedback control (Figure 1)? An anatomically plausible rapid cerebellar feedback loop might involve collateral projections from LRN neurons to deep cerebellar nuclei, which excite reticulospinal projections to motor neurons [69,71–73] (Figure 2d). The extent to which this short pathway might correspond to forward model implementation remains unclear, but one advantage of such rapid subcortical positive feedback might be increased motor stability, as has been suggested for oculomotor control [74]. Additional longer latency feedback loops could conceivably involve the recruitment of cerebellar cortical circuits that use PN copy information to implement forward model predictions (Figure 2d). Moreover, a polysynaptic pathway from the dentate deep cerebellar nucleus to posterior parietal cortex and motor cortex, via thalamus, might provide a substrate for state estimation, facilitating the flexible updating of motor commands [14,16,31–33] (Figure 2d). Thus, while these ideas are speculative, we suspect that PN feedback loops are likely to be functionally diverse, further emphasizing the need for selective circuit access to resolve the grain of internal feedback control.
Future Directions
Closing the divide between theory and neural implementation in the study of forelimb internal copy pathways will require increasingly precise means for anatomical and functional dissection of neural circuits. We raise two of the central questions that remain, and suggest that the best way forward is through greater experimental and conceptual overlap between the behavioral and physiological precision of primate studies, and the expanding ability to characterize and manipulate discrete neural populations in behaving mice.
What types of motor information do internal copies convey? They could, for example, transmit kinematic (e.g. limb trajectory) or kinetic signals (e.g. muscle force). With the ability to perform fine-grained genetic dissection of internal copy pathways [69,70], combined with population-scale quantification of neural activity during behavior [47,48], mice provide one of the more promising models for discerning the information carried by internal copy pathways. These experiments, however, require more precise mouse behavioral assays combined with electromyography and kinematic quantification of forelimb movements to correlate internal copy circuit activity with specific aspects of motor behavior. The degree to which mouse behavioral methods can approach the precision of primate and cat studies, and whether mice exhibit similar abilities to correct and adapt their limb movements, are open questions that will ultimately decide how useful mice will be for the functional dissection of internal copy circuits.
How do internal copy pathways contribute to online correction and adaptation? Resolving the functional role of internal feedback requires not only defining how neural activity correlates with behavior, but also manipulating internal copy circuits during movement. Gain- and loss-of-function studies, in which internal copy pathways are activated and inactivated with high temporal precision during forelimb tasks, can help clarify how internal feedback modifies cerebellar activity and affects motor output. Optogenetic approaches in mice provide one way to achieve this interventional specificity [50,69]. Yet much progress can also be made in other species by expanding the use of viral tools to manipulate anatomically segregated neural pathways without the need for transgenic techniques [67]. Thus increasing the degree of experimental crosstalk between animal models offers substantial promise for a more complete description of the neural basis of forelimb motor control.
Highlights.
Refinement of forelimb movement is thought to rely on forward model predictions
Forward models use internal copies of motor commands to predict movement outcome
Internal copy-based predictions can support online correction and adaptation of limb movements
Mouse genetic tools are helping to resolve neural substrates of internal copy pathways
Acknowledgments
We thank T.M. Jessell, J.W. Krakauer, M. Dimitriou and J.A. Pruszynski for helpful discussions and feedback. E.A. was supported by a National Institutes of Health K99 award (NS088193). B.A. was supported by grants from the Swedish Research Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Morasso P. Spatial control of arm movements. Exp Brain Res. 1981;42:223–227. doi: 10.1007/BF00236911. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein NA. The co-ordination and regulation of movements. Oxford, New York: Pergamon Press; 1967. ed 1st English. [Google Scholar]
- 3.Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14:3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci. 2006;26:3642–3645. doi: 10.1523/JNEUROSCI.5317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. 1998;394:780–784. doi: 10.1038/29528. [DOI] [PubMed] [Google Scholar]
- 6.Jones KE, Hamilton AF, Wolpert DM. Sources of signal-dependent noise during isometric force production. J Neurophysiol. 2002;88:1533–1544. doi: 10.1152/jn.2002.88.3.1533. [DOI] [PubMed] [Google Scholar]
- 7.Xu-Wilson M, Zee DS, Shadmehr R. The intrinsic value of visual information affects saccade velocities. Exp Brain Res. 2009;196:475–481. doi: 10.1007/s00221-009-1879-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haith AM, Krakauer JW. Theoretical models of motor control and motor learning. In: G A, T W, BN J, editors. The Routledge Handbook of Motor Control and Motor Learning. Routledge; 2013. pp. 7–28. [Google Scholar]
- 9.Prablanc C, Martin O. Automatic control during hand reaching at undetected two-dimensional target displacements. J Neurophysiol. 1992;67:455–469. doi: 10.1152/jn.1992.67.2.455. [DOI] [PubMed] [Google Scholar]
- 10.Alstermark B, Lundberg A. The C3–C4 propriospinal system: target-reaching and food-taking. In: Jami L, Pierrot-Deseilligny E, Z D, editors. Muscle afferents and spinal control of movement. Pergamon; 1992. pp. 327–354. [Google Scholar]
- 11.Messier J, Kalaska JF. Comparison of variability of initial kinematics and endpoints of reaching movements. Exp Brain Res. 1999;125:139–152. doi: 10.1007/s002210050669. [DOI] [PubMed] [Google Scholar]
- 12.Wolpert DM, Miall RC. Forward Models for Physiological Motor Control. Neural Netw. 1996;9:1265–1279. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- 13.Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci. 2000;4:423–431. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- 14.Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci. 2010;33:89–108. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- 16.Scott SH. Optimal feedback control and the neural basis of volitional motor control. Nat Rev Neurosci. 2004;5:532–546. doi: 10.1038/nrn1427. [DOI] [PubMed] [Google Scholar]
- 17.Fuentes CT, Bastian AJ. Where is your arm? Variations in proprioception across space and tasks. J Neurophysiol. 2010;103:164–171. doi: 10.1152/jn.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Bhanpuri NH, Okamura AM, Bastian AJ. Predictive modeling by the cerebellum improves proprioception. J Neurosci. 2013;33:14301–14306. doi: 10.1523/JNEUROSCI.0784-13.2013. The authors find that cerebellar patients exhibit deficits in the precision of their proprioceptive sensation during active but not passive limb movement when compared to control subjects. Moreover, when control subjects are exposed to unpredictable forces during active movement, reducing the utility of predictive estimates, their proprioceptive precision returns to levels observed during passive movements. This study suggests that internal copy motor signals conveyed to the cerebellum during active movement can increase the fidelity of proprioception and improve the estimate of limb state. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci. 2002;5:1226–1235. doi: 10.1038/nn963. [DOI] [PubMed] [Google Scholar]
- 20.Nashed JY, Crevecoeur F, Scott SH. Influence of the behavioral goal and environmental obstacles on rapid feedback responses. J Neurophysiol. 2012;108:999–1009. doi: 10.1152/jn.01089.2011. [DOI] [PubMed] [Google Scholar]
- 21•.Nashed JY, Crevecoeur F, Scott SH. Rapid online selection between multiple motor plans. J Neurosci. 2014;34:1769–1780. doi: 10.1523/JNEUROSCI.3063-13.2014. Using an obstacle avoidance reaching paradigm in humans, the authors find long-latency feedback responses (<60 ms after a limb perturbation) that redirect limb trajectory around an obstacle, consistent with the use of an estimate of the new position of the limb to update movement. Moreover, slightly longer latency feedback responses are capable of redirecting the limb to an entirely new target after perturbation. These findings support the idea that an internal model of the limb is merged with delayed sensory feedback information to extrapolate an estimate of limb state, enabling new motor strategies to be rapidly implemented. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Dimitriou M, Wolpert DM, Franklin DW. The temporal evolution of feedback gains rapidly update to task demands. J Neurosci. 2013;33:10898–10909. doi: 10.1523/JNEUROSCI.5669-12.2013. The authors quantify visuomotor feedback gain in humans performing reaching movements, finding that gains are modulated throughout the course of movement according to the distance between the visual position of the hand and target. Moreover, feedback gains are rapidly recomputed if target location is suddenly switched during movement. These findings are consistent with the predictions of optimal feedback control in which limb state estimates and cost minimization are computed throughout the movement, resulting in appropriate feedback gain levels that evolve over the course of a goal-directed reach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haith AM, Krakauer JW. Model-based and model-free mechanisms of human motor learning. In: R MJ, R MA, S K, editors. Progress in Motor Control VII: Neural Computational and Dynamic Approaches. 2013. pp. 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez Castro LN, Monsen CB, Smith MA. The binding of learning to action in motor adaptation. PLoS Comput Biol. 2011;7:e1002052. doi: 10.1371/journal.pcbi.1002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sing GC, Orozco SP, Smith MA. Limb motion dictates how motor learning arises from arbitrary environmental dynamics. J Neurophysiol. 2013;109:2466–2482. doi: 10.1152/jn.00497.2011. [DOI] [PubMed] [Google Scholar]
- 26.Izawa J, Shadmehr R. Learning from sensory and reward prediction errors during motor adaptation. PLoS Comput Biol. 2011;7:e1002012. doi: 10.1371/journal.pcbi.1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marko MK, Haith AM, Harran MD, Shadmehr R. Sensitivity to prediction error in reach adaptation. J Neurophysiol. 2012;108:1752–1763. doi: 10.1152/jn.00177.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magescas F, Urquizar C, Prablanc C. Two modes of error processing in reaching. Exp Brain Res. 2009;193:337–350. doi: 10.1007/s00221-008-1629-9. [DOI] [PubMed] [Google Scholar]
- 29.Yousif N, Diedrichsen J. Structural learning in feedforward and feedback control. J Neurophysiol. 2012;108:2373–2382. doi: 10.1152/jn.00315.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang VS, Haith A, Mazzoni P, Krakauer JW. Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron. 2011;70:787–801. doi: 10.1016/j.neuron.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersen RA, Snyder LH, Bradley DC, Xing J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci. 1997;20:303–330. doi: 10.1146/annurev.neuro.20.1.303. [DOI] [PubMed] [Google Scholar]
- 32.Mulliken GH, Musallam S, Andersen RA. Forward estimation of movement state in posterior parietal cortex. Proc Natl Acad Sci U S A. 2008;105:8170–8177. doi: 10.1073/pnas.0802602105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pruszynski JA, Kurtzer I, Nashed JY, Omrani M, Brouwer B, Scott SH. Primary motor cortex underlies multi-joint integration for fast feedback control. Nature. 2011;478:387–390. doi: 10.1038/nature10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.London BM, Miller LE. Responses of somatosensory area 2 neurons to actively and passively generated limb movements. J Neurophysiol. 2013;109:1505–1513. doi: 10.1152/jn.00372.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albus JS. A theory of cerebellar function. Mathematical Biosciences. 1971;10:25–61. [Google Scholar]
- 37.Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Miall RC, Christensen LO, Cain O, Stanley J. Disruption of state estimation in the human lateral cerebellum. PLoS Biol. 2007;5:e316. doi: 10.1371/journal.pbio.0050316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol. 2007;98:54–62. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- 40.Bastian AJ. Moving, sensing and learning with cerebellar damage. Curr Opin Neurobiol. 2011;21:596–601. doi: 10.1016/j.conb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herzfeld DJ, Pastor D, Haith AM, Rossetti Y, Shadmehr R, O’Shea J. Contributions of the cerebellum and the motor cortex to acquisition and retention of motor memories. Neuroimage. 2014;98:147–158. doi: 10.1016/j.neuroimage.2014.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ekerot CF, Jörntell H. Synaptic integration in cerebellar granule cells. Cerebellum. 2008;7:539–541. doi: 10.1007/s12311-008-0064-6. [DOI] [PubMed] [Google Scholar]
- 43•.Huang CC, Sugino K, Shima Y, Guo C, Bai S, Mensh BD, Nelson SB, Hantman AW. Convergence of pontine and proprioceptive streams onto multimodal cerebellar granule cells. Elife (Cambridge) 2013;2:e00400. doi: 10.7554/eLife.00400. Using a combination of genetic and viral tools for cell-type-specific tracing, the authors find that basilar pontine and proprioceptive pathways associated with upper body motor and sensory signaling, respectively, converge onto individual granule cells. Moreover, they provide evidence that these basilar pontine circuits receive input from internal copy pathways originating in motor cortex. These findings offer a detailed anatomical map of multimodal projections to the cerebellum, providing insight into how internal model input pathways might be organized. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Popa LS, Hewitt AL, Ebner TJ. Predictive and feedback performance errors are signaled in the simple spike discharge of individual Purkinje cells. J Neurosci. 2012;32:15345–15358. doi: 10.1523/JNEUROSCI.2151-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medina JF. The multiple roles of Purkinje cells in sensori-motor calibration: to predict, teach and command. Curr Opin Neurobiol. 2011;21:616–622. doi: 10.1016/j.conb.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Yang Y, Lisberger SG. Purkinje-cell plasticity and cerebellar motor learning are graded by complex-spike duration. Nature. 2014;510:529–532. doi: 10.1038/nature13282. Using electrophysiological recording in the cerebellar floccular complex of monkeys performing smooth-pursuit eye movements, the authors reveal that Purkinje cell plasticity and motor learning occur in a graded manner, dependent on the duration of the Purkinje cell complex spike response. These findings reveal that errors are not signaled as all-or-nothing events, and more broadly, they provide finer detail into the physiological basis for motor adaptation and learning within cerebellar circuits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Najafi F, Giovannucci A, Wang SS, Medina JF. Sensory-driven enhancement of calcium signals in individual Purkinje cell dendrites of awake mice. Cell Rep. 2014;6:792–798. doi: 10.1016/j.celrep.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Najafi F, Giovannucci A, Wang SS, Medina JF. Coding of stimulus strength via analog calcium signals in Purkinje cell dendrites of awake mice. Elife. 2014;3:e03663. doi: 10.7554/eLife.03663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen-Vu TD, Kimpo RR, Rinaldi JM, Kohli A, Zeng H, Deisseroth K, Raymond JL. Cerebellar Purkinje cell activity drives motor learning. Nat Neurosci. 2013;16:1734–1736. doi: 10.1038/nn.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Kimpo RR, Rinaldi JM, Kim CK, Payne HL, Raymond JL. Gating of neural error signals during motor learning. Elife. 2014;3:e02076. doi: 10.7554/eLife.02076. The authors use optogenetic tools in mice to compare vestibulo-ocular reflex motor learning paradigms that direct either an increase or a decrease in the motor response. They find that imposing equivalent climbing fiber error signals via optogenetic photostimulation drives learning in the gain-increase but not decrease paradigm, suggesting that downstream processes in Purkinje neurons, potentially influenced by mossy fiber-granule cell input pathways, can regulate motor adaptation efficacy. Moreover, this study highlights the potential for circuit specific manipulations in mice to explore causality in the relationship between circuit activity and motor output. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cullen KE. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 2012;35:185–196. doi: 10.1016/j.tins.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laurens J, Meng H, Angelaki DE. Computation of linear acceleration through an internal model in the macaque cerebellum. Nat Neurosci. 2013;16:1701–1708. doi: 10.1038/nn.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53••.Brooks JX, Cullen KE. The primate cerebellum selectively encodes unexpected self-motion. Curr Biol. 2013;23:947–955. doi: 10.1016/j.cub.2013.04.029. The authors record from deep cerebellar nuclei neurons in monkeys during active and passively applied movements that elicit vestibular and proprioceptive feedback. They find that individual neurons that normally encode unexpected sensory stimuli during passive movements are unresponsive when these same movements are self-generated. Moreover, during simultaneous active and passive motion, neurons selectively respond to the unexpected component of sensory feedback, encoding its time course in detail. These findings provide compelling experimental evidence that cerebellar output reflects the computation of sensory prediction error, a hallmark of forward model adaptation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spanne A, Jörntell H. Processing of Multi-dimensional Sensorimotor Information in the Spinal and Cerebellar Neuronal Circuitry: A New Hypothesis. PLoS Comput Biol. 2013;9:e1002979. doi: 10.1371/journal.pcbi.1002979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poulet JF, Hedwig B. New insights into corollary discharges mediated by identified neural pathways. Trends Neurosci. 2007;30:14–21. doi: 10.1016/j.tins.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Requarth T, Sawtell NB. Plastic corollary discharge predicts sensory consequences of movements in a cerebellum-like circuit. Neuron. 2014;82:896–907. doi: 10.1016/j.neuron.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Uckermann G, Le Ray D, Combes D, Straka H, Simmers J. Spinal Efference Copy Signaling and Gaze Stabilization during Locomotion in Juvenile Xenopus Frogs. J Neurosci. 2013;33:4253–4264. doi: 10.1523/JNEUROSCI.4521-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lundberg A. Function of the ventral spinocerebellar tract. A new hypothesis. Exp Brain Res. 1971;12:317–330. doi: 10.1007/BF00237923. [DOI] [PubMed] [Google Scholar]
- 59.Arshavsky YI, Gelfand IM, Orlovsky GN, Pavlova GA. Messages conveyed by spinocerebellar pathways during scratching in the cat. II. Activity of neurons of the ventral spinocerebellar tract. Brain Res. 1978;151:493–506. doi: 10.1016/0006-8993(78)91082-x. [DOI] [PubMed] [Google Scholar]
- 60.Jankowska E, Hammar I. Interactions between spinal interneurons and ventral spinocerebellar tract neurons. J Physiol. 2013;591:5445–5451. doi: 10.1113/jphysiol.2012.248740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arshavsky YI, Gelfand IM, Orlovsky GN. Cerebellum and rhythmical movements. Berlin; New York: Springer-Verlag; 1986. [Google Scholar]
- 62.Bosco G, Poppele RE. Proprioception from a spinocerebellar perspective. Physiol Rev. 2001;81:539–568. doi: 10.1152/physrev.2001.81.2.539. [DOI] [PubMed] [Google Scholar]
- 63.Fedirchuk B, Stecina K, Kristensen KK, Zhang M, Meehan CF, Bennett DJ, Hultborn H. Rhythmic activity of feline dorsal and ventral spinocerebellar tract neurons during fictive motor actions. J Neurophysiol. 2013;109:375–388. doi: 10.1152/jn.00649.2012. [DOI] [PubMed] [Google Scholar]
- 64.Hantman AW, Jessell TM. Clarke’s column neurons as the focus of a corticospinal corollary circuit. Nat Neurosci. 2010;13:1233–1239. doi: 10.1038/nn.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alstermark B, Isa T. Circuits for skilled reaching and grasping. Annu Rev Neurosci. 2012;35:559–578. doi: 10.1146/annurev-neuro-062111-150527. [DOI] [PubMed] [Google Scholar]
- 66.Alstermark B, Ekerot CF. The Lateral Reticular Nucleus: a precerebellar center providing the Cerebellum with overview and integration of motor functions at systems level. A new hypothesis. J Physiol. 2013;591:5453–5458. doi: 10.1113/jphysiol.2013.256669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67••.Kinoshita M, Matsui R, Kato S, Hasegawa T, Kasahara H, Isa K, Watakabe A, Yamamori T, Nishimura Y, Alstermark B, et al. Genetic dissection of the circuit for hand dexterity in primates. Nature. 2012;487:235–238. doi: 10.1038/nature11206. Using a combinatorial tetanus toxin viral approach in monkeys to selectively and reversibly silence PNs, the authors reveal a disruption of reaching and grasping movements. This study implicates primate PNs in an indirect pathway from neocortex to forelimb motor neurons involved in the control of dexterous limb and hand movements. More broadly, the authors demonstrate the effective use of viral tools for circuit specific functional dissection in primates. [DOI] [PubMed] [Google Scholar]
- 68.Giboin LS, Lackmy-Vallee A, Burke D, Marchand-Pauvert V. Enhanced propriospinal excitation from hand muscles to wrist flexors during reach-to-grasp in humans. J Neurophysiol. 2012;107:532–543. doi: 10.1152/jn.00774.2011. [DOI] [PubMed] [Google Scholar]
- 69••.Azim E, Jiang J, Alstermark B, Jessell TM. Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature. 2014;508:357–363. doi: 10.1038/nature13021. The authors use mouse genetic tools to identify a population of excitatory PNs within the genetically defined class of V2a spinal interneurons, and find that cervical V2a interneuron ablation selectively disrupts reaching. Moreover, optogenetic activation of only the PN internal copy axon branch activates forelimb motor neurons and modifies reaching through a short latency cerebellar-motor feedback loop. These findings provide direct evidence that a forelimb internal copy-cerebellar pathway can modulate motor output with rapidity suitable for the online updating of forelimb movement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70••.Pivetta C, Esposito MS, Sigrist M, Arber S. Motor-circuit communication matrix from spinal cord to brainstem neurons revealed by developmental origin. Cell. 2014;156:537–548. doi: 10.1016/j.cell.2013.12.014. Using intersectional genetic and viral tools in mice, the authors reveal that diverse classes of excitatory and inhibitory spinal interneurons send bifurcating projections to forelimb motor neurons and the LRN, anatomical features characteristic of PNs implicated in the control of reaching movements. Moreover, PN axon terminals are segregated into distinct LRN territories based on neuronal subtype identity, suggesting that copy signals are organized into discrete channels, potentially reflecting the diverse internal feedback demands of forelimb motor circuits. [DOI] [PubMed] [Google Scholar]
- 71.Schultz W, Montgomery EB, Jr, Marini R. Proximal limb movements in response to microstimulation of primate dentate and interpositus nuclei mediated by brain-stem structures. Brain. 1979;102:127–146. doi: 10.1093/brain/102.1.127. [DOI] [PubMed] [Google Scholar]
- 72.Wu HS, Sugihara I, Shinoda Y. Projection patterns of single mossy fibers originating from the lateral reticular nucleus in the rat cerebellar cortex and nuclei. J Comp Neurol. 1999;411:97–118. doi: 10.1002/(sici)1096-9861(19990816)411:1<97::aid-cne8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 73.Alstermark B, Ogawa J. In vivo recordings of bulbospinal excitation in adult mouse forelimb motoneurons. J Neurophysiol. 2004;92:1958–1962. doi: 10.1152/jn.00092.2004. [DOI] [PubMed] [Google Scholar]
- 74.Lisberger SG. Internal models of eye movement in the floccular complex of the monkey cerebellum. Neuroscience. 2009;162:763–776. doi: 10.1016/j.neuroscience.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]


