Key Points
NET formation is stimulated by platelet or soluble P-selectin.
Abstract
Neutrophil extracellular traps (NETs) can be released in the vasculature. In addition to trapping microbes, they promote inflammatory and thrombotic diseases. Considering that P-selectin induces prothrombotic and proinflammatory signaling, we studied the role of this selectin in NET formation. NET formation (NETosis) was induced by thrombin-activated platelets rosetting with neutrophils and was inhibited by anti-P-selectin aptamer or anti-P-selectin glycoprotein ligand-1 (PSGL-1) inhibitory antibody but was not induced by platelets from P-selectin−/− mice. Moreover, NETosis was also promoted by P-selectin–immunoglobulin fusion protein but not by control immunoglobulin. We isolated neutrophils from mice engineered to overproduce soluble P-selectin (P-selectinΔCT/ΔCT mice). Although the levels of circulating DNA and nucleosomes (indicative of spontaneous NETosis) were normal in these mice, basal neutrophil histone citrullination and presence of P-selectin on circulating neutrophils were elevated. NET formation after stimulation with platelet activating factor, ionomycin, or phorbol 12-myristate 13-acetate was significantly enhanced, indicating that the P-selectinΔCT/ΔCT neutrophils were primed for NETosis. In summary, P-selectin, cellular or soluble, through binding to PSGL-1, promotes NETosis, suggesting that this pathway is a potential therapeutic target for NET-related diseases.
Introduction
Neutrophil extracellular traps (NETs) are extracellular DNA fibers comprising histones and neutrophil antimicrobial proteins.1 Although NETs trap pathogens and may play a beneficial role against infections,1-3 a growing body of evidence indicates that NETs are harmful in many inflammatory and thrombotic diseases, including sepsis,4 coronary artery disease,5 and microvascular4 and deep vein thrombosis,6 highlighting NETs as a potential new target for therapeutic intervention. Platelets, through P-selectin, were shown to activate neutrophil integrins,7 and activated platelets were implicated in NET formation (NETosis).3,8 Considering that platelet-neutrophil interactions involve cell-to-cell cross talk mainly mediated by P-selectin,9 we studied the role of P-selectin in NETosis.
Study design
For a full description of all methods, see supplemental Materials and methods, available on the Blood Web site.
Animals
Eight- to 10-week-old male or female mice, all on a C57BL/6J background, were used in the study. P-selectinΔCT/ΔCT 10 and P-selectin−/− 11 mouse colonies were maintained in-house at Boston Children’s Hospital and were routinely crossed to the C57BL/6J background. Wild-type (WT) C57BL/6J mice were purchased from The Jackson Laboratory. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Boston Children’s Hospital (protocol number 14-03-2631R).
NET induction in vitro
Isolated peripheral blood neutrophils were allowed to adhere and incubated with thrombin-activated or resting platelets in the presence or absence of the P-selectin blocking aptamer ARC5690,12 anti-PSGL-1 blocking antibody (4RA10; BD Pharmingen), or the reactive oxygen species (ROS) scavenger (TEMPOL), purified mouse P-selectin–immunoglobulin fusion protein (P-sel-Ig; BD Pharmingen), platelet activating factor (PAF; Calbiochem), ionomycin (Invitrogen), or phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich). Cells were stained with anti-citrullinated histone H3 (H3Cit; Abcam) and Hoechst-33342 (Invitrogen), and NETosis was analyzed by microscopy.13
Flow cytometry studies
Isolated peripheral blood neutrophils were stained with allophycocyanin (APC)-conjugated anti-Ly6G and fluorescein isothiocyanate (FITC)–conjugated anti-P-selectin to detect P-selectin staining on neutrophils or APC-conjugated anti-Ly6G and CellROX Green to detect ROS generation by flow cytometry.
NET determination in vivo
Plasma levels of DNA (Quant-iT PicoGreen dsDNA; Invitrogen)13 and nucleosomes (Cell Death Detection ELISA; Roche) were measured according to manufacturers’ instructions.
Statistics
Data were analyzed using GraphPad Prism software by 2-sided Student t test or 2-sided Mann-Whitney U test performed between groups. Results were expressed as mean ± standard error of the mean and were considered significant at P < .05.
Results and discussion
P-selectin on activated platelets is required for platelet-mediated NET induction
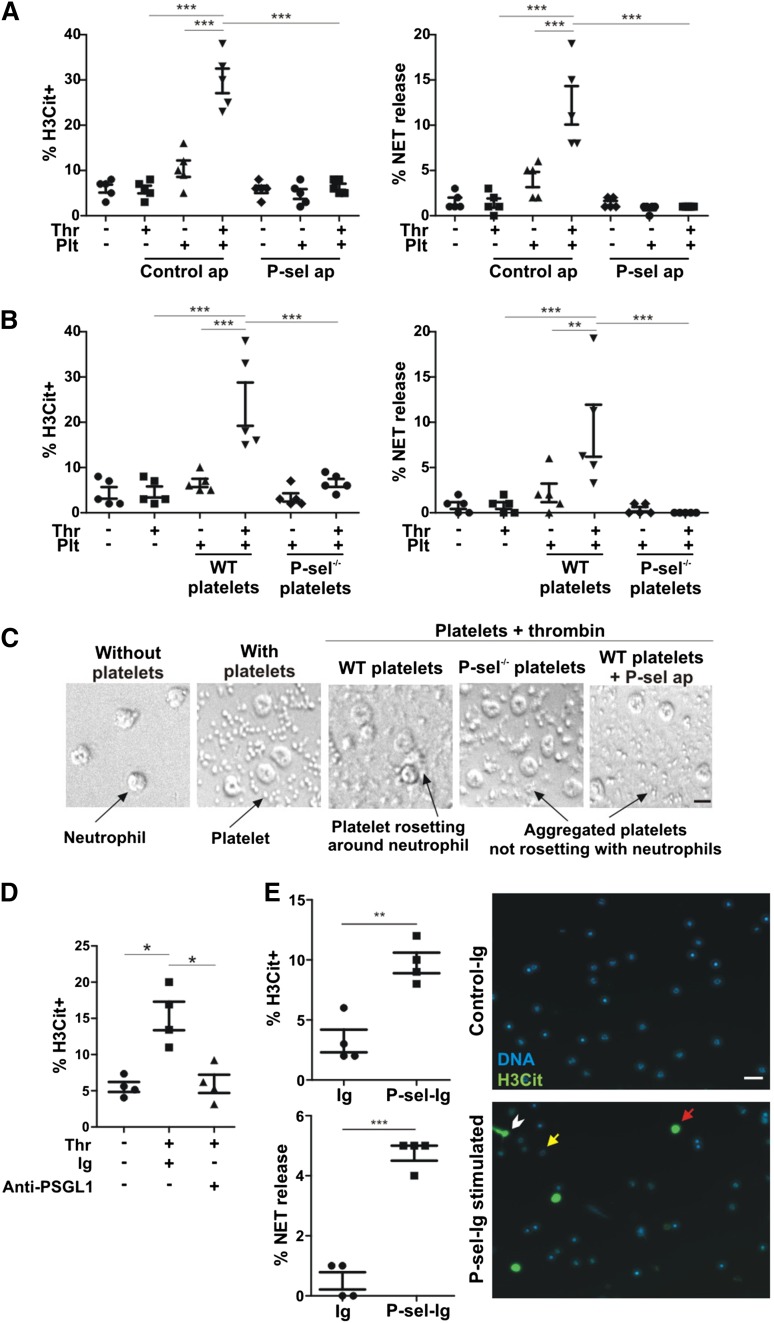
To study the role of platelet P-selectin in NETosis, platelets were stimulated with thrombin and then overlaid on adherent neutrophils. Although thrombin alone or unstimulated platelets failed to induce NETosis, neutrophil incubation with thrombin-stimulated platelets resulted in a significant increase in the percentage of both H3Cit-positive (H3Cit+) cells and neutrophils releasing NETs (Figure 1A). NETosis mediated by activated WT platelets was completely inhibited by the P-selectin blocking aptamer, and not by the control aptamer (Figure 1A). Platelets from P-selectin−/− mice did not induce H3Cit or NETosis (Figure 1B). Differential interference contrast microscopy showed that although unstimulated WT platelets remained on the well bottom without interacting with each other or neutrophils, thrombin stimulation promoted WT platelet rosetting with the neutrophils (Figure 1C), a process known to be mediated by P-selectin.14 Although thrombin-stimulated P-selectin−/− platelets or WT platelets treated with the P-selectin aptamer formed homotypic aggregates, we confirmed that they did not interact with neutrophils (Figure 1C). In addition, blocking of neutrophil PSGL-1 completely inhibited the histone H3 citrullination induced by activated platelets, indicating that the signaling leading to histone citrullination was mediated by P-selectin/PSGL-1 interaction (Figure 1D).
Figure 1.
P-selectin is a stimulant of NET formation. (A) Neutrophils were incubated with unstimulated or thrombin (Thr)-stimulated platelets (Plt) in the presence of P-selectin aptamer inhibitor ARC5690 (P-sel ap) or ARC5694 as control aptamer (Control ap). H3Cit+ cells and NET formation were evaluated (n = 5; ***P < .001). (B) H3Cit+ cells and NET formation were quantified in neutrophils incubated with unstimulated or thrombin-stimulated platelets from WT or P-selectin−/− mice (P-sel−/−) (n = 5; **P < .01, ***P < .001). (C) The behavior of platelets in panels A and B was visualized by differential interference contrast microscopy. The images are representative from 5 experiments (scale bar represents 5 μm). (D) NET formation was examined after incubation with unstimulated or thrombin-stimulated WT platelets in the presence of anti-PSGL-1–blocking antibody or its immunoglobulin control (Ig). The percentage of H3Cit+ neutrophils was quantified (n = 4; *P < .05). (E) Left: WT neutrophils were stimulated with P-sel-Ig or with the same class of immunoglobulin as a control (n = 4; **P < .01, ***P < .001). Right: Representative fluorescence microscopy images of control immunoglobulin (Control-Ig) or P-sel-Ig treatments showing H3Cit-negative neutrophils (yellow arrow), H3Cit+ neutrophils without NET release (red arrow), and H3Cit+ neutrophils releasing NETs (white arrowhead). Scale bar represents 20 μm.
P-sel-Ig chimera or elevated plasma soluble P-selectin promotes NETosis
To examine whether P-selectin is sufficient to stimulate NETosis, murine neutrophils were incubated in vitro with P-sel-Ig, a molecule previously shown to induce production of tissue factor–containing microparticles from monocytes.15 Recombinant P-selectin was also shown to trigger protein tyrosine phosphorylation in neutrophils and the tyrosine kinase–dependent function of αMβ2 integrin.16,17 A significant increase in the percentage of H3Cit+ neutrophils and cells forming NETs was observed after the addition of the P-sel-Ig to WT neutrophils, whereas control immunoglobulin had no such effects (Figure 1E). Although activated platelets and P-sel-Ig both induced NETosis, our results indicate that activated platelets were more potent stimulants than P-sel-Ig. This could be due to differences in the local concentration achieved and quality of the P-selectin presented to the neutrophils in these experiments. Alternatively, it is possible that after the signaling through P-selectin/PSGL-1 is established, platelets may provide other co-stimulatory signals to the neutrophils, leading to enhanced NETosis. These could be receptor mediated, such as through the binding of platelet glycoprotein Ib-α to activated αMβ2 integrin,18 or by soluble factors released by the platelets interacting with the neutrophils.
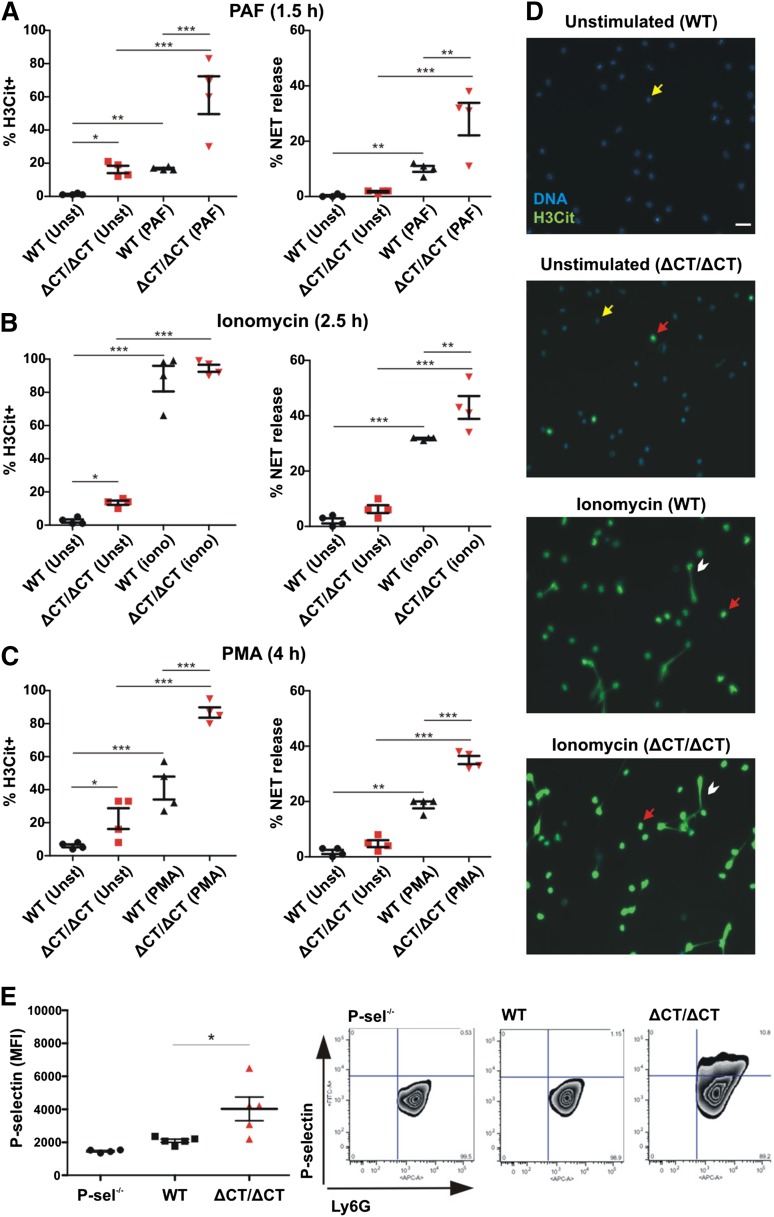
To study whether in vivo released soluble P-selectin (sP-sel) in mice may also have a stimulatory effect on circulating neutrophils, we evaluated P-selectinΔCT/ΔCT mice that have fivefold elevated plasma sP-sel.10 These mice express P-selectin without the cytoplasmic domain, leading to proteolytic shedding of P-selectin from endothelium.10 To evaluate NETosis, we first examined plasma DNA and nucleosome levels in these mice and found that they were similar to those of WT (DNA WT = 92 ± 10 ng/mL vs P-selectinΔCT/ΔCT = 84 ± 2 ng/mL, P = .43; nucleosomes WT = 0.05 ± 0.01 vs P-selectinΔCT/ΔCT = 0.042 ± 0.01 (absorbance 405nm, P = .68), indicating that at steady state, in either genotype, NETs do not appear to be spontaneously released into the circulation. However, knowing that the P-selectinΔCT/ΔCT mice have a worse phenotype than WT mice in many pathological conditions known to produce NETs,19,20 we wondered whether their neutrophils could be primed for NETosis. We isolated neutrophils from WT and P-selectinΔCT/ΔCT mice and incubated them in the absence or presence of different stimulants for NETosis (Figure 2). Indeed, even without stimulation, the levels of H3Cit+ neutrophils were higher when obtained from the P-selectinΔCT/ΔCT mice, indicating that the chromatin modification leading to NETosis occurred more frequently in the P-selectinΔCT/ΔCT neutrophils as compared to WT (Figures 2A-D), although neither genotype produced NETs without additional stimulation (Figures 2A-D). The percentage of cells forming NETs upon neutrophil stimulation by PAF (Figure 2A), ionomycin (Figure 2B), or PMA (Figure 2C) were all significantly higher in neutrophils from P-selectinΔCT/ΔCT compared with WT mice, confirming that neutrophils from the P-selectinΔCT/ΔCT mice are primed to release NETs. Furthermore, the circulating neutrophils of P-selectinΔCT/ΔCT mice, but not the neutrophils from WT or control P-selectin−/− mice, had P-selectin bound to their surface (Figure 2E), suggesting that it could be constitutively priming these neutrophils. Hazeldine et al recently concluded that the induction of ROS generation is one of the mechanisms by which tumor necrosis factor-α primes neutrophils.21 We also were able to show that P-selectin promotes ROS generation in neutrophils and that NET formation induced by this selectin is completely blocked by pharmacologically scavenging ROS using TEMPOL (supplemental Figure 1). Our results, based on the P-sel-Ig treatment and the genetically engineered mice overexpressing sP-sel, indicate that sP-sel is the priming factor in the P- selectinΔCT/ΔCT mice; however, we do not rule out that other circulating inflammatory factors such as the monocyte-derived procoagulant microparticles22 may also contribute to the neutrophil priming. Our data suggest that increased levels of sP-sel, seen in many chronic thrombotic and inflammatory conditions, may sensitize neutrophils for NETosis, which may further aggravate the pathology. Indeed, the increase in sP-sel in P-selectinΔCT/ΔCT mice is associated with a procoagulant and proinflammatory phenotype19,22 that results in larger thrombi in a deep vein thrombosis model,20 more extensive infarcts in the middle cerebral artery ischemia/reperfusion stroke model,19 and increased susceptibility to atherosclerosis on an ApoE−/− background.19 We demonstrate in this study that sP-sel is also priming neutrophils for NETosis, a new mechanism likely contributing to the phenotype of the P-selectinΔCT/ΔCT mice. Our finding supports the notion that sP-sel is an important biomarker predictive of future thrombotic and inflammatory events.
Figure 2.
Neutrophils of P-selectinΔCT/ΔCT mice are primed to release NETs in vitro. Neutrophils isolated from peripheral blood of WT and P-selectinΔCT/ΔCT (ΔCT/ΔCT) mice were kept unstimulated (Unst) or stimulated with PAF (A), ionomycin (iono) (B), or PMA (C). The percentage of H3Cit+ neutrophils and NET release were quantified (n = 4; *P < .05, **P < .01, ***P < .001). (D) Representative fluorescence microscopy images of unstimulated or ionomycin-stimulated cells showing H3Cit-negative neutrophils (yellow arrow), H3Cit+ neutrophils without NET release (red arrow), and H3Cit+ neutrophils releasing NETs (white arrowhead). Scale bar represents 20 μm. (E) Left: Neutrophils isolated from peripheral blood of P-selectin−/−, WT, or P-selectinΔCT/ΔCT mice were double stained with APC-conjugated anti-Ly6G and FITC-conjugated anti-P-selectin, and the mean fluorescence intensity (MFI) of P-selectin-positive cells was analyzed in the neutrophil gate (Ly6G-positive cells) by flow cytometry (n = 4-5; *P < .05). Right: Representative fluorescence-activated cell sorter plots for each genotype.
Upon their adhesion to leukocytes, activated platelets are known to have many stimulating effects on the leukocytes themselves and even on surrounding cell types such as endothelium.9 Recently, activated platelets were shown to enhance NETosis in mouse models such as acute lung injury8 and sepsis.23 In one study, the high mobility group box 1 presented by activated platelets to the neutrophils was implicated in the NETotic process.24 P-selectin is the most important molecule mediating platelet-leukocyte interaction, triggering activating signaling into leukocytes primarily through PSGL-1. We now show that platelet P-selectin primes neutrophils for NETosis. It is likely that E-selectin expressed by inflamed endothelium, and which also binds to PSGL-1,9 has the same effect. Interestingly, PSGL-1 blockade was recently shown to reduce NET biomarkers (myeloperoxidase-DNA) in plasma in acute lung inflammation and sepsis models.25
Although the relevance of our experimental data in mice remains to be determined in clinical settings, increased levels of sP-sel and platelet-leukocyte complexes have been reported in many human diseases.26,27 The present finding that P-selectin promotes NETosis further supports the clinical development of P-selectin/PSGL-1 inhibitors as therapeutic agents to reduce pathological thrombosis and inflammation.
Acknowledgments
The authors thank Melanie Demers for helpful discussions. The P-selectin inhibitory and control aptamers were generously provided by Dr Robert Schaub (Archemix, Cambridge, MA).
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute grant R01HL102101 (D.D.W.). J.E. was the recipient of a Reach-the-World Fellowship from the International Society on Thrombosis and Haemostasis.
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.E. performed and designed the research, performed the experiments, analyzed and interpreted the data, and wrote the manuscript; K.M. obtained the preliminary results on P-selectin ΔCT/ΔCT mice and performed the DNA and nucleosome experiments, P-selectin staining on neutrophils, and NETosis mediated by platelets in the presence of anti-PSGL-1 blocking antibody; S.L.W. and S.M.C. taught and assisted J.E. with the experimental methodology; K.M., S.L.W., M.S., and D.D.W. helped to analyze and interpret the results; M.S. contributed to writing the manuscript; D.D.W. designed the objectives of the work, provided a critical and substantive review of the intellectual work, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Denisa D. Wagner, Program in Cellular and Molecular Medicine, Boston Children’s Hospital, 3 Blackfan Circle, Third Floor, Boston, MA 02115; e-mail: denisa.wagner@childrens.harvard.edu.
References
- 1.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan JT, Simpson AJ, Aziz RK, et al. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16(4):396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 3.Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13(4):463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 4.Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15(11):1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borissoff JI, Joosen IA, Versteylen MO, et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol. 2013;33(8):2032–2040. doi: 10.1161/ATVBAHA.113.301627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107(36):15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evangelista V, Manarini S, Sideri R, et al. Platelet/polymorphonuclear leukocyte interaction: P-selectin triggers protein-tyrosine phosphorylation-dependent CD11b/CD18 adhesion: role of PSGL-1 as a signaling molecule. Blood. 1999;93(3):876–885. [PubMed] [Google Scholar]
- 8.Caudrillier A, Kessenbrock K, Gilliss BM, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122(7):2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner DD, Frenette PS. The vessel wall and its interactions. Blood. 2008;111(11):5271–5281. doi: 10.1182/blood-2008-01-078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartwell DW, Mayadas TN, Berger G, et al. Role of P-selectin cytoplasmic domain in granular targeting in vivo and in early inflammatory responses. J Cell Biol. 1998;143(4):1129–1141. doi: 10.1083/jcb.143.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74(3):541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 12.Gutsaeva DR, Parkerson JB, Yerigenahally SD, et al. Inhibition of cell adhesion by anti-P-selectin aptamer: a new potential therapeutic agent for sickle cell disease. Blood. 2011;117(2):727–735. doi: 10.1182/blood-2010-05-285718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinod K, Demers M, Fuchs TA, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci USA. 2013;110(21):8674–8679. doi: 10.1073/pnas.1301059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen E, Celi A, Gilbert GE, et al. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989;59(2):305–312. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- 15.Hrachovinová I, Cambien B, Hafezi-Moghadam A, et al. Interaction of P-selectin and PSGL-1 generates microparticles that correct hemostasis in a mouse model of hemophilia A. Nat Med. 2003;9(8):1020–1025. doi: 10.1038/nm899. [DOI] [PubMed] [Google Scholar]
- 16.Piccardoni P, Sideri R, Manarini S, et al. Platelet/polymorphonuclear leukocyte adhesion: a new role for SRC kinases in Mac-1 adhesive function triggered by P-selectin. Blood. 2001;98(1):108–116. doi: 10.1182/blood.v98.1.108. [DOI] [PubMed] [Google Scholar]
- 17.de Gaetano G, Cerletti C, Evangelista V. Recent advances in platelet-polymorphonuclear leukocyte interaction. Haemostasis. 1999;29(1):41–49. doi: 10.1159/000022459. [DOI] [PubMed] [Google Scholar]
- 18.Simon DI, Chen Z, Xu H, et al. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J Exp Med. 2000;192(2):193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kisucka J, Chauhan AK, Zhao BQ, et al. Elevated levels of soluble P-selectin in mice alter blood-brain barrier function, exacerbate stroke, and promote atherosclerosis. Blood. 2009;113(23):6015–6022. doi: 10.1182/blood-2008-10-186650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers DD, Hawley AE, Farris DM, et al. P-selectin and leukocyte microparticles are associated with venous thrombogenesis. J Vasc Surg. 2003;38(5):1075–1089. doi: 10.1016/s0741-5214(03)01033-4. [DOI] [PubMed] [Google Scholar]
- 21.Hazeldine J, Harris P, Chapple IL, et al. Impaired neutrophil extracellular trap formation: a novel defect in the innate immune system of aged individuals. Aging Cell. 2014;13(4):690–698. doi: 10.1111/acel.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.André P, Hartwell D, Hrachovinová I, Saffaripour S, Wagner DD. Pro-coagulant state resulting from high levels of soluble P-selectin in blood. Proc Natl Acad Sci USA. 2000;97(25):13835–13840. doi: 10.1073/pnas.250475997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12(3):324–333. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Maugeri N, Campana L, Gavina M, et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J Thromb Haemost. 2014;12(12):2074–2088. doi: 10.1111/jth.12710. [DOI] [PubMed] [Google Scholar]
- 25.Sreeramkumar V, Adrover JM, Ballesteros I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science. 2014;346(6214):1234–1238. doi: 10.1126/science.1256478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludwig RJ, Schön MP, Boehncke WH. P-selectin: a common therapeutic target for cardiovascular disorders, inflammation and tumour metastasis. Expert Opin Ther Targets. 2007;11(8):1103–1117. doi: 10.1517/14728222.11.8.1103. [DOI] [PubMed] [Google Scholar]
- 27.Fuentes Q E, Fuentes Q F, Andrés V, Pello OM, Font de Mora J, Palomo G I. Role of platelets as mediators that link inflammation and thrombosis in atherosclerosis. Platelets. 2013;24(4):255–262. doi: 10.3109/09537104.2012.690113. [DOI] [PubMed] [Google Scholar]