Abstract
Pituitary adenomas are a common feature of a subset of endocrine neoplasia syndromes, which have otherwise highly variable disease manifestations. We provide here a review of the clinical features and human molecular genetics of multiple endocrine neoplasia type 1 and 4 (MEN1 and MEN4, respectively) and Carney complex (CNC). MEN1, MEN4 and CNC are hereditary autosomal dominant syndromes that can present with pituitary adenomas. MEN1 is caused by inactivating mutations in the MEN1 gene, whose product menin is involved in multiple intracellular pathways contributing to transcriptional control and cell proliferation. MEN1 clinical features include primary hyperparathyroidism, pancreatic neuroendocrine tumours and prolactinomas and other pituitary adenomas. A subset of patients with pituitary adenomas and other MEN1 features have mutations in the CDKN1B gene; their disease has been called MEN type 4 (MEN4). Inactivating mutations in the type 1α regulatory subunit of protein kinase A (PKA) (the PRKAR1A gene), that lead to dysregulation and activation of the PKA pathway, are the main genetic cause of CNC, which is clinically characterised by primary pigmented adrenocortical disease (PPNAD), spotty skin pigmentation (lentigines), cardiac and other myxomas and acromegaly due to somatotropinomas or somatotrope hyperplasia.
Keywords: Multiple endocrine neoplasias, pituitary adenomas, pituitary hyperplasia, protein kinase A, menin, genetics
Introduction
About 5 % of pituitary adenomas occur in a familial setting [1]. The majority of these familial pituitary tumours are due to multiple endocrine neoplasia (MEN) type 1 (MEN1) [2]. Other genetic causes include Carney complex (CNC), MEN type 4 (MEN4), mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene leading to familial isolated pituitary adenomas (FIPAs) and McCune-Albright syndrome (MAS). Recently mutations in succinate dehydrogenase subunits (SDHx) and DICER1 as well as Xq26.3 microduplications have also been associated with pituitary tumours. Genetic conditions (germline or somatic) leading to pituitary tumours are summarised in table 1.
Table 1.
Genetic conditions that lead to pituitary adenomas.
| Genetic cause | General pathology | Endocrine pathology | |
|---|---|---|---|
| Multiple endocrine neoplasia type 1 (MEN1) | MEN1 (>80%) |
|
|
| Carney complex (CNC) | PRKAR1A (>70%) |
|
|
| MEN4 | CDKN1B (>90%) |
|
|
| McCune-Albright syndrome (MAS) | GNAS (>90%) |
|
|
| Familial isolated pituitary adenoma (FIPA) | AIP (approximately 20%) | None |
|
| Familial pheochromocytoma/ paraganglioma syndromes | SDHx (approximately 40%) |
|
|
| DICER1 syndrome (pleuropulmonary blastoma-familial tumour and dysplasia syndrome) | DICER1 (>90%) |
|
|
| X-linked acrogigantism (X-LAG) | Xq26.3 microduplication (100%) |
|
Despite the relative rarity of syndromic pituitary adenomas, the study of their genetic causes has contributed significantly to the understanding of pituitary tumourigenesis in general. MAS and CNC are caused by mutations in members of the cAMP-dependent protein kinase (protein kinase A or PKA) pathway [3, 4]; similarly, the PKA pathway, which contributes to pituitary proliferation and hormone secretion, is also altered in approximately 40% of sporadic somatotropinomas [5–10].
On the other hand, no single consistent genetic mechanism for pituitary tumourigenesis has emerged yet. Classic tumour suppressor genes, such as TP53 or RB1, or oncogenes, including HRAS, are all involved but not thought to be major contributors to germline predisposition to pituitary tumourigenesis [11–14]. MEN1 and CDKN1B, whose mutations can cause pituitary tumour formation in the context of multiple endocrine neoplasia syndromes, were shown to be mutated or downregulated only in few sporadic tumours [15–19]. Similarly, mutations in AIP and PRKAR1A, which lead to familial pituitary tumours in the context of FIPA and CNC, respectively, do not seem to contribute significantly to sporadic pituitary tumours [10, 20–24], although AIP expression is reduced in some sporadic somatotropinomas in the absence of AIP mutations [25], probably due to the actions of different microRNAs [26, 27]. The variable and incomplete penetrance of these hereditary pituitary tumour syndromes suggests that additional circumstances are required for pituitary tumourigenesis. These have not been clearly defined yet, even though some candidate loci have been reported for AIP-associated tumours [28, 29], and a possible association was suggested between a CDKN1B variant and tumour multiplicity in MEN1 [30]. However these additional circumstances required for pituitary tumourigenesis in the context of genetic syndromes may well involve the same pathways controlling cell proliferation and hormone secretion that are also dysfunctional in sporadic pituitary adenomas.
MAS is a genetic but not hereditary condition caused by somatic, postzygotic mutations in the α-subunit of the Gs protein (GNAS), leading to somatotrope or somatolactotrope hyperplasia and growth hormone (GH) hypersecretion in approximately 20% of patients [3, 31, 32]. Inactivating germline mutations in AIP were recently discovered to be the genetic cause in about 20% of FIPA patients [33]. These patients have mostly somatotropinomas or prolactinomas (and sometimes non-functioning pituitary adenomas and rarely corticotropinomas), and no other recurrent clinical manifestations [33, 34]. MAS, FIPA due to AIP mutations and their contribution to pituitary pathology are described in detail elsewhere [3, 31–34].
Mutations in different subunits of SDH can lead to hereditary pheochromocytoma and paraganglioma syndromes [35] and recently SDHx mutations were also reported to be associated with pituitary adenomas [36–40]. Pituitary blastoma, manifesting mostly in early childhood and leading to Cushing’s syndrome, was recently described [41, 42]. Germline mutations in DICER1 were reported in almost all cases of pituitary blastoma [41–43]; interestingly, germline DICER1 mutations also cause other early childhood tumours summarised as pleuropulmonary blastoma-familial tumour and dysplasia syndrome (see table 1). However, in the few cases that have been reported the manifestation of another DICER1-related tumour in association with pituitary blastoma is rare [43].
We have recently reported Xq26.3 microduplications in association with early childhood-onset gigantism, termed X-linked acrogigantism (X-LAG) [44]. This is probably due to overexpression of GPR101, a G-protein coupled orphan receptor that is located in this region, and downstream PKA pathway activation; interestingly a recurring GPR101 variant is found in some cases of sporadic acromegaly [44]. A possible association was suggested between neurofibromatosis type 1 and acromegaly or gigantism in a few case reports [45]; this growth hormone excess is due to optic pathway tumours that are hypothesised to suppress hypothalamic somatostatin secretion, and hence will not be discussed in detail here.
In this review, we present an update on the clinical manifestations and human molecular genetics of three of the above referenced diseases, all caused by genetic defects in the germline, MEN1, MEN4 and CNC.
Multiple endocrine neoplasia type 1
Multiple endocrine neoplasia type 1 (MEN1, MIM*131100) is an autosomal dominant disorder, leading to parathyroid neoplasms, pancreatic neuroendocrine tumours and pituitary adenomas [46]. Other MEN1-associated endocrine and non-endocrine neoplasms, including adrenocortical tumours, carcinoids and facial angiofibromas, may also occur [46, 47]. The prevalence of MEN1 is about 1:30,000 [46], but geographical clustering due to a founder effect can be observed [48, 49]. There is considerable phenotypic variability of tumour type manifestations even within the same family [50], suggesting the possible involvement of modifier genes or epigenetic changes.
The penetrance of MEN1 is generally high, with biochemical signs present in >95% and clinical signs in 80% of patients by the fifth decade of life [50–52]; for instance, the age-related penetrance at 50 years is 73–75 % for primary hyperparathyroidism, 31–48 % for pituitary adenomas and for 45–49 % islet cell tumours [52, 53]. However the age of presentation of specific tumour types is highly variable, and may range from 9–25 for the earliest to 68–77 years for the latest tumour manifestation [52]. MEN1 patients have a decreased life expectancy and MEN1-associated mortality is mostly due to enteropancreatic malignancy [52–56]. MEN1-associated mortality has improved since the 1980s due to more intense screening programs, better perioperative survival and, since protein pump inhibitors became available, reduced mortality due to gastrinoma-associated gastric ulcer perforation and haemorrhage [55].
Primary Hyperparathyroidism
Primary hyperparathyroidism (PHPT) is frequently the presenting feature of MEN1 [50] and also the most common MEN1-associated clinical manifestation that occurs in more than 90 % of mutation carriers [47, 50, 52]. MEN1-associated PHPT develops approximately 30 years earlier than sporadic PHPT, and has an almost even gender ratio whereas sporadic PHPT has a 75% female preponderance [57]. PHPT in the context of MEN1 is associated with higher severity of bone involvement with borderline rather than elevated parathyroid hormone levels and only mildly elevated serum calcium [58]. Usually MEN1-associated PHPT is due to multiglandular hyperplasia whilst there is more often one evident adenoma in sporadic PHPT [59–62]. Consequently surgery is often more challenging, involving intraoperative identification and removal of all four glands, and recurrence rates are high [63, 64]. In addition, supernumerary or ectopic parathyroid glands occur in up to 20 % of patients [60] and their identification is crucial to prevent recurrence. In conjunction with open bilateral neck exploration, total thymectomy is also often performed since this is the most common location for ectopic parathyroid glands; intraoperative parathyroid hormone measurement can aid to determine successful removal of all overactive glands [65].
Pancreatic neuroendocrine tumours
Pancreatic neuroendocrine tumours (PNET) are also a frequent feature of MEN1 in up to 75 % of patients [47, 50, 52], but patients are often asymptomatic and the real prevalence may be higher [47, 66]. These tumours are most often gastrinomas, insulinomas or nonfunctioning PNETs, occasionally glucagonomas, VIPomas or somatostatinomas [47, 52, 55]. Most MEN1 patients have multiple microadenomas in pancreas and duodenum, only few of which become clinically relevant [67, 68], consequently metastases are frequently present (30–50 %) at the time of appearance of symptoms [68, 69]. Gastrinomas associated with excessive gastric acid production and gastric ulceration, referred to as Zollinger-Ellison syndrome, are a major contributor to MEN1-associated mortality [49, 70]. MEN1-associated gastrinomas are usually located in the duodenum, and are small and multicentric compared to the larger and mostly singular sporadic gastrinomas [71–73]; consequently the surgical resection of a single tumour is not likely to be curative and therapy of small non-metastatic tumours is primarily symptomatic [47]. Importantly, gastrinomas are relatively rare in the general population, and 20 % of patients with gastrinomas have MEN1 [73]. Non-functioning PNETs have recently attracted attention due to the finding that their prevalence and associated mortality are higher than previously thought [55, 74]. Their prognosis is worse than that of functioning PNETs and clinical assessment is difficult due to the absence of specific symptoms or biochemical markers [55, 74]. Insulinomas are the first manifestation of MEN1 in 10 % of patients, and even though there is often more than one tumour, surgery is recommended [65]. VIPomas, glucagonomas and somatostatinomas are rare, however if present they have a high risk of malignancy [68, 75], and surgery should be performed in the absence of distant metastases [75].
Pituitary adenomas
The frequency of pituitary adenomas (PAs) in MEN1 is around 40% depending on the patient population [47, 50, 52, 76]; conversely, only 3% of patients with PA have MEN1 [2]. Mean age of presentation is around 38 years, but can vary between 5 years and the ninth decade of life [76, 77]. PAs are the first MEN1 manifestation in about 20 % of patients [53, 76]. PAs are most commonly prolactinomas, followed by non-functioning PAs, somatotropinomas and corticotropinomas; this distribution is approximately the same as that seen in sporadic PAs. However in MEN1 PAs there is a higher incidence of multiple hormone expression and multiple adenomas [76, 78]. MEN1-associated PAs are more aggressive than sporadic PAs, are macroadenomas in 85 % of cases compared to 40 % in sporadic PAs, and they may infiltrate surrounding tissues more frequently [76, 78]. This is accompanied by a worse rate of hormonal control in MEN1 PAs [76]: 44 % of MEN1-associated prolactinomas are resistant to dopamine agonist therapy [76, 79]. The presence of pituitary enlargement in imaging studies does not preclude elevated hormone production elsewhere, and in rare cases, PNETs that secrete hypothalamic hormones and lead to excessive secretion of pituitary hormones have been encountered [78, 80, 81]. Sporadic PAs and MEN1 PAs are similarly more common in females than in males (approximately 70 % female in MEN1 patients); there is currently no explanation for this although oestrogen was hypothesised to stimulate the proliferation of pituitary cells, as seen experimentally [76, 82].
Other MEN1-associated features
Approximately 40% of MEN1 patients develop additional functioning or non-functioning endocrine tumours [52]. Most commonly these are benign non-functioning adrenocortical lesions, but primary aldosteronism, adrenocorticotropic hormone (ACTH)-independent Cushing’s syndrome or (more rarely) adrenal hyperandrogenism due to adrenal adenomas are found in about 15 % [83]. This is significantly higher than the rate of endocrine activity among sporadic adrenal incidentalomas, indicating that adrenocortical tumours or bilateral hyperplasia in MEN1 are frequently functioning [83]. Facial angiofibromas occur in up to 88% of patients and collagenomas in up to 72 % [84, 85], and their presence may aid diagnosis (the presence of three facial angiofibromas and one collagenoma is 75 % sensitive and 95 % specific for MEN1) [85]. Pheochromocytoma is rarely observed (<1 %) [83]. Interestingly, MEN1 patients have impaired fasting glucose (17 % vs. 6 % in controls) that cannot be adequately explained by the presence of hormone secreting tumours or previous pancreatic surgery, and may contribute to cardiovascular mortality [86, 87].
Molecular genetics
The genetic cause of MEN1 was initially localized to 11q13 and later identified as the tumour suppressor gene MEN1, which consists of 10 exons and codes for the protein menin [88–90]. Loss of heterozygosity (LOH) of the MEN1 locus is frequently found in MEN1 tumours [88, 91, 92].
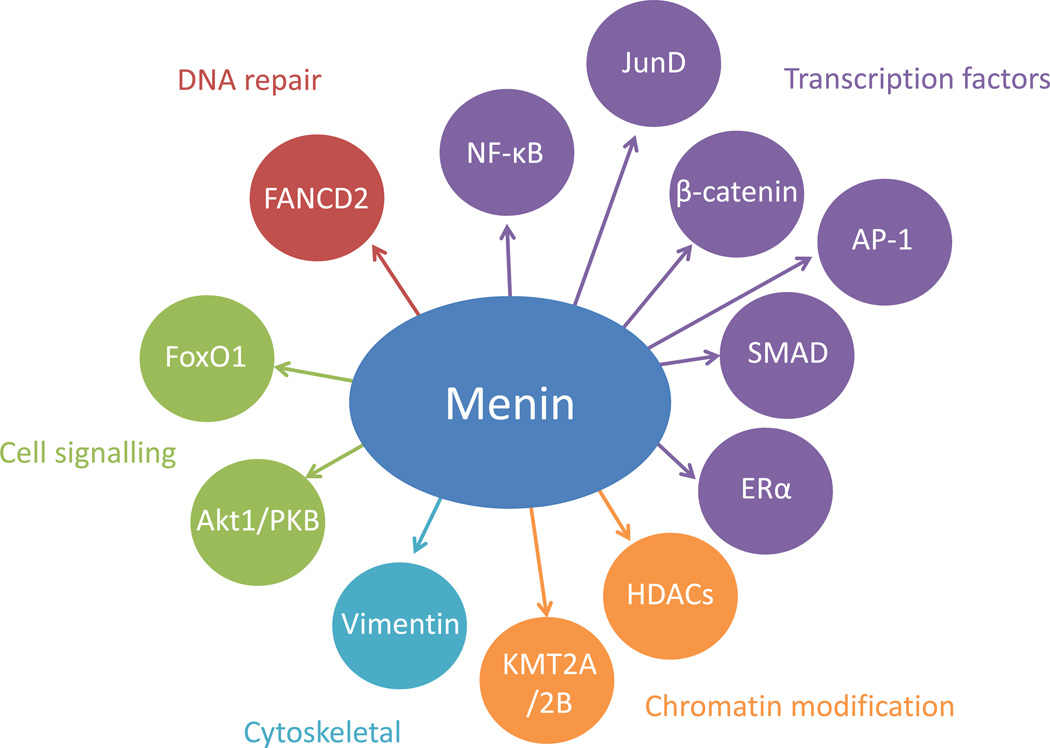
Menin is a 610 amino acid protein with no homology to other known proteins; its expression is ubiquitous and the mechanism of how loss of function of menin leads to MEN1 is still unclear [89, 90, 93]. Menin predominantly localises to the nucleus, containing two classical nuclear localisation signals (NLSs) and at least one further non-classical NLS in its C-terminus [94, 95]. In the nucleus it can associate with chromatin [96], dsDNA [97], the lysine-specific histone methyltransferases KMT2A and KMT2B [98, 99] and components of a transcriptional repressor complex also including histone deacetylases (HDACs) [100]. Menin interacts with transcription factors including activating protein-1 (AP-1), JunD, nuclear factor-κB (NF-κB), β-catenin, mothers against decapentaplegic (SMAD) family members and oestrogen receptor α (ERα) [98, 101–107]. Menin binds to cytoskeletal proteins, e.g. vimentin [108], and cytoplasmic cell signalling mediators including Akt1/protein kinase B (PKB) and forkhead box protein O1 (FoxO1) [109, 110]. Some of the known menin interaction partners are depicted in Fig. 1. Menin was shown to play a role in cell proliferation [111–113], apoptosis [114, 115] and genome integrity [116]. Menin and KMT2A in complex regulate expression of several Hox genes as well as CDKN1B, and they interact with ERα to coactivate ERα-mediated transcription [98, 107, 117, 118]. Interestingly, chromosomal rearrangements involving KMT2A lead to mixed-lineage leukaemia, and in this context menin was shown to be required for KMT2A-dependent oncogenic transformation [119]. This illustrates the functional versatility of menin in different tissues that may also help to understand the yet unexplained tissue selectivity of MEN1-associated tumours even in the presence of the same mutation.
Figure 1.
Interacting partners of menin. Menin interacts with transcription factors including nuclear factor-κB (NF-κB), JunD, β-catenin, activator protein-1 (AP-1), mothers against decapentaplegic (SMAD) family members and oestrogen receptor α (Erα); with proteins regulating chromatin structure including histone deacetylases (HDACs) and the histone methyltransferases KMT2A and KMT2B; with cytoskeletal proteins such as vimentin, with cytoplasmic cell signalling mediators including forkhead box protein O1 (FoxO1) and Akt1/protein kinase B (PKB); and with DNA repair proteins including Fanconi anaemia group D2 protein (FANCD2).
Hundreds of MEN1 mutations have been described, which are located along the whole coding region and splice sites of the gene [120]. While most MEN1 cases are familial, 10 % of cases occur in a non-familial context and are due to de novo MEN1 mutations [120]. The majority of mutations leading to MEN1 are frameshift deletions or insertions and nonsense mutations leading to truncation or absence of the protein [52, 120]. Missense mutations leading to single amino acid substitutions were assumed to cause less severely impaired protein function, but no notable difference was observed in clinical manifestation of those patients [52]. Some mutations leading to single amino acid substitutions were demonstrated to lead to proteasomal degradation and hence markedly reduced protein levels [121], while other mutations lead to nonsense-mediated mRNA decay [122]. A reduction of interaction capacity of menin with its binding partners was also shown for some mutations leading to single amino acid substitutions [98, 101]. Some intronic mutations were demonstrated to lead to alternative splicing, suggesting that they are causative for MEN1 [123–125]. Interestingly, in approximately 10 % of patients with clinical MEN1, no MEN1 gene mutations could be identified [120, 126–128]. In a small number (1 %) of these cases, large deletions of one exon or more could be detected using multiple ligation-dependent probe amplification (MLPA) or longrange PCR amplification [129, 130]. In the remaining cases, the phenotype may be due to intronic mutations that are not detected by routine sequencing, however the involvement of mutations in other genes cannot be excluded.
Genotype-phenotype correlation
Due to the large number of different mutations in combination with the heterogeneity of disease manifestations it has proved difficult to establish subtle genotype-phenotype correlations in MEN1. One study found that all patients with frameshift mutations have PNETs [131], while another study showed a higher rate of malignant tumours for mutations in MEN1 exons 2, 9 and 10 [132]. However, no genotype-phenotype correlation could be consistently confirmed in different patient populations [52, 120]. In addition, studies of unrelated kindreds with the same mutation showed large variability of different MEN1 associated tumours [51, 133], and there are reports of identical twins with different MEN1 manifestations [134–136]. Remarkably, some families with particular MEN1 mutations develop only isolated hyperparathyroidism, while the same mutations in other families lead to full MEN1 [120]. Epigenetic mechanisms caused by environmental factors may influence disease phenotype in patients carrying the same MEN1 mutation [137]. Recently, a specific variant of the CDKN1B gene was demonstrated to be disease modifying in MEN1 patients with truncating MEN1 mutations, causing a higher number of MEN1 related tumours [30].
MEN4
In the approximately 10 % of patients with a MEN1-like phenotype where no MEN1 mutations could be detected, other genes were suspected to be responsible for the clinical manifestation [120, 126–128]. A rat model displaying a MEN1-like phenotype was discovered to harbour a mutation in the CDKN1B gene, leading to premature termination [138]. CDKN1B transcription is regulated by menin [117, 139]. CDKN1B encodes for the cyclin-dependent kinase inhibitor p27Kip1, which participates in cell cycle regulation by interaction with cyclin-dependent kinases [140], and in turn, p27Kip1 levels are regulated via the mitogen-activated protein kinase (MAPK) and the phosphatidyl inositol-3 kinase (PI3K) pathways [141, 142]. In a small number (up to 3 %) of MEN1 mutation-negative patients fulfilling the diagnostic criteria for MEN1, mutations in CDKN1B have been detected and the corresponding clinical syndrome has been termed MEN4 (MIM#610755) [138]. Mutations in some of those patients were shown to either lead to decreased cellular levels of p27Kip1 by reduced translation or proteasomal degradation, or to functional defects causing reduced binding to interacting partners or decreased nuclear localisation [138, 143–145]. Interestingly, a novel mechanism of CDKN1B loss of function leading to MEN4 was recently discovered: a 4 bp deletion in an upstream ORF within the CDKN1B 5’UTR led to decreased translation reinitiation and decreased p27Kip1 levels [146].
PHPT is present in all the MEN4 patients reported so far, but the clinical and histological manifestations seem to be more variable than in MEN1, and due to the small number of patients so far reported, a comprehensive phenotype has not been yet established [147]. PAs (corticotropinomas, somatotropinomas and nonfunctioning PAs) as well as neuroendocrine tumours, uterine neoplasms, adrenocortical masses and thyroid tumours have also been described in the MEN4 context [138, 143–145, 148].
Carney complex
Carney complex (CNC, MIM#160980) is a rare endocrine tumour syndrome with currently about 750 documented cases worldwide [149, 150]. Initially described as the complex of myxomas, spotty skin pigmentation (lentigines), and endocrine overactivity, the familial syndrome is inherited in an autosomal dominant fashion [151, 152]. Further manifestations include primary pigmented nodular adrenocortical disease (PPNAD) leading to Cushing’s syndrome, PAs, thyroid nodules, testicular neoplasms, ovarian cysts, psammomatous melanotic schwannomas, ductal breast adenomas and osteochondromyxomas [153]. Most CNC patients initially present with ACTH-independent Cushing’s syndrome due to PPNAD or heart myxomas, although abnormal skin pigmentation may be present at birth and is most often the first manifestation [153–155]. The majority of CNC cases are caused by inactivating germline mutations in the type 1α regulatory subunit of protein kinase A (PRKAR1A) gene [156], and those mutations lead to CNC with a penetrance close to 100 % [149, 150]. About 70 % of CNC cases occur in a familial context [149].
Cushing’s syndrome due to PPNAD
PPNAD is most frequently seen in the context of CNC, and 60 % of CNC patients have PPNAD [149, 157]. Conversely, of all patients with PPNAD, about 80 % have CNC while 20 % have isolated PPNAD, where no other CNC-associated lesions could be detected [149]. PPNAD typically manifests at a young age (median age 34 years) and leads to ACTH-independent adrenocortical Cushing’s syndrome [149]. Diagnosis can be challenging in cases of cyclical (14 %) or subclinical Cushing’s syndrome (19 %) [157]. It should be noted that most CNC patients with PPNAD have a paradoxical increase of cortisol secretion after dexamethasone administration, which is diagnostically particularly useful in patients with normal baseline cortisol levels [157].
Pituitary adenomas
While approximately 75% of CNC patients have abnormal GH, insulin-like growth factor 1 (IGF-1) or prolactin levels basally or during dynamic testing, PAs can only be detected in about 10 % [149, 153, 158, 159]. This may be due to prolonged periods of somatolactotrope hyperplasia preceding adenoma formation [153, 159–161]. CNC-associated PAs are mostly positive for GH or GH and prolactin, and can lead to acromegaly or gigantism, and rarely to clinically significant hyperprolactinaemia [152, 158–162]. A minority of adenomas also stained positive for thyroid-stimulating hormone (TSH), luteinising hormone (LH) or α-subunit [160, 162] but these do not cause a clinical phenotype. CNC-related adenomas are often multiple, surrounded by hyperplasia, and mostly microadenomas, but there are also cases of very aggressive and invasive macroadenomas [160].
Cardiac myxomas
CNC-associated cardiac myxomas can be found in 30 % of CNC patients [149]. They have an even age and gender distribution and occur anywhere in the heart as opposed to sporadic cardiac myxomas more frequently occurring in the left atrium and in older females [153]. Tumours can be multiple and can recur after removal [149]. The age of manifestation varies between 3–67 years [149]. Although these are benign neoplasms, they can lead to serious complications including cardiac insufficiency, stroke or pulmonary embolism [153].
Skin manifestations
Lentiginosis (present in 70 % of the patients) and other pigmented cutaneous lesions (blue, Spitz and compound naevi and café-au-lait spots, 50 %) are frequently observed and may be present at birth or appear in early childhood [149, 153]. Cutaneous myxomas are found in 20 % of CNC patients [149]. Twenty percent of affected women have mammary myxomas [149]. Other skin tumours include lipomas, collagenomas and ear canal trichofolliculoepitheliomas.
Other CNC-associated tumours
Psammomatous melanotic schwannomas are a relatively rare manifestation of CNC; however, they cause significant morbidity and can even be the cause of death of CNC patients, because they can (depending on their location) cause significant neurologic deficits, obstructive pulmonary disease, or increased intracranial pressure. Finally, they can become malignant and when they do they are aggressive tumours with frequent lung or cerebral metastases [153].
Testicular neoplasms occur in more than two thirds of male patients (by ultrasonography) and are mostly large cell calcifying Sertoli cell tumours [149]. Thyroid nodules can also be observed frequently, but thyroid cancer is rare [149]. Rarely patients also develop ovarian cancer [163], liver cancer [164] and pancreatic neoplasms [165].
Molecular genetics
The most frequent genetic cause (in about 73% of the patients) of CNC is a PRKAR1A defect [4, 149, 166]. PRKAR1A acts as a tumour suppressor by haploinsufficiency, although loss of the wild-type allele is also found in most CNC-associated tumours [4, 153, 166].
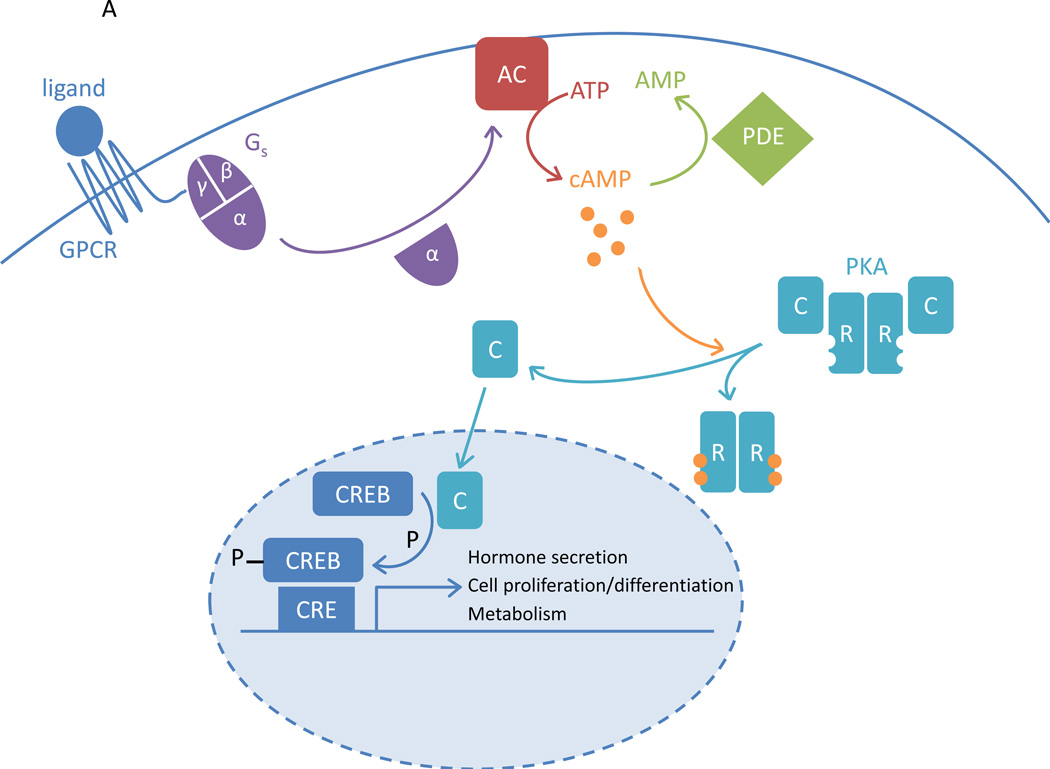
PRKAR1A encodes for the type 1α regulatory subunit (R1α) of PKA. PKA is a heterotetramer composed of two catalytic subunits (Cα, Cβ, Cγ or Cx) and two regulatory subunits (R1α, R1β, R2α or R2β). Stimulation of the Gs protein leads to activation of adenylyl cyclase that produces cAMP. cAMP is then bound by PKA regulatory subunits, which leads to activation of PKA by dissociation of the regulatory subunits from the active sites of the catalytic subunits. The active catalytic subunits are then free to act as a serine/threonine kinases phosphorylating downstream targets both in the cytoplasm and in the nucleus. These downstream targets include CREB (cAMP responsive element binding protein), which in turn mediates CRE (cAMP responsive element)-dependent transcription [167, 168] (Figure 2A).
Figure 2.
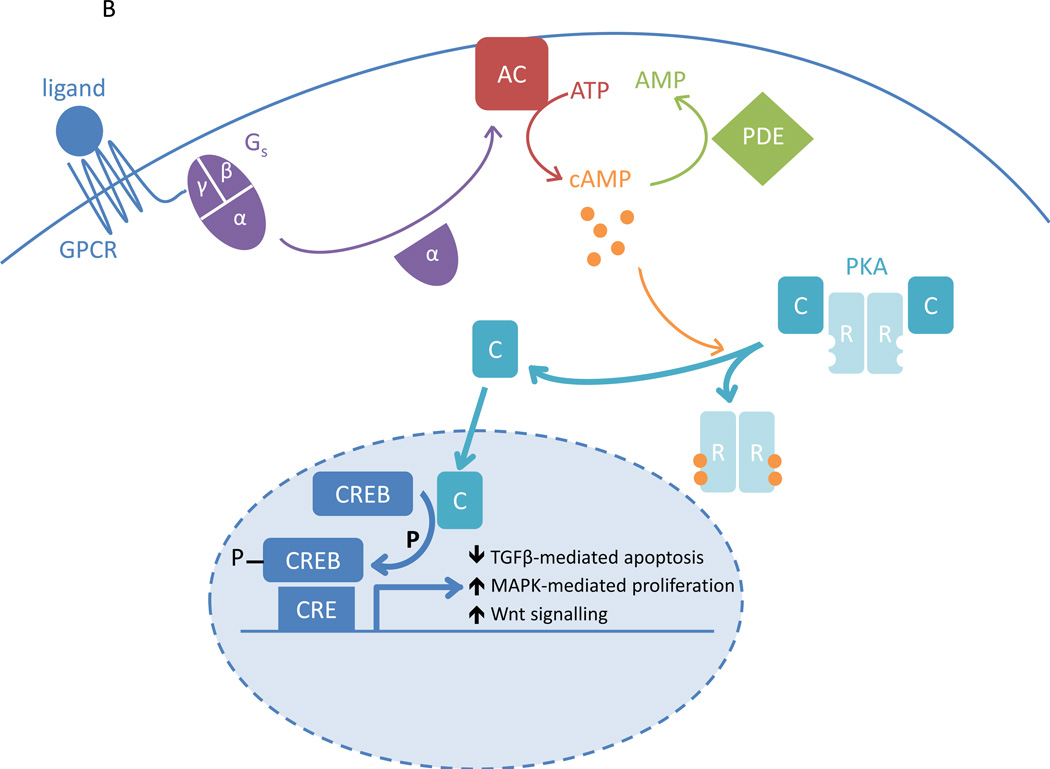
The PKA pathway. A. Ligand activation of the G-protein coupled receptor (GPCR) leads to activation of the stimulatory G protein (Gs) and its α-subunit; this is followed by activation of adenylyl cyclase (AC). AC converts ATP into cAMP. In the basal state, PKA consists of two regulatory subunits (R) bound to two catalytic subunits (C). cAMP-binding to R causes dissociation from C, which is now free to act as a serine/threonine kinase. It can activate cAMP-responsive element binding protein (CREB) by phosphorylation, which mediates transcription of genes with cAMP-responsive element (CRE)-containing promoters. The PKA pathway contributes to the control of cell proliferation and differentiation, metabolism and hormone secretion. The phosphodiesterases (PDEs) hydrolyse cAMP, thereby reducing PKA pathway activity. B. In Carney complex, R1α levels are reduced, leading to increased PKA activation, reduced transforming growth factor β (TGFβ)-mediated apoptosis, increased mitogen activated protein kinase (MAPK)-dependent proliferation and a stimulated Wnt signalling pathway.
A reduction of R1α levels therefore causes disinhibition of PKA, leading to an increase of cAMP-stimulated PKA activity [4, 169–171] (Figure 2B). R1α deficiency leads to decreased SMAD3 expression, thereby reducing transforming growth factor β (TGFβ)-mediated apoptosis in adrenocortical cells [172]. In addition, MAPK pathway activity was shown to increase in response to inactivating PRKAR1A mutations, causing increased cell proliferation [169, 173, 174]. R1α deficiency also leads to an up-regulation of different components of the Wnt signalling pathway [175, 176]. Interestingly, some CNC patients with PPNAD have somatic mutations in the β-catenin gene (CTNNB1) within the adrenal nodules, which is the main effector of the Wnt signalling pathway [177, 178].
More than 120 different PRKAR1A mutations have been identified to date in CNC patients [149, 156, 166, 171]. Sanger sequencing is used for the large majority of routine clinical sequence analysis and recently, large deletions have been identified in about 20 % of those patients previously thought to be PRKAR1A mutation-negative by array-based comparative genomic hybridisation (aCGH) [179]. Almost all mutations generate a premature stop codon, either directly or by frameshift, leading to nonsense-mediated mRNA decay and absence of the R1α protein [4, 156], although some PRKAR1A mutations in CNC were demonstrated to lead to expressed R1α that had lost its inhibitory effect on PKA signalling [170, 171].
Genotype-phenotype correlation
No obvious genotype-phenotype correlation could initially be detected in CNC patients with different PRKAR1A mutations; most different mutations invariably lead to the absence of the R1α protein [156]. However, a small intronic deletion in PRKAR1A which has been identified in CNC patients can also be found in some cases of isolated PPNAD; this mutation also leads to nonsense-mediated decay but is associated with lower penetrance than CNC [180]. In addition, PRKAR1A mutation carriers manifest with myxomas, thyroid tumours, schwannomas and Sertoli cell tumours more frequently and generally present earlier than CNC patients where no PRKAR1A mutations were found [149]. Patients with large deletions of PRKAR1A were suggested to present with CNC at an earlier age (14 years vs. 20 years) [150, 179].
Conclusions
The multifactorial and heterogeneous pathogenesis of pituitary tumours is reflected by the multitude of different tumour manifestations in genetic syndromes that also cause pituitary adenomas, such as MEN1 and CNC. The study of such rare diseases can contribute immensely to the faster diagnosis and better monitoring of affected patients, as reflected by the improvement of MEN1-associated mortality over the last decades. Moreover, the study of multiple endocrine neoplasia syndromes will also aid our understanding of endocrine physiology and tumourigenesis.
Interestingly, pituitary adenomas are mostly benign and only very rarely acquire malignant properties. MEN1-associated pituitary adenomas and FIPAs due to AIP mutations are more aggressive than sporadic PAs, with more frequent macroadenomas and a younger age at manifestation in AIP-associated FIPAs [76, 181, 182]. Conversely, pituitary hyperplasia is presumed to precede adenoma formation; an example of this has been observed in an AIP-associated PA [183]. In MAS and CNC, a PAs is rarely observed; however, there are prolonged periods of pituitary hyperplasia in conjunction with abnormal GH, IGF-1 or prolactin levels [160]. Each of these diseases can therefore serve as a model for the understanding of the steps leading to pituitary tumourigenesis.
Acknowledgements
M.H.S.-R. is supported by the Austrian Science Fund (FWF) J-3482; this work was supported mainly by the Intramural Research Program (IRP) of NICHD, NIH.
Footnotes
Disclosure Statement: The authors have nothing to disclose
References
- 1.Daly AF, Jaffrain-Rea ML, Ciccarelli A, Valdes-Socin H, Rohmer V, Tamburrano G, et al. Clinical characterization of familial isolated pituitary adenomas. J Clin Endocrinol Metab. 2006;91:3316–3323. doi: 10.1210/jc.2005-2671. [DOI] [PubMed] [Google Scholar]
- 2.Scheithauer BW, Laws ER, Jr, Kovacs K, Horvath E, Randall RV, Carney JA. Pituitary adenomas of the multiple endocrine neoplasia type I syndrome. Semin Diagn Pathol. 1987;4:205–211. [PubMed] [Google Scholar]
- 3.Vortmeyer AO, Glasker S, Mehta GU, Abu-Asab MS, Smith JH, Zhuang Z, et al. Somatic GNAS mutation causes widespread and diffuse pituitary disease in acromegalic patients with McCune-Albright syndrome. J Clin Endocrinol Metab. 2012;97:2404–2413. doi: 10.1210/jc.2012-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, et al. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 5.Vallar L, Spada A, Giannattasio G. Altered Gs and adenylate cyclase activity in human GH-secreting pituitary adenomas. Nature. 1987;330:566–568. doi: 10.1038/330566a0. [DOI] [PubMed] [Google Scholar]
- 6.Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989;340:692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- 7.Landis CA, Harsh G, Lyons J, Davis RL, McCormick F, Bourne HR. Clinical characteristics of acromegalic patients whose pituitary tumors contain mutant Gs protein. J Clin Endocrinol Metab. 1990;71:1416–1420. doi: 10.1210/jcem-71-6-1416. [DOI] [PubMed] [Google Scholar]
- 8.Buchfelder M, Fahlbusch R, Merz T, Symowski H, Adams EF. Clinical correlates in acromegalic patients with pituitary tumors expressing GSP oncogenes. Pituitary. 1999;1:181–185. doi: 10.1023/a:1009905131334. [DOI] [PubMed] [Google Scholar]
- 9.Freda PU, Chung WK, Matsuoka N, Walsh JE, Kanibir MN, Kleinman G, et al. Analysis of GNAS mutations in 60 growth hormone secreting pituitary tumors: correlation with clinical and pathological characteristics and surgical outcome based on highly sensitive GH and IGF-I criteria for remission. Pituitary. 2007;10:275–282. doi: 10.1007/s11102-007-0058-2. [DOI] [PubMed] [Google Scholar]
- 10.Yamasaki H, Mizusawa N, Nagahiro S, Yamada S, Sano T, Itakura M, et al. GH-secreting pituitary adenomas infrequently contain inactivating mutations of PRKAR1A and LOH of 17q23–24. Clin Endocrinol (Oxf) 2003;58:464–470. doi: 10.1046/j.1365-2265.2003.01740.x. [DOI] [PubMed] [Google Scholar]
- 11.Levy A, Hall L, Yeudall WA, Lightman SL. p53 gene mutations in pituitary adenomas: rare events. Clin Endocrinol (Oxf) 1994;41:809–814. doi: 10.1111/j.1365-2265.1994.tb02797.x. [DOI] [PubMed] [Google Scholar]
- 12.Karga HJ, Alexander JM, Hedley-Whyte ET, Klibanski A, Jameson JL. Ras mutations in human pituitary tumors. J Clin Endocrinol Metab. 1992;74:914–919. doi: 10.1210/jcem.74.4.1312542. [DOI] [PubMed] [Google Scholar]
- 13.Honda S, Tanaka-Kosugi C, Yamada S, Sano T, Matsumoto T, Itakura M, et al. Human pituitary adenomas infrequently contain inactivation of retinoblastoma 1 gene and activation of cyclin dependent kinase 4 gene. Endocr J. 2003;50:309–318. doi: 10.1507/endocrj.50.309. [DOI] [PubMed] [Google Scholar]
- 14.Vandeva S, Jaffrain-Rea ML, Daly AF, Tichomirowa M, Zacharieva S, Beckers A. The genetics of pituitary adenomas. Best Pract Res Clin Endocrinol Metab. 2010;24:461–476. doi: 10.1016/j.beem.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Zhuang Z, Ezzat SZ, Vortmeyer AO, Weil R, Oldfield EH, Park WS, et al. Mutations of the MEN1 tumor suppressor gene in pituitary tumors. Cancer Res. 1997;57:5446–5451. [PubMed] [Google Scholar]
- 16.Evans CO, Brown MR, Parks JS, Oyesiku NM. Screening for MEN1 tumor suppressor gene mutations in sporadic pituitary tumors. J Endocrinol Invest. 2000;23:304–309. doi: 10.1007/BF03343727. [DOI] [PubMed] [Google Scholar]
- 17.Korbonits M, Chahal HS, Kaltsas G, Jordan S, Urmanova Y, Khalimova Z, et al. Expression of phosphorylated p27(Kip1) protein and Jun activation domain-binding protein 1 in human pituitary tumors. J Clin Endocrinol Metab. 2002;87:2635–2643. doi: 10.1210/jcem.87.6.8517. [DOI] [PubMed] [Google Scholar]
- 18.Prezant TR, Levine J, Melmed S. Molecular characterization of the men1 tumor suppressor gene in sporadic pituitary tumors. J Clin Endocrinol Metab. 1998;83:1388–1391. doi: 10.1210/jcem.83.4.4859. [DOI] [PubMed] [Google Scholar]
- 19.Newey PJ, Nesbit MA, Rimmer AJ, Head RA, Gorvin CM, Attar M, et al. Whole-exome sequencing studies of nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 2013;98:E796–E800. doi: 10.1210/jc.2012-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwata T, Yamada S, Mizusawa N, Golam HM, Sano T, Yoshimoto K. The aryl hydrocarbon receptor-interacting protein gene is rarely mutated in sporadic GH-secreting adenomas. Clin Endocrinol (Oxf) 2007;66:499–502. doi: 10.1111/j.1365-2265.2007.02758.x. [DOI] [PubMed] [Google Scholar]
- 21.Raitila A, Georgitsi M, Karhu A, Tuppurainen K, Makinen MJ, Birkenkamp-Demtroder K, et al. No evidence of somatic aryl hydrocarbon receptor interacting protein mutations in sporadic endocrine neoplasia. Endocr Relat Cancer. 2007;14:901–906. doi: 10.1677/ERC-07-0025. [DOI] [PubMed] [Google Scholar]
- 22.DiGiovanni R, Serra S, Ezzat S, Asa SL. AIP Mutations are not identified in patients with sporadic pituitary adenomas. Endocr Pathol. 2007;18:76–78. doi: 10.1007/s12022-007-0010-z. [DOI] [PubMed] [Google Scholar]
- 23.Kaltsas GA, Kola B, Borboli N, Morris DG, Gueorguiev M, Swords FM, et al. Sequence analysis of the PRKAR1A gene in sporadic somatotroph and other pituitary tumours. Clin Endocrinol (Oxf) 2002;57:443–448. doi: 10.1046/j.1365-2265.2002.01643.x. [DOI] [PubMed] [Google Scholar]
- 24.Sandrini F, Kirschner LS, Bei T, Farmakidis C, Yasufuku-Takano J, Takano K, et al. PRKAR1A, one of the Carney complex genes, and its locus (17q22–24 are rarely altered in pituitary tumours outside the Carney complex. J Med Genet. 2002;39:e78. doi: 10.1136/jmg.39.12.e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasuki L, Vieira Neto L, Wildemberg LE, Colli LM, de Castro M, Takiya CM, et al. AIP expression in sporadic somatotropinomas is a predictor of the response to octreotide LAR therapy independent of SSTR2 expression. Endocr Relat Cancer. 2012;19:L25–L29. doi: 10.1530/ERC-12-0020. [DOI] [PubMed] [Google Scholar]
- 26.Trivellin G, Butz H, Delhove J, Igreja S, Chahal HS, Zivkovic V, et al. MicroRNA miR-107 is overexpressed in pituitary adenomas and inhibits the expression of aryl hydrocarbon receptor-interacting protein in vitro. Am J Physiol Endocrinol Metab. 2012;303:E708–E719. doi: 10.1152/ajpendo.00546.2011. [DOI] [PubMed] [Google Scholar]
- 27.Denes J, Kasuki L, Trivellin G, Colli LM, Takiya CM, Stiles CE, et al. Regulation of aryl hydrocarbon receptor interacting protein (AIP) protein expression by miR-34a in sporadic somatotropinomas. PLoS One. 2015;10:e0117107. doi: 10.1371/journal.pone.0117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toledo RA, Mendonca BB, Fragoso MC, Soares IC, Almeida MQ, Moraes MB, et al. Isolated familial somatotropinoma: 11q13-loh and gene/protein expression analysis suggests a possible involvement of aip also in non-pituitary tumorigenesis. Clinics (Sao Paulo) 2010;65:407–415. doi: 10.1590/S1807-59322010000400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khoo SK, Pendek R, Nickolov R, Luccio-Camelo DC, Newton TL, Massie A, et al. Genome-wide scan identifies novel modifier loci of acromegalic phenotypes for isolated familial somatotropinoma. Endocr Relat Cancer. 2009;16:1057–1063. doi: 10.1677/ERC-08-0287. [DOI] [PubMed] [Google Scholar]
- 30.Longuini VC, Lourenco DM, Jr, Sekiya T, Meirelles O, Goncalves TD, Coutinho FL, et al. Association between the p27 rs2066827 variant and tumor multiplicity in patients harboring MEN1 germline mutations. Eur J Endocrinol. 2014;171:335–342. doi: 10.1530/EJE-14-0130. [DOI] [PubMed] [Google Scholar]
- 31.de Sanctis C, Lala R, Matarazzo P, Balsamo A, Bergamaschi R, Cappa M, et al. McCune-Albright syndrome: a longitudinal clinical study of 32 patients. J Pediatr Endocrinol Metab. 1999;12:817–826. doi: 10.1515/jpem.1999.12.6.817. [DOI] [PubMed] [Google Scholar]
- 32.Gadelha MR, Trivellin G, Hernandez Ramirez LC, Korbonits M. Genetics of pituitary adenomas. Front Horm Res. 2013;41:111–140. doi: 10.1159/000345673. [DOI] [PubMed] [Google Scholar]
- 33.Beckers A, Aaltonen LA, Daly AF, Karhu A. Familial isolated pituitary adenomas (FIPA) and the pituitary adenoma predisposition due to mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocr Rev. 2013;34:239–277. doi: 10.1210/er.2012-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, et al. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science. 2006;312:1228–1230. doi: 10.1126/science.1126100. [DOI] [PubMed] [Google Scholar]
- 35.Kantorovich V, King KS, Pacak K. SDH-related pheochromocytoma and paraganglioma. Best Pract Res Clin Endocrinol Metab. 2010;24:415–424. doi: 10.1016/j.beem.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xekouki P, Pacak K, Almeida M, Wassif CA, Rustin P, Nesterova M, et al. Succinate dehydrogenase (SDH) D subunit (SDHD) inactivation in a growth-hormone- producing pituitary tumor: a new association for SDH? J Clin Endocrinol Metab. 2012;97:E357–E366. doi: 10.1210/jc.2011-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dwight T, Mann K, Benn DE, Robinson BG, McKelvie P, Gill AJ, et al. Familial SDHA mutation associated with pituitary adenoma and pheochromocytoma/paraganglioma. J Clin Endocrinol Metab. 2013;98:E1103–E1108. doi: 10.1210/jc.2013-1400. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Jimenez E, de Campos JM, Kusak EM, Landa I, Leskela S, Montero-Conde C, et al. SDHC mutation in an elderly patient without familial antecedents. Clin Endocrinol (Oxf) 2008;69:906–910. doi: 10.1111/j.1365-2265.2008.03368.x. [DOI] [PubMed] [Google Scholar]
- 39.Xekouki P, Stratakis CA. Succinate dehydrogenase (SDHx) mutations in pituitary tumors: could this be a new role for mitochondrial complex II and/or Krebs cycle defects? Endocr Relat Cancer. 2012;19:C33–C40. doi: 10.1530/ERC-12-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denes J, Swords F, Rattenberry E, Stals K, Owens M, Cranston T, et al. Heterogeneous genetic background of the association of pheochromocytoma/paraganglioma and pituitary adenoma – results from a large patient cohort. J Clin Endocrinol Metab. 2014:jc20143399. doi: 10.1210/jc.2014-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheithauer BW, Kovacs K, Horvath E, Kim DS, Osamura RY, Ketterling RP, et al. Pituitary blastoma. Acta Neuropathol. 2008;116:657–666. doi: 10.1007/s00401-008-0388-9. [DOI] [PubMed] [Google Scholar]
- 42.Scheithauer BW, Horvath E, Abel TW, Robital Y, Park SH, Osamura RY, et al. Pituitary blastoma: a unique embryonal tumor. Pituitary. 2012;15:365–373. doi: 10.1007/s11102-011-0328-x. [DOI] [PubMed] [Google Scholar]
- 43.de Kock L, Sabbaghian N, Plourde F, Srivastava A, Weber E, Bouron-Dal Soglio D, et al. Pituitary blastoma: a pathognomonic feature of germ-line DICER1 mutations. Acta Neuropathol. 2014;128:111–122. doi: 10.1007/s00401-014-1285-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trivellin G, Daly AF, Faucz FR, Yuan B, Rostomyan L, Larco DO, et al. Gigantism and Acromegaly Due to Xq26 Microduplications and GPR101 Mutation. N Engl J Med. 2014 doi: 10.1056/NEJMoa1408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Josefson J, Listernick R, Fangusaro JR, Charrow J, Habiby R. Growth hormone excess in children with neurofibromatosis type 1-associated and sporadic optic pathway tumors. J Pediatr. 2011;158:433–436. doi: 10.1016/j.jpeds.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Agarwal SK. Multiple endocrine neoplasia type 1. Front Horm Res. 2013;41:1–15. doi: 10.1159/000345666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marx S, Spiegel AM, Skarulis MC, Doppman JL, Collins FS, Liotta LA. Multiple endocrine neoplasia type 1: clinical and genetic topics. Ann Intern Med. 1998;129:484–494. doi: 10.7326/0003-4819-129-6-199809150-00011. [DOI] [PubMed] [Google Scholar]
- 48.Kytola S, Villablanca A, Ebeling T, Nord B, Larsson C, Hoog A, et al. Founder effect in multiple endocrine neoplasia type 1 (MEN 1) in Finland. J Med Genet. 2001;38:185–189. doi: 10.1136/jmg.38.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vierimaa O, Ebeling TM, Kytola S, Bloigu R, Eloranta E, Salmi J, et al. Multiple endocrine neoplasia type 1 in Northern Finland; clinical features and genotype phenotype correlation. Eur J Endocrinol. 2007;157:285–294. doi: 10.1530/EJE-07-0195. [DOI] [PubMed] [Google Scholar]
- 50.Trump D, Farren B, Wooding C, Pang JT, Besser GM, Buchanan KD, et al. Clinical studies of multiple endocrine neoplasia type 1 (MEN1) QJM. 1996;89:653–669. doi: 10.1093/qjmed/89.9.653. [DOI] [PubMed] [Google Scholar]
- 51.Bassett JH, Forbes SA, Pannett AA, Lloyd SE, Christie PT, Wooding C, et al. Characterization of mutations in patients with multiple endocrine neoplasia type 1. Am J Hum Genet. 1998;62:232–244. doi: 10.1086/301729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Machens A, Schaaf L, Karges W, Frank-Raue K, Bartsch DK, Rothmund M, et al. Age-related penetrance of endocrine tumours in multiple endocrine neoplasia type 1 (MEN1): a multicentre study of 258 gene carriers. Clin Endocrinol (Oxf) 2007;67:613–622. doi: 10.1111/j.1365-2265.2007.02934.x. [DOI] [PubMed] [Google Scholar]
- 53.Carty SE, Helm AK, Amico JA, Clarke MR, Foley TP, Watson CG, et al. The variable penetrance and spectrum of manifestations of multiple endocrine neoplasia type 1. Surgery. 1998;124:1106–1113. doi: 10.1067/msy.1998.93107. [DOI] [PubMed] [Google Scholar]
- 54.Doherty GM, Olson JA, Frisella MM, Lairmore TC, Wells SA, Jr, Norton JA. Lethality of multiple endocrine neoplasia type I. World J Surg. 1998;22:581–586. doi: 10.1007/s002689900438. [DOI] [PubMed] [Google Scholar]
- 55.Goudet P, Murat A, Binquet C, Cardot-Bauters C, Costa A, Ruszniewski P, et al. Risk factors and causes of death in MEN1 disease. A GTE (Groupe d'Etude des Tumeurs Endocrines) cohort study among 758 patients. World J Surg. 2010;34:249–255. doi: 10.1007/s00268-009-0290-1. [DOI] [PubMed] [Google Scholar]
- 56.Dean PG, van Heerden JA, Farley DR, Thompson GB, Grant CS, Harmsen WS, et al. Are patients with multiple endocrine neoplasia type I prone to premature death? World J Surg. 2000;24:1437–1441. doi: 10.1007/s002680010237. [DOI] [PubMed] [Google Scholar]
- 57.Twigt BA, Scholten A, Valk GD, Rinkes IH, Vriens MR. Differences between sporadic and MEN related primary hyperparathyroidism; clinical expression, preoperative workup, operative strategy and follow-up. Orphanet J Rare Dis. 2013;8:50. doi: 10.1186/1750-1172-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eller-Vainicher C, Chiodini I, Battista C, Viti R, Mascia ML, Massironi S, et al. Sporadic and MEN1-related primary hyperparathyroidism: differences in clinical expression and severity. J Bone Miner Res. 2009;24:1404–1410. doi: 10.1359/jbmr.090304. [DOI] [PubMed] [Google Scholar]
- 59.Lourenco DM, Jr, Coutinho FL, Toledo RA, Montenegro FL, Correia-Deur JE, Toledo SP. Early-onset, progressive, frequent, extensive, and severe bone mineral and renal complications in multiple endocrine neoplasia type 1-associated primary hyperparathyroidism. J Bone Miner Res. 2010;25:2382–2391. doi: 10.1002/jbmr.125. [DOI] [PubMed] [Google Scholar]
- 60.Waldmann J, Lopez CL, Langer P, Rothmund M, Bartsch DK. Surgery for multiple endocrine neoplasia type 1-associated primary hyperparathyroidism. Br J Surg. 2010;97:1528–1534. doi: 10.1002/bjs.7154. [DOI] [PubMed] [Google Scholar]
- 61.Arnalsteen LC, Alesina PF, Quiereux JL, Farrel SG, Patton FN, Carnaille BM, et al. Long-term results of less than total parathyroidectomy for hyperparathyroidism in multiple endocrine neoplasia type 1. Surgery. 2002;132:1119–1124. doi: 10.1067/msy.2002.128607. [DOI] [PubMed] [Google Scholar]
- 62.Kraimps JL, Duh QY, Demeure M, Clark OH. Hyperparathyroidism in multiple endocrine neoplasia syndrome. Surgery. 1992;112:1080–1086. [PubMed] [Google Scholar]
- 63.Rizzoli R, Green J, 3rd, Marx SJ. Primary hyperparathyroidism in familial multiple endocrine neoplasia type I. Long-term follow-up of serum calcium levels after parathyroidectomy. Am J Med. 1985;78:467–474. doi: 10.1016/0002-9343(85)90340-7. [DOI] [PubMed] [Google Scholar]
- 64.Salmeron MD, Gonzalez JM, Sancho Insenser J, Goday A, Perez NM, Zambudio AR, et al. Causes and treatment of recurrent hyperparathyroidism after subtotal parathyroidectomy in the presence of multiple endocrine neoplasia 1. World J Surg. 2010;34:1325–1331. doi: 10.1007/s00268-010-0605-2. [DOI] [PubMed] [Google Scholar]
- 65.Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1) J Clin Endocrinol Metab. 2012;97:2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 66.Anlauf M, Schlenger R, Perren A, Bauersfeld J, Koch CA, Dralle H, et al. Microadenomatosis of the endocrine pancreas in patients with and without the multiple endocrine neoplasia type 1 syndrome. Am J Surg Pathol. 2006;30:560–574. doi: 10.1097/01.pas.0000194044.01104.25. [DOI] [PubMed] [Google Scholar]
- 67.Kloppel G, Willemer S, Stamm B, Hacki WH, Heitz PU. Pancreatic lesions and hormonal profile of pancreatic tumors in multiple endocrine neoplasia type I. An immunocytochemical study of nine patients. Cancer. 1986;57:1824–1832. doi: 10.1002/1097-0142(19860501)57:9<1824::aid-cncr2820570920>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 68.Akerstrom G, Hessman O, Hellman P, Skogseid B. Pancreatic tumours as part of the MEN-1 syndrome. Best Pract Res Clin Gastroenterol. 2005;19:819–830. doi: 10.1016/j.bpg.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 69.Skogseid B, Oberg K, Eriksson B, Juhlin C, Granberg D, Akerstrom G, et al. Surgery for asymptomatic pancreatic lesion in multiple endocrine neoplasia type I. World J Surg. 1996;20:872–876. doi: 10.1007/s002689900133. discussion 7. [DOI] [PubMed] [Google Scholar]
- 70.Berna MJ, Annibale B, Marignani M, Luong TV, Corleto V, Pace A, et al. A prospective study of gastric carcinoids and enterochromaffin-like cell changes in multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: identification of risk factors. J Clin Endocrinol Metab. 2008;93:1582–1591. doi: 10.1210/jc.2007-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruszniewski P, Podevin P, Cadiot G, Marmuse JP, Mignon M, Vissuzaine C, et al. Clinical, anatomical, and evolutive features of patients with the Zollinger-Ellison syndrome combined with type I multiple endocrine neoplasia. Pancreas. 1993;8:295–304. doi: 10.1097/00006676-199305000-00003. [DOI] [PubMed] [Google Scholar]
- 72.Pipeleers-Marichal M, Somers G, Willems G, Foulis A, Imrie C, Bishop AE, et al. Gastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. N Engl J Med. 1990;322:723–727. doi: 10.1056/NEJM199003153221103. [DOI] [PubMed] [Google Scholar]
- 73.Fendrich V, Langer P, Waldmann J, Bartsch DK, Rothmund M. Management of sporadic and multiple endocrine neoplasia type 1 gastrinomas. Br J Surg. 2007;94:1331–1341. doi: 10.1002/bjs.5987. [DOI] [PubMed] [Google Scholar]
- 74.Triponez F, Dosseh D, Goudet P, Cougard P, Bauters C, Murat A, et al. Epidemiology data on 108 MEN 1 patients from the GTE with isolated nonfunctioning tumors of the pancreas. Ann Surg. 2006;243:265–272. doi: 10.1097/01.sla.0000197715.96762.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akerstrom G, Hellman P. Surgery on neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007;21:87–109. doi: 10.1016/j.beem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 76.Verges B, Boureille F, Goudet P, Murat A, Beckers A, Sassolas G, et al. Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. J Clin Endocrinol Metab. 2002;87:457–465. doi: 10.1210/jcem.87.2.8145. [DOI] [PubMed] [Google Scholar]
- 77.Stratakis CA, Schussheim DH, Freedman SM, Keil MF, Pack SD, Agarwal SK, et al. Pituitary macroadenoma in a 5-year-old: an early expression of multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 2000;85:4776–4780. doi: 10.1210/jcem.85.12.7064. [DOI] [PubMed] [Google Scholar]
- 78.Trouillas J, Labat-Moleur F, Sturm N, Kujas M, Heymann MF, Figarella- Branger D, et al. Pituitary tumors and hyperplasia in multiple endocrine neoplasia type 1 syndrome (MEN1): a case-control study in a series of 77 patients versus 2509 non-MEN1 patients. Am J Surg Pathol. 2008;32:534–543. doi: 10.1097/PAS.0b013e31815ade45. [DOI] [PubMed] [Google Scholar]
- 79.Daly AF, Tichomirowa MA, Beckers A. The epidemiology and genetics of pituitary adenomas. Best Pract Res Clin Endocrinol Metab. 2009;23:543–554. doi: 10.1016/j.beem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 80.Saleem TF, Santhanam P, Hamoudeh E, Hassan T, Faiz S. Acromegaly caused by growth hormone releasing hormone (GHRH) secreting tumor in multiple endocrine neoplasia (MEN-1) W V Med J. 2012;108:26–30. [PubMed] [Google Scholar]
- 81.Ramsay JA, Kovacs K, Asa SL, Pike MJ, Thorner MO. Reversible sellar enlargement due to growth hormone-releasing hormone production by pancreatic endocrine tumors in a acromegalic patient with multiple endocrine neoplasia type I syndrome. Cancer. 1988;62:445–450. doi: 10.1002/1097-0142(19880715)62:2<445::aid-cncr2820620233>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 82.Shull JD, Birt DF, McComb RD, Spady TJ, Pennington KL, Shaw-Bruha CM. Estrogen induction of prolactin-producing pituitary tumors in the Fischer 344 rat: modulation by dietary-energy but not protein consumption. Mol Carcinog. 1998;23:96–105. [PubMed] [Google Scholar]
- 83.Gatta-Cherifi B, Chabre O, Murat A, Niccoli P, Cardot-Bauters C, Rohmer V, et al. Adrenal involvement in MEN1. Analysis of 715 cases from the Groupe d'etude des Tumeurs Endocrines database. Eur J Endocrinol. 2012;166:269–279. doi: 10.1530/EJE-11-0679. [DOI] [PubMed] [Google Scholar]
- 84.Darling TN, Skarulis MC, Steinberg SM, Marx SJ, Spiegel AM, Turner M. Multiple facial angiofibromas and collagenomas in patients with multiple endocrine neoplasia type 1. Arch Dermatol. 1997;133:853–857. [PubMed] [Google Scholar]
- 85.Asgharian B, Turner ML, Gibril F, Entsuah LK, Serrano J, Jensen RT. Cutaneous tumors in patients with multiple endocrine neoplasm type 1 (MEN1) and gastrinomas: prospective study of frequency and development of criteria with high sensitivity and specificity for MEN1. J Clin Endocrinol Metab. 2004;89:5328–5336. doi: 10.1210/jc.2004-0218. [DOI] [PubMed] [Google Scholar]
- 86.van Wijk JP, Dreijerink KM, Pieterman CR, Lips CJ, Zelissen PM, Valk GD. Increased prevalence of impaired fasting glucose in MEN1 gene mutation carriers. Clin Endocrinol (Oxf) 2012;76:67–71. doi: 10.1111/j.1365-2265.2011.04166.x. [DOI] [PubMed] [Google Scholar]
- 87.McCallum RW, Parameswaran V, Burgess JR. Multiple endocrine neoplasia type 1 (MEN 1) is associated with an increased prevalence of diabetes mellitus and impaired fasting glucose. Clin Endocrinol (Oxf) 2006;65:163–168. doi: 10.1111/j.1365-2265.2006.02563.x. [DOI] [PubMed] [Google Scholar]
- 88.Larsson C, Skogseid B, Oberg K, Nakamura Y, Nordenskjold M. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature. 1988;332:85–87. doi: 10.1038/332085a0. [DOI] [PubMed] [Google Scholar]
- 89.Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–407. doi: 10.1126/science.276.5311.404. [DOI] [PubMed] [Google Scholar]
- 90.Lemmens I, Van de Ven WJ, Kas K, Zhang CX, Giraud S, Wautot V, et al. Identification of the multiple endocrine neoplasia type 1 (MEN1) gene. The European Consortium on MEN1. Hum Mol Genet. 1997;6:1177–1183. doi: 10.1093/hmg/6.7.1177. [DOI] [PubMed] [Google Scholar]
- 91.Friedman E, Sakaguchi K, Bale AE, Falchetti A, Streeten E, Zimering MB, et al. Clonality of parathyroid tumors in familial multiple endocrine neoplasia type 1. N Engl J Med. 1989;321:213–218. doi: 10.1056/NEJM198907273210402. [DOI] [PubMed] [Google Scholar]
- 92.Thakker RV, Bouloux P, Wooding C, Chotai K, Broad PM, Spurr NK, et al. Association of parathyroid tumors in multiple endocrine neoplasia type 1 with loss of alleles on chromosome 11. N Engl J Med. 1989;321:218–224. doi: 10.1056/NEJM198907273210403. [DOI] [PubMed] [Google Scholar]
- 93.Wautot V, Khodaei S, Frappart L, Buisson N, Baro E, Lenoir GM, et al. Expression analysis of endogenous menin, the product of the multiple endocrine neoplasia type 1 gene, in cell lines and human tissues. Int J Cancer. 2000;85:877–881. doi: 10.1002/(sici)1097-0215(20000315)85:6<877::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 94.Guru SC, Goldsmith PK, Burns AL, Marx SJ, Spiegel AM, Collins FS, et al. Menin, the product of the MEN1 gene, is a nuclear protein. Proc Natl Acad Sci U S A. 1998;95:1630–1634. doi: 10.1073/pnas.95.4.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.La P, Desmond A, Hou Z, Silva AC, Schnepp RW, Hua X. Tumor suppressor menin: the essential role of nuclear localization signal domains in coordinating gene expression. Oncogene. 2006;25:3537–3546. doi: 10.1038/sj.onc.1209400. [DOI] [PubMed] [Google Scholar]
- 96.Jin S, Mao H, Schnepp RW, Sykes SM, Silva AC, D'Andrea AD, et al. Menin associates with FANCD2, a protein involved in repair of DNA damage. Cancer Res. 2003;63:4204–4210. [PubMed] [Google Scholar]
- 97.La P, Silva AC, Hou Z, Wang H, Schnepp RW, Yan N, et al. Direct binding of DNA by tumor suppressor menin. J Biol Chem. 2004;279:49045–49054. doi: 10.1074/jbc.M409358200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hughes CM, Rozenblatt-Rosen O, Milne TA, Copeland TD, Levine SS, Lee JC, et al. Menin associates with a trithorax family histone methyltransferase complex and with the hoxc8 locus. Mol Cell. 2004;13:587–597. doi: 10.1016/s1097-2765(04)00081-4. [DOI] [PubMed] [Google Scholar]
- 99.Huang J, Gurung B, Wan B, Matkar S, Veniaminova NA, Wan K, et al. The same pocket in menin binds both MLL and JUND but has opposite effects on transcription. Nature. 2012;482:542–546. doi: 10.1038/nature10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Busygina V, Kottemann MC, Scott KL, Plon SE, Bale AE. Multiple endocrine neoplasia type 1 interacts with forkhead transcription factor CHES1 in DNA damage response. Cancer Res. 2006;66:8397–8403. doi: 10.1158/0008-5472.CAN-06-0061. [DOI] [PubMed] [Google Scholar]
- 101.Agarwal SK, Guru SC, Heppner C, Erdos MR, Collins RM, Park SY, et al. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell. 1999;96:143–152. doi: 10.1016/s0092-8674(00)80967-8. [DOI] [PubMed] [Google Scholar]
- 102.Kaji H, Canaff L, Lebrun JJ, Goltzman D, Hendy GN. Inactivation of menin, a Smad3-interacting protein, blocks transforming growth factor type beta signaling. Proc Natl Acad Sci U S A. 2001;98:3837–3842. doi: 10.1073/pnas.061358098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.La P, Schnepp RW, C DP, A CS, Hua X. Tumor suppressor menin regulates expression of insulin-like growth factor binding protein 2. Endocrinology. 2004;145:3443–3450. doi: 10.1210/en.2004-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heppner C, Bilimoria KY, Agarwal SK, Kester M, Whitty LJ, Guru SC, et al. The tumor suppressor protein menin interacts with NF-kappaB proteins and inhibits NF-kappaB-mediated transactivation. Oncogene. 2001;20:4917–4925. doi: 10.1038/sj.onc.1204529. [DOI] [PubMed] [Google Scholar]
- 105.Inoue Y, Hendy GN, Canaff L, Seino S, Kaji H. Menin interacts with beta-catenin in osteoblast differentiation. Horm Metab Res. 2011;43:183–187. doi: 10.1055/s-0030-1270527. [DOI] [PubMed] [Google Scholar]
- 106.Imachi H, Murao K, Dobashi H, Bhuyan MM, Cao X, Kontani K, et al. Menin, a product of the MENI gene, binds to estrogen receptor to enhance its activity in breast cancer cells: possibility of a novel predictive factor for tamoxifen resistance. Breast Cancer Res Treat. 2010;122:395–407. doi: 10.1007/s10549-009-0581-0. [DOI] [PubMed] [Google Scholar]
- 107.Dreijerink KM, Mulder KW, Winkler GS, Hoppener JW, Lips CJ, Timmers HT. Menin links estrogen receptor activation to histone H3K4 trimethylation. Cancer Res. 2006;66:4929–4935. doi: 10.1158/0008-5472.CAN-05-4461. [DOI] [PubMed] [Google Scholar]
- 108.Lopez-Egido J, Cunningham J, Berg M, Oberg K, Bongcam-Rudloff E, Gobl A. Menin's interaction with glial fibrillary acidic protein and vimentin suggests a role for the intermediate filament network in regulating menin activity. Exp Cell Res. 2002;278:175–183. doi: 10.1006/excr.2002.5575. [DOI] [PubMed] [Google Scholar]
- 109.Wuescher L, Angevine K, Hinds T, Ramakrishnan S, Najjar SM, Mensah-Osman EJ. Insulin regulates menin expression, cytoplasmic localization, and interaction with FOXO1. Am J Physiol Endocrinol Metab. 2011;301:E474–E483. doi: 10.1152/ajpendo.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Y, Ozawa A, Zaman S, Prasad NB, Chandrasekharappa SC, Agarwal SK, et al. The tumor suppressor protein menin inhibits AKT activation by regulating its cellular localization. Cancer Res. 2011;71:371–382. doi: 10.1158/0008-5472.CAN-10-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim YS, Burns AL, Goldsmith PK, Heppner C, Park SY, Chandrasekharappa SC, et al. Stable overexpression of MEN1 suppresses tumorigenicity of RAS. Oncogene. 1999;18:5936–5942. doi: 10.1038/sj.onc.1203005. [DOI] [PubMed] [Google Scholar]
- 112.Ratineau C, Bernard C, Poncet G, Blanc M, Josso C, Fontaniere S, et al. Reduction of menin expression enhances cell proliferation and is tumorigenic in intestinal epithelial cells. J Biol Chem. 2004;279:24477–24484. doi: 10.1074/jbc.M401835200. [DOI] [PubMed] [Google Scholar]
- 113.Schnepp RW, Hou Z, Wang H, Petersen C, Silva A, Masai H, et al. Functional interaction between tumor suppressor menin and activator of S-phase kinase. Cancer Res. 2004;64:6791–6796. doi: 10.1158/0008-5472.CAN-04-0724. [DOI] [PubMed] [Google Scholar]
- 114.Schnepp RW, Mao H, Sykes SM, Zong WX, Silva A, La P, et al. Menin induces apoptosis in murine embryonic fibroblasts. J Biol Chem. 2004;279:10685–10691. doi: 10.1074/jbc.M308073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sayo Y, Murao K, Imachi H, Cao WM, Sato M, Dobashi H, et al. The multiple endocrine neoplasia type 1 gene product, menin, inhibits insulin production in rat insulinoma cells. Endocrinology. 2002;143:2437–2440. doi: 10.1210/endo.143.6.8950. [DOI] [PubMed] [Google Scholar]
- 116.Busygina V, Suphapeetiporn K, Marek LR, Stowers RS, Xu T, Bale AE. Hypermutability in a Drosophila model for multiple endocrine neoplasia type 1. Hum Mol Genet. 2004;13:2399–2408. doi: 10.1093/hmg/ddh271. [DOI] [PubMed] [Google Scholar]
- 117.Milne TA, Hughes CM, Lloyd R, Yang Z, Rozenblatt-Rosen O, Dou Y, et al. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc Natl Acad Sci U S A. 2005;102:749–754. doi: 10.1073/pnas.0408836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yokoyama A, Wang Z, Wysocka J, Sanyal M, Aufiero DJ, Kitabayashi I, et al. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol Cell Biol. 2004;24:5639–5649. doi: 10.1128/MCB.24.13.5639-5649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yokoyama A, Somervaille TC, Smith KS, Rozenblatt-Rosen O, Meyerson M, Cleary ML. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell. 2005;123:207–218. doi: 10.1016/j.cell.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 120.Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29:22–32. doi: 10.1002/humu.20605. [DOI] [PubMed] [Google Scholar]
- 121.Yaguchi H, Ohkura N, Takahashi M, Nagamura Y, Kitabayashi I, Tsukada T. Menin missense mutants associated with multiple endocrine neoplasia type 1 are rapidly degraded via the ubiquitin-proteasome pathway. Mol Cell Biol. 2004;24:6569–6580. doi: 10.1128/MCB.24.15.6569-6580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Luzi E, Marini F, Tognarini I, Carbonell Sala S, Galli G, Falchetti A, et al. Ribozyme-mediated compensatory induction of menin-oncosuppressor function in primary fibroblasts from MEN1 patients. Cancer Gene Ther. 2010;17:814–825. doi: 10.1038/cgt.2010.39. [DOI] [PubMed] [Google Scholar]
- 123.Turner JJ, Leotlela PD, Pannett AA, Forbes SA, Bassett JH, Harding B, et al. Frequent occurrence of an intron 4 mutation in multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 2002;87:2688–2693. doi: 10.1210/jcem.87.6.8607. [DOI] [PubMed] [Google Scholar]
- 124.Nagamura Y, Yamazaki M, Shimazu S, Sano K, Tsukada T, Sakurai A. A novel splice site mutation of the MEN1 gene identified in a patient with primary hyperparathyroidism. Endocr J. 2012;59:523–530. doi: 10.1507/endocrj.ej12-0037. [DOI] [PubMed] [Google Scholar]
- 125.Lemos MC, Harding B, Shalet SM, Thakker RV. A novel MEN1 intronic mutation associated with multiple endocrine neoplasia type 1. Clin Endocrinol (Oxf) 2007;66:709–713. doi: 10.1111/j.1365-2265.2007.02806.x. [DOI] [PubMed] [Google Scholar]
- 126.Ellard S, Hattersley AT, Brewer CM, Vaidya B. Detection of an MEN1 gene mutation depends on clinical features and supports current referral criteria for diagnostic molecular genetic testing. Clin Endocrinol (Oxf) 2005;62:169–175. doi: 10.1111/j.1365-2265.2005.02190.x. [DOI] [PubMed] [Google Scholar]
- 127.Klein RD, Salih S, Bessoni J, Bale AE. Clinical testing for multiple endocrine neoplasia type 1 in a DNA diagnostic laboratory. Genet Med. 2005;7:131–138. doi: 10.1097/01.gim.0000153663.62300.f8. [DOI] [PubMed] [Google Scholar]
- 128.Burgess JR, Nord B, David R, Greenaway TM, Parameswaran V, Larsson C, et al. Phenotype and phenocopy: the relationship between genotype and clinical phenotype in a single large family with multiple endocrine neoplasia type 1 (MEN 1) Clin Endocrinol (Oxf) 2000;53:205–211. doi: 10.1046/j.1365-2265.2000.01032.x. [DOI] [PubMed] [Google Scholar]
- 129.Owens M, Ellard S, Vaidya B. Analysis of gross deletions in the MEN1 gene in patients with multiple endocrine neoplasia type 1. Clin Endocrinol (Oxf) 2008;68:350–354. doi: 10.1111/j.1365-2265.2007.03045.x. [DOI] [PubMed] [Google Scholar]
- 130.Tham E, Grandell U, Lindgren E, Toss G, Skogseid B, Nordenskjold M. Clinical testing for mutations in the MEN1 gene in Sweden: a report on 200 unrelated cases. J Clin Endocrinol Metab. 2007;92:3389–3395. doi: 10.1210/jc.2007-0476. [DOI] [PubMed] [Google Scholar]
- 131.Kouvaraki MA, Lee JE, Shapiro SE, Gagel RF, Sherman SI, Sellin RV, et al. Genotype-phenotype analysis in multiple endocrine neoplasia type 1. Arch Surg. 2002;137:641–647. doi: 10.1001/archsurg.137.6.641. [DOI] [PubMed] [Google Scholar]
- 132.Bartsch DK, Langer P, Wild A, Schilling T, Celik I, Rothmund M, et al. Pancreaticoduodenal endocrine tumors in multiple endocrine neoplasia type 1: surgery or surveillance? Surgery. 2000;128:958–966. doi: 10.1067/msy.2000.109727. [DOI] [PubMed] [Google Scholar]
- 133.Thakker RV. Multiple endocrine neoplasia--syndromes of the twentieth century. J Clin Endocrinol Metab. 1998;83:2617–2620. doi: 10.1210/jcem.83.8.5045. [DOI] [PubMed] [Google Scholar]
- 134.Flanagan DE, Armitage M, Clein GP, Thakker RV. Prolactinoma presenting in identical twins with multiple endocrine neoplasia type 1. Clin Endocrinol (Oxf) 1996;45:117–120. [PubMed] [Google Scholar]
- 135.Namihira H, Sato M, Miyauchi A, Ohye H, Matsubara S, Bhuiyan MM, et al. Different phenotypes of multiple endocrine neoplasia type 1 (MEN1) in monozygotic twins found in a Japanese MEN1 family with MEN1 gene mutation. Endocr J. 2000;47:37–43. doi: 10.1507/endocrj.47.37. [DOI] [PubMed] [Google Scholar]
- 136.Concolino P, Rossodivita A, Carrozza C, Raffaelli M, Lombardi CP, Rigante D, et al. A novel MEN1 frameshift germline mutation in two Italian monozygotic twins. Clin Chem Lab Med. 2008;46:824–826. doi: 10.1515/CCLM.2008.165. [DOI] [PubMed] [Google Scholar]
- 137.Lips CJ, Dreijerink KM, Hoppener JW. Variable clinical expression in patients with a germline MEN1 disease gene mutation: clues to a genotype-phenotype correlation. Clinics (Sao Paulo) 2012;67(Suppl 1):49–56. doi: 10.6061/clinics/2012(Sup01)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pellegata NS, Quintanilla-Martinez L, Siggelkow H, Samson E, Bink K, Hofler H, et al. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci U S A. 2006;103:15558–15563. doi: 10.1073/pnas.0603877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Karnik SK, Hughes CM, Gu X, Rozenblatt-Rosen O, McLean GW, Xiong Y, et al. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc Natl Acad Sci U S A. 2005;102:14659–14664. doi: 10.1073/pnas.0503484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.James MK, Ray A, Leznova D, Blain SW. Differential modification of p27Kip1 controls its cyclin D-cdk4 inhibitory activity. Mol Cell Biol. 2008;28:498–510. doi: 10.1128/MCB.02171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Donovan JC, Milic A, Slingerland JM. Constitutive MEK/MAPK activation leads to p27(Kip1) deregulation and antiestrogen resistance in human breast cancer cells. J Biol Chem. 2001;276:40888–40895. doi: 10.1074/jbc.M106448200. [DOI] [PubMed] [Google Scholar]
- 142.Andreu EJ, Lledo E, Poch E, Ivorra C, Albero MP, Martinez-Climent JA, et al. BCR-ABL induces the expression of Skp2 through the PI3K pathway to promote p27Kip1 degradation and proliferation of chronic myelogenous leukemia cells. Cancer Res. 2005;65:3264–3272. doi: 10.1158/0008-5472.CAN-04-1357. [DOI] [PubMed] [Google Scholar]
- 143.Agarwal SK, Mateo CM, Marx SJ. Rare germline mutations in cyclindependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J Clin Endocrinol Metab. 2009;94:1826–1834. doi: 10.1210/jc.2008-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Molatore S, Marinoni I, Lee M, Pulz E, Ambrosio MR, degli Uberti EC, et al. A novel germline CDKN1B mutation causing multiple endocrine tumors: clinical, genetic and functional characterization. Hum Mutat. 2010;31:E1825–E1835. doi: 10.1002/humu.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Malanga D, De Gisi S, Riccardi M, Scrima M, De Marco C, Robledo M, et al. Functional characterization of a rare germline mutation in the gene encoding the cyclin-dependent kinase inhibitor p27Kip1 (CDKN1B) in a Spanish patient with multiple endocrine neoplasia-like phenotype. Eur J Endocrinol. 2012;166:551–560. doi: 10.1530/EJE-11-0929. [DOI] [PubMed] [Google Scholar]
- 146.Occhi G, Regazzo D, Trivellin G, Boaretto F, Ciato D, Bobisse S, et al. A novel mutation in the upstream open reading frame of the CDKN1B gene causes a MEN4 phenotype. PLoS Genet. 2013;9:e1003350. doi: 10.1371/journal.pgen.1003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pellegata NS. MENX and MEN4. Clinics (Sao Paulo) 2012;67(Suppl 1):13–18. doi: 10.6061/clinics/2012(Sup01)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Georgitsi M, Raitila A, Karhu A, van der Luijt RB, Aalfs CM, Sane T, et al. Germline CDKN1B/p27Kip1 mutation in multiple endocrine neoplasia. J Clin Endocrinol Metab. 2007;92:3321–3325. doi: 10.1210/jc.2006-2843. [DOI] [PubMed] [Google Scholar]
- 149.Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, et al. Mutations in regulatory subunit type 1A of cyclic adenosine 5'-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab. 2009;94:2085–2091. doi: 10.1210/jc.2008-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab. 2001;86:4041–4046. doi: 10.1210/jcem.86.9.7903. [DOI] [PubMed] [Google Scholar]
- 151.Carney JA, Hruska LS, Beauchamp GD, Gordon H. Dominant inheritance of the complex of myxomas, spotty pigmentation, and endocrine overactivity. Mayo Clin Proc. 1986;61:165–172. doi: 10.1016/s0025-6196(12)61843-6. [DOI] [PubMed] [Google Scholar]
- 152.Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 1985;64:270–283. doi: 10.1097/00005792-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 153.Stergiopoulos SG, Stratakis CA. Human tumors associated with Carney complex and germline PRKAR1A mutations: a protein kinase A disease! FEBS Lett. 2003;546:59–64. doi: 10.1016/s0014-5793(03)00452-6. [DOI] [PubMed] [Google Scholar]
- 154.Atherton DJ, Pitcher DW, Wells RS, MacDonald DM. A syndrome of various cutaneous pigmented lesions, myxoid neurofibromata and atrial myxoma: the NAME syndrome. Br J Dermatol. 1980;103:421–429. doi: 10.1111/j.1365-2133.1980.tb07266.x. [DOI] [PubMed] [Google Scholar]
- 155.Rhodes AR, Silverman RA, Harrist TJ, Perez-Atayde AR. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: the "LAMB" syndrome. J Am Acad Dermatol. 1984;10:72–82. doi: 10.1016/s0190-9622(84)80047-x. [DOI] [PubMed] [Google Scholar]
- 156.Kirschner LS, Sandrini F, Monbo J, Lin JP, Carney JA, Stratakis CA. Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the carney complex. Hum Mol Genet. 2000;9:3037–3046. doi: 10.1093/hmg/9.20.3037. [DOI] [PubMed] [Google Scholar]
- 157.Stratakis CA, Sarlis N, Kirschner LS, Carney JA, Doppman JL, Nieman LK, et al. Paradoxical response to dexamethasone in the diagnosis of primary pigmented nodular adrenocortical disease. Ann Intern Med. 1999;131:585–591. doi: 10.7326/0003-4819-131-8-199910190-00006. [DOI] [PubMed] [Google Scholar]
- 158.Raff SB, Carney JA, Krugman D, Doppman JL, Stratakis CA. Prolactin secretion abnormalities in patients with the "syndrome of spotty skin pigmentation, myxomas, endocrine overactivity and schwannomas" (Carney complex) J Pediatr Endocrinol Metab. 2000;13:373–379. [PubMed] [Google Scholar]
- 159.Watson JC, Stratakis CA, Bryant-Greenwood PK, Koch CA, Kirschner LS, Nguyen T, et al. Neurosurgical implications of Carney complex. J Neurosurg. 2000;92:413–418. doi: 10.3171/jns.2000.92.3.0413. [DOI] [PubMed] [Google Scholar]
- 160.Pack SD, Kirschner LS, Pak E, Zhuang Z, Carney JA, Stratakis CA. Genetic and histologic studies of somatomammotropic pituitary tumors in patients with the "complex of spotty skin pigmentation, myxomas, endocrine overactivity and schwannomas" (Carney complex) J Clin Endocrinol Metab. 2000;85:3860–3865. doi: 10.1210/jcem.85.10.6875. [DOI] [PubMed] [Google Scholar]
- 161.Stratakis CA, Matyakhina L, Courkoutsakis N, Patronas N, Voutetakis A, Stergiopoulos S, et al. Pathology and molecular genetics of the pituitary gland in patients with the 'complex of spotty skin pigmentation, myxomas, endocrine overactivity and schwannomas' (Carney complex) Front Horm Res. 2004;32:253–264. doi: 10.1159/000079049. [DOI] [PubMed] [Google Scholar]
- 162.Stergiopoulos SG, Abu-Asab MS, Tsokos M, Stratakis CA. Pituitary pathology in Carney complex patients. Pituitary. 2004;7:73–82. doi: 10.1007/s11102-005-5348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stratakis CA, Papageorgiou T, Premkumar A, Pack S, Kirschner LS, Taymans SE, et al. Ovarian lesions in Carney complex. clinical genetics and possible predisposition to malignancy. J Clin Endocrinol Metab. 2000;85:4359–4366. doi: 10.1210/jcem.85.11.6921. [DOI] [PubMed] [Google Scholar]
- 164.Gennari M, Stratakis CA, Horvath A, Pirazzoli P, Cicognani A. A novel PRKAR1A mutation associated with hepatocellular carcinoma in a young patient and a variable Carney complex phenotype in affected subjects in older generations. Clin Endocrinol (Oxf) 2008;69:751–755. doi: 10.1111/j.1365-2265.2008.03286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gaujoux S, Tissier F, Ragazzon B, Rebours V, Saloustros E, Perlemoine K, et al. Pancreatic ductal and acinar cell neoplasms in Carney complex. a possible new association. J Clin Endocrinol Metab. 2011;96:E1888–E1895. doi: 10.1210/jc.2011-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Veugelers M, Wilkes D, Burton K, McDermott DA, Song Y, Goldstein MM, et al. Comparative PRKAR1A genotype-phenotype analyses in humans with Carney complex and prkar1a haploinsufficient mice. Proc Natl Acad Sci U S A. 2004;101:14222–14227. doi: 10.1073/pnas.0405535101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bossis I, Stratakis CA. Minireview: PRKAR1A: normal and abnormal functions. Endocrinology. 2004;145:5452–5458. doi: 10.1210/en.2004-0900. [DOI] [PubMed] [Google Scholar]
- 168.Yu B, Ragazzon B, Rizk-Rabin M, Bertherat J. Protein kinase A alterations in endocrine tumors. Horm Metab Res. 2012;44:741–748. doi: 10.1055/s-0032-1316292. [DOI] [PubMed] [Google Scholar]
- 169.Robinson-White A, Meoli E, Stergiopoulos S, Horvath A, Boikos S, Bossis I, et al. PRKAR1A Mutations and protein kinase A interactions with other signaling pathways in the adrenal cortex. J Clin Endocrinol Metab. 2006;91:2380–2388. doi: 10.1210/jc.2006-0188. [DOI] [PubMed] [Google Scholar]
- 170.Groussin L, Kirschner LS, Vincent-Dejean C, Perlemoine K, Jullian E, Delemer B, et al. Molecular analysis of the cyclic AMP-dependent protein kinase A (PKA) regulatory subunit 1A (PRKAR1A) gene in patients with Carney complex and primary pigmented nodular adrenocortical disease (PPNAD) reveals novel mutations and clues for pathophysiology: augmented PKA signaling is associated with adrenal tumorigenesis in PPNAD. Am J Hum Genet. 2002;71:1433–1442. doi: 10.1086/344579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Horvath A, Bossis I, Giatzakis C, Levine E, Weinberg F, Meoli E, et al. Large deletions of the PRKAR1A gene in Carney complex. Clin Cancer Res. 2008;14:388–395. doi: 10.1158/1078-0432.CCR-07-1155. [DOI] [PubMed] [Google Scholar]
- 172.Ragazzon B, Cazabat L, Rizk-Rabin M, Assie G, Groussin L, Fierrard H, et al. Inactivation of the Carney complex gene 1 (protein kinase A regulatory subunit 1A) inhibits SMAD3 expression and TGF beta-stimulated apoptosis in adrenocortical cells. Cancer Res. 2009;69:7278–7284. doi: 10.1158/0008-5472.CAN-09-1601. [DOI] [PubMed] [Google Scholar]
- 173.Robinson-White AJ, Leitner WW, Aleem E, Kaldis P, Bossis I, Stratakis CA. PRKAR1A inactivation leads to increased proliferation and decreased apoptosis in human B lymphocytes. Cancer Res. 2006;66:10603–10612. doi: 10.1158/0008-5472.CAN-06-2200. [DOI] [PubMed] [Google Scholar]
- 174.Robinson-White A, Hundley TR, Shiferaw M, Bertherat J, Sandrini F, Stratakis CA. Protein kinase-A activity in PRKAR1A-mutant cells, and regulation of mitogen-activated protein kinases ERK1/2. Hum Mol Genet. 2003;12:1475–1484. doi: 10.1093/hmg/ddg160. [DOI] [PubMed] [Google Scholar]
- 175.Almeida MQ, Muchow M, Boikos S, Bauer AJ, Griffin KJ, Tsang KM, et al. Mouse Prkar1a haploinsufficiency leads to an increase in tumors in the Trp53+/− or Rb1+/− backgrounds and chemically induced skin papillomas by dysregulation of the cell cycle and Wnt signaling. Hum Mol Genet. 2010;19:1387–1398. doi: 10.1093/hmg/ddq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tsang KM, Starost MF, Nesterova M, Boikos SA, Watkins T, Almeida MQ, et al. Alternate protein kinase A activity identifies a unique population of stromal cells in adult bone. Proc Natl Acad Sci U S A. 2010;107:8683–8688. doi: 10.1073/pnas.1003680107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tadjine M, Lampron A, Ouadi L, Horvath A, Stratakis CA, Bourdeau I. Detection of somatic beta-catenin mutations in primary pigmented nodular adrenocortical disease (PPNAD) Clin Endocrinol (Oxf) 2008;69:367–373. doi: 10.1111/j.1365-2265.2008.03273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gaujoux S, Tissier F, Groussin L, Libe R, Ragazzon B, Launay P, et al. Wnt/beta-catenin and 3',5'-cyclic adenosine 5'-monophosphate/protein kinase A signaling pathways alterations and somatic beta-catenin gene mutations in the progression of adrenocortical tumors. J Clin Endocrinol Metab. 2008;93:4135–4140. doi: 10.1210/jc.2008-0631. [DOI] [PubMed] [Google Scholar]
- 179.Salpea P, Horvath A, London E, Faucz FR, Vetro A, Levy I, et al. Deletions of the PRKAR1A locus at 17q24.2-q24.3 in Carney complex: genotype-phenotype correlations and implications for genetic testing. J Clin Endocrinol Metab. 2014;99:E183–E188. doi: 10.1210/jc.2013-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Groussin L, Horvath A, Jullian E, Boikos S, Rene-Corail F, Lefebvre H, et al. A PRKAR1A mutation associated with primary pigmented nodular adrenocortical disease in 12 kindreds. J Clin Endocrinol Metab. 2006;91:1943–1949. doi: 10.1210/jc.2005-2708. [DOI] [PubMed] [Google Scholar]
- 181.Cuny T, Pertuit M, Sahnoun-Fathallah M, Daly A, Occhi G, Odou MF, et al. Genetic analysis in young patients with sporadic pituitary macroadenomas: besides AIP don't forget MEN1 genetic analysis. Eur J Endocrinol. 2013;168:533–541. doi: 10.1530/EJE-12-0763. [DOI] [PubMed] [Google Scholar]
- 182.Daly AF, Tichomirowa MA, Petrossians P, Heliovaara E, Jaffrain-Rea ML, Barlier A, et al. Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. J Clin Endocrinol Metab. 2010;95:E373–E383. doi: 10.1210/jc.2009-2556. [DOI] [PubMed] [Google Scholar]
- 183.Villa C, Lagonigro MS, Magri F, Koziak M, Jaffrain-Rea ML, Brauner R, et al. Hyperplasia-adenoma sequence in pituitary tumourigenesis related to aryl hydrocarbon receptor interacting protein gene mutation. Endocr Relat Cancer. 2011;18:347–356. doi: 10.1530/ERC-11-0059. [DOI] [PubMed] [Google Scholar]