Key Points
Hematopoietic cell transplantation results in long-term survival.
Primary graft failure is very high and the predominant cause of death.
Abstract
We report the international experience in outcomes after related and unrelated hematopoietic transplantation for infantile osteopetrosis in 193 patients. Thirty-four percent of transplants used grafts from HLA-matched siblings, 13% from HLA-mismatched relatives, 12% from HLA-matched, and 41% from HLA-mismatched unrelated donors. The median age at transplantation was 12 months. Busulfan and cyclophosphamide was the most common conditioning regimen. Long-term survival was higher after HLA-matched sibling compared to alternative donor transplantation. There were no differences in survival after HLA-mismatched related, HLA-matched unrelated, or mismatched unrelated donor transplantation. The 5- and 10-year probabilities of survival were 62% and 62% after HLA-matched sibling and 42% and 39% after alternative donor transplantation (P = .01 and P = .002, respectively). Graft failure was the most common cause of death, accounting for 50% of deaths after HLA-matched sibling and 43% of deaths after alternative donor transplantation. The day-28 incidence of neutrophil recovery was 66% after HLA-matched sibling and 61% after alternative donor transplantation (P = .49). The median age of surviving patients is 7 years. Of evaluable surviving patients, 70% are visually impaired; 10% have impaired hearing and gross motor delay. Nevertheless, 65% reported performance scores of 90 or 100, and in 17%, a score of 80 at last contact. Most survivors >5 years are attending mainstream or specialized schools. Rates of veno-occlusive disease and interstitial pneumonitis were high at 20%. Though allogeneic transplantation results in long-term survival with acceptable social function, strategies to lower graft failure and hepatic and pulmonary toxicity are urgently needed.
Introduction
Osteopetrosis refers to a heterogeneous group of inherited conditions characterized by dysfunctional osteoclasts, the multinucleated cells that resorb bone.1 The absence of functional osteoclasts in autosomal-recessive osteopetrosis results in a bone marrow cavity insufficient to support hematopoiesis.2 The ensuing extramedullary hematopoiesis leads to massive hepatosplenomegaly, frontal bossing, and macrocephaly. Bony overgrowth is often associated with encroachment of nerve foramina in the skull, resulting in cranial nerve dysfunction.2 Infants with recessive osteopetrosis often have their vision severely affected very early in life, and may present with nystagmus and/or an inability to follow people and objects.2 Other clinical manifestations of bony overgrowth include nasal obstruction and obstructive sleep apnea, abnormal dentition, and gross motor delays.2 Untreated, autosomal recessive osteopetrosis has a mortality rate of ∼70% by 6 years of age, primarily due to complications from bone marrow failure due to insufficient hematopoiesis. As osteoclasts are hematopoietically derived, intrinsic osteoclast defects can be treated through the establishment of an allogeneic graft.3-5 Differentiation of functional osteoclasts following transplantation can lead to bony remodeling and reversal of pancytopenia and extramedullary hematopoiesis. In this report, we report long-term survival after related and unrelated donor transplantation for infantile osteopetrosis.
Patients and methods
Data collection
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a working group of over 400 transplant centers worldwide that voluntarily contributes data on their autologous and allogeneic transplants. Participating centers register consecutive transplants, and detailed demographic, disease, and transplant characteristics and outcomes are collected on a subset of registered patients using a weighted randomization scheme. All patients are followed longitudinally and compliance monitored with onsite audits. The institutional review boards of the Medical College of Wisconsin and the National Marrow Donor Program approved the study.
Inclusion criteria
Included are patients with infantile osteopetrosis transplanted between 1990 and 2011 at 65 transplant centers and reported to the CIBMTR. The diagnosis was established by treating physicians; data on genotype are not collected on CIBMTR’s standardized reporting forms. As the study population covers over 2 decades, genotypic data are not available in the current analysis. However, based on the ages at transplantation virtually all of the included patients would be expected to have a severe recessive genotype.
End points
The primary end point was survival. Death from any cause was considered an event and surviving patients were censored at last follow-up. Hematopoietic recovery was defined as achieving an absolute neutrophil count 0.5 × 109/L or greater for 3 consecutive days, and platelet counts, 20 × 109/L or greater, unsupported for 7 days. The diagnosis of acute and chronic graft-versus-host disease (GVHD) was assigned using standard criteria.6-8
Statistical analysis
The probability of overall survival was calculated using the Kaplan-Meier estimator.9 The probabilities of neutrophil and platelet recovery and acute and chronic GVHD were calculated using the cumulative incidence estimator; death without an event was considered the competing risk.10 Confidence intervals (CIs) were calculated using log transformation.
Preliminary analysis of survival rates among recipients of alternative donor transplantation failed to show differences between the groups. Within the cohort receiving unrelated donor grafts, an analysis on HLA-matching was not considered as the majority were HLA-mismatched. The 5-year probabilities of overall survival after mismatched related, adult unrelated donor, and umbilical cord blood transplants were 51% (95% CI, 31-70), 43% (95% CI, 30-56), and 38% (95% CI, 25-53), respectively (P = .75). All umbilical cord blood units were from unrelated donors except 1 from a HLA-mismatched sibling. Therefore, subsequent analysis considered donor type as 2 categories: HLA-matched sibling and alternative donors. Cox regression model was built to identify risk factors associated with mortality.11 Logistic regression model was built to identify risk factors associated with neutrophil recovery.12 Factors considered in model building included the following: age at transplantation (<6 vs 6-12 vs >12 months), donor type (HLA-matched sibling vs alternative donor), and transplant period (1990-2004 vs 2005-2011). A P value of .05 or less was considered statistically significant and all P values are 2-sided. All analyses were performed using the statistical package SAS version 9.2 (SAS Institute).
Results
Patient, disease, and transplant characteristics
Patient, disease, and transplant characteristics are shown in Table 1. Although about 70% of patients were transplanted within the first year of life regardless of whether an HLA-matched sibling or an alternative donor was available, HLA-matched sibling transplants were more common for those who received transplants when younger than 6 months of age. There were no differences in the proportion of those transplanted older than 12 months by donor source. At time of transplantation, 45% of patients were reported to have a Lansky score of 90 or 100 and 27% reported scores of 80 or 70. Thirty-four percent of transplants used grafts from HLA-matched siblings (n = 65), 13% from HLA-mismatched relatives (n = 25), 12% from HLA-matched (n = 24), and 41% from HLA-mismatched (n = 79) unrelated donors. All but 10 patients received myeloablative transplant conditioning and busulfan with cyclophosphamide was the predominant regimen. Total body irradiation was included in only 15% of transplantation regimens. Transplant conditioning regimens for alternative donor transplantation were more likely to include in vivo T-cell depletion approaches (antithymocyte globulin or alemtuzumab). Bone marrow was the predominant graft source accounting for 66% of transplants; cord blood grafts were used in 25% of transplants. Unrelated donor transplants accounted for over half of transplants (102 or 193; 53%) and of these, 77% were HLA-mismatched. Most cord blood transplants occurred after 1999. Almost all patients received calcineurin inhibitor containing GVHD prophylaxis and 10% received bone marrow grafts that were T-cell depleted (ex vivo methods).
Table 1.
Patient and transplant characteristic
| HLA-matched sibling* | Alternative donor† | |
|---|---|---|
| Number | 65 | 128 |
| Age, median (range), y | 1 (<1-6) | 1 (<1-7) |
| Age, no. (%) | ||
| <6 mo | 29 (45) | 43 (34) |
| 6-12 mo | 18 (28) | 48 (38) |
| 13-24 mo | 12 (18) | 29 (23) |
| 25-60 mo | 6 (9) | 8 (6) |
| Sex, male (%) | 32 (49) | 75 (59) |
| Lansky performance score, no. (%) | ||
| 90-100 | 35 (54) | 52 (41) |
| 70-80 | 13 (20) | 39 (30) |
| ≤60 | 11 (17) | 25 (20) |
| Not reported | 6 (9) | 12 (9) |
| Visual impairment at diagnosis, no. (%) | ||
| Present | 37 (57) | 77 (60) |
| Absent | 12 (18) | 28 (22) |
| Not reported | 16 (25) | 23 (18) |
| Auditory impairment at diagnosis, no. (%) | ||
| Present | 11 (17) | 31 (24) |
| Absent | 38 (58) | 66 (52) |
| Not reported | 16 (25) | 31 (24) |
| Gross motor delay at diagnosis, no. (%) | ||
| Present | 12 (18) | 50 (39) |
| Absent | 34 (53) | 49 (38) |
| Not reported | 19 (29) | 29 (23) |
| Conditioning regimen, no. (%) | ||
| Myeloablative | ||
| TBI + busulfan + cyclophosphamide | — | 16 (12) |
| TBI + cyclophosphamide | — | 7 (5) |
| Busulfan + cyclophosphamide | 56 (86) | 83 (65) |
| Busulfan + melphalan | 8 (12) | 1 (1) |
| Busulfan + fludarabine | 9 (7) | |
| Melphalan + fludarabine | — | 3 (2) |
| Reduced intensity | ||
| TBI + chemotherapeutic agent | — | 3 (3) |
| TLI + cyclophosphamide | — | 1 (1) |
| Busulfan + fludarabine | 1 (2) | 3 (2) |
| Melphalan + fludarabine | — | 2 (2) |
| GVHD prophylaxis | ||
| Ex vivo T-cell depletion | — | 20 (16) |
| Cyclosporine-containing | 62 (95) | 100 (78) |
| Tacrolimus-containing | 3 (5) | 8 (6) |
| In vivo T-cell depletion, no. (%) | 22 (34) | 87 (68) |
| Transplant period, no. (%) | ||
| 1990-2004 | 56 (86) | 94 (73) |
| 2005-2011 | 9 (14) | 34 (27) |
| Follow up, median (range), mo | 67 (3-172) | 81 (3-223) |
TBI, total body irradiation; TLI, total lymphoid irradiation.
N = 62 received bone marrow grafts and N = 3, peripheral blood.
N = 25 mismatched relative (bone marrow, n = 20; peripheral blood, n = 4; cord blood, n = 1), N = 55 unrelated adult donor (bone marrow, n = 46; 8 were HLA-matched and 38 HLA-mismatched; peripheral blood, n = 9, 6 were HLA-matched and 3 HLA-mismatched), N = 48 umbilical cord blood (10 HLA-matched and 38, HLA-mismatched).
Overall survival
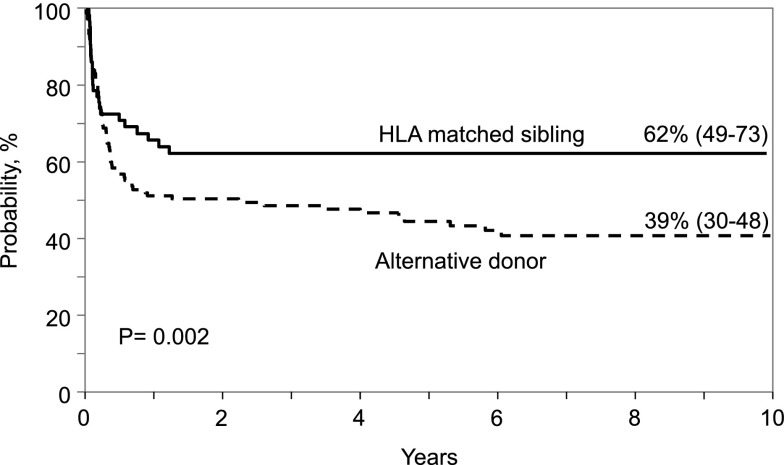
With a median follow up of 6 years, 94 of 193 patients (48.7%) are alive at last follow-up. Of these, 24 (25%) have been followed for 10 years or longer. The median age at last follow-up was 7 years (range, 0.5-18). There were no differences in day-100 survival after HLA-matched sibling compared with alternative donor transplantation. The day-100 probability of overall survival after HLA-matched sibling transplantation was 72% (95% CI, 61-82) compared with 67% (95% CI, 59-75) for alternative donor transplantation (P = .46). However, survival differed thereafter, with higher survival rates observed after HLA-matched sibling compared with alternative donor transplantation. The 1-year and 5-year probabilities of survival were 65% (95% CI, 53-77) and 62% (95% CI, 49 - 75) after HLA-matched sibling transplants. The corresponding 1-year and 5-year probabilities of survival after alternative donor transplants were 50% (95% CI, 41-58) and 42% (95% CI, 34-51), P = .04 and P = .01, respectively. The 10-year probabilities of overall survival after HLA-matched sibling and alternative donor transplantation were 62% (95% CI, 49-73) and 39% (95% CI, 30-48), respectively, P = .002 (Figure 1). In multivariate analysis, donor type was the only factor associated with mortality; mortality risks were higher after alternative donor compared with HLA-matched sibling donor transplantation (hazard ratio [HR] 1.65; 95% CI, 1.04-2.62; P = .03). We also explored the effect of age (≤6 vs >6 months) at transplantation and found none (HR 1.05; 95% CI, 0.69-1.58; P = .83). Among recipients of HLA-matched sibling transplantation, the 5-year probabilities of survival for those transplanted aged younger than 6 months vs older than 6 months was 50% (95% CI, 31-68) and 71% (95% CI, 56-85), P = .08. The corresponding probabilities of survival after alternative donor transplantation were 48% (95% CI, 33-63) and 40% (95% CI, 29-51), P = .37.
Figure 1.
Probability of overall survival by donor type.
The overall rate of veno-occlusive disease was 19% and interstitial pneumonitis, 21%. Veno-occulsive disease was most frequent after mismatched related donor transplant, occurring in 11 of 25 recipients (44%) compared with 3 of 24 (12.5%) recipients of HLA-matched unrelated donor grafts, 12 of 79 (15%) recipients of HLA-mismatched unrelated donor transplants, and 10 of 65 (16%) recipients of HLA-matched sibling donor transplants (P = .05). Rates of interstitial pneumonitis were higher after alternative donor (34 of 128; 27%) compared with HLA-matched sibling donor transplantation (7 of 65; 11%), P = .01. Neither veno-occlusive disease nor interstitial pneumonitis was associated with transplant period.
Among the 94 patients alive at last contact, 77 patients (82%) reported performance scores of 80 (n = 16), 90 (n = 21), or 100 (n = 39). The remaining patients reported scores of 60 or 70 (n = 10) or lower (n = 5). A performance score was not reported for 2 patients, although 1 is attending school and the other patient was aged 2 years at last contact.
Among the 55 patients who are now aged 6 to 17 years, 45 are attending school (mainstream or special education). Three patients are now 18 years or older and all attended school. The reporting forms do not distinguish between school types.
For 73 of the 94 patients (78%) who are alive, data on extramedullary hematopoiesis, cranial nerve dysfunction, and motor function were available. Complete resolution of hepatosplenomegaly was reported, and serum calcium was within the normal range for these patients within 1 to 2 years after transplantation. Visual impairment was common, occurring in 51 of 73 patients (70%). Fifteen patients reported normal visual acuity pre- and posttransplant and in 7 patients, visual acuity improved posttransplantation. For the remaining patients, there was persistent visual impairment despite sustained engraftment.
In contrast, hearing was reported as normal for most patients (57 of 73; 78%). Hearing was impaired in 16 patients pretransplant with improvement in only 8 patients posttransplant. Pre- and posttransplant gross motor milestones were in keeping with chronologic age for most patients (48 of 68; 70%). Gross motor milestones were delayed in 20 patients pretransplant but 14 of these patients demonstrated improvement posttransplant. Dentition was reported as normal in 62 patients (85%) pre- and posttransplant. Of the 11 patients with abnormal dentition pretransplant, there was improvement for 6 patients.
Chimerism data were available for 64 of 94 patients. Chimerism testing was more frequent after alternative donor transplant (46 of 53 patients) compared with HLA-matched sibling donor transplant (18 of 41). Among the 46 recipients of alternative donor transplant, 29 (63%) were reported to be 96% to 100% donor, 6 (13%) were reported 10% to 90% donor, and 11 (24%), 5% or lower donor chimerism. Among the 18 recipients of HLA-matched sibling donor transplant, 7 (39%) reported 96% to 100% donor, 7 (39%) were reportedly 10% to 90% donor, and 4 (22%), 5% or lower donor chimerism. All patients with mixed chimerism are alive with sustained hematopoietic recovery; their median follow-up was 6 years (range, 2-12).
Causes of death
Ninety-nine of 193 patients died (Table 2). Most deaths (86 of 99; 89%) occurred within the first year after transplantation. There were 10 deaths between 1 and 5 years after transplantation and 3 deaths beyond 5 years. In both donor groups, graft failure was the predominant cause of death, accounting for 50% of deaths (12 of 24) after HLA-matched sibling and 43% of deaths (32 of 75) after alternative donor transplants. There were another 12 deaths (infection, n = 3; interstitial pneumonitis/adult respiratory distress syndrome, n = 2; veno-occlusive disease, n = 5; GVHD, n = 1; not reported, n = 1) after HLA-matched sibling transplant and 42 deaths in patients transplanted with alternative donor sources (GVHD, n = 8; interstitial pneumonitis/adult respiratory distress syndrome, n = 3; infection, n = 10; veno-occlusive disease, n = 8; and organ failure, n = 11). Of the 3 late deaths (beyond 5 years), 2 had received alternative donor transplants and died of GVHD and organ failure; the remaining patient, who received an HLA-matched sibling transplant, died of GVHD.
Table 2.
Causes of death
| HLA-matched sibling | Alternative donor | |||
|---|---|---|---|---|
| ≤12 mo | >12 mo | ≤12 mo | >12 mo | |
| No. | 22 | 2 | 64 | 11 |
| Graft failure | 12 | — | 29 | 3 |
| GVHD | — | 1 | 6 | 2 |
| Interstitial pneumonitis/ARDS | 2 | — | 3 | 0 |
| Infection | 3 | — | 8 | 2 |
| Veno-occlusive disease | 5 | — | 8 | — |
| Organ failure | — | — | 7 | 4 |
| Other | — | 1 | 2 | — |
Hematopoietic recovery
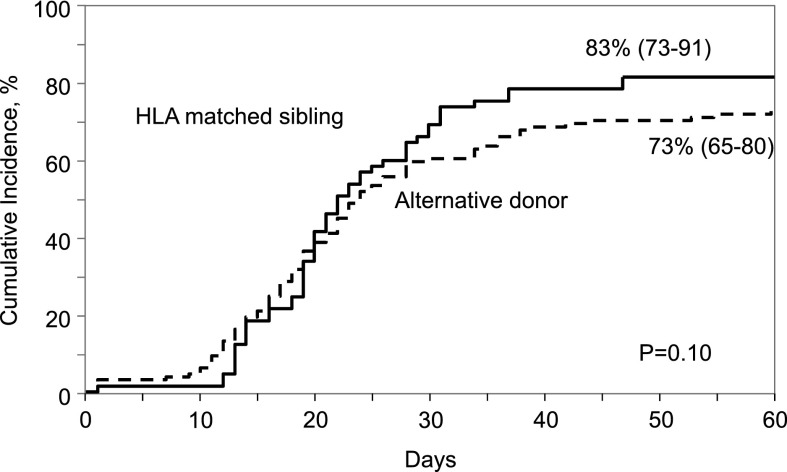
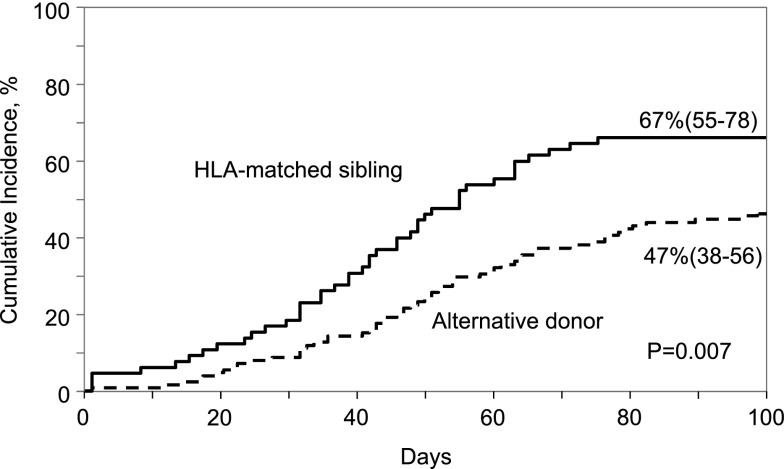
Neutrophil recovery was slow after both HLA-matched sibling and alternative donor transplantation but did not differ between donor groups. The median time to neutrophil recovery after HLA-matched sibling and alternative donor transplantation was 20 days. The day-28 probabilities of neutrophil recovery after HLA-matched sibling and alternative donor transplantation were 66% (95% CI, 54-76) and 61% (95% CI, 53-60), respectively, P = .49 (Figure 2). The corresponding day-60 probabilities of recovery were 83% (95% CI, 73-91) and 73% (95% CI, 65-80), P = .10. Similarly, platelet recovery was less than optimal. The day-100 probability of platelet recovery was higher after HLA-matched sibling transplant compared with alternative donor transplant; 67% (95% CI, 55-78) and 47% (95% CI, 38-56), P = .007 (Figure 3). The median time to recovery after HLA-matched sibling transplant was 41 days compared with 50 days after alternative donor transplant. In multivariate analysis, age at transplantation (odds ratio [OR], 0.78; 95% CI, 0.43-1.42; P = .42), donor type (OR, 1.18; 95% CI, 0.64-2.21; P = .59), and transplant period (OR, 1.26; 95% CI, 0.64-2.48; P = .50) were not associated with neutrophil recovery.
Figure 2.
Cumulative incidence of neutrophil recovery by donor type.
Figure 3.
Cumulative incidence of platelet recovery by donor type.
Forty-two patients had primary graft failure (defined as an inability to achieve an absolute neutrophil count ≥0.5 × 109/L) and 25 patients, secondary graft failure (defined as sustained loss of absolute neutrophil count <0.5 × 109/L), and/or donor chimerism <5%. Therefore, the number of patients experiencing either primary or secondary graft failure totaled 67. The 1-year probability of primary or secondary graft failure was 32% (95% CI, 25-39); there were 4 very late graft failures that occurred at 3.3, 4, 4.4, and 7 years after transplantation.
Twenty-two of 67 patients with graft failure underwent second transplantation. Most second transplants (n = 13) occurred within 3 months after the first transplant, whereas 5 transplants occurred between 3 and 12 months after the first transplant and the remaining 4 transplants occurred at 4 years (n = 2), 5 years (n = 1), and 8 years (n = 1). A variety of conditioning regimens was used and data were reported for 16 of 22 patients: antithymocyte globulin alone or with fludarabine (n = 6); cyclophosphamide with total body irradiation (n = 4); busulfan and cyclophosphamide (n = 2); cyclophosphamide with thiotepa or fludarabine or total lymphoid irradiation (n = 3); melphalan with antithymocyte globulin (n = 1). Data were not available for 6 patients. Only 7 patients (32%) are alive; median follow-up after second transplantation was 6 years (4-16). Among the 15 patients who died after second transplantation, graft failure was the predominant cause (n = 11; 73%), with organ failure (n = 3) and infection (n = 1). Although all 4 patients with late secondary graft failure underwent second transplantation, only 2 are alive; 1 patient did not engraft and died and the other patient died of multiorgan failure.
GVHD
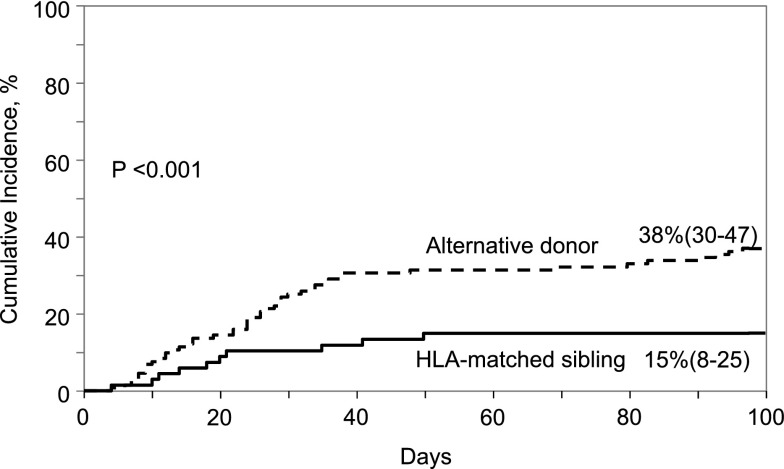
The day-100 cumulative incidence of grade 2-4 and grade 3-4 acute GVHD were lower after HLA-matched sibling compared with alternative donor transplant; 15% (95% CI, 8-25) compared with 38% (95% CI, 30-47), P < .0001 and 8% (95% CI, 3-15) compared with 17% (95% CI, 11-24), P = .04, respectively (Figure 4). The incidence of chronic GVHD was low and did not differ between the donor groups. The 1-year cumulative incidence of chronic GVHD was 7% (95% CI, 2-14) and 9% (95% CI, 4-14) after HLA-matched sibling and alternative donor transplant, respectively (P = .62). The corresponding 5-year cumulative incidence of chronic GVHD was 7% (95% CI, 2-14) and 12% (95% CI, 7-19), P = .19. Furthermore, the severity of chronic GVHD did not differ by treatment group; 2 of 5 patients (40%) after HLA-matched sibling and 7 of 17 (41%) after alternative donor transplant reported extensive chronic GVHD (P = .92). None of the patients with mixed chimerism developed chronic GVHD.
Figure 4.
Cumulative incidence of grade II- IV acute GVHD by donor type.
Reduced-intensity transplants
Ten patients received reduced intensity conditioning regimens and only 3 are alive. Of the 7 deaths, 4 were attributed to graft failure, 2 to organ failure, and 1 to infection. With respect to the conditioning regimen, 5 patients received busulfan and fludarabine; 3 of these patients received antithymyocyte globulin and 1 patient, total lymphoid irradiation. Two patients are alive at 42 months and 81 months after transplantation (1 received antithymyocyte globulin in addition to busulfan with fludarabine). Four patients received melphalan with fludarabine; 2 of these patients also received total body irradiation 200 cGy. Two of these patients also received alemtuzumab and 1 patient, antithymocyte globulin. One patient is alive at 54 months. The remaining patient received cyclophosphamide, total lymphoid irradiation, and antithymocyte globulin and is dead.
Discussion
We report survival rates for the largest cohort of patients with infantile osteopetrosis complied to date. The follow-up period is as late as 10 years. Although survival is significantly better after HLA-matched sibling transplantation, when such a donor is not available, transplantation using grafts from an HLA-mismatched relative, an adult unrelated donor or umbilical cord blood is a reasonable approach for an otherwise lethal disease.5,13-17 Despite the relatively modest long-term survival and the fact that several of these patients are visually impaired, they appear to be engaged socially based on functional scores and the reported school attendance. Mortality rates within the first year after transplantation were high, with graft failure and early transplant-related complications accounting for most deaths. Our findings extend those reported earlier by Driessen and colleagues.18 The current analysis failed to show differences in survival after HLA-mismatched related, adult unrelated donor and umbilical cord blood transplantation. This is not entirely unexpected as primary graft failure is high after transplantation for osteopetrosis regardless of donor type. It is presumed that the difficulty in achieving engraftment in osteopetrosis is related to abnormalities in the marrow microenvironment and/or homing. There is evidence in the oc/oc osteopetrotic mouse that the marrow mesenchymal compartment is much less able to support stem cell homing and retention.19 In addition, these mice, which have the comparable genotype to the most commonly observed genetic abnormality in recessive osteopetrosis (TCIRG1), had impaired localization of infused hematopoietic stem cells to the marrow, whereas donor undifferentiated cells were more likely to be present in the spleen. There have been a number of genotypes identified as associated with human osteopetrosis.20 However, whether there is a difference in the ability to achieve engraftment or other outcomes in the various human genotypes could not be addressed in this analysis, as the data are not available.
The natural history of osteopetrosis is such that the absence of functional osteoclasts results in a bone marrow cavity that is unable to support hematopoiesis.21 The transplantation of functional hematopoietic progenitor cells is intended to reverse the process by providing functional osteoclasts, but remodeling and sustained hematopoiesis requires time. Consequently, early morbidity and mortality are high. Therefore, in this setting we hypothesize that among the available donor and graft options, HLA-matched sibling and unrelated adult donor grafts (bone marrow or peripheral blood) offer the advantage of faster hematopoietic recovery relative to umbilical cord blood. Our data suggest most patients with primary graft failure expired. Salvage with a subsequent transplant for graft failure was less than optimal. Only a third of patients survived and graft failure the predominant cause of failure after the second transplant.
There is a definite need to develop transplantation strategies to improve engraftment. Ninety-four percent of recipients in the current analysis received a myeloablative transplant conditioning regimen and busulfan with cyclophosphamide was the predominant regimen. Others have shown an association between busulfan exposure and graft failure.22,23 As the current analyses span over 2 decades, a substantial number of patients would not have had busulfan pharmacokinetics with dose adjustments. Although the influence of busulfan kinetics could not be directly explored, a surrogate analysis examined outcomes based on transplant period, making the assumption that after 2004 most busulfan would be administered using dose targeting. We did not observe differences for engraftment or survival by transplant period. Nevertheless, we recommend busulfan pharmacokinetics and dose adjustments in an effort to improve engraftment and lower end-organ toxicity, particularly veno-occlusive disease. Clearly, other novel strategies are needed to overcome the unusually high rates of graft failure. Reduced-intensity transplant conditioning regimens potentially lower end-organ toxicity. However, when used for osteopetrosis, graft failure was high and the predominant cause of treatment failure. As only about a third of patients survived it is impossible to gauge the effects of reduced-intensity conditioning on veno-occlusive disease and interstitial pneumonitis, the 2 most common transplant-related complications.
Better HLA-matching between adult unrelated donors and their recipients lowers graft failure and improves overall survival.24 The modest population in the current analysis prohibits us from examining for an effect of HLA matching on graft failure and survival. However, in keeping with the findings of the effect of donor-recipient HLA matching on graft failure, selecting the best HLA-matched unrelated adult donor would seem important.24 Umbilical cord blood grafts accounted for about half of unrelated donor transplants in the current analysis. The modest sample size prohibits us from a detailed analysis of the effect of graft source on engraftment and survival. Reports on cord blood unit selection for hematologic malignancy support selecting units that are better HLA-matched to recipients and contain adequate cell dose to facilitate hematopoietic recovery.25-27 It is noteworthy that graft failure rates were high after HLA-matched sibling transplants, which would support the hypothesis that the microenvironment of the marrow plays a prominent role in modulating graft failure. Alternatively, it is possible that an enhanced immunologic-based rejection of allogeneic cells occurs in osteopetrosis. This would seem unlikely, as decreased immunity has been described in this patient population. It is certainly possible that due to the heterogeneity of the disease, specific subgroups of patients may be at higher risk for graft failure, either related to immune function or to differences in the marrow microenvironment or homing. Genetic data are not available for our population as the data presented were reported to an observational registry over a period of 20 years. We addressed the potential effect of the transplant period on survival and found none.
The unique clinical aspects of osteopetrosis are important to consider in regards to transplantation. There are patients that develop hydrocephalus or evidence of increased intracranial pressure that may relate to vascular drainage, although the specifics and prevalence of this is not well described.28,29 Rapid loss of functional vision is an important consideration that may necessitate moving quickly to transplantation to provide the best opportunity to maintain eyesight. Although optic decompression has been described as a means of increasing the size of optic foramen,28,30 the procedure is associated with morbidity, and may delay transplantation. Airway issues and choanal atresia may result in abnormal sleep studies, and can also complicate the care of these patients.31-33 Veno-occlusive disease was common especially after mismatched related donor transplantation. Most HLA-matched sibling and mismatched related donor transplants occurred prior to 2000, making it impossible to test for a period effect. However, for recipients of unrelated donor transplantation rates of veno-occlusive disease did not differ by transplant period (prior to 2005 compared with later years) implying the observed higher rate of veno-occlusive disease is most likely related to the disease process. Interstitial pneumonitis was also common, especially after alternative donor transplantation, and was not associated with transplant period. This lends support to the hypothesis that the underlying disease exacerbates pulmonary toxicity. Severe pulmonary hypertension has been reported as a frequent complication after transplantation for infantile osteopetrosis.34,35 It has been reported in approximately a third of patients34 and may be linked to a specific variant of malignant infantile osteopetrosis.35 We do not have data specifically related to pulmonary hypertension, and on that basis pulmonary hypertension may have contributed to what was reported as interstitial pneumonitis.
Driessen and colleagues have documented that most children attend school with about a third receiving specialized education.18 We also observed that most patients attend school, whereas documentation as to whether they attend mainstream or specialized schools is lacking. As 70% of evaluable survivors are visually impaired, several are likely to be receiving specialized education on this basis. Persistence of visual impairment is not surprising as several of these patients were reported to have optic atrophy prior to transplantation. A substantially lower proportion (about 10%) of survivors have hearing impairment, abnormal dentition, or gross motor delay. We do not have data on height and orthopedic conservation in survivors.
In summary, allogeneic transplantation for infantile osteopetrosis remains a challenge. Allogeneic transplantation provides the opportunity for long-term cure, and survival rates are best after HLA-matched sibling transplantation but alternative donor transplantation also extends survival. There is an urgent need to improve engraftment by developing novel strategies that target the microenvironment and study the association between genetic variants of osteopetrosis and transplantation outcomes. Our data suggest use of reduced-intensity conditioning regimens to lower transplant-related morbidity resulted in high rates of graft failure and death. The outcomes of second transplantation as a salvage option for graft failure is unlikely to be successful. However, there are measures that can be undertaken with our current knowledge to improve care: (1) early referral may lower rates of visual impairment; (2) careful monitoring/intervention for veno-occlusive disease, interstitial pneumonitis, and pulmonary hypertension; and (3) optimization of donor-recipient HLA matching and a preference for bone marrow or peripheral blood grafts.
Acknowledgments
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the US government.
The Center for International Blood and Marrow Transplant Research (CIBMTR) is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Institutes of Health National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; contract HHSH250201200016C with Health Resources and Services Administration (HRSA/Department of Health and Human Services [DHHS]); two grants (N00014-12-1-0142 and N00014-13-1-0039) from the Office of Naval Research; and grants from Actinium Pharmaceuticals (Corporate Member); Allos Therapeutics, Inc.; Amgen, Inc (Corporate Member); anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association (Corporate Member); Celgene Corporation (Corporate Member); Chimerix, Inc; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc; Gamida Cell Teva Joint Venture Ltd (Corporate Member); Genentech, Inc; Gentium SpA (Corporate Member); Genzyme Corporation; GlaxoSmithKline; Health Research, Inc Roswell Park Cancer Institute; HistoGenetics, Inc; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc; Millennium: The Takeda Oncology Co; Milliman USA, Inc (Corporate Member); Miltenyi Biotec, Inc (Corporate Member); National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc; Osiris Therapeutics, Inc; Otsuka America Pharmaceutical, Inc; PerkinElmer, Inc; Remedy Informatics (Corporate Member); Sanofi US (Corporate Member); Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc; St Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co; Stemsoft Software, Inc; Swedish Orphan Biovitrum; Tarix Pharmaceuticals (Corporate Member); TerumoBCT (Corporate Member); Teva Neuroscience, Inc (Corporate Member); THERAKOS, Inc (Corporate Member); University of Minnesota; University of Utah; and Wellpoint, Inc (Corporate Member).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.J.O., M.E., and W.H. designed the study; W.H. prepared the dataset; W.H. and J.L.R. analyzed the data; P.J.O. and M.E. interpreted the data and drafted the manuscript; A.L.F., J.J.B., E.M.H., A.A.-S., M.A., C.M.B., F.B., T.L., D.K.B., N.K., T.A.O., M.A.D.P., and P.A.V. critically reviewed and edited the manuscript; and all authors approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul J. Orchard, Department of Pediatrics, Division of Blood and Marrow Transplantation, University of Minnesota, Mayo Mail Code 366, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: orcha001@umn.edu.
References
- 1.Tolar J, Teitelbaum SL, Orchard PJ. Osteopetrosis. N Engl J Med. 2004;351(27):2839–2849. doi: 10.1056/NEJMra040952. [DOI] [PubMed] [Google Scholar]
- 2.Gerritsen EJ, Vossen JM, van Loo IHG, et al. Autosomal recessive osteopetrosis: variability of findings at diagnosis and during the natural course. Pediatrics. 1994;93(2):247–253. [PubMed] [Google Scholar]
- 3.Coccia PF, Krivit W, Cervenka J, et al. Successful bone-marrow transplantation for infantile malignant osteopetrosis. N Engl J Med. 1980;302(13):701–708. doi: 10.1056/NEJM198003273021301. [DOI] [PubMed] [Google Scholar]
- 4.Sieff CA, Chessells JM, Levinsky RJ, et al. Allogeneic bone-marrow transplantation in infantile malignant osteopetrosis. Lancet. 1983;1(8322):437–441. doi: 10.1016/s0140-6736(83)91438-1. [DOI] [PubMed] [Google Scholar]
- 5.Gerritsen EJ, Vossen JM, Fasth A, et al. Bone marrow transplantation for autosomal recessive osteopetrosis. A report from the Working Party on Inborn Errors of the European Bone Marrow Transplantation Group. J Pediatr. 1994;125(6 Pt 1):896–902. doi: 10.1016/s0022-3476(05)82004-9. [DOI] [PubMed] [Google Scholar]
- 6.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 7.Flowers ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematol Oncol Clin North Am. 1999;13(5):1091–1112, viii-ix. doi: 10.1016/s0889-8588(05)70111-8. [DOI] [PubMed] [Google Scholar]
- 8.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 10.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 11.Cox DR. Regression model and life tables. J R Stat Soc B. 1972;34:187–200. [Google Scholar]
- 12.Agresti A. Categorical Data Analysis. 2nd ed. New York, NY: John Wiley & Sons; 2002. [Google Scholar]
- 13.Locatelli F, Beluffi G, Giorgiani G, et al. Transplantation of cord blood progenitor cells can promote bone resorption in autosomal recessive osteopetrosis. Bone Marrow Transplant. 1997;20(8):701–705. doi: 10.1038/sj.bmt.1700946. [DOI] [PubMed] [Google Scholar]
- 14.Eapen M, Davies SM, Ramsay NK, Orchard PJ. Hematopoietic stem cell transplantation for infantile osteopetrosis. Bone Marrow Transplant. 1998;22(10):941–946. doi: 10.1038/sj.bmt.1701474. [DOI] [PubMed] [Google Scholar]
- 15.Schulz AS, Classen CF, Mihatsch WA, et al. HLA-haploidentical blood progenitor cell transplantation in osteopetrosis. Blood. 2002;99(9):3458–3460. doi: 10.1182/blood.v99.9.3458. [DOI] [PubMed] [Google Scholar]
- 16.Peters C, Steward CG National Marrow Donor Program; International Bone Marrow Transplant Registry; Working Party on Inborn Errors, European Bone Marrow Transplant Group. Hematopoietic cell transplantation for inherited metabolic diseases: an overview of outcomes and practice guidelines. Bone Marrow Transplant. 2003;31(4):229–239. doi: 10.1038/sj.bmt.1703839. [DOI] [PubMed] [Google Scholar]
- 17.Steward CG. Hematopoietic stem cell transplantation for osteopetrosis. Pediatr Clin North Am. 2010;57(1):171–180. doi: 10.1016/j.pcl.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Driessen GJA, Gerritsen EJ, Fischer A, et al. Long-term outcome of haematopoietic stem cell transplantation in autosomal recessive osteopetrosis: an EBMT report. Bone Marrow Transplant. 2003;32(7):657–663. doi: 10.1038/sj.bmt.1704194. [DOI] [PubMed] [Google Scholar]
- 19.Mansour A, Abou-Ezzi G, Sitnicka E, Jacobsen SE, Wakkach A, Blin-Wakkach C. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J Exp Med. 2012;209(3):537–549. doi: 10.1084/jem.20110994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol. 2013;9(9):522–536. doi: 10.1038/nrendo.2013.137. [DOI] [PubMed] [Google Scholar]
- 21.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 22.McCune JS, Gooley T, Gibbs JP, et al. Busulfan concentration and graft rejection in pediatric patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;30(3):167–173. doi: 10.1038/sj.bmt.1703612. [DOI] [PubMed] [Google Scholar]
- 23.Bartelink IH, Bredius RG, Belitser SV, et al. Association between busulfan exposure and outcome in children receiving intravenous busulfan before hematologic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(2):231–241. doi: 10.1016/j.bbmt.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Horan J, Wang T, Haagenson M, et al. Evaluation of HLA matching in unrelated hematopoietic stem cell transplantation for nonmalignant disorders. Blood. 2012;120(14):2918–2924. doi: 10.1182/blood-2012-03-417758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood. 2010;115(9):1843–1849. doi: 10.1182/blood-2009-07-231068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eapen M, Klein JP, Ruggeri A, et al. Center for International Blood and Marrow Transplant Research, Netcord, Eurocord, and the European Group for Blood and Marrow Transplantation. Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123(1):133–140. doi: 10.1182/blood-2013-05-506253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruggeri A, Labopin M, Sormani MP, et al. Eurocord; Cord Blood Committee EBMT; Netcord. Engraftment kinetics and graft failure after single umbilical cord blood transplantation using a myeloablative conditioning regimen. Haematologica. 2014;99(9):1509–1515. doi: 10.3324/haematol.2014.109280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steward CG. Neurological aspects of osteopetrosis. Neuropathol Appl Neurobiol. 2003;29(2):87–97. doi: 10.1046/j.1365-2990.2003.00474.x. [DOI] [PubMed] [Google Scholar]
- 29.Al-Tamimi YZ, Tyagi AK, Chumas PD, Crimmins DW. Patients with autosomal-recessive osteopetrosis presenting with hydrocephalus and hindbrain posterior fossa crowding. J Neurosurg Pediatr. 2008;1(1):103–106. doi: 10.3171/PED-08/01/103. [DOI] [PubMed] [Google Scholar]
- 30.Haines SJ, Erickson DL, Wirtschafter JD. Optic nerve decompression for osteopetrosis in early childhood. Neurosurgery. 1988;23(4):470–475. doi: 10.1227/00006123-198810000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Stocks RM, Wang WC, Thompson JW, Stocks MC, II, Horwitz EM. Malignant infantile osteopetrosis: otolaryngological complications and management. Arch Otolaryngol Head Neck Surg. 1998;124(6):689–694. doi: 10.1001/archotol.124.6.689. [DOI] [PubMed] [Google Scholar]
- 32.Kasow KA, Stocks RM, Kaste SC, et al. Airway evaluation and management in 7 children with malignant infantile osteopetrosis before hematopoietic stem cell transplantation. J Pediatr Hematol Oncol. 2008;30(3):225–229. doi: 10.1097/MPH.0b013e318162c463. [DOI] [PubMed] [Google Scholar]
- 33.Burgoyne LL, Kaur A, Billups CA, et al. Complications of anesthesia for children with malignant infantile osteopetrosis before and after hematopoietic stem cell transplantation. Paediatr Anaesth. 2010;20(11):1046–1051. doi: 10.1111/j.1460-9592.2010.03425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steward CG, Pellier I, Mahajan A, et al. Working Party on Inborn Errors of the European Blood and Marrow Transplantation Group. Severe pulmonary hypertension: a frequent complication of stem cell transplantation for malignant infantile osteopetrosis. Br J Haematol. 2004;124(1):63–71. doi: 10.1046/j.1365-2141.2003.04739.x. [DOI] [PubMed] [Google Scholar]
- 35.Kasow KA, Bonfim C, Asch J, et al. Malignant infantile osteopetrosis and primary pulmonary hypertension: a new combination? Pediatr Blood Cancer. 2004;42(2):190–194. doi: 10.1002/pbc.10455. [DOI] [PubMed] [Google Scholar]