Figure 1.
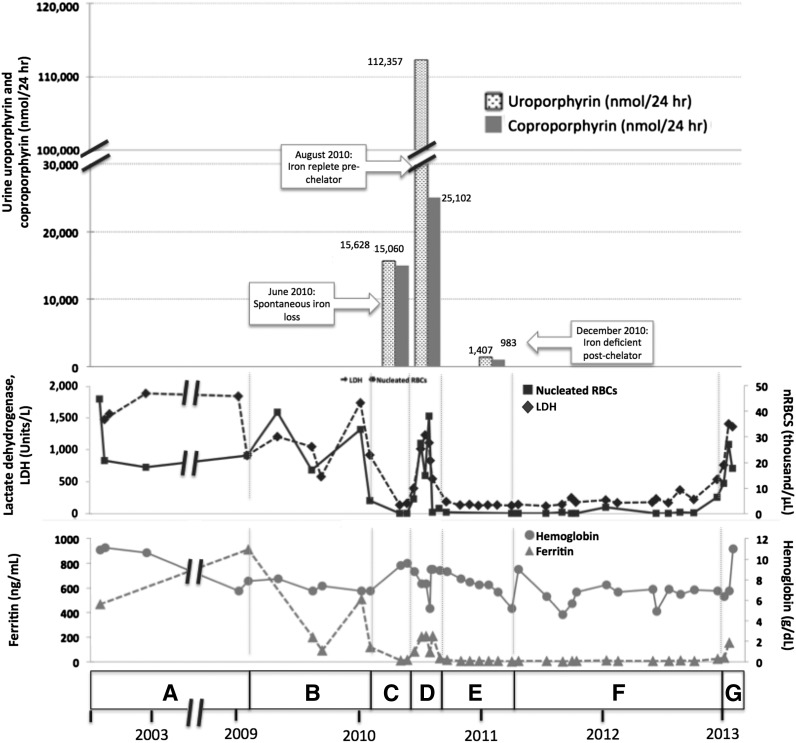
Correlation of clinical and laboratory findings in patient 1. (A) A female of Alaskan Native descent (patient 1) was diagnosed with CEP at 12 months of age after presenting with red urine, discolored teeth, and blisters. A younger sister (patient 2) would also later be diagnosed with CEP. Genetic testing revealed compound heterozygosity for previously described C73R and A104V mutations in UROS. Since childhood, disease complications included chronic hemolysis and severe photosensitivity with scarring, which were managed with sun avoidance and supportive measures including blood transfusions. Patient 1 underwent laparoscopic splenectomy in February 2002 at the age of 25 years. Baseline laboratory parameters include an LDH that ranged from 906 to 1885 U/L (reference range, 80-190 U/L), nucleated red blood cells (nRBCs) between 17.1 and 44.9 × 103 cells/μL (reference, 0.0 × 103 cells/μL), and a reticulocyte count of 195 billion cells/L (reference, 20-65 billion cells/L), reflecting chronic hemolysis. The hemoglobin level ranged from 6.9 to 10.9 g/dL (reference range, 11.5-15.5 g/dL), and the patient’s ferritin level was 470 ng/mL in 2002 (reference range, 10-180 ng/mL). Laboratory values are provided in detail in supplemental Table 1. (B) In February 2009, the ferritin level increased to 910 ng/mL as a result of blood transfusions. Deferasirox was administered to treat iron overload and was discontinued after ferritin level decreased to 93 ng/mL in September 2009. The ferritin level increased again to 508 ng/mL by January 2010. (C) In May 2010, a spontaneous reduction in serum ferritin level to 13 ng/mL was noted and was attributed to occult gastrointestinal (GI) bleeding. Concurrently, the patient reported a dramatic improvement in photosensitivity and a normalization in urine color. Markers of hemolysis also improved, as shown, with a reduction in LDH level to 138 U/L and a marked reduction in circulating nRBCs to 0.2 × 103 cells/μL. Hemoglobin level increased from 6.9 to 9.4. Reticulocyte count decreased to 55 billion cells/L (data not shown). Quantitative analysis of urine porphyrins demonstrated uroporphyrin at 15 628 nmol/24 hours and coproporphyrin at 15 060 nmol/24 hours. Prior values are not available for comparison, and full data regarding the porphyrin fractions are available in supplemental Table 2. (D) Resolution of GI bleeding was accompanied by a rise in serum ferritin level to 208 ng/mL and a concordant worsening of photosensitivity and darkening of urine color. Similarly, LDH level increased to 540 U/L, reticulocyte count increased markedly to 163 billion cells/L, and nRBCs increased to 4.3 × 103 cells/μL. Repeat quantitative analysis of urine porphyrins at this time demonstrated a significant increase in both uroporphyrin (112 357 nmol/24 hours) and coproporphyrin (25 102 nmol/24 hours). (E) In August 2010, based on the correlation between iron status, laboratory parameters, and photosensitivity, we initiated a trial of deferasirox to induce and maintain an iron-deficient state. Deferasirox was initially dosed at 500 mg daily and then in November 2010 was adjusted to 500 mg 3 times weekly to target a ferritin level range of 10 to 15 ng/mL. With deferasirox, the serum ferritin level dropped to 16 ng/mL over 2 months, and the patient again reported an improvement in her quality of life with reduced photosensitivity. As further evidence of the efficacy of deferasirox, an impressive reduction in uroporphyrin to 1470 nmol/24 hours and coproporphyrin to 983 nmol/24 hours was seen on repeat quantitative analysis of urine porphyrins 4 months after chelation began. In addition, total (unfractionated) urine porphyrins decreased from 108 364 μg/24 hours in June 2010 to 5896.3 μg/24 hours in December 2010. Concurrently, LDH normalized to a level of 135 U/L, reticulocyte count decreased to 47 billion/L, nRBCs remained between 0.14 and 0.68 × 103 cells/μL, and hemoglobin level remained between 6.8 and 9.0 g/dL without transfusional support. (F) In April 2011, patient 1 developed recurrent upper GI bleeding, deferasirox was discontinued, and red blood cell transfusions were administered. Over the ensuing 2 years, numerous repeat endoscopies showed persistent gastric erosions and ulcerations despite ongoing use of a proton pump inhibitor. As a result of the ongoing iron losses, ferritin level remained <15 ng/mL, and there was continued control of CEP symptoms and stabilization of laboratory markers of hemolysis. (G) In January 2013, the patient’s serum ferritin increased abruptly, consistent with resolution of GI bleeding. Her photosensitivity suddenly worsened, accompanied by a rise in LDH level to 1403 U/L, nRBCs to 6.4 × 103 cells/μL, and reticulocytes to 118 billion cells/L. Although not known to be a complication of CEP, the patient developed liver disease. Workup, including liver biopsy, failed to discern its etiology. This contributed to a delay in restarting deferasirox when her iron levels began to increase again. In early 2013, at age 35, the patient died of complications of liver failure, hepatorenal syndrome, and hemolysis after enjoying nearly 3 years of reduced symptom severity and a considerable improvement in her quality of life. Microscopic examination of the liver at the time of autopsy demonstrated areas of marked sinusoidal congestion and dilatation containing aggregates of erythroid precursors consistent with diffuse intrasinusoidal extramedullary hematopoiesis. Extensive patchy fibrosis was present, without regenerative nodules or cirrhosis. Importantly, there was no evidence of polarizable material to suggest porphyrin metabolite accumulation. Sadly, the patient’s sister (patient 2) also died, in 2011, from sudden cardiac death in the setting of pulmonary hypertension. A similar trial of chelation was not initiated in her case because she was unable to obtain insurance coverage for the off-label use of deferasirox. Patient 2’s clinical course is further described in supplemental Appendix 1.