Abstract
Background
Brugada syndrome (BrS) is among the more common familial arrhythmia syndromes, with an estimated prevalence of 1 to 5 per 10 000 persons. It is characterized by a right ventricular conduction delay, dynamic or persistent ST-segment elevations in the precordial leads V1–3, and an elevated risk of syncope and sudden cardiac death in young adults without structural heart disease.
Methods
This article is based on original and review articles on BrS that appeared in English from 2010 onward and were retrieved by a selective search in PubMed, with special attention to international consensus publications on inherited arrhythmogenic diseases.
Results
According to the new diagnostic criteria, the diagnosis of BrS requires typical ECG changes in only one precordial lead. This will likely increase sensitivity, but may also lead to an increase in asymptomatic patients. Established risk markers include sudden cardiac arrest and a spontaneous type 1 ECG with arrhythmic syncope. Patients with these findings benefit from the implantation of a cardioverter-defibrillator. There is no validated algorithm for risk stratification of asymptomatic patients. Because of the low prevalence of BrS, there have been no randomized controlled trials (RCTs) in this disease, and all recommendations are based on expert opinion. BrS is usually inherited in an autosomal dominant manner. Recently discovered gene polymorphisms modify the risk of BrS, challenging the conception of BrS as a monogenetic disease. Electro-anatomic mapping studies have revealed, for the first time, an arrhythmogenic substrate over the right ventricular outflow tract in BrS patients.
Conclusion
BrS is one important differential diagnosis to consider in patients presenting with syncope or sudden cardiac arrest. The goal of current research is to achieve a deeper understanding of the genetic and electrophysiological changes underlying BrS. Further insights in these areas will probably enable better risk stratification of asymptomatic BrS patients in the future.
In 1992, the Spanish brothers Pedro and Josep Brugada described a new disease entity seen in eight patients. Its characteristics were right bundle branch block, persistent ST-segment elevation, and sudden cardiac death (1). In the following years, it was to become known as “Brugada syndrome” (2). With an estimated prevalence of 1 to 5 in 10 000, Brugada syndrome (BrS) is one of the commoner forms of inherited arrhythmogenic disease (3, 4). Characteristic features are:
Right ventricular conduction delay
Dynamic or persistent ST-segment elevations in precordial leads V1–3, and
Considerably increased risk of syncope and sudden cardiac death due to polymorphic ventricular tachycardia (VT) and ventricular fibrillation (VF) in young adults without structural heart disease (1– 6, e1).
After sudden cardiac arrest the risk of VT/VF recurrence is approximately 50% in the next 5 years (7). Men are considerably more often affected than women (8) and usually show a more severe phenotype (9), although many patients are asymptomatic at first diagnosis and in the course of the disease (10). BrS typically manifests during the third to fifth decades of life, but the disease can occur at any age (1, 8, e2). Symptoms range from palpitations and dizziness to recurrent syncope, nocturnal agonal respiration, and (aborted) sudden cardiac death.
Ventricular fibrillation in BrS typically occurs at night (11) or in resting phases during periods of increased vagal tone, and can be initiated by monomorphic ventricular extrasystoles (Figure 1) from the right ventricular outflow tract (RVOT) (e3– e5). Supraventricular arrhythmias (especially atrial fibrillation) are found in 15% to 30% of patients (e6– e9). Among the known triggers for the occurrence of cardiac arrhythmias in BrS are fever, which must be reduced immediately, and certain drugs (www.brugadadrugs.org) (1, 5– 6).
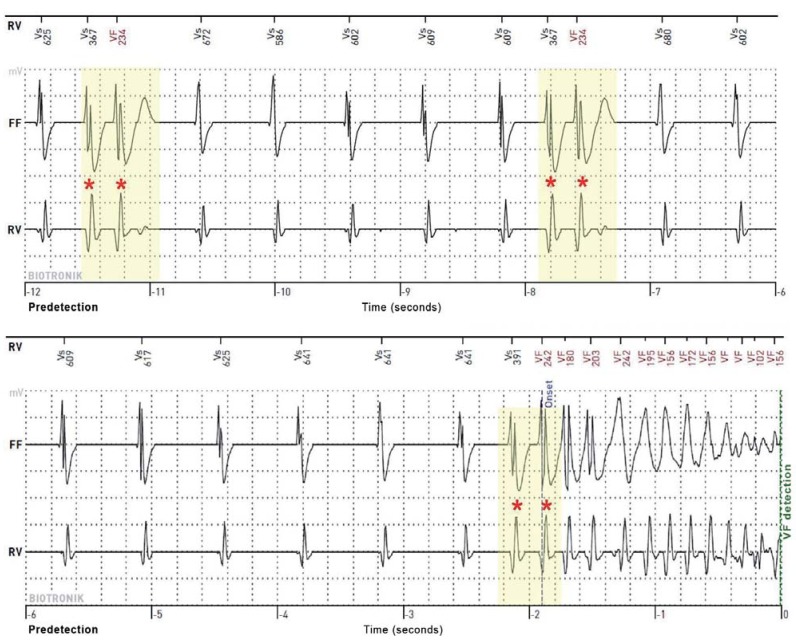
Figure 1.
Spontaneous ventricular fibrillation in a 50-year-old man with sudden cardiac arrest as the first manifestation of Brugada syndrome. The recording of the implantable cardioverter–defibrillator (ICD) consists of three channels. The marker channel (RV) shows the interpretation of sensed events (Vs = ventricular sensing) by the ICD. The far field (FF) and the right ventricular (RV) electrograms (EGM) are derived between the tip of the electrode and the ICD can (FF) and between the distal tip and proximal ring of the ICD electrode (bipolar, RV), respectively. The predetection time is given in seconds. The defibrillator EGM shows monomorphic ventricular extrasystoles (as couplets, asterisks) with identical coupling intervals (in milliseconds), which eventually trigger ventricular fibrillation (VF).
Since there are no randomized, controlled and/or blinded studies for inherited arrhythmogenic diseases such as BrS, all the recommendations given in this text are based on expert opinion (evidence level C) and should be understood as follows (3– 6):
Class I recommendation: the therapeutic intervention is recommended.
Class IIa recommendation: the therapeutic intervention may be useful/effective.
Class IIb recommendation: the therapeutic intervention may be considered.
Class III recommendation: the therapeutic intervention is not recommended.
Pathophysiology
As the site of origin of malignant arrhythmias, the right ventricle has been described as the weak point and the RVOT as the Achilles heel of BrS (e10, e11). Whether BrS is caused by a disturbance of depolarization, repolarization, or cardiac development in this region of the heart is a question under debate (12, 13). Some studies show reduced conduction velocity in the RVOT (14, 15), reduced gap junction expression (e12), and increased fibrosis (14, e12), suggesting disturbed depolarization.
The repolarization hypothesis postulates an unbalance between depolarizing inward currents (sodium and L-type calcium currents, INa and ICa,L) and the prominent transient outward potassium current (Ito) during early repolarization in the epicardial RVOT (13). Animal studies have recently demonstrated reduced conduction reserve in the fetal and the adult RVOT (e13).
Diagnosis
To date, the diagnosis of BrS has required a typical type 1 ECG (“coved type”) (Figure 2) in at least two precordial leads (V1–3) and the presence of one clinical criterion:
Figure 2.
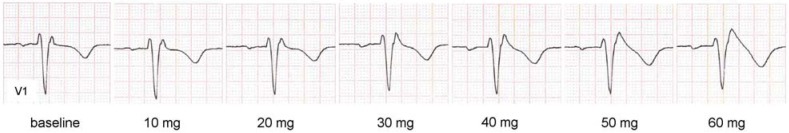
Dynamic ST-segment elevation during fractionated administration of ajmaline. After 12 minutes and a total of 60 mg ajmaline, V1 (2nd intercostal space) shows a type 1 ECG with coved ST-segment elevation (ST-segment elevation ≥2 mm with descending ST segment, and inverted T wave, “coved” pattern [e23]).
V1 = precordial lead; ECG = electrocardiogram
Documented ventricular arrhythmia: Polymorphic VT or VF
Arrhythmia-related symptom: Syncope, seizure, or nocturnal agonal respiration
Positive family history: Sudden cardiac death before age 45 or type 1 ECG in relatives (8, 11).
New diagnostic criteria
To increase diagnostic sensitivity, the expert consensus statement of 2013 on inherited arrhythmogenic diseases (5, 6) omits any clinical criterion (e14) and demands diagnostic ECG changes in only one right precordial lead (e15). Once any possible differential diagnoses with similar ECG changes (“phenocopies”) (e16)—for example, electrolyte disturbances, pericarditis, acute myocardial infarction, or pulmonary embolism (8)—and arrhythmogenic right ventricular cardiomyopathy (e17) have all been excluded, BrS is definitively diagnosed if a type 1 ECG is observed in V1 or V2, either spontaneously or induced by Na+-channel blockade (e.g., using ajmaline) (e18– e20). The precordial leads V1 and V2 may be placed in the standard position or higher, up to the second intercostal space (ICS) (Figure 2) (5, 6). Both echocardiographically (e21) and by magnetic resonance imaging (MRI) (e22), a correlation has been demonstrated between the anatomical location of the RVOT and the intercostal space in which diagnostic ECG changes occur. The new diagnostic criteria have already been validated in a first case series of patients with known BrS (e21).
The saddle-back type 2 and type 3 ECG patterns are suspicious for, but not diagnostic of BrS (8) and the two patterns have now been grouped together into one type 2 ECG (saddle-back pattern) (Figure 3) (e23). When encountering a type 2 ECG the diagnosis of BrS may only be made after drug-induced conversion to a type 1 ECG (5, 6). Because of frequent fluctuations between diagnostic, non-diagnostic, and normal ECGs without ST-segment changes, repeated ECG recordings should be performed for accurate risk stratification (16, e24). In asymptomatic patients, other ECG changes may support the diagnosis (5), such as:
Figure 3.
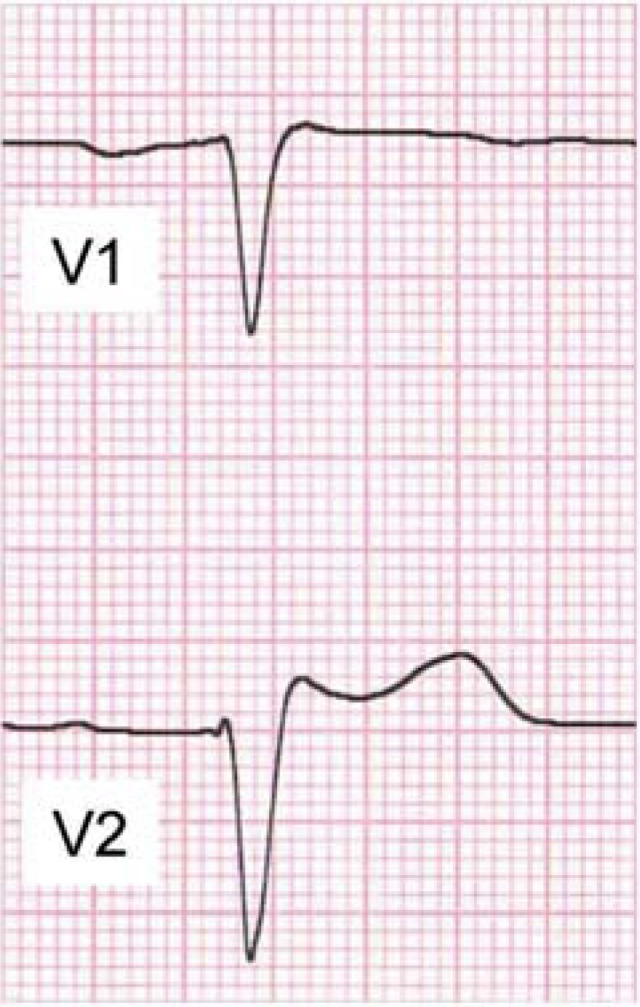
Type 2 ECG with saddle-back type ST-segment elevation in V2,
suspicious for Brugada syndrome:
high take-off of r’ ≥2 mm
with ST segment elevation ≥0.5 mm
and positive T wave,
where Tmax > STmin > 0,
saddle-back pattern (e23)
First-degree atrioventricular block (e25)
Right bundle branch block (e25)
Fragmented QRS complex (17)
Increased ST-segment elevation during exercise (e26) or during the recovery phase after exercise (e27– e29)
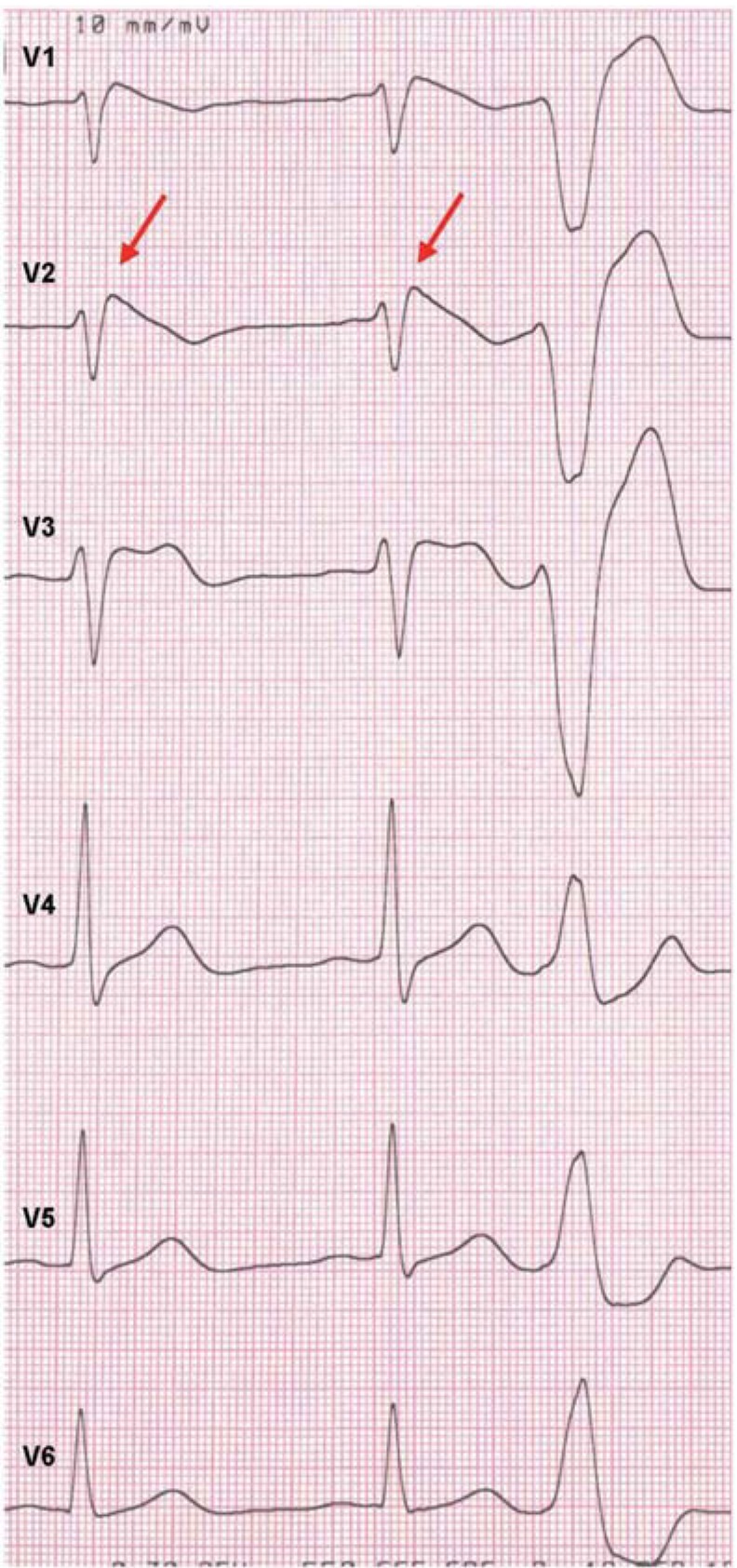
Ventricular extrasystoles with left bundle branch block pattern (Figure 4) (e3, e4)
Atrial fibrillation (e14).
Figure 4.
Ventricular extrasystole with left bundle branch block pattern from the right ventricular outflow tract in a patient with Brugada syndrome with spontaneous type 1 ECG in V2 (arrows) and type 2 ECG in V3
Genetics
BrS is a genetic disease with an autosomal dominant pattern of inheritance and incomplete penetrance (3– 4). To date, changes in 22 genes have been linked with BrS (18, e30– e32). Of practical relevance is the most frequent mutation in the Na+ channel gene SCN5A, which has been shown to be present in 20% to 30% of patients (e33, e34). The other involved genes represent rare sporadic cases or individual BrS families (18, 19, e35). In functional terms, the mutations show either a loss-of-function effect on depolarizing currents (INa or ICa,L) or a gain-of-function effect on repolarizing currents (Ito and ATP-sensitive potassium current, IK-ATP) (19). Whether these aforementioned gene mutations are actually causative of BrS or only have a modifying role is a matter of ongoing debate (19, 20). More recent data show frequent genetic variants (single nucleotide polymorphisms, SNPs) in SCN5A, SCN10A, and HEY2 (SCN5A codes for the alpha subunit of the voltage-gated Na+ channel Nav 1.5; SCN10A codes for the voltage-gated Nav 1.8; HEY2 codes for the transcription factor HEY2). These gene polymorphisms influence individual disease risk and may in the future allow the development of a genetic risk score that could be of practical clinical use (21).
At present, molecular genetic testing is indicated for all patients with a type 1 ECG (class IIa recommendation) (3– 4, 18). Because the interpretation of the genetic results may be challenging—especially in SCN5A-related “overlap” syndromes (long QT syndrome type 3, progressive cardiac conduction defect, J-wave syndrome, and others) (e36)—genetic counseling should take place in collaboration with a center with proven expertise (3, 4).
Risk stratification
With the clinical diagnostic criterion no longer being used, risk stratification (Figure 5) plays a critical role, both to identify patients at high risk of sudden cardiac death, who actually would benefit from placement of an implantable cardioverter–defibrillator (ICD), and to identify asymptomatic patients with a very low risk, in order to spare these patients from undergoing a probably unnecessary ICD placement (5– 6, 19) and potential ICD-related complications (7, e37– e41).
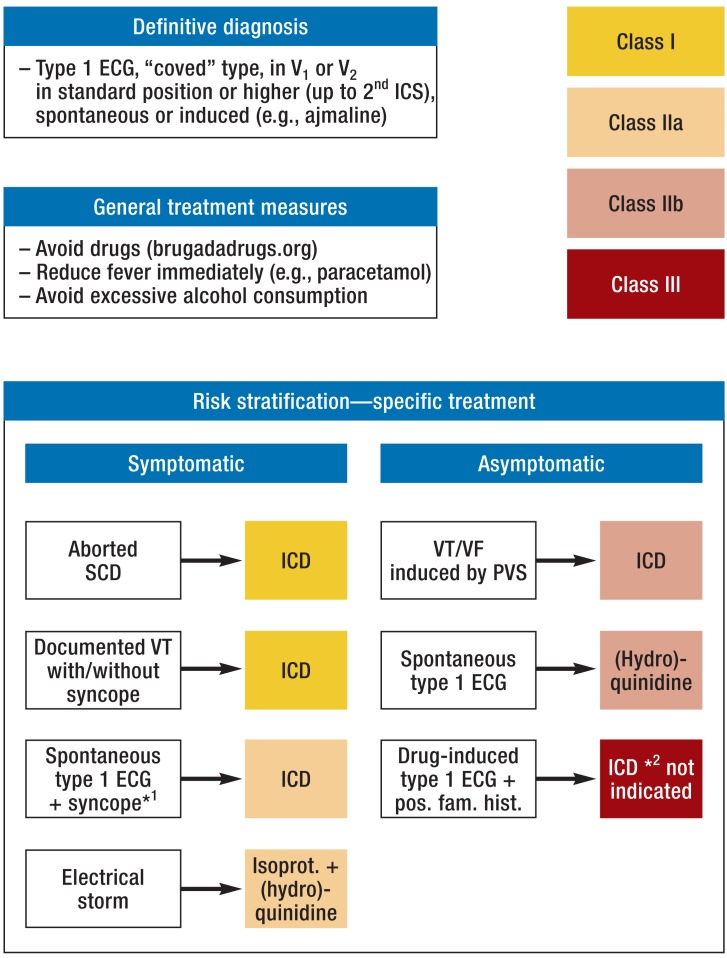
Figure 5.
Algorithm for diagnosis, risk stratification, and treatment of Brugada syndrome (adapted from Priori et al. [5, 6])
*1Arrhythmic;
*2For explanation see text, Risk stratification and Treatment
ICS, intercostal space; SCD, sudden cardiac death; ICD, implantable cardioverter– defibrillator; VT, ventricular tachycardia; VF, ventricular fibrillation; PVS, programmed ventricular stimulation; ECG, electrocardiogram; pos. fam. hist., positive family history; isoprot., isoproterenol
Classical risk markers
Since BrS was first described (1), numerous markers for risk stratification have been identified, but only symptoms (sudden cardiac arrest and syncope), and a spontaneous type 1 ECG have consistently been associated with a raised VT/VF risk in all studies and thus have prognostic impact (10, 19, 22, 23). Established risk markers are sudden cardiac arrest and a spontaneous type 1 ECG in association with the occurrence of arrhythmic syncope (5, 6). Nonarrhythmic syncope (e.g., vasovagal or orthostatic syncope), which is also often seen in BrS patients, has no prognostic impact, and it is therefore important to distinguish between these two entities during history-taking (24).
Asymptomatic patients with BrS show, over the follow-up periods reported to date, a very low incidence of malignant arrhythmias (e.g., 0.5% per year in the largest BrS registry [10], with 1029 patients and a follow-up of 14–54 months) (19). As a method of assessing individual risk of ventricular arrhythmias, the current expert consensus (5, 6) mentions programmed ventricular stimulation, which tests the inducibility of VF in the setting of an electrophysiological examination. However, there is still disagreement between the initiators of the Brugada registry and other groups regarding the prognostic impact of VF inducibility (25– 28). In the Brugada registry, VT/VF induction in previously asymptomatic BrS patients was associated with a markedly higher risk of ventricular arrhythmias in the future (27), but two meta-analyses reported no prognostic impact of VT/VF inducibility (22, 26). The meta-analyses results were confirmed in the FINGER (France, Italy, Netherlands, Germany) study (10) and in the prospective Italian PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) study (23), and the former class IIa recommendation for ICD implantation in patients with inducible VF (11) has been downgraded to IIb in the recent expert consensus statement (5, 6) (Figure 5). In one study, a combination of programmed ventricular stimulation and clinical parameters was used for risk stratification of asymptomatic patients (29), and Giustetto (e42) and Brugada et al. (27) emphasize the high negative predictive value of programmed ventricular stimulation. Asymptomatic patients should be reassessed at regular intervals; in particular, a spontaneous type 1 ECG indicates an elevated risk for ventricular arrhythmias (16).
Novel risk markers
A relatively new risk marker and sign of disturbed depolarization has been described in patients with BrS: a fragmented QRS complex (fQRS) (Fgure 6) (17). fQRS is defined by two or more spikes within the QRS complex in leads V1–V3 (23). QRS fragmentation, initially identified as a risk marker after myocardial infarction (e43), appears, according to early study results, to be associated with a markedly higher risk of VF in BrS patients (17, 23, 30). The question of whether fQRS in V1–V3 reflects the arrhythmogenic substrate (see Catheter ablation) has not yet been investigated. The ECG filters need to be adjusted to detect the high-frequency QRS fragmentation (17).
Figure 6.
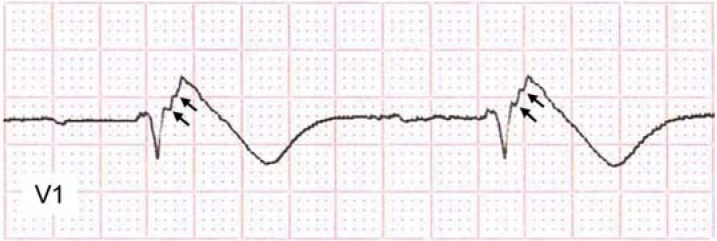
Fragmented QRS complex (fQRS) with two spikes (arrows) within the QRS complex in V1 in a patient with Brugada syndrome with type 1 ECG
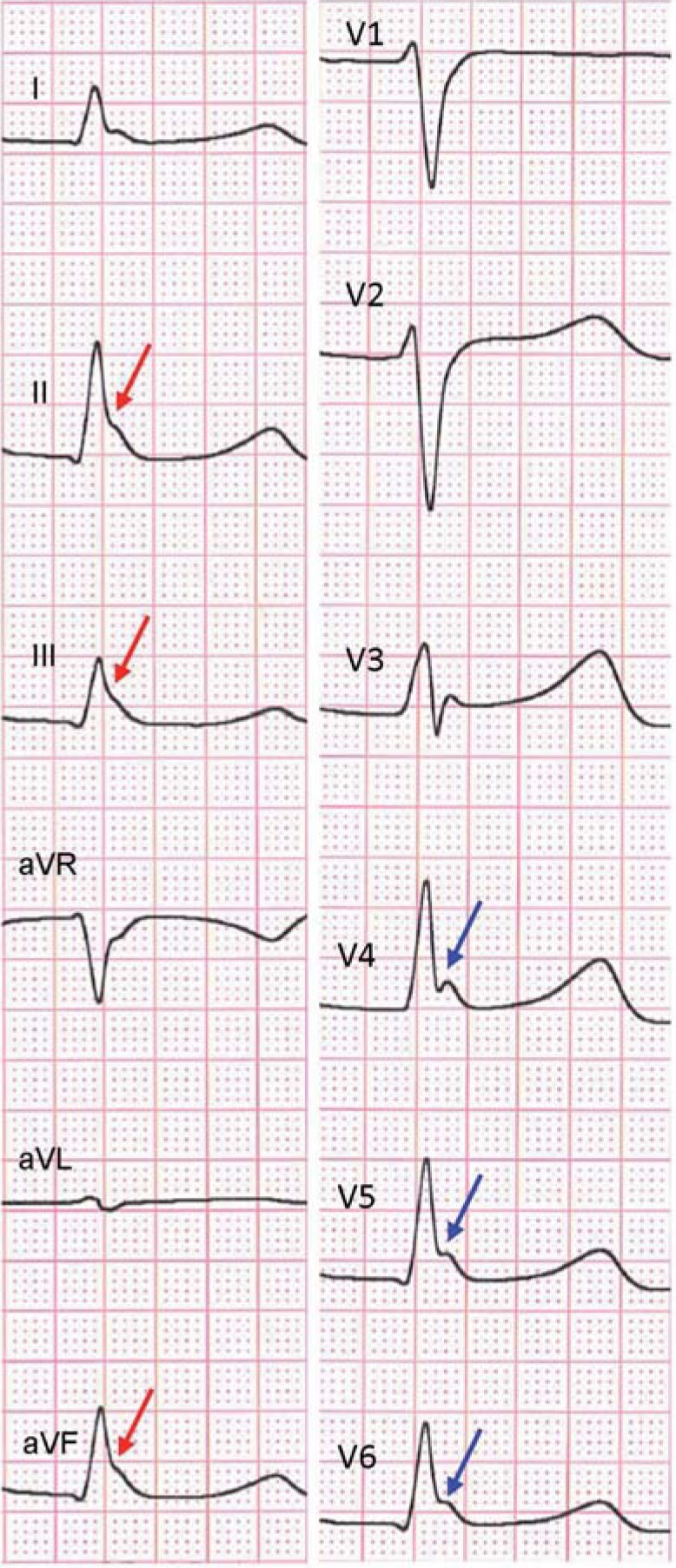
About 10% of BrS patients (a higher percentage than in the normal population) show signs of early repolarization (ER) in the inferolateral leads (e44) (Figure 7). These ECG changes, long regarded as benign (e45), can be associated with an increased risk of sudden cardiac death and the occurrence of VF, now known as ER syndrome (e46– e48). In early studies, BrS patients with signs of early repolarization show a more severe phenotype and a considerably increased risk of VF, respectively (30– 33). In addition, there is an increased risk of an “electrical storm” (≥3 VT/VF episodes within 24 hours) if additional signs of ER are present (33, 34). Patients with right precordial fQRS and inferolateral ER appear to have a particularly high risk of VF (30).
Figure 7.
Early repolarization pattern in the form of QRS “slurring”
(red arrows) and “notching” (blue arrows) in the inferior (II, III, aVF) and lateral (V4–6) leads in a 49-year-old man with sudden cardiac arrest as the first manifestation of Brugada syndrome
In two studies, paroxysmal atrial fibrillation was associated with more frequent syncope and/or ventricular fibrillation (e7, e8).
Not relevant to risk stratification
According to the study results (10, 22, e49), a family history of sudden cardiac death or the presence of a SCN5A mutation has no prognostic impact and is therefore currently not included in risk stratification (5, 6).
Treatment
Implantable cardioverter–defibrillator
In symptomatic BrS patients (aborted sudden cardiac death, documented VT with or without syncope), implantation of an ICD is clearly indicated (class I recommendation) (5, 6) (Figure 5). Because of the high risk of ventricular arrhythmias, ICD implantation is also indicated in symptomatic BrS patients with arrhythmic syncope and a spontaneous type 1 ECG (class IIa recommendation) (5– 6, 35) (Figure 5). ICD implantation is the only treatment shown in studies to be effective in preventing sudden cardiac death in BrS patients (7). To avoid inappropriate ICD shocks, a single VF detection zone with a long detection time can be programmed (7, e50). The role of the subcutaneous ICD (S-ICD) is about to be evaluated in a multicenter study (S-ICD Brugada; NCT02344277).
For asymptomatic BrS patients, individual risk assessment including consideration of other risk factors (age, sex, baseline ECG, and inducibility) is recommended (5, 6). Because of the very low risk of ventricular arrhythmias in asymptomatic BrS patients (10) without spontaneous type I ECG (19), primary prophylactic ICD implantation on the basis of only a drug-induced type I ECG and a positive family history of sudden cardiac death is currently not recommended (class III recommendation) (5, 6) (Figure 5). It is important to realize that these recommendations represent a snapshot and that the current follow-up times do not allow any definite conclusions about the long-term risk in this patient group (e51). The most recent data from the Brugada registry show for example VT/VF-related ICD shocks in 13% of initially asymptomatic patients with BrS (mean follow-up: 7 years) (e41).
General therapeutic measures
In patients with BrS, many substances in addition to class IC antiarrhythmics can have a proarrhythmic effect and need to be avoided. These include certain beta-blockers, various psychoactive and anesthetic drugs, antihistamines, cocaine, and alcohol consumed in excessive quantities. Fever, another important trigger of ECG changes and VT/VF in BrS patients, must be treated immediately with an antipyretic, e.g., paracetamol (class I recommendation) (5, 6) (Figure 5).
A current list of contraindicated drugs and further information about how to act in cases of fever, anesthesia, and recurrent ventricular arrhythmias, is available at www.brugadadrugs.org.
Drug therapy
In case of electrical storm (≥ 3 VT/VF episodes within 24 hours), beta-sympathomimetics such as isoproterenol and Ito-blockers such as (hydro)quinidine are used (class IIa recommendation) (5, 6, e3, e52) (Figure 5). Any precipitating factors such as fever or hypokalemia must be treated at the same time (e3, e52). Isoproterenol can result in immediate reduction of the typical ST elevation (e53) and reduction of (VF-initiating) ventricular extrasystoles with VF suppression (e54). After treatment with (hydro)quinidine, ST elevation may also decrease, in some cases to the point of normalization of the ECG (e55), and VF suppression has been reported in about 85% of patients (36). In addition to its known gastrointestinal side effects, especially a significantly prolonged QTc time may limit (hydro)quinidine treatment (19).
Treatment with (hydro)quinidine can also be considered in hitherto asymptomatic BrS patients with a spontaneous type 1 ECG (class IIb recommendation) (5, 6) (Figure 5). One study that was terminated early (NCT00927732) (e56) and one study that is still recruiting (NCT00789165) (e57, e58), using (hydro)quinidine in asymptomatic patients with BrS, show good long-term drug tolerability and a low incidence of arrhythmias, although patient numbers are still small (about 200 patients in total).
Catheter ablation
For the first time, the current expert consensus includes catheter ablation in its therapeutic recommendations for BrS. Catheter ablation may now be considered in BrS patients with a history of electrical storms or repeated appropriate ICD shocks in whom other treatments have failed (class IIb recommendation) (5, 6). Haïssaguerre et al. and other groups have already demonstrated successful endocardial catheter ablation of the VF triggers (RVOT extrasystoles) in a few BrS patients (37, e59). However, these triggers occur extremely rarely and thus a substrate-based approach has been developed. For the first time, Nademanee et al. have identified fractionated electrograms (a typical feature of disturbed depolarization) over the anterior RVOT as an arrhythmogenic substrate in nine highly symptomatic BrS patients. After epicardial catheter ablation of these potentials, the ECG normalized and there was no further VF episode in eight of nine patients off any antiarrhythmic drugs during a mean follow-up of 20 months (38). Similar results were obtained by Cortez-Dias et al. who performed epicardial catheter ablation in a highly symptomatic woman with BrS (39). As a further indication of disturbed depolarization in BrS, Sacher et al. showed that a drug-induced ECG conversion (type 2 to type 1 ECG) correlated with a further increase of the duration of the epicardial fractionated potentials (40).
Key Messages.
Brugada syndrome is an important differential diagnosis for syncope and (aborted) sudden cardiac death in young adults without structural heart disease.
Based on the new diagnostic criteria of 2013, Brugada syndrome is diagnosed in patients with spontaneous or drug-induced type 1 ECG in V1 or V2 (positioned in the 4th, 3rd, or up to the 2nd intercostal space).
Symptomatic patients with sudden cardiac arrest or a spontaneous type 1 ECG plus arrhythmic syncope are at high risk of sudden cardiac death and should be provided with an implantable cardioverter–defibrillator (ICD).
Novel noninvasive risk markers may be a fragmented QRS complex in the right precordial leads and signs of early repolarization in the inferolateral leads.
Using electro-anatomic mapping techniques, an arrhythmogenic substrate of the right ventricular outflow tract (RVOT) has for the first time been demonstrated in patients with Brugada syndrome. Catheter ablation of these fractionated potentials can prevent recurrence of ventricular fibrillation in highly symptomatic patients in whom other treatments have failed.
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 2.Miyazaki T, Mitamura H, Miyoshi S, Soejima K, Aizawa Y, Ogawa S. Autonomic and antiarrhythmic drug modulation of ST segment elevation in patients with Brugada syndrome. J Am Coll Cardiol. 1996;27:1061–1070. doi: 10.1016/0735-1097(95)00613-3. [DOI] [PubMed] [Google Scholar]
- 3.Ackerman MJ, Priori SG, Willems S, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA) Heart Rhythm. 2011;8:1308–1339. doi: 10.1016/j.hrthm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Ackerman MJ, Priori SG, Willems S, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA) Europace. 2011;13:1077–1109. doi: 10.1093/europace/eur245. [DOI] [PubMed] [Google Scholar]
- 5.Priori SG, Wilde AA, Horie M, et al. HRS/EHRA/APHRS Expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: Document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Europace. 2013;15:1389–1406. doi: 10.1093/europace/eut272. [DOI] [PubMed] [Google Scholar]
- 7.Sacher F, Probst V, Maury P, et al. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: a multicenter study-part 2. Circulation. 2013;128:1739–1747. doi: 10.1161/CIRCULATIONAHA.113.001941. [DOI] [PubMed] [Google Scholar]
- 8.Wilde AA, Antzelevitch C, Borggrefe M, et al. Proposed diagnostic criteria for the Brugada syndrome: consensus report. Circulation. 2002;106:2514–2519. doi: 10.1161/01.cir.0000034169.45752.4a. [DOI] [PubMed] [Google Scholar]
- 9.Benito B, Sarkozy A, Mont L, et al. Gender differences in clinical manifestations of Brugada syndrome. J Am Coll Cardiol. 2008;52:1567–1573. doi: 10.1016/j.jacc.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 10.Probst V, Veltmann C, Eckardt L, et al. Long-term prognosis of patients diagnosed with Brugada syndrome: Results from the FINGER Brugada Syndrome Registry. Circulation. 2010;121:635–643. doi: 10.1161/CIRCULATIONAHA.109.887026. [DOI] [PubMed] [Google Scholar]
- 11.Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 12.Hoogendijk MG, Opthof T, Postema PG, Wilde AA, de Bakker JM, Coronel R. The Brugada ECG pattern: a marker of channelopathy, structural heart disease, or neither? Toward a unifying mechanism of the Brugada syndrome. Circ Arrhythm Electrophysiol. 2010;3:283–290. doi: 10.1161/CIRCEP.110.937029. [DOI] [PubMed] [Google Scholar]
- 13.Wilde AA, Postema PG, Di Diego JM, et al. The pathophysiological mechanism underlying Brugada syndrome: depolarization versus repolarization. J Mol Cell Cardiol. 2010;49:543–553. doi: 10.1016/j.yjmcc.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coronel R, Casini S, Koopmann TT, et al. Right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada syndrome: a combined electrophysiological, genetic, histopathologic, and computational study. Circulation. 2005;112:2769–2777. doi: 10.1161/CIRCULATIONAHA.105.532614. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Sacher F, Hoffmayer K, et al. Cardiac electrophysiologic substrate underlying the ECG phenotype and electrogram abnormalities in Brugada syndrome patients. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.114.013698. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richter S, Sarkozy A, Veltmann C, et al. Variability of the diagnostic ECG pattern in an ICD patient population with Brugada syndrome. J Cardiovasc Electrophysiol. 2009;20:69–75. doi: 10.1111/j.1540-8167.2008.01282.x. [DOI] [PubMed] [Google Scholar]
- 17.Morita H, Kusano KF, Miura D, et al. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008;118:1697–1704. doi: 10.1161/CIRCULATIONAHA.108.770917. [DOI] [PubMed] [Google Scholar]
- 18.Campuzano O, Allegue C, Iglesias A, Brugada R. Genetic basis of Brugada syndrome. J Genet Syndr Gene Ther. 2013;4 [Google Scholar]
- 19.Mizusawa Y, Wilde AA. Brugada syndrome. Circ Arrhythm Electrophysiol. 2012;5:606–616. doi: 10.1161/CIRCEP.111.964577. [DOI] [PubMed] [Google Scholar]
- 20.Probst V, Wilde AA, Barc J, et al. SCN5A mutations and the role of genetic background in the pathophysiology of Brugada syndrome. Circ Cardiovasc Genet. 2009;2:552–557. doi: 10.1161/CIRCGENETICS.109.853374. [DOI] [PubMed] [Google Scholar]
- 21.Bezzina CR, Barc J, Mizusawa Y, et al. Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat Genet. 2013;45:1044–1049. doi: 10.1038/ng.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gehi AK, Duong TD, Metz LD, Gomes JA, Mehta D. Risk stratification of individuals with the Brugada electrocardiogram: a meta-analysis. J Cardiovasc Electrophysiol. 2006;17:577–583. doi: 10.1111/j.1540-8167.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 23.Priori SG, Gasparini M, Napolitano C, et al. Risk stratification in Brugada syndrome: results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J Am Coll Cardiol. 2012;59:37–45. doi: 10.1016/j.jacc.2011.08.064. [DOI] [PubMed] [Google Scholar]
- 24.Olde Nordkamp LR, Vink AS, Wilde AA, et al. Syncope in Brugada syndrome: Prevalence, clinical significance, and clues from history taking to distinguish arrhythmic from nonarrhythmic causes. Heart Rhythm. 2015;12:367–375. doi: 10.1016/j.hrthm.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Brugada P, Brugada R, Brugada J. Should patients with an asymptomatic Brugada electrocardiogram undergo pharmacological and electrophysiological testing? Circulation. 2005;112:279–292. doi: 10.1161/CIRCULATIONAHA.104.485326. discussion 279-92. [DOI] [PubMed] [Google Scholar]
- 26.Paul M, Gerss J, Schulze-Bahr E, et al. Role of programmed ventricular stimulation in patients with Brugada syndrome: a meta-analysis of worldwide published data. Eur Heart J. 2007;28:2126–2133. doi: 10.1093/eurheartj/ehm116. [DOI] [PubMed] [Google Scholar]
- 27.Brugada J, Brugada R, Brugada P. Electrophysiologic testing predicts events in Brugada syndrome patients. Heart Rhythm. 2011;8:1595–1597. doi: 10.1016/j.hrthm.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Wilde AA, Viskin S. EP testing does not predict cardiac events in Brugada syndrome. Heart Rhythm. 2011;8:1598–1600. doi: 10.1016/j.hrthm.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Delise P, Allocca G, Marras E, et al. Risk stratification in individuals with the Brugada type 1 ECG pattern without previous cardiac arrest: usefulness of a combined clinical and electrophysiologic approach. Eur Heart J. 2011;32:169–176. doi: 10.1093/eurheartj/ehq381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tokioka K, Kusano KF, Morita H, et al. Electrocardiographic parameters and fatal arrhythmic events in patients with brugada syndrome: combination of depolarization and repolarization abnormalities. J Am Coll Cardiol. 2014;63:2131–2138. doi: 10.1016/j.jacc.2014.01.072. [DOI] [PubMed] [Google Scholar]
- 31.Sarkozy A, Chierchia GB, Paparella G, et al. Inferior and lateral electrocardiographic repolarization abnormalities in Brugada syndrome. Circ Arrhythm Electrophysiol. 2009;2:154–161. doi: 10.1161/CIRCEP.108.795153. [DOI] [PubMed] [Google Scholar]
- 32.Kamakura S, Ohe T, Nakazawa K, et al. Long-term prognosis of probands with Brugada-pattern ST-elevation in leads V1-V3. Circ Arrhythm Electrophysiol. 2009;2:495–503. doi: 10.1161/CIRCEP.108.816892. [DOI] [PubMed] [Google Scholar]
- 33.Kawata H, Morita H, Yamada Y, et al. Prognostic significance of early repolarization in inferolateral leads in Brugada patients with documented ventricular fibrillation: a novel risk factor for Brugada syndrome with ventricular fibrillation. Heart Rhythm. 2013;10:1161–1168. doi: 10.1016/j.hrthm.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Kaneko Y, Horie M, Niwano S, et al. Electrical storm in patients with Brugada syndrome is associated with early repolarization. Circ Arrhythm Electrophysiol. 2014;7:1122–1128. doi: 10.1161/CIRCEP.114.001806. [DOI] [PubMed] [Google Scholar]
- 35.Priori SG, Napolitano C, Gasparini M, et al. Natural history of Brugada syndrome: insights for risk stratification and management. Circulation. 2002;105:1342–1347. doi: 10.1161/hc1102.105288. [DOI] [PubMed] [Google Scholar]
- 36.Marquez MF, Bonny A, Hernandez-Castillo E, et al. Long-term efficacy of low doses of quinidine on malignant arrhythmias in Brugada syndrome with an implantable cardioverter-defibrillator: a case series and literature review. Heart Rhythm. 2012;9:1995–2000. doi: 10.1016/j.hrthm.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 37.Haïssaguerre M, Extramiana F, Hocini M, et al. Mapping and ablation of ventricular fibrillation associated with long-QT and Brugada syndromes. Circulation. 2003;108:925–928. doi: 10.1161/01.CIR.0000088781.99943.95. [DOI] [PubMed] [Google Scholar]
- 38.Nademanee K, Veerakul G, Chandanamattha P, et al. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123:1270–1279. doi: 10.1161/CIRCULATIONAHA.110.972612. [DOI] [PubMed] [Google Scholar]
- 39.Cortez-Dias N, Placido R, Marta L, et al. Epicardial ablation for prevention of ventricular fibrillation in a patient with Brugada syndrome. Rev Port Cardiol. 2014;33(305):e1–e7. doi: 10.1016/j.repc.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Sacher F, Jesel L, Jais P, Haissaguerre M. Insight into the mechanism of Brugada syndrome: epicardial substrate and modification during ajmaline testing. Heart Rhythm. 2014;11:732–734. doi: 10.1016/j.hrthm.2013.05.023. [DOI] [PubMed] [Google Scholar]
- e1.Perez-Riera AR, Ferreira Filho C, de Abreu LC, et al. Do patients with electrocardiographic Brugada type 1 pattern have associated right bundle branch block? A comparative vectorcardiographic study. Europace. 2012;14:889–897. doi: 10.1093/europace/eur395. [DOI] [PubMed] [Google Scholar]
- e2.Probst V, Denjoy I, Meregalli PG, et al. Clinical aspects and prognosis of Brugada syndrome in children. Circulation. 2007;115:2042–2048. doi: 10.1161/CIRCULATIONAHA.106.664219. [DOI] [PubMed] [Google Scholar]
- e3.Maury P, Hocini M, Haissaguerre M. Electrical storms in Brugada syndrome: review of pharmacologic and ablative therapeutic options. Indian Pacing Electrophysiol J. 2005;5:25–34. [PMC free article] [PubMed] [Google Scholar]
- e4.Morita H, Nagase S, Miura D, et al. Differential effects of cardiac sodium channel mutations on initiation of ventricular arrhythmias in patients with Brugada syndrome. Heart Rhythm. 2009;6:487–492. doi: 10.1016/j.hrthm.2009.01.031. [DOI] [PubMed] [Google Scholar]
- e5.Zuberi Z, Jogiya R, Behr ER. VERP in Brugada syndrome - Very effective risk predictor? Int J Cardiol. 2015;184C:270–271. doi: 10.1016/j.ijcard.2015.02.044. [DOI] [PubMed] [Google Scholar]
- e6.Eckardt L, Kirchhof P, Loh P, et al. Brugada syndrome and supraventricular tachyarrhythmias: a novel association? J Cardiovasc Electrophysiol. 2001;12:680–685. doi: 10.1046/j.1540-8167.2001.00680.x. [DOI] [PubMed] [Google Scholar]
- e7.Morita H, Kusano-Fukushima K, Nagase S, et al. Atrial fibrillation and atrial vulnerability in patients with Brugada syndrome. J Am Coll Cardiol. 2002;40:1437–1444. doi: 10.1016/s0735-1097(02)02167-8. [DOI] [PubMed] [Google Scholar]
- e8.Kusano KF, Taniyama M, Nakamura K, et al. Atrial fibrillation in patients with Brugada syndrome relationships of gene mutation, electrophysiology, and clinical backgrounds. J Am Coll Cardiol. 2008;51:1169–1175. doi: 10.1016/j.jacc.2007.10.060. [DOI] [PubMed] [Google Scholar]
- e9.Richter S, Brugada P. Remote monitoring of a high-risk patient with Brugada syndrome: association of different arrhythmic manifestations. Herzschrittmacherther Elektrophysiol. 2013;24:275–279. doi: 10.1007/s00399-013-0292-4. [DOI] [PubMed] [Google Scholar]
- e10.Elizari MV, Levi R, Acunzo RS, et al. Abnormal expression of cardiac neural crest cells in heart development: a different hypothesis for the etiopathogenesis of Brugada syndrome. Heart Rhythm. 2007;4:359–365. doi: 10.1016/j.hrthm.2006.10.026. [DOI] [PubMed] [Google Scholar]
- e11.Brugada P. On the intriguing phenotypic manifestations of Brugada syndrome and the diagnostic value of the electrocardiogram. J Am Coll Cardiol. 2011;58:2299–2300. doi: 10.1016/j.jacc.2011.09.008. [DOI] [PubMed] [Google Scholar]
- e12.Raju H, de Noronha SV, Rothery S, et al. The Brugada syndrome and cardiomyopathy: altered collagen and gap junction expression. Europace. 2014;16 [Google Scholar]
- e13.Boukens BJ, Sylva M, de Gier-de Vries C, et al. Reduced sodium channel function unmasks residual embryonic slow conduction in the adult right ventricular outflow tract. Circ Res. 2013;113:137–41. doi: 10.1161/CIRCRESAHA.113.301565. [DOI] [PubMed] [Google Scholar]
- e14.Sarkozy A, Paparella G, Boussy T, et al. The usefulness of the consensus clinical diagnostic criteria in Brugada syndrome. Int J Cardiol. 2013;167:2700–2704. doi: 10.1016/j.ijcard.2012.06.115. [DOI] [PubMed] [Google Scholar]
- e15.Richter S, Sarkozy A, Paparella G, et al. Number of electrocardiogram leads displaying the diagnostic coved-type pattern in Brugada syndrome: a diagnostic consensus criterion to be revised. Eur Heart J. 2010;31:1357–1364. doi: 10.1093/eurheartj/ehq049. [DOI] [PubMed] [Google Scholar]
- e16.Anselm DD, Gottschalk BH, Baranchuk A. Brugada phenocopies: consideration of morphologic criteria and early findings from an international registry. Can J Cardiol. 2014;30:1511–1515. doi: 10.1016/j.cjca.2014.09.023. [DOI] [PubMed] [Google Scholar]
- e17.Hoogendijk MG. Diagnostic dilemmas: overlapping features of brugada syndrome and arrhythmogenic right ventricular cardiomyopathy. Front Physiol. 2012;3 doi: 10.3389/fphys.2012.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e18.Rolf S, Bruns HJ, Wichter T, et al. The ajmaline challenge in Brugada syndrome: diagnostic impact, safety, and recommended protocol. Eur Heart J. 2003;24:1104–1112. doi: 10.1016/s0195-668x(03)00195-7. [DOI] [PubMed] [Google Scholar]
- e19.Wolpert C, Echternach C, Veltmann C, et al. Intravenous drug challenge using flecainide and ajmaline in patients with Brugada syndrome. Heart Rhythm. 2005;2:254–260. doi: 10.1016/j.hrthm.2004.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e20.Arnalsteen-Dassonvalle E, Hermida JS, Kubala M, et al. Ajmaline challenge for the diagnosis of Brugada syndrome: which protocol? Arch Cardiovasc Dis. 2010;103:570–578. doi: 10.1016/j.acvd.2010.10.007. [DOI] [PubMed] [Google Scholar]
- e21.Savastano S, Rordorf R, Vicentini A, et al. A comprehensive electrocardiographic, molecular, and echocardiographic study of Brugada syndrome: validation of the 2013 diagnostic criteria. Heart Rhythm. 2014;11:1176–1183. doi: 10.1016/j.hrthm.2014.04.010. [DOI] [PubMed] [Google Scholar]
- e22.Veltmann C, Papavassiliu T, Konrad T, et al. Insights into the location of type I ECG in patients with Brugada syndrome: correlation of ECG and cardiovascular magnetic resonance imaging. Heart Rhythm. 2012;9:414–421. doi: 10.1016/j.hrthm.2011.10.032. [DOI] [PubMed] [Google Scholar]
- e23.Bayes de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45:433–442. doi: 10.1016/j.jelectrocard.2012.06.004. [DOI] [PubMed] [Google Scholar]
- e24.Veltmann C, Schimpf R, Echternach C, et al. A prospective study on spontaneous fluctuations between diagnostic and non-diagnostic ECGs in Brugada syndrome: implications for correct phenotyping and risk stratification. Eur Heart J. 2006;27:2544–2552. doi: 10.1093/eurheartj/ehl205. [DOI] [PubMed] [Google Scholar]
- e25.Maury P, Rollin A, Sacher F, et al. Prevalence and prognostic role of various conduction disturbances in patients with the Brugada syndrome. Am J Cardiol. 2013;112:1384–1389. doi: 10.1016/j.amjcard.2013.06.033. [DOI] [PubMed] [Google Scholar]
- e26.Amin AS, de Groot EA, Ruijter JM, Wilde AA, Tan HL. Exercise-induced ECG changes in Brugada syndrome. Circ Arrhythm Electrophysiol. 2009;2:531–539. doi: 10.1161/CIRCEP.109.862441. [DOI] [PubMed] [Google Scholar]
- e27.Grimster A, Segal OR, Behr ER. Type I Brugada electrocardiogram pattern during the recovery phase of exercise testing. Europace. 2008;10:897–898. doi: 10.1093/europace/eun101. [DOI] [PubMed] [Google Scholar]
- e28.Papadakis M, Petzer E, Sharma S. Unmasking of the Brugada phenotype during exercise testing and its association with ventricular arrhythmia on the recovery phase. Heart. 2009;95 doi: 10.1136/hrt.2009.174052. [DOI] [PubMed] [Google Scholar]
- e29.Makimoto H, Nakagawa E, Takaki H, et al. Augmented ST-segment elevation during recovery from exercise predicts cardiac events in patients with Brugada syndrome. J Am Coll Cardiol. 2010;56:1576–1584. doi: 10.1016/j.jacc.2010.06.033. [DOI] [PubMed] [Google Scholar]
- e30.Hu D, Barajas-Martinez H, Pfeiffer R, et al. Mutations in SCN10A are responsible for a large fraction of cases of Brugada syndrome. J Am Coll Cardiol. 2014;64:66–79. doi: 10.1016/j.jacc.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e31.Hu D, Barajas-Martinez H, Terzic A, et al. ABCC9 is a novel Brugada and early repolarization syndrome susceptibility gene. Int J Cardiol. 2014;171:431–442. doi: 10.1016/j.ijcard.2013.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e32.Juang JM, Lu TP, Lai LC, et al. Disease-targeted sequencing of ion channel genes identifies de novo mutations in patients with non-familial Brugada syndrome. Sci Rep. 2014;4 doi: 10.1038/srep06733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e33.Chen Q, Kirsch GE, Zhang D, et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293–296. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- e34.Kapplinger JD, Tester DJ, Alders M, et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7:33–46. doi: 10.1016/j.hrthm.2009.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e35.Le Scouarnec S, Karakachoff M, Gourraud JB, et al. Testing the burden of rare variation in arrhythmia-susceptibility genes provides new insights into molecular diagnosis for Brugada syndrome. Hum Mol Genet 2015. doi: 10.1093/hmg/ddv036. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- e36.Remme CA, Wilde AA, Bezzina CR. Cardiac sodium channel overlap syndromes: different faces of SCN5A mutations. Trends Cardiovasc Med. 2008;18:78–87. doi: 10.1016/j.tcm.2008.01.002. [DOI] [PubMed] [Google Scholar]
- e37.Sacher F, Probst V, Iesaka Y, et al. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: a multicenter study. Circulation. 2006;114:2317–2324. doi: 10.1161/CIRCULATIONAHA.106.628537. [DOI] [PubMed] [Google Scholar]
- e38.Sarkozy A, Boussy T, Kourgiannides G, et al. Long-term follow-up of primary prophylactic implantable cardioverter-defibrillator therapy in Brugada syndrome. Eur Heart J. 2007;28:334–344. doi: 10.1093/eurheartj/ehl450. [DOI] [PubMed] [Google Scholar]
- e39.Rosso R, Glick A, Glikson M, et al. Outcome after implantation of cardioverter defibrillator [corrected] in patients with Brugada syndrome: a multicenter Israeli study (ISRABRU) Isr Med Assoc J. 2008;10:435–439. [PubMed] [Google Scholar]
- e40.Miyazaki S, Uchiyama T, Komatsu Y, et al. Long-term complications of implantable defibrillator therapy in Brugada syndrome. Am J Cardiol. 2013;111:1448–1451. doi: 10.1016/j.amjcard.2013.01.295. [DOI] [PubMed] [Google Scholar]
- e41.Conte G, Sieira J, Ciconte G, et al. Implantable cardioverter-defibrillator therapy in brugada syndrome: a 20-year single-center experience. J Am Coll Cardiol. 2015;65:879–888. doi: 10.1016/j.jacc.2014.12.031. [DOI] [PubMed] [Google Scholar]
- e42.Giustetto C, Drago S, Demarchi PG, et al. Risk stratification of the patients with Brugada type electrocardiogram: a community-based prospective study. Europace. 2009;11:507–513. doi: 10.1093/europace/eup006. [DOI] [PubMed] [Google Scholar]
- e43.Das MK, Saha C, El Masry H, et al. Fragmented QRS on a 12-lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007;4:1385–1392. doi: 10.1016/j.hrthm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- e44.Letsas KP, Sacher F, Probst V, et al. Prevalence of early repolarization pattern in inferolateral leads in patients with Brugada syndrome. Heart Rhythm. 2008;5:1685–1689. doi: 10.1016/j.hrthm.2008.09.021. [DOI] [PubMed] [Google Scholar]
- e45.Wellens HJ. Early repolarization revisited. N Engl J Med. 2008;358:2063–2065. doi: 10.1056/NEJMe0801060. [DOI] [PubMed] [Google Scholar]
- e46.Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- e47.Tikkanen JT, Anttonen O, Junttila MJ, et al. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- e48.Sinner MF, Reinhard W, Muller M, et al. Association of early repolarization pattern on ECG with risk of cardiac and all-cause mortality: a population-based prospective cohort study (MONICA/KORA) PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e49.Sarkozy A, Sorgente A, Boussy T, et al. The value of a family history of sudden death in patients with diagnostic type I Brugada ECG pattern. Eur Heart J. 2011;32:2153–2160. doi: 10.1093/eurheartj/ehr129. [DOI] [PubMed] [Google Scholar]
- e50.Veltmann C, Kuschyk J, Schimpf R, et al. Prevention of inappropriate ICD shocks in patients with Brugada syndrome. Clin Res Cardiol. 2010;99:37–44. doi: 10.1007/s00392-009-0075-4. [DOI] [PubMed] [Google Scholar]
- e51.Viskin S, Rosso R. Risk of sudden death in asymptomatic Brugada syndrome: not as high as we thought and not as low as we wished .but the contrary. J Am Coll Cardiol. 2010;56:1585–1588. doi: 10.1016/j.jacc.2010.07.019. [DOI] [PubMed] [Google Scholar]
- e52.Veerakul G, Nademanee K. Treatment of electrical storms in Brugada syndrome. J Arrhythm. 2013;29:117–124. [Google Scholar]
- e53.Tanaka H, Kinoshita O, Uchikawa S, et al. Successful prevention of recurrent ventricular fibrillation by intravenous isoproterenol in a patient with Brugada syndrome. Pacing Clin Electrophysiol. 2001;24:1293–1294. doi: 10.1046/j.1460-9592.2001.01293.x. [DOI] [PubMed] [Google Scholar]
- e54.Maury P, Couderc P, Delay M, Boveda S, Brugada J. Electrical storm in Brugada syndrome successfully treated using isoprenaline. Europace. 2004;6:130–133. doi: 10.1016/j.eupc.2003.11.009. [DOI] [PubMed] [Google Scholar]
- e55.Alings M, Dekker L, Sadee A, Wilde A. Quinidine induced electrocardiographic normalization in two patients with Brugada syndrome. Pacing Clin Electrophysiol. 2001;24:1420–1422. doi: 10.1046/j.1460-9592.2001.01420.x. [DOI] [PubMed] [Google Scholar]
- e56.Probst V, Leenhardt A, Babuty D, et al. Clinical presentation and short-term effect of hydroquinidine in Brugada syndrome patients included in the QUIDAM study. Eur Heart J. 2013;34([Suppl 1]) [Google Scholar]
- e57.Viskin S, Wilde AA, Tan HL, Antzelevitch C, Shimizu W, Belhassen B. Empiric quinidine therapy for asymptomatic Brugada syndrome: time for a prospective registry. Heart Rhythm. 2009;6:401–404. doi: 10.1016/j.hrthm.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e58.Adler A, Nordkamp LO, Crotti L, et al. Empiric quinidine for asymptomatic Brugada syndrome - preliminary results of an international registry. Heart Rhythm. 2012;9:1918–1919. [Google Scholar]
- e59.Nakagawa E, Takagi M, Tatsumi H, Yoshiyama M. Successful radiofrequency catheter ablation for electrical storm of ventricular fibrillation in a patient with Brugada syndrome. Circ J. 2008;72:1025–1029. doi: 10.1253/circj.72.1025. [DOI] [PubMed] [Google Scholar]