Abstract
Electronic shared medical records (SMR) are emerging healthcare technologies that allow patients to engage in their healthcare by communicating with providers, refilling prescriptions, scheduling appointments, and viewing portions of medical records. We conducted a pre-post cohort study of HIV-positive adults who used and did not use SMR in two integrated healthcare systems. We compared the difference in antiretroviral refill adherence between SMR users and age- and sex-frequency matched non-users from the 12-month period prior to SMR use to the 12-month period starting six months after initiation of SMR use. High adherence was maintained among SMR users (change=−0.11%) but declined among non-users (change=−2.05%; p=0.003). Among SMR users, there was a steady improvement in adherence as monthly frequency of SMR use increased (p=0.009). SMR use, particularly more frequent use, is associated with maintaining high adherence and non-use is associated with declines in adherence over time among patients with access to these online services.
Keywords: HIV, electronic health records, medication adherence, antiretroviral therapy, integrated healthcare system
INTRODUCTION
The biggest threat to successful HIV treatment is non-adherence to antiretroviral therapy (ART), as non-adherence remains one of the strongest predictors of progression to AIDS and death1–3. Additionally, poor engagement in HIV care has been associated with delayed initiation of ART and non-adherence4,5. In the U.S., ART non-adherence is estimated to be in the 10% to 50% range6,7 and has been reported to account for $1.8 billion in annual avoidable costs8.
Prior research has shown that people living with HIV who used the internet for health-related purposes were significantly more likely to adhere to their ART regimen in the past week than those who did not use the internet for health-related purposes9. Additionally, self-care technology-based methods have the potential for improving engagement in care and enhanced adherence10. Therefore, healthcare systems that provide technology-based methods that enable patients to effectively and easily communicate with their healthcare providers, access laboratory test results, and request medication refills may result in improved engagement in care and adherence.
Patient websites or portals that provide secure access to sections of electronic medical records that are shared between patients and healthcare providers, also known as shared medical records (SMR), are emerging healthcare technologies. SMRs are a component of electronic medical records that allow patients to communicate with providers, refill medications, schedule appointments, and view portions of their medical record, including laboratory test results. An increasing number of healthplans are anticipated to offer SMR services in order to qualify for Stage 2 Meaningful Use Incentive Program under the Affordable Care Act11. Prior research has examined the efficiencies and positive impact of SMR in primary care12,13 and other chronic conditions, including diabetes14,15, hypertension16, and depression17. Therefore, these online services may help meet ongoing healthcare needs of HIV-positive patients in many circumstances, such as when initiating a new ART regimen or experiencing adverse effects.SMR may ultimately improve engagement in HIV care and ART adherence and may be valuable in supporting disease management and self-care. Although the use of SMR by HIV-positive individuals has been previously described18, the association between SMR use and HIV-related outcomes has not been examined. Thus, our objective was to determine whether SMR use (versus no use) and the frequency of SMR use were associated with changes in ART adherence in HIV-positive individuals.
METHODS
Design
We conducted a pre-post cohort study of HIV-positive adults who used SMR within two years of initial SMR rollout in two large integrated healthcare systems, Kaiser Permanente Northern California (KPNC) and Group Health Cooperative (GHC). We compared changes in ART refill adherence from the 12-month period prior to SMR use (pre-interval) to the 12-month period starting six months after initiation of SMR use (post-interval). The six-month period post-SMR rollout was considered a“confirmation stage in adoption”19 of this emerging technology, and therefore excluded from adherence calculations. This helped to ensure that we were measuring adherence in the post-interval most likely to be influenced by SMR use, allowing enough time for SMR users to both gain confidence in use of the SMR features and to establish a personal SMR use routine.
Our primary objective was to compare refill adherence change between SMR users and age- and sex-frequency matched non-users pre- and post-SMR use (or a randomly assigned reference date in SMR non-users). Additionally, among SMR users, we evaluated the association between mean frequency of SMR use (i.e., mean number of days per month using any SMR service over a six-month period) and refill adherence change, as well as the factors associated with changes in adherence pre- and post-SMR use.
Setting and Participants
GHC is an integrated healthcare delivery system with over half a million members in Washington and North Idaho. KPNC is a large integrated healthcare delivery system that provides comprehensive medical services to over three million members in Northern California. In total, these two organizations provide healthcare to more than 15,500 people living with HIV in the U.S. and maintain a similar confidential HIV registry of these individuals, as well as hospital, pharmacy, laboratory, and administrative databases. Over 90% of members obtain their prescription medications from KPNC and GHC pharmacies20,21. These healthcare systems have robust and comprehensive HIV care programs that have demonstrated previous success with high levels of ART adherence and viral suppression among their members22,23. Key elements of this successful care have been the multidisciplinary care team and electronic health records22,24.
SMR became available to all patients at GHC (MyGroupHealth.org) and KPNC (KP.org) in August 2003 and November 2005, respectively. As these healthplans were early adopters of SMRs, they offer the ideal settings to evaluate the effectiveness of this emerging technology on health outcomes. SMR in these healthplans have seven common features, including: secure messaging with healthcare providers; requesting medication refills; scheduling appointments with healthcare providers; and viewing after-visit summaries, allergies, immunizations, and laboratory test results. The descriptions of these web services have previously been reported18,25–27.
In our study, we first identified a cohort of SMR users consisting of HIV-positive adults (≥18 years) who had: 1) completed the enrollment process to use the online services at MyGroupHealth.org or KP.org; 2) used one or more of the seven SMR functions sometime during the first 24 months post-SMR rollout (8/1/2003 for GHC and 11/1/2005 for KPNC); 3) enrolled in the healthplan at least 12 months prior to the date of first SMR use and maintained enrollment for at least 18 months after the date of first SMR use (to ensure sufficient length of time for adherence calculation in the pre- and post-intervals); and 4) started ART at least 12 months prior to the first SMR use. We examined the association between mean frequency of SMR use and refill adherence change pre- and post-interval, as well as the factors associated with changes in adherence in this cohort of SMR users.
We first compared refill adherence change between SMR users and age- and sex-frequency matched non-users. SMR non-users were HIV-positive adults who met the abovementioned inclusion criteria, except for the fact that they had not registered for SMR during the first 36 months post-SMR rollout. The 36-month period was chosen to ensure a sufficient timeframe to establish SMR non-use. For this comparison, to ensure comparability with the extended 36-month timeframe of SMR non-use, we created a restricted SMR user group that included members of the SMR user cohort with the additional inclusion criteria of at least 36 months healthplan membership post-SMR rollout. SMR non-users were frequency matched to the restricted SMR user group according to age group (i.e., 18–29, 30–39, 40–49, and ≥50 years), sex, and number of months since SMR rollout. We randomly assigned an index date for non-users within the month they were selected as a non-user, in order to compute pre- and post-interval adherence measurements, analogous to pre- and post-SMR adherence measurements for users.
Institutional Review Board approval including waivers of informed consent was obtained from both institutions.
Main Measures
The difference in refill adherence change pre- and post-SMR use (for users) or before and after a randomly assigned reference date (for non-users) constituted our primary outcome measure. The pharmacy databases provided refill dates for each antiretroviral medication. Our refill adherence measure was computed using previously described methods28–30 and involved computing percent refill adherence for each antiretroviral during the specified 12-month pre- and post-intervals. Specifically, for individual antiretrovirals, we first computed the continuous measure of medication gaps29 using a numerator of days’ supply dispensed from first fill to end of interval, and a denominator of total days between first fill to end of interval. Such an approach assumes that any observed gap in medication coverage prior to the end of an interval (i.e., terminal gap) is due to non-adherence. Therefore, we looked for evidence to more accurately distinguish between terminal gaps that represented true non-adherence or a change in ART, similar to a validated approach previously described30. Briefly, if the original antiretroviral was re-prescribed within 60 days of the start of the terminal gap, we did not adjust the denominator and assumed that the terminal gap did represent non-adherence. However, if a new antiretroviral was prescribed, we assumed that there was a medication change, and adjusted the denominator to either: (a) the end of the last fill of the original antiretroviral if the new antiretroviral was prescribed within 60 days of the start of the terminal gap; or (b) the start of the new antiretroviral fill if the new antiretroviral was prescribed more than 60 days of the start of the terminal gap. Finally, we computed the overall refill adherence during an interval as the mean refill adherence across all individual antiretrovirals31.
Our predictors of interest included: (1) SMR use versus no use and (2) frequency of SMR use (mean SMR use <0.5, 0.5 to <1, 1 to <2, or ≥2 times per month over a six-month period). Potential confounders considered in analyses included age (18–39, 40–49, 50–59, and ≥60 years of age), sex (women versus men), race/ethnicity (Asian/Pacific Islander, Black, Hispanic, White, and other/unknown), baseline CD4+ cell count (<200, 200–499, and ≥500 cells/mm3), baseline plasma HIV RNA (≤500, 501–9,999, and ≥10,000 copies/mL), and healthplan membership (GHC versus KPNC).
Analysis
Initially, we used descriptive statistics to characterize demographics of SMR user sandnon-users. Next, we estimated “refill adherence change” for the restricted SMR user group and SMR non-users, calculated by subtracting refill adherence pre-interval from refill adherence post-interval in each group. A linear regression model was then used to estimate the unadjusted “difference” in refill adherence change between SMR users and non-users, interpreted as refill adherence change of the restricted SMR user group minus refill adherence change of SMR non-users. We then employed multivariable linear regression, adjusted for potential confounders, to compute an adjusted difference in refill adherence comparing users and non-users. Next, among the SMR users and non-users, we estimated refill adherence change and the difference in refill adherence change stratified by baseline CD4+ cell count (≥200 versus <200 cells/mm3) and plasma HIV RNA (<500 versus ≥500 copies/mL). Lastly, among SMR users only, using linear regression, we evaluated factors associated with refill adherence changes, including mean frequency of SMR use, age, sex, race/ethnicity, baseline CD4 and HIV RNA, and healthplan.
For all analyses, a p-value <0.05 was deemed statistically significant. We used SAS software version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
We identified 1,638 HIV-positive patients in the SMR user cohort, who had a mean age of 49 years and were primarily men (94%) and White (76%). At baseline, mean ART refill adherence was 90% (standard deviation [SD]= 13%), mean CD4+ cell count was 524 cells/mm3, and 91% had plasma HIV RNA below the limit of quantification. Ninety percent were enrolled in KPNC and 10% at GHC. In the restricted SMR user group, we included 1,453 individuals with at least 36 months membership post SMR-rollout, and identified 1,014 age- and sex-frequency matched SMR non-users (Table 1). Baseline adherence was slightly lower among non-users compared with users (88% versus 90%; P<0.001). Race/ethnicity, healthplan, and baseline CD4+ cell count were also significantly different between the two groups.
Table 1.
Baseline characteristics comparing the restricted SMR user group* and SMR non-users
| Characteristic | SMR non-users (N = 1,014) |
Restricted SMR user group (N = 1,453) |
p-value |
|---|---|---|---|
| Age, mean years (SD) | 49 (10) | 49 (9) | 0.38 |
| Men, % | 94 | 94 | 0.83 |
| KPNC/GHC, % | 96/4 | 91/9 | <0.001 |
| Race/ethnicity, % | <0.001 | ||
| Asian/Pacific Islander | 5 | 3 | |
| Black | 21 | 8 | |
| Latino | 19 | 10 | |
| White | 52 | 76 | |
| Other | 3 | 3 | |
| ART refill adherence, mean % (SD) | 88 (15) | 90 (13) | <0.001 |
| CD4+ cell count, mean cells/mm3 (SD) | 493 (267) | 525 (261) | 0.003 |
| Plasma HIV RNA <75 copies/mL, % | 90 | 91 | 0.74 |
SMR users restricted to those with ≥36 months of health plan enrollment following SMR rollout
Table 2 displays the refill adherence changes and differences in refill adherence change between the pre- and post-intervals for the restricted SMR user group and SMR non-users, both overall and stratified by CD4+ cell counts and plasma HIV RNA. Between the pre- and post-intervals, high adherence was maintained among the restricted group of SMR users (refill adherence change= −0.11%; 95% CI= −0.83, 0.62); however, mean adherence declined among SMR non-users (refill adherence change = −2.05%; 95% CI = −2.92, −1.18). The corresponding difference in refill adherence change when comparing SMR users and nonusers was 1.94% (95% CI = 0.81, 3.07; p<0.001) in unadjusted models and 1.80% (95% CI = 0.62, 2.98; p = 0.003) with adjustment for potential confounders. Among those with CD4+ cell count ≥200 cells/mm3, SMR users maintained high levels of adherence (refill adherence change = −0.22%; 95% CI = −0.94, 0.50) but SMR non-users had a reduction in refill adherence (refill adherence change = −2.31%; 95% CI = −3.20, −1.42). This reduction corresponds to an unadjusted refill adherence change of 2.09% (95% CI = 0.95, 3.23; p<0.001) and adjusted refill adherence change of 1.91% (95% CI = 0.72, 3.10; p = 0.002). Similarly, among those with plasma HIV RNA <500 copies/mL, SMR users maintained high levels of adherence (refill adherence change = −0.36%; 95% CI = −1.08, 0.35) but SMR non-users had a decline in refill adherence (refill adherence change = −2.30%; 95% CI = −3.16, −1.44), corresponding with an unadjusted difference in refill adherence change of 1.94% (95% CI = 0.82, 3.05; p<0.001), and adjusted refill adherence change of 1.78% (95% CI = 0.62, 2.95; p = 0.003). There was no difference in refill adherence change between the restricted group of SMR users and non-users who had a baseline CD4+ cell count <200 cells/mm3 or plasma HIV RNA ≥500 cells/mL.
Table 2.
Adjusted1 and unadjusted pre- to post-interval percent refill adherence changes2 and differences in percent refill adherence change3 among the restricted SMR user group4 and non-users overall and stratified by baseline CD4+ cell count and plasma HIV RNA
| Unadjusted | ||||
|---|---|---|---|---|
| Percent Refill Adherence Change2 (95% CI) |
Differences in Percent Refill Adherence Change3 (95% CI) |
p-value | Differences Refill |
|
| Overall | ||||
| SMR non-users (N = 1,014) | −2.05 (−2.92, −1.18) | Reference | Reference | |
| SMR users (N = 1,453) | −0.11 (−0.83, 0.62) | 1.94 (0.81, 3.07) | <0.001 | 1.80 |
| Baseline CD4+ <200 cells/mm3 | ||||
| SMR non-users (N = 116) | −0.01 (−3.51, 3.49) | Reference | Reference | |
| SMR users (N = 101) | 1.40 (−2.35, 5.15) | 1.42 (−3.68, 6.51) | 0.59 | 1.33 |
| Baseline CD4+ cell count ≥200 cells/mm3 | ||||
| SMR non-users (N = 898) | −2.31 (−3.20, −1.42) | Reference | Reference | |
| SMR users (N = 1,352) | −0.22 (−0.94, 0.50) | 2.09 (0.95, 3.23) | 0.0003 | 1.91 |
| Baseline plasma HIV RNA <500 copies/mL | ||||
| SMR non-users (N = 915) | −2.30 (−3.16, −1.44) | Reference | Reference | |
| SMR users (N = 1,317) | −0.36 (−1.08, 0.35) | 1.94 (0.82, 3.05) | <0.001 | 1.78 |
| Baseline plasma HIV RNA ≥500 copies/mL | ||||
| SMR non-users (N = 99) | 0.31 (−3.80, 4.41) | Reference | Reference | |
| SMR users (N = 136) | 2.38 (−1.12, 5.88) | 2.07 (−3.30, 7.44) | 0.45 | 1.50 |
Adjusted for age, sex, health plan, and race/ethnicity, baseline CD4+ cell count (excluding CD4+ cell count stratified), and plasma HIV RNA (excluding plasma HIV RNA stratified)
Refill adherence changes calculated based on post-interval adherence minus pre-interval adherence
Differences in refill adherence change calculated based on comparison of the pre- and post-interval adherence changes between SMR users and non-users
SMR users restricted to those with ≥36 months of health plan enrollment following SMR rollout
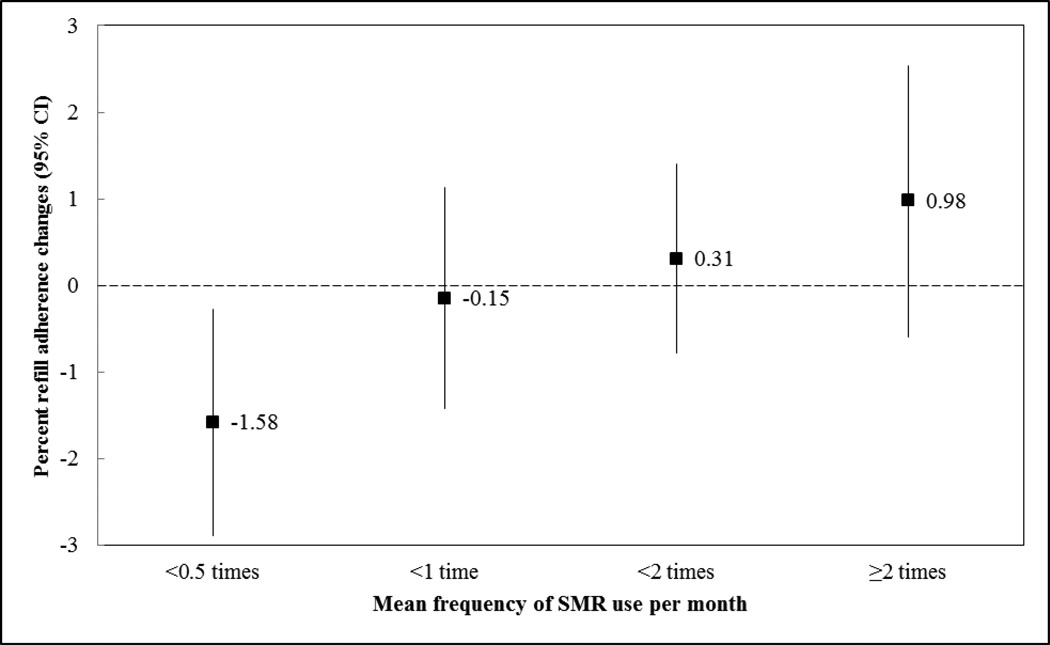
When comparing refill adherence in the pre- and post-intervals in the SMR user cohort, those who used SMR at a mean frequency of <0.5 times, 0.5 to <1 time, 1 to <2 times, and ≥2 times per month had a −1.58%, −0.15%, 0.31%, and 0.98% mean refill adherence change, respectively, as seen in the Figure (unadjusted overall p = 0.009). These changes correspond to a difference in refill adherence change of 1.43% (95% CI = 0.40, 3.26; p = 0.12), 1.89% (95% CI= 0.18, 3.60; p = 0.03), and 2.56% (95% CI = 0.52, 4.60; p = 0.01) for mean SMR use of 0.5 to <1 time, 1 to <2 times, and ≥2 times, respectively, compared to <0.5 times. After adjustment for potential confounders, these changes across categories remained statistically significant (adjusted overall p = 0.007), with corresponding differences in refill adherence change of 1.39% (95% CI = −0.43, 3.22), 2.03% (95% CI = 0.32, 3.74), and 2.60% (95% CI = 0.54, 4.66), respectively (Table 3).
Table 3.
Factors1 associated with the difference in percent refill adherence change2 among the SMR user cohort (N=1,638)3
| Factors | Difference in Percent Refill Adherence Change2 | 95% CI | p-value |
|---|---|---|---|
| Mean frequency of SMR use per month | |||
| <0.5 times | Reference | - | - |
| 0.5 to <1 time | 1.39 | −0.43, 3.22 | 0.14 |
| 1 to <2 times | 2.03 | 0.32, 3.74 | 0.02 |
| ≥2 times | 2.60 | 0.54, 4.66 | 0.01 |
| Age, years | |||
| 18−39 | Reference | - | - |
| 40−49 | −0.66 | −2.61, 1.28 | 0.51 |
| 50−59 | 0.74 | −1.31, 2.79 | 0.48 |
| ≥60 | −0.34 | −2.94, 2.26 | 0.80 |
| Sex | |||
| Men | Reference | - | - |
| Women | 2.90 | 0.14, 5.67 | 0.04 |
| Race/ethnicity | |||
| White | Reference | - | - |
| Asian/Pacific Islander | 1.40 | −2.36, 5.16 | 0.47 |
| Black | −1.52 | −4.01, 0.96 | 0.23 |
| Hispanic | 0.27 | −1.94, 2.48 | 0.81 |
| Other\unknown | −2.96 | −6.51, 0.59 | 0.10 |
| Baseline CD4+ cell count, cells\mm3 | |||
| <200 | Reference | - | - |
| 200–499 | −2.04 | −4.72,0.64 | 0.14 |
| ≥500 | −0.53 | −3.23, 2.18 | 0.70 |
| Baseline plasma HIV RNA, copies/mL | |||
| ≥10,000 | Reference | - | - |
| 501–9,999 | 0.34 | −4.03, 4.71 | 0.88 |
| ≤500 | −2.36 | −5.78, 1.06 | 0.18 |
Model adjusted for all confounders in table and health plan
Refill adherence changes calculated based on post-interval adherence minus pre-interval adherence
Includes all SMR users, not limited to those with ≥36 months of health plan enrollment following SMR rollout
In addition to higher mean frequency of SMR use per month, female sex was the only other factor associated with improved refill adherence among the SMR user cohort, with a difference in refill adherence change of 2.90% (95% CI = 0.14, 5.67; Table 3) compared to male sex. Age, healthplan, race/ethnicity, CD4+ cell count, and plasma HIV RNA were not associated with difference in refill adherence change among SMR users.
DISCUSSION
Our research demonstrates that SMR use is associated with maintenance of high levels of ART adherence and SMR non-use is associated with declines in adherence over time among HIV-positive patients enrolled in integrated healthcare systems with access to SMR. The benefits of SMR use versus no use were particularly evident in those with better controlled HIV disease (i.e., CD4+ cell count ≥200 cells/mm3 and those with plasma HIV RNA <500 copies/mL). Among SMR users, we noted a positive “dose-response” relationship between frequency of SMR use per month and ART adherence. However, changes in mean refill adherence between the pre- and post-intervals were similar among SMR users regardless of age, race/ethnicity, CD4+ cell count, and plasma HIV RNA.
HIV adherence research is increasingly supporting the use of bidirectional communication between patients and healthcare providers10,32, personalized message content32, and the use of tools that are practical and can be used in patients’ daily lives10. Additionally, according to an internet-based survey of HIV-positive online social media users, the use of the internet for healthcare engagement purposes (including emailing providers, refilling medications online, or making medical appointments online) was significantly associated with higher odds of self-reported ART adherence and maximal virologic control even after controlling for potential confounders33. Internet use for health-related purpose has been associated with significantly lower likelihood of non-adherence9 and greater patient self-confidence in adhering to ART34. Correspondingly, SMR allows for the use of the internet for direct and personal communication between patients and healthcare providers, refilling medications, and making medical appointments. These SMR functions may support better ART adherence among SMR users compared to non-users. However, it is unclear whether SMR use results in a higher level of engagement in care, which in turn is related to ART adherence4,5, or if those who are already more engaged in their HIV care are more likely to become SMR users.
We have previously described our cohort of HIV-positive patients at KPNC and GHC who are web-based SMR users18. During the first 36 months following the implementation of SMR, more than half of HIV-positive patients studied used SMR, primarily the SMR’s medication refill function, secure messaging of healthcare providers, viewing medical test results, and requesting appointments. Initial SMR users were more likely to identify as non-Latino and White. In our study, we did not note any racial/ethnic disparities in changes in refill adherence pre- and post-intervals among SMR users. This may be due to the fact that among individuals with similar access to care35, the association between SMR use and adherence is not modified by race/ethnicity.
In our study, women who used SMR had a larger improvement in mean ART adherence in comparison to men; however, in our prior descriptive study, women were about half as likely to use SMR compared to men18. Women are reported to have worse HIV-related health outcomes, lower rates of ART initiation and adherence, and higher likelihood of discontinuing ART23,36–38. Although further research is warranted, it is possible that targeted campaigns to increase SMR use among HIV-positive women may result in improved HIV clinical outcomes in this group.
Our study has several limitations. First, while timely refill of ART has been associated with plasma HIV RNA39, this adherence measure only represents receipt of medication and not actual medication ingestion. However, using pharmacy refill to calculate adherence also has a number of advantages; these data can easily be collected, are not influenced by patients’ ability to recall, are relatively inexpensive to acquire, and are readily obtainable from computerized records. Additionally, because we calculated mean adherence over a 12-month time-frame, short periods of ART non-adherence may have been masked, leading to a potential overestimation of ART adherence.
A second important limitation is that we conducted an observational study with which we cannot establish causality. Although randomized trials would allow causal evaluation of SMR use on adherence, the feasibility of these trials is limited due to the implementation of federal meaningful use criteria for electronic health records40. These criteria include some of the functions of the SMR studied here such as secure messaging with healthcare providers and viewing portions of the medical record. Third, we evaluated SMR use in HIV-positive patients in two integrated healthcare delivery systems; therefore, the generalizability of results to uninsured HIV-positive individuals or those not enrolled in an integrated healthcare system may be limited. Fourth, we were unable to assess internet access among our study population which has previously been shown to be a key barrier for the use of patient portals among HIV-infected populations41.
A final limitation was our use of a pre-post cohort design was that it could not account for secular changes in adherence occurring concurrently with the rollout of SMR. To account for possible secular trends, we conducted a two-group pre-post within-person longitudinal design comparing changes in adherence among SMR users and non-users. We acknowledge that potential selection biases may still exist and we were unable to account for unmeasured variables such as participants’ personality traits and level of proactivity and vigilance in their own health care. However, it is noteworthy that our finding of maintenance of adherence with SMR use was observed in both the analysis of the restricted group of SMR users versus non-users and in the analysis of SMR use frequency among the complete cohort of SMR users. Given little data regarding this important area of research and clinical care for HIV patients, future studies should evaluate the benefits of specific features of SMR prospectively or in randomized clinical trials and examine its cost-effectiveness.
In summary, in two healthplans which were early adopters of SMRs, we observed stable adherence over time among SMR users, compared with small declines among non-users. This difference is likely to have significant health impact, given prior research indicating that ART adherence typically declines over time and that even small reductions in adherence are associated with increased mortality risk42. In addition, an increasing number of healthplans are anticipated to provide SMRs with implementation of the Affordable Care Act. Thus, based on our data and other studies demonstrating the relationship between HIV clinical outcomes and use of online services for healthcare engagement33, we believe that healthcare systems should adopt and promote access to SMR use for all HIV-positive patients in order to improve communication between patients and providers and increase patients’ engagement in their HIV care.
Figure 1.
Unadjusted1 pre- to post-interval percent refill adherence changes based on mean frequency of SMR use among the SMR user cohort2
1Unadjusted p-value= 0.009
2Includes all SMR users, not limited to those with ≥36 months of healthplan enrollment following SMR rollout
▪ Represents the unadjusted point estimate of post-interval minus pre-interval ART refill adherence
| Represents the 95% confidence interval (CI) for the unadjusted change in ART refill adherence
… Represents no change in ART refill adherence from pre-interval to post-interval
Acknowledgements
The authors would like to thank Christine Mahoney and Courtney G. Ellis for project management, and Steven M. Carzasty, MSW for his key early input regarding study design and analysis. This research was supported by National Institutes of Health grant numbers R01MH081750 and K23MH097649.
REFERENCES
- 1.Garcia de Olalla P, Knobel H, Carmona A, Guelar A, Lopez-Colomes JL, Cayla JA. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr. 2002 May 1;30(1):105–110. doi: 10.1097/00042560-200205010-00014. [DOI] [PubMed] [Google Scholar]
- 2.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. Aids. 2001 Jun 15;15(9):1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 3.Hogg RS, Heath K, Bangsberg D, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. Aids. 2002 May 3;16(7):1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 4.Horstmann E, Brown J, Islam F, Buck J, Agins BD. Retaining HIV-infected patients in care: Where are we? Where do we go from here? Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010 Mar 1;50(5):752–761. doi: 10.1086/649933. [DOI] [PubMed] [Google Scholar]
- 5.Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 Oct;57(8):1164–1171. doi: 10.1093/cid/cit420. [DOI] [PubMed] [Google Scholar]
- 6.Cooke CE, Lee HY, Xing S. Adherence to antiretroviral therapy in managed care members in the United States: a retrospective claims analysis. Journal of managed care pharmacy : JMCP. 2014 Jan;20(1):86–92. doi: 10.18553/jmcp.2014.20.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heckman BD, Catz SL, Heckman TG, Miller JG, Kalichman SC. Adherence to antiretroviral therapy in rural persons living with HIV disease in the United States. AIDS care. 2004 Feb;16(2):219–230. doi: 10.1080/09540120410001641066. [DOI] [PubMed] [Google Scholar]
- 8.Aitken M, Valkova S. Avoidable Costs in U.S. Healthcare: The 200 Billion Opportunity from Using Medicines More Responsibly. 2013 [Google Scholar]
- 9.Kalichman SC, Cain D, Cherry C, Pope H, Eaton L, Kalichman MO. Internet use among people living with HIV/AIDS: Coping and health-related correlates. Aids Patient Care St. 2005 Jul;19(7):439–448. doi: 10.1089/apc.2005.19.439. [DOI] [PubMed] [Google Scholar]
- 10.Saberi P, Johnson MO. Technology-based self-care methods of improving antiretroviral adherence: a systematic review. PloS one. 2011;6(11):e27533. doi: 10.1371/journal.pone.0027533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.EHR Incentives Program. Eligible Professional's Guide to Stage 2 of the EHR Incentive Programs. [Accessed 5/23/2014];2013 http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/Stage2_Guide_EPs_9_23_13.pdf.
- 12.Zhou YY, Garrido T, Chin HL, Wiesenthal AM, Liang LL. Patient access to an electronic health record with secure messaging: impact on primary care utilization. The American journal of managed care. 2007 Jul;13(7):418–424. [PubMed] [Google Scholar]
- 13.Chen C, Garrido T, Chock D, Okawa G, Liang L. The Kaiser Permanente Electronic Health Record: transforming and streamlining modalities of care. Health affairs. 2009 Mar-Apr;28(2):323–333. doi: 10.1377/hlthaff.28.2.323. [DOI] [PubMed] [Google Scholar]
- 14.Weppner WG, Ralston JD, Koepsell TD, et al. Use of a shared medical record with secure messaging by older patients with diabetes. Diabetes care. 2010 Nov;33(11):2314–2319. doi: 10.2337/dc10-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris LT, Koepsell TD, Haneuse SJ, Martin DP, Ralston JD. Glycemic Control Associated With Secure Patient-Provider Messaging Within a Shared Electronic Medical Record: A longitudinal analysis. Diabetes care. 2013 Sep;36(9):2726–2733. doi: 10.2337/dc12-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green BB, Cook AJ, Ralston JD, et al. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2008 Jun 25;299(24):2857–2867. doi: 10.1001/jama.299.24.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon GE, Ralston JD, Savarino J, Pabiniak C, Wentzel C, Operskalski BH. Randomized trial of depression follow-up care by online messaging. Journal of general internal medicine. 2011 Jul;26(7):698–704. doi: 10.1007/s11606-011-1679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ralston JD, Silverberg MJ, Grothaus L, et al. Use of Web-Based Shared Medical Records Among Patients With HIV. American Journal of Managed Care. 2013 Apr;19(4):E114–E124. [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers EM. Diffusion of Innovations. 5th edition. New York: Simon and Schuster; 2003. [Google Scholar]
- 20.Saunders KW, Davis RL, Stergachis A. Group Health Cooperative. In: Strom BL, editor. Pharmacoepidemiology. John Wiley & Sons, Ltd; 2005. [Google Scholar]
- 21.Selby J, Smith DH, Johnson ES, Raebel MA, Friedman GD, McFarland BH. Kaiser Permanente Medical Care Program. In: Strom BL, editor. Pharmacoepidemiology. John Wiley & Sons, Ltd; 2005. [Google Scholar]
- 22.Horberg M, Hurley L, Towner W, et al. HIV quality performance measures in a large integrated health care system. AIDS patient care and STDs. 2011 Jan;25(1):21–28. doi: 10.1089/apc.2010.0315. [DOI] [PubMed] [Google Scholar]
- 23.Horberg M, Hurley L, Towner W, et al. HIV Spectrum of Engagement Cascade in a Large Integrated Care System by Gender, Age and Methodologies (Abstract 1033). Paper presented at: Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 2013. [Google Scholar]
- 24.Horberg MA, Hurley LB, Towner WJ, et al. Determination of optimized multidisciplinary care team for maximal antiretroviral therapy adherence. J Acquir Immune Defic Syndr. 2012 Jun 1;60(2):183–190. doi: 10.1097/QAI.0b013e31824bd605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ralston JD, Coleman K, Reid RJ, Handley MR, Larson EB. Patient experience should be part of meaningful-use criteria. Health affairs. 2010 Apr;29(4):607–613. doi: 10.1377/hlthaff.2010.0113. [DOI] [PubMed] [Google Scholar]
- 26.Silvestre AL, Sue VM, Allen JY. If you build it, will they come? The Kaiser Permanente model of online health care. Health affairs. 2009 Mar-Apr;28(2):334–344. doi: 10.1377/hlthaff.28.2.334. [DOI] [PubMed] [Google Scholar]
- 27.Ralston JD, Carrell D, Reid R, Anderson M, Moran M, Hereford J. Patient web services integrated with a shared medical record: patient use and satisfaction. Journal of the American Medical Informatics Association : JAMIA. 2007 Nov-Dec;14(6):798–806. doi: 10.1197/jamia.M2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steiner JF, Koepsell TD, Fihn SD, Inui TS. A General-Method of Compliance Assessment Using Centralized Pharmacy Records - Description and Validation. Med Care. 1988 Aug;26(8):814–823. doi: 10.1097/00005650-198808000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. Journal of clinical epidemiology. 1997 Jan;50(1):105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 30.Karter AJ, Parker MM, Moffet HH, Ahmed AT, Schmittdiel JA, Selby JV. New Prescription Medication Gaps: A Comprehensive Measure of Adherence to New Prescriptions. Health Serv Res. 2009 Oct;44(5):1640–1661. doi: 10.1111/j.1475-6773.2009.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choudhry NK, Shrank WH, Levin RL, et al. Measuring concurrent adherence to multiple related medications. The American journal of managed care. 2009 Jul;15(7):457–464. [PMC free article] [PubMed] [Google Scholar]
- 32.Finitsis DJ, Pellowski JA, Johnson BT. Text Message Intervention Designs to Promote Adherence to Antiretroviral Therapy (ART): A Meta-Analysis of Randomized Controlled Trials. PloS one. 2014;9(2):e88166. doi: 10.1371/journal.pone.0088166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saberi P, Johnson MO. Correlation of Internet use for healthcare utilization purposes and HIV clinical outcomes among HIV-positive individuals using online social media (Abstract No. 282); 9th International Conference on HIV Treatment and Prevention Adherence; Miami, FL. 2014. [Google Scholar]
- 34.Kalichman SC, Weinhardt L, Benotsch E, Cherry C. Closing the digital divide in HIV/AIDS care: development of a theory-based intervention to increase Internet access. AIDS care. 2002 Aug;14(4):523–537. doi: 10.1080/09540120208629670. [DOI] [PubMed] [Google Scholar]
- 35.Silverberg MJ, Leyden W, Quesenberry CP, Jr, Horberg MA. Race/ethnicity and risk of AIDS and death among HIV-infected patients with access to care. Journal of general internal medicine. 2009 Sep;24(9):1065–1072. doi: 10.1007/s11606-009-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Losina E, Schackman BR, Sadownik SN, et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the united states: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009 Nov 15;49(10):1570–1578. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood E, Montaner JS, Tyndall MW, Schechter MT, O'Shaughnessy MV, Hogg RS. Prevalence and correlates of untreated human immunodeficiency virus type 1 infection among persons who have died in the era of modern antiretroviral therapy. The Journal of infectious diseases. 2003 Oct 15;188(8):1164–1170. doi: 10.1086/378703. [DOI] [PubMed] [Google Scholar]
- 38.Gebo KA, Fleishman JA, Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005 Jan 1;38(1):96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 39.Grossberg R, Zhang Y, Gross R. A time-to-prescription-refill measure of antiretroviral adherence predicted changes in viral load in HIV. Journal of clinical epidemiology. 2004 Oct;57(10):1107–1110. doi: 10.1016/j.jclinepi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 40. HealthIT.gov. Meaningful Use Regulations. [Accessed 5/1/2014];Health IT Regulations. 2014 http://www.healthit.gov/policy-researchers-implementers/meaningful-use-regulations.
- 41.van Keken A, Winters P, Keefer MC, Sanders M, Fiscella K. Barriers and Facilitators of Online Patient Portals to Personal Health Records Among Persons Living With HIV: Formative Research. Journal of Medical Internet Research. 2013;15(1):8–8. doi: 10.2196/resprot.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lima VD, Harrigan R, Bangsberg DR, et al. The Combined Effect of Modern Highly Active Antiretroviral Therapy Regimens and Adherence on Mortality Over Time. Jaids-J Acq Imm Def. 2009 Apr 15;50(5):529–536. doi: 10.1097/QAI.0b013e31819675e9. [DOI] [PMC free article] [PubMed] [Google Scholar]