Background: Factors that govern peripheral neuropathy associated with Schwann cell dysfunction are not fully understood.
Results: Under hyperglycemic conditions, Schwann cells de-differentiate into immature cells via sorbitol accumulation and Igf1 down-regulation.
Conclusion: Schwann cell de-differentiation promotes neuropathy development under hyperglycemic conditions.
Significance: These findings reveal new mechanisms underlying neuropathy seen in diabetes mellitus via Schwann cell de-differentiation leading to de-myelination.
Keywords: cell differentiation, cell metabolism, diabetes, differentiation, Schwann cells, vitamin D
Abstract
Diabetes mellitus (DM) is frequently accompanied by complications, such as peripheral nerve neuropathy. Schwann cells play a pivotal role in regulating peripheral nerve function and conduction velocity; however, changes in Schwann cell differentiation status in DM are not fully understood. Here, we report that Schwann cells de-differentiate into immature cells under hyperglycemic conditions as a result of sorbitol accumulation and decreased Igf1 expression in those cells. We found that de-differentiated Schwann cells could be re-differentiated in vitro into mature cells by treatment with an aldose reductase inhibitor, to reduce sorbitol levels, or with vitamin D3, to elevate Igf1 expression. In vivo DM models exhibited significantly reduced nerve function and conduction, Schwann cell de-differentiation, peripheral nerve de-myelination, and all conditions were significantly rescued by aldose reductase inhibitor or vitamin D3 administration. These findings reveal mechanisms underlying pathological changes in Schwann cells seen in DM and suggest ways to treat neurological conditions associated with this condition.
Introduction
Diabetes mellitus (DM)2 is characterized by continuously elevated systemic glucose levels (1). DM patients are classified as either type I or type II; type I patients frequently show reduced insulin levels due to loss of β-cells resulting from viral infection or autoimmune disease, and type II patients exhibit insulin resistance (1). Most DM patients are diagnosed as type II (2). Currently, in the United States more than 25.8 million people are estimated to suffer from DM, a number that is increasing (2). Three major complications of DM, known collectively as triopathy, include diabetic neuropathy, nephropathy, and retinopathy; these conditions often promote additional complications such as sensory/motor loss, renal failure, and blindness, respectively (3). In DM patients, polyneuropathy develops in peripheral sensory, motor, and autonomic nerves and is one of the first triopathy symptoms observed (4). Neurological dysfunction seen in DM patients is frequently associated with wounds or wound deterioration due to sensory deficits or falls accompanied by fractures due to loss of motor nerve function or bathyanesthesia (5). Thus, protecting DM patients from polyneuropathy is crucial to prevent development of further complications (6).
Nerves can be myelinated or nonmyelinated, and nerve function and conduction velocity (NCV) is approximately 10 times faster in the former (7). Peripheral nerve myelination is accomplished by Schwann cells, which produce myelin-associated proteins such as myelin-associated glycoprotein (MAG), myelin basic protein (MBP), and myelin protein zero (P0) (8). Immature Schwann cells express high levels of the neurotrophin receptor P75 and low levels of MAG, MBP, and P0, whereas mature Schwann cells down-regulate P75 and up-regulate MAG, MBP, and P0 as they become functional myelinating cells (8). Schwann cells undergo de-differentiation following injury such as denervation (9); however, Schwann cell de-differentiation by other means has not been demonstrated.
Hyperglycemia-dependent tissue damage occurring in DM is often indirect and results from disturbances in blood flow or blood vessel perturbation (10). Hyperglycemia also frequently promotes accumulation of advanced glycation end products and reactive oxygen species, leading to cellular and tissue damage (11, 12). Glucose is taken up by cells through insulin-dependent or -independent pathways, the latter of which are activated under high glucose conditions (13). Increasing levels of intracellular glucose occurring under hyperglycemia activate the polyol pathway, which is driven by aldose reductase (AR) and sorbitol dehydrogenase activities (14). As a result, intracellular glucose is converted to sorbitol by AR, and sorbitol is then converted to fructose sorbitol dehydrogenase (14). When AR activity exceeds that of sorbitol dehydrogenase, sorbitol accumulates in cells, elevates osmotic pressure, and produces cellular damage (15). Although activation of the polyol pathway occurs in various cell types under high glucose conditions (16–18), the effects of these activities on cellular differentiation have not been fully characterized.
Vitamin D3 is a lipid-soluble vitamin required for calcium uptake from the intestine (19). Lack of the vitamin D receptor (VDR) or low vitamin D3 intake causes skeletal diseases like rickets, and low vitamin D3 is a risk factor for fracture in osteoporosis patients (20, 21). Vitamin D3 is also thought to play a role in preventing falls (22), although how this occurs remains unclear. Insulin-like growth factor 1 (Igf1) is similar to insulin in structure and function and regulates various aspects of growth and development of individuals (23). Igf1 is produced primarily in the liver following growth hormone stimulation (23), and vitamin D3 level is reportedly correlated with serum Igf1 levels (24).
Here, we report that Schwann cells de-differentiate into immature cells under hyperglycemic conditions due to sorbitol accumulation and reduced Igf1 expression. We found that treatment of Schwann cells with an AR inhibitor (ARI) reduced intracellular sorbitol levels. Likewise, treatment of Schwann cells with vitamin D3 increased Igf1 levels, even under high glucose conditions. Either treatment improved NCV and rescued Schwann cell de-differentiation and peripheral nerve de-myelination in DM model mice. We propose that loss of peripheral function in DM patients is due in part to direct effects of hyperglycemia on Schwann cell de-differentiation and subsequent peripheral nerve de-myelination and is a condition potentially treatable by ARI or vitamin D3.
Experimental Procedures
Cell and Sciatic Nerve Culture
Primary Schwann cells isolated from rat dorsal root ganglia, IMS32 cells, or sciatic nerves isolated from control, STZ, or db/db mice were cultured for 48 h in DMEM (Sigma) containing 3% (v/v) heat-inactivated FBS (JRH Biosciences, Lenexa, KS) and GlutaMAX (Invitrogen) under different glucose conditions (100, 300, or 540 mg/dl) in the presence or absence of ARI (Epalrestat) (1.0 μm, provided by Ono Pharmaceutical Co., Ltd., Osaka, Japan), ED71 (0.1 μm, provided by Chugai Pharmaceutical Co., Ltd., Tokyo, Japan), 1,25(OH)2D3 (0.1 μm, Wako Pure Chemicals Industries, Osaka, Japan), or Igf1 (10 ng/ml, R&D Systems, Minneapolis, MN) with or without anti-Igf1 (1.0 μg/ml). Cells or sciatic nerve tissues were then subjected to real time PCR or immunohistochemical analysis.
Quantitative PCR Analysis
Total RNA was isolated from IMS32 cells or sciatic nerves using TRIzol reagent (Invitrogen), and cDNA was synthesized using oligo(dT) primers and reverse transcriptase (Wako Pure Chemicals Industries). Quantitative PCR was performed using the SYBR Premix ExTaq II reagent and a DICE Thermal cycler (Takara Bio Inc., Tokyo, Japan), following the manufacturer's instructions.
β-Actin (Actb) expression served as an internal control. Primers for MAG, MBP, P0, P75, Igf1, and Actb were as follows: MAG-s, 5′-AGAGAGCCACTGCCTTCAAC, and MAG-As, 5′-CGGGTTGGATTTTACCACAC; MBP-s; 5′-TCAAGAACATTGTGACACCTCGAA, and MBP-As, 5′-GTGAGCCGATTTATAGTCGGAAGC; P0-s, 5′-GGCAAGACCTCTCAGGTCAC, and P0-As, 5′-AGCCAGCAGTACCGAATCAG; P75-s, 5′-CTGCTGCTGCTGCTGCTTCT, and P75-As, 5′-TTGCAGGCTTTGCAGCACTC; Igf1-s, 5′-CCACCAATTCATTTCCAGACTTTG, and Igf1-As, 5′-CCAGGTAGAAGAGGTGTGAAGACG; and Actb-forward, 5′-TGAGAGGGAAATCGTGCGTGAC-3′, and Actb-reverse, 5′-AAGAAGGAAGGCTGGAAAAGAG-3′.
Immunohistochemistry
Primary rat Schwann cells cultured under various glucose concentrations were fixed in 4% paraformaldehyde at room temperature for 20 min and then stained with mouse anti-rat MAG antibody (clone 513, Millipore, Temecula, CA) at 4 °C overnight. Cells were then stained with Alexa488-conjugated goat anti-mouse antibody (Invitrogen) at room temperature for 60 min and observed under a fluorescence microscope (Biorevo, Keyence Corp.).
Sorbitol Assay
Sciatic nerves isolated either from mice or cultured cells were harvested and placed on filter paper to remove adherent fluid, weighed, and then homogenized in 1.0 ml of cold 8% perchloric acid. Cells or nerves were sedimented by centrifugation (5500 × g, 10 min), washed with saline, and precipitated with 3 volumes of cold 8% perchloric acid. Homogenates or extracts were centrifuged at 5500 × g for 10 min, and supernatants were neutralized at 4 °C with 1.0 ml of 2 m K2CO3. Neutralized extracts were re-centrifuged, and supernatants were assayed enzymatically for sorbitol using a Multi-Detection Microplate Reader (Ds Pharma Biomedical, Tokyo, Japan) and the d-Sorbitol/Xylitol Colorimetric Method (Roche Applied Science/R-Biopharm, Tokyo, Japan).
ARI and ED71 Treatment in Vivo
Wild-type C57BL/6 mice were obtained from CLEA Japan, Inc. (Tokyo, Japan), and db/db mice were from Oriental Yeast Co., Ltd. (Tokyo, Japan). Wild-type mice were treated with or without STZ administered intraperitoneally (250 mg/kg) at 4 weeks of age to generate type I diabetic model mice or control mice, respectively. Starting at 1 week after STZ injection, body weight and blood glucose levels were checked once a week, and mice were treated or not treated with Epalrestat (ARI) (2.5 mg/kg/day, by oral administration). Mice were also intraperitoneally treated with or without ED71 (0.05 μg/kg/day), and 4 weeks later, mice underwent ROTA-ROD, von Frey, and nerve conduction velocity tests, as described below. Similar experiments were performed in db/db mice starting at 5 weeks of age. Animals were maintained under specific pathogen-free conditions in animal facilities certified by the Keio University School of Medicine animal care committee, and animal protocols were approved by that committee.
ROTA-ROD Test
Motor function of type I or II diabetic model mice was evaluated using a Rotarod treadmill apparatus (Muromachi Kikai Co., Ltd., Tokyo, Japan). For this analysis, mice were evaluated by monitoring the time (latency) that an animal spends on a rod rotating at 20 rpm in a 2-min session. Three trials were conducted, and the average number of seconds spent on the rod was recorded.
Gait Analysis
Quadrupedal gait dynamics were evaluated based on mouse footprints using a DigiGait imaging system (Mouse Specifics Inc, (Framingham, MA), as described previously (25). Stride lengths of hind limbs were assessed at a speed of 8 cm/s. Three trials were conducted to evaluate average stride lengths.
von Frey Test
To quantify sensitivity to a tactile stimulus, paw withdrawal time in response to a tactile stimulus was measured using von Frey filaments (North Coast Medical, Morgan Hill, CA) with 0.16-g bending forces. Each filament was applied to the hind paw plantar surface for 3 s, and testing was repeated three times. Hind paws were tested individually. Response scores were evaluated as follows: 0, no response; 1, slow and/or slight response; 2, quick withdrawal from the stimulus without flinching or licking; 3, intense withdrawal from the stimulus with brisk flinching and/or licking. Paw withdrawal in response to each filament was determined as the average of two scores per paw. Paw movements associated with locomotion or weight shifting were not counted as a response. Left and right paws were measured alternately with a 3-min interval between measurements. Before testing, mice were habituated on an elevated nylon mesh floor where testing would occur for at least 1 h.
NCV Analysis
Conduction velocity was measured using a commercially available electromyogram device (Neuropack S1 MEB-9402, Nihon-Kohden, Tokyo, Japan). A needle pick-up electrode was inserted into the interosseous muscle, and the ground electrode was placed on the tail. Waves were triggered by gradually increasing stimulus intensity from 0.1 to 2.0 mA. Stimuli were applied for 1 ms at 1 Hz. Compound motor action potentials were recorded.
Electron Microscopy
Sciatic nerves from wild-type or db/db mice were removed and immersed in a mixture of 4% paraformaldehyde, 2.5% glutaraldehyde solution for 24 h at 4 °C after euthanasia. Specimens were post-fixed in 1% osmium tetroxide in 0.1 m cacodylate buffer for 4 h at 4 °C, dehydrated in ascending acetone solutions, and embedded in epoxy resin (Epon 812, Taab, Berkshire, UK). Ultrathin sections were prepared using an ultramicrotome and stained with uranyl acetate and lead citrate for transmission electron microscopy (Hitachi H-7100 Hitachi Co. Ltd., Tokyo, Japan) at 80 kV. G ratios, namely the ratio of the mean inner diameter of an axon to the mean diameter of the fiber (including myelin), were calculated as described (26, 27). Sciatic nerve myelination, de-myelination, and re-myelination were assessed by electron microscopy, as described previously (28).
Statistical Analysis
Statistical analyses were performed using the unpaired two-tailed Student's t test or analysis of variance (*, p < 0.05; **, p < 0.01; ***, p < 0.005; NS, not significant throughout). All data are expressed as the mean ± S.D.
Results
High Glucose Concentrations Induce Schwann Cell De-differentiation
DM patients frequently suffer from peripheral nerve polyneuropathy. Because Schwann cells regulate peripheral nerve function and conduction velocity through myelination, we analyzed changes in Schwann cells ex vivo under high glucose concentrations. To do so, we cultured peripheral sciatic nerves dissected from wild-type mice in the presence of normal (100 mg/dl), moderate (300 mg/dl), or high (540 mg/dl) glucose and then used real time PCR to analyze tissue for expression of mature Schwann cell markers such as MAG, MBP, and P0, as well for a marker of immature cells, P75 (Fig. 1A). Mature Schwann cell markers were significantly down-regulated, whereas P75 expression was significantly up-regulated in a glucose-dependent manner in sciatic nerves (Fig. 1A), suggesting that high glucose conditions promote Schwann cell de-differentiation. Because sciatic nerve tissue is heterogeneous, we cultured primary Schwann cells isolated from rat dorsal root ganglia under varying glucose concentrations, and we observed similar glucose-dependent reduction of MAG protein expression by immunohistochemical analysis (Fig. 1B). We also observed glucose-dependent Schwann cell de-differentiation, as evidenced by reduced MAG, MBP, and P0 expression and concomitant P75 up-regulation in the clonal Schwann cell line IMS32 (Fig. 1C).
FIGURE 1.
High glucose conditions induce Schwann cell de-differentiation. Sciatic nerves dissected from wild-type mice (A), rat primary Schwann cells isolated from rat dorsal root ganglia (B), or cells of the Schwann line IMS32 (C) were cultured in 100 mg/dl (normal), 300 mg/dl (moderate), or 540 mg/dl (high) glucose for 48 h. MAG, MBP, P0, and P75 expression relative to Actb was evaluated by real time PCR (A and C), and MAG expression was analyzed by immunohistochemistry (B). Data represent means ± S.D of (MAG, MBP, P0. or P75)/Actb levels (*, p < 0.05; **, p < 0.01; ***, p < 0.001. NS, not significant; n = 5 wells each). Representative data of at least three independent experiments are shown.
To determine whether Schwann cells undergo de-differentiation in high glucose conditions in vivo, we utilized the STZ-induced mouse model of type I DM (the STZ mouse), as well as db/db mice as a model of type II DM. Significantly higher blood glucose concentrations were evident in both STZ and db/db mice compared with control mice (Fig. 2, A and B). Sciatic nerves were dissected from control, STZ, or db/db mice, and Schwann cell differentiation status was analyzed by real time PCR analysis of tissues (Fig. 2, C and D). STZ and db/db mouse sciatic nerve tissues exhibited a MAGlowMBPlowP0lowP75high gene expression pattern relative to controls, similar to results obtained in in vitro (Fig. 2, C and D), suggesting that hyperglycemia promotes Schwann cell de-differentiation. To confirm this idea, we undertook electron microscopic analysis of db/db versus control mouse nerves. db/db mice showed significant defects in myelination based on G ratio analysis, which is determined by the area of axon and the entire myelinated fiber, as well as increased density of de-myelinated fibers relative to controls (Fig. 2, E–G).
FIGURE 2.
High glucose conditions induce Schwann cell de-differentiation in type I and II DM model mice in vivo. A, blood glucose levels at the indicated ages of STZ-injected (250 mg/kg) mice, which are models of type I DM, were evaluated. B, blood glucose levels at the indicated ages of db/db mice, which are models of type II DM, were evaluated. C and D, sciatic nerves were dissected from STZ mice 4 weeks after STZ injection (C) or from 9-week-old db/db mice (D), and MAG, MBP, P0, and P75 expression relative to Actb was evaluated. NC, negative controls. Data represent means ± S.D. of blood glucose levels (mg/dl) or (MAG, MBP, P0, or p75)/Actb levels (*, p < 0.05; **, p < 0.01; ***, p < 0.001. NS, not significant; n = 5 mice each). E and F, electron microscopy analysis of sciatic nerves from control (upper panel, NC) and db/db (lower panel, DM) mice (E). Myelination was evaluated by determination of the G ratio (F) and de-myelination index, defined as the number of demyelinated fibers per mm2 (G). Representative data of at least two independent experiments are shown.
Schwann Cells Accumulate Sorbitol in High Glucose Conditions
Hyperglycemia activates the polyol pathway and promotes AR-dependent sorbitol accumulation in tissues and cells (14). Indeed, we observed significantly higher sorbitol levels in either sciatic nerves or in Schwann cells cultured in high glucose concentrations compared with samples cultured in normal glucose (Fig. 3A and data not shown), suggesting polyol pathway activation in Schwann cells. Interestingly, sorbitol accumulation in sciatic nerves or Schwann cells cultured in high glucose was blocked by a treatment of tissues or cells with Epalrestat, an AR inhibitor (Fig. 3A and data not shown). To determine whether the polyol pathway activation is required for Schwann cell de-differentiation in high glucose, we undertook similar analysis and examined gene expression patterns (Fig. 3B). De-differentiation of sciatic nerve tissue in high glucose, as evidenced by relatively lower expression of MAG, MBP, and P0 and higher P75 gene expression, was completely abrogated by ARI treatment (Fig. 3B).
FIGURE 3.

ARI or vitamin D treatment antagonizes Schwann cell de-differentiation and induces re-differentiation. A and B, sciatic nerves dissected from wild-type mice were cultured in 100 mg/dl (Normal) or 540 mg/dl (H, High) glucose with or without ARI (1.0 μm), the vitamin D3 agonist ED71 (ED; 0.1 μm) or 1,25(OH)2D3 (D3; 0.1 μm) for 48 h. Intracellular sorbitol levels (A) or MAG, MBP, P0 and P75 expression relative to Actb (B) were analyzed. Data represent means ± S.D. of sorbitol levels (mg/liter) or (MAG, MBP, P0, or P75)/Actb levels (*, p < 0.05; **, p < 0.01; ***, p < 0.001, NS, not significant, relative to culture in high glucose; n = 5 nerves each). C, ARI (1.0 μm) or ED71 (ED; 0.1 μm) was added to IMS32 cell culture in 540 mg/dl glucose from days 0 to 1 (days 0–1) or days 1 to 2 (days 1–2), and MAG, MBP, P0, and P75 expression relative to Actb was analyzed. Data represent means ± S.D. of (MAG, MBP, P0, or p75)/Actb levels (*, p < 0.05; **, p < 0.01; ***, p < 0.001, NS, not significant, relative to culture in high glucose; n = 5 wells each). Representative data of at least three independent experiments are shown.
DM promotes peripheral nerve dysfunction and is a frequent cause of falls (29), although vitamin D3 treatment reportedly plays a role in preventing falls (22). Thus, we hypothesized that Schwann cells were critical targets of DM and that vitamin D3 might regulate their activity. Thus, we next asked whether vitamin D3 treatment would reverse Schwann cell de-differentiation in high glucose (Fig. 3B). To do so, we utilized the active vitamin D3 metabolite 1,25(OH)2D3 or the vitamin D3 agonist ED71. Sciatic nerves or Schwann cells were cultured in high glucose conditions with or without 1,25(OH)2D3, or ED71, and intracellular sorbitol levels and gene expression patterns were analyzed. Although intracellular sorbitol levels were unchanged by either treatment (Fig. 3A, and data not shown), treatment with either 1,25(OH)2D3 or ED71 completely blocked Schwann cell de-differentiation in sciatic nerves cultured in high glucose (Fig. 3B). We confirmed these findings using cultures of IMS32 Schwann cells; IMS32 cell de-differentiation in high glucose was blocked by treatment with either 1,25(OH)2D3 or ED71 (data not shown). To test whether de-differentiation was reversible, we de-differentiated cultured Schwann cells in high glucose and then treated them with ARI or vitamin D3 (Fig. 3C). Schwann cell de-differentiation was evident after 1 day in high glucose, based on the MAGlowMBPlowP0lowP75high gene expression profile (Fig. 3C). However, treatment with either ARI or vitamin D3 at day 1, even in the presence of high glucose, reversed that signature to MAGhighMBPhighP0highP75low (Fig. 3C). Overall, these results indicate that polyol pathway activation is required for Schwann cell de-differentiation in high glucose, and it is blocked by ARI. Moreover, high glucose condition-induced Schwann cell de-differentiation can be blocked or even reversed by either ARI or vitamin D3 treatment.
Sorbitol Accumulation in Sciatic Nerves Accompanies Reduced Nerve Conduction Velocity and De-myelination Seen in DM Model Mice
Next, we assessed potential therapeutic effects of ARI and vitamin D3 in vivo in DM mouse models. Increased sorbitol levels in STZ mouse sciatic nerves were rescued by ARI but not by vitamin D3 treatment, as seen in in vitro and ex vivo analyses (Fig. 4A). However, reduced exercise and sensory capacity characteristics of STZ mice, as analyzed by rota-rod performance (Fig. 4B), and sensory score, analyzed by von Frey test (Fig. 4C), were rescued by either ARI or vitamin D3 administration, as was reduced NCV seen in these mice (Fig. 4D). ARI or vitamin D3 treatment also promoted Schwann cell re-differentiation to a MAGhighMBPhighP0highP75low versus a MAGlowMBPlowP0lowP75high signature, the latter seen in STZ mice (Fig. 4E).
FIGURE 4.
ARI or vitamin D treatment rescues diabetic neuropathy in type I or type II DM model mice in vivo. STZ (A–E) or db/db (F–J) mice were treated with ARI, ED71 (ED), or vehicle for 4 weeks. Subsequently, sorbitol levels in sciatic nerves were analyzed (A and F), and motor or sensory capacity was tested by ROTA-ROD or a DigiGate system (B and G) or von Frey (C and H) tests, respectively. Nerve conduction velocity was also analyzed (D and I). Data represent means ± S.D. of sorbitol (mg/liter, A and F), time on the ROTA-ROD (seconds, B), stride length (relative to WT, G), tactile threshold (seconds, C and H), or NCV (m/s, D and I). E and J, MAG, MBP, P0, and P75 expression relative to Actb was analyzed in sciatic nerves of STZ (E) or db/db (J) mice. Data represent means ± S.D. of (MAG, MBP, P0, or p75)/Actb levels (*, p < 0.05; **, p < 0.01; ***, p < 0.001, NS, not significant; n = 5 nerves each). Electron microscopy analysis of sciatic nerves from control (NC) or db/db mice (DM) treated with or without ARI or ED71 (K). The extent of myelination was measured by G ratio determination (L) and calculation of density of myelinated (M) or de-myelinated (N) fibers. Representative data of at least two independent experiments are shown.
Like STZ mice, db/db mice also showed increased sorbitol levels in sciatic nerves, an outcome rescued by treatment with ARI but not vitamin D3 (Fig. 4F). Both reduced exercise capacity, as assessed by stride length, or down-regulated sensory levels, and NCV evident in db/db mice was rescued by either ARI or vitamin D3 administration (Fig. 4, G–I). Likewise, either ARI or vitamin D3 administration to db/db mice promoted Schwann cell de-differentiation, as evidenced by restoration of MAGhighMBPhighP0highP75low status (Fig. 4J). Consistent with these observations, electron microscopic analysis demonstrated that reduced myelination seen in db/db compared with control mouse sciatic nerve was significantly rescued by ARI or vitamin D3 administration (Fig. 4, K–M). Furthermore, ARI or vitamin D3 treatment promoted significantly greater levels of re-myelination compared with vehicle treatment in db/db mouse nerve fibers (Fig. 4N).
Increased Igf1 Expression Promoted by Vitamin D3 Treatment Enables Schwann Cell Re-differentiation in High Glucose Conditions
Finally, we asked what vitamin D3 targets were responsible for Schwann cell re-differentiation in high glucose conditions, given that sorbitol levels remain high in those circumstances. Igf1 levels are reportedly correlated with vitamin D3 levels and are up-regulated in sera by vitamin D3 treatment (24). Interestingly, we found that expression of Igf1, which encodes a factor that maintains tissue homeostasis (30), was significantly down-regulated in sciatic nerves and clonal Schwann cells under high glucose conditions but that its expression was rescued by either ARI or vitamin D3 treatment (Fig. 5A, and data not shown). Igf1 expression in sciatic nerves was significantly inhibited in both STZ and db/db mice relative to controls but was rescued by ARI or vitamin D3 administration in vivo (Fig. 5, B and C). Furthermore, addition of recombinant Igf1 protein to cultured sciatic nerves or cells was sufficient to promote Schwann cell re-differentiation in high glucose in the absence of ARI and vitamin D3 (Fig. 5D, and data not shown). Interestingly, addition of an Igf1-neutralizing antibody abolished rescue of de-differentiation by vitamin D3 in high glucose conditions in either sciatic nerves or cells (Fig. 5E, and data not shown), as evidenced by maintenance of MAGlowMBPlowP0lowP75high status; however, similar treatment with an Igf1 antibody had no effect on Schwann cell re-differentiation promoted by ARI (Fig. 5E, and data not shown).
FIGURE 5.
ARI or vitamin D treatment rescues suppressed Igf1 expression in Schwann cells grown in high glucose. A, sciatic nerves dissected from wild-type were cultured in 100 (N, normal) or 540 (H, high) mg/dl glucose with or without ARI (1. 0 μm), ED71 (ED, 0.1 μm) or 1,25(OH)2D3 (D3, 0.1 μm) for 48 h, and Igf1 expression relative to Actb was analyzed. B and C, sciatic nerves were dissected from wild-type, STZ (B), or db/db (C) mice treated with or without ARI or ED71 (ED), and Igf1 expression relative to Actb was analyzed. D, sciatic nerves dissected from wild-type mice were cultured in 100 (N, normal) or 540 (H, high) mg/dl glucose with or without Igf1 (10 ng/ml) for 48 h, and MAG, MBP, P0, and P75 expression relative to Actb was analyzed. E, sciatic nerves dissected from wild-type mice were cultured in 100 (N, normal, white bars) or 540 (H, high, black bars) mg/dl glucose with or without ARI (1.0 μm), ED71 (ED; 0.1 μm) or 1,25(OH)2D3 (D3; 0.1 μm) in the presence or absence of Igf1 neutralizing antibody (1.0 μg/ml) for 48 h, and MAG, MBP, P0, and P75 expression relative to Actb was analyzed. F and G, sciatic nerves dissected from wild-type (black bars) or VDR-deficient (white bars) mice were cultured in 100 or 540 mg/dl glucose with or without ARI (1.0 μm), ED71 (ED; 0.1 μm), or 1,25(OH)2D3 (D3; 0.1 μm) for 48 h, and MAG, MBP, P0, and P75 (F) or Igf1 (G) expression relative to Actb was analyzed. Data represent means ± S.D. of (Igf1, MAG, MBP, P0, or p75)/β-actin levels (*, p < 0.05; **, p < 0.01; ***, p < 0.001, NS, not significant, relative to culture in high glucose; n = 5 nerves each). Representative data of at least three independent experiments are shown.
The VDR is required for vitamin D3 biological activity. To determine whether the VDR is required for 1,25(OH)2D3 or ED71 activity in Schwann cells, we utilized VDR-deficient mice (VDR-KO). Sciatic nerves were isolated from wild-type (WT) or VDR-KO mice, cultured in high glucose with or without ARI, 1,25(OH)2D3, or ED71, and assessed for gene expression patterns indicative of Schwann cell differentiation (Fig. 5, F and G). Schwann cell re-differentiation by either 1,25(OH)2D3 or ED71 but not by ARI in high glucose condition was abrogated in VDR-KO sciatic nerves, suggesting that the VDR is required for re-differentiation from a de-differentiated status in high glucose in the presence of vitamin D3 but not ARI (Fig. 5F). Igf1 expression was significantly elevated by treatment with ARI but not vitamin D3 in VDR-deficient Schwann cells (Fig. 5G), suggesting that vitamin D3/VDR activity is required to elevate Igf1 levels and promote Schwann cell re-differentiation in high glucose.
In summary, we report that sorbitol accumulation via AR activity underlies Schwann cell de-differentiation and that reduction of these levels by an ARI can promote Schwann cell re-differentiation, even in high glucose. By contrast, vitamin D3 treatment did not reduce sorbitol levels but rather elevated Igf1 levels via the VDR to promote Schwann cell re-differentiation in high glucose.
Discussion
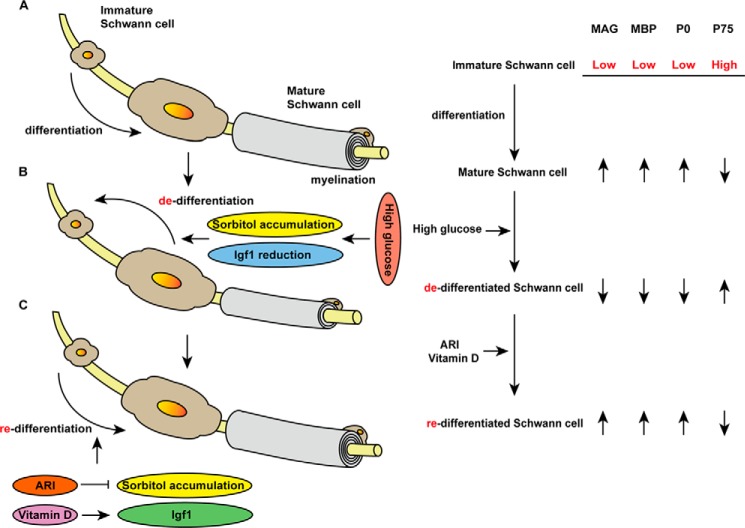
Diabetic neuropathy is one of the three major complications of DM and appears earlier and more frequently than the other two, nephropathy and retinopathy (31). Diabetic neuropathy develops in peripheral nerves and, in most cases, becomes polyneuropathy (32). Polyneuropathy often causes sensory/motor disturbance, dysautonomia, and bathyanesthesia, which in turn promotes poor prognosis and further complications such as gangrene or falls and associated fractures (32). Thus, understanding the pathogenesis of neuropathy in DM patients is necessary to devise effective treatments. Diabetic polyneuropathy reportedly develops due to various factors, including perturbed blood flow, oxidative stress, accumulation of advanced glycation end products, or neuronal damage, making its treatment complex (11, 33). We report here that, at least in part, Schwann cell de-differentiation due to sorbitol accumulation and reduced Igf1 expression under hyperglycemia underlies the type of neurological dysfunction seen in DM patients, and in mice, ARI and vitamin D3 are therapeutically effective in reversing these perturbations and blocking peripheral nerve de-myelination through Schwann cell re-differentiation (Fig. 6).
FIGURE 6.
Schematic model of Schwann cell differentiation, de-differentiation and re-differentiation. A, differentiation of immature (MAGlowMBPlowP0lowP75high) to mature (MAGhighMBPhighP0highP75low) Schwann cells, which myelinate peripheral nerves. B, high glucose conditions promote Schwann cell de-differentiation through sorbitol accumulation and reduced Igf1 expression, leading to peripheral nerve de-myelination. C, ARI or vitamin D3 treatment promotes Schwann cell re-differentiation and peripheral nerve re-myelination. ARI inhibits sorbitol accumulation, although vitamin D3 increases Igf1 expression in Schwann cells.
Schwann cells regulate peripheral nerve function by increasing NCV through myelination (34). Disturbances in this activity are seen in diseases marked peripheral nerve dysfunction, such as Charcot-Marie-Tooth disease (35). Thus understanding the basis of these dysmyelinating diseases is required to develop therapeutic approaches to promote or restore myelination. Previously, Ho et al. (36) reported that oxidative stress occurs in peripheral nerves of DM mice in an AR-dependent manner. Here, we found that Schwann cell myelinating activity is perturbed by high glucose, which activates polyol pathway signaling via AR activity.
Sorbitol accumulation following polyol pathway activation reportedly promotes degeneration and apoptosis of several cell types in high glucose conditions (37). In our study, hyperglycemia promoted sorbitol accumulation followed by Schwann cell de-differentiation, which also occurs following peripheral nerve injury.
Modulation of two different pathways, either sorbitol accumulation (using ARI) or Igf1-down-regulation (using vitamin D3), produced identical effects on Schwann cell de-differentiation. Blocking sorbitol accumulation antagonized this process, even in the presence of an Igf1-neutralizing antibody, suggesting that sorbitol accumulation is the primary driver of Schwann cell de-differentiation in this context. By contrast, vitamin D3 promoted Schwann cell re-differentiation in high glucose without reducing sorbitol levels, and Igf1 was required for that rescue. These results suggest that sorbitol accumulation triggers Schwann cell de-differentiation and that sorbitol down-regulation can antagonize this process. Meanwhile, sorbitol-induced Schwann cell de-differentiation can be rescued by elevated Igf1 levels, even if sorbitol levels remain unchanged, and thus Igf1 is crucial for Schwann cell re-differentiation under high sorbitol conditions. Reagents that up-regulate Igf1, such as vitamin D3, could be therapeutically beneficial to block sorbitol-induced Schwann cell de-differentiation. At present, there are no reports of regulation of Igf1 expression by sorbitol in any cells. We hypothesize that Igf1 levels are low in immature Schwann cells but up-regulated upon their differentiation; thus, Schwann cell re-differentiation by ARI may correlate with high Igf1 expression. Levels of circulating Igf1 are up-regulated by vitamin D3 (24), strongly suggesting that D3 promotes Igf1 expression in Schwann cells as well. Further studies are needed to confirm this possibility. Vitamin D3 treatment reportedly decreases osteoporotic fractures (38); however, administration of an active form of vitamin D3 does not increase bone mineral density in osteoporosis patients (39), and some investigators propose that vitamin D3 treatment prevents fractures by decreasing the likelihood of falls (22). Our study indicates that Schwann cells are vitamin D3 target cells and that their subsequent re-differentiation occurs through Igf1 up-regulation. Igf1 is reportedly produced in liver following growth hormone stimulation (23); however, our data demonstrate that local Igf1 production in Schwann cells is also effective in maintaining homeostasis.
In conclusion, we show that ARI or vitamin D3 administration improves nerve function in DM mouse models. However, advanced diabetic polyneuropathy is reportedly refractory to treatment and is irreversible in patients (5). Our work suggests that, because diabetic polyneuropathy develops relatively earlier than other complications, early treatment with ARI and vitamin D3 might antagonize neurological disorders and prevent the complications that follow.
Acknowledgments
IMS32 cells were provided by Dr. Kazuhiko Watabe (Tokyo Metropolitan Institute for Neuroscience), and VDR KO mice were provided by Dr. Shigeaki Kato (Soma Central Hospital).
This work was supported by a grant-in-aid for scientific research. The authors declare that they have no conflicts of interest with the contents of this article.
- DM
- diabetes mellitus
- NCV
- nerve function and conduction velocity
- STZ
- streptozotocin
- MBP
- myelin basic protein
- MAG
- myelin-associated glycoprotein
- AR
- aldose reductase
- ARI
- AR inhibitor
- VDR
- vitamin D receptor.
References
- 1. Alberti K. G., Zimmet P. Z. (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Med. 15, 539–553 [DOI] [PubMed] [Google Scholar]
- 2. NIDDK (2011) National Diabetes Statistics. NIH Publication 11-3892, National Diabetes Information Clearinghouse, Bethesda [Google Scholar]
- 3. Fowler M. J. (2008) Microvascular and macrovascular complications of diabetes. Clin. Diabet. 26, 77–82 [Google Scholar]
- 4. Boulton A. J., Gries F. A., Jervell J. A. (1998) Guidelines for the diagnosis and outpatient management of diabetic peripheral neuropathy. Diabet. Med. 15, 508–514 [DOI] [PubMed] [Google Scholar]
- 5. Said G. (2007) Diabetic neuropathy—a review. Nat. Clin. Pract. Neurol. 3, 331–340 [DOI] [PubMed] [Google Scholar]
- 6. Greene D. A., Sima A. A., Stevens M. J., Feldman E. L., Lattimer S. A. (1992) Complications: neuropathy, pathogenetic considerations. Diabetes Care 15, 1902–1925 [DOI] [PubMed] [Google Scholar]
- 7. Sato A., Sato Y., Suzuki H. (1985) Aging effects on conduction velocities of myelinated and unmyelinated fibers of peripheral nerves. Neurosci. Lett. 53, 15–20 [DOI] [PubMed] [Google Scholar]
- 8. Felitsyn N., Stacpoole P. W., Notterpek L. (2007) Dichloroacetate causes reversible demyelination in vitro: potential mechanism for its neuropathic effect. J. Neurochem. 100, 429–436 [DOI] [PubMed] [Google Scholar]
- 9. Lee H. K., Shin Y. K., Jung J., Seo S. Y., Baek S. Y., Park H. T. (2009) Proteasome inhibition suppresses Schwann cell dedifferentiation in vitro and in vivo. GLIA 57, 1825–1834 [DOI] [PubMed] [Google Scholar]
- 10. Rambhade S., Chakraborty A. K., Patil1 U. K., Rambhade A. (2010) Diabetes mellitus–its complications, factors influencing complications, and prevention–an overview. J. Chem. Pharm. Res. 2, 7–25 [Google Scholar]
- 11. Peppa M., Vlassara H. (2005) Advanced glycation end products and diabetic complications: a general overview. Hormones 4, 28–37 [DOI] [PubMed] [Google Scholar]
- 12. Busik J. V., Mohr S., Grant M. B. (2008) Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes 57, 1952–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kahn S. E., Prigeon R. L., McCulloch D. K., Boyko E. J., Bergman R. N., Schwartz M. W., Neifing J. L., Ward W. K., Beard J. C., Palmer J. P. (1994) The contribution of insulin-dependent and insulin-independent glucose uptake to intravenous glucose tolerance in healthy human subjects. Diabetes 43, 587–592 [DOI] [PubMed] [Google Scholar]
- 14. Brownlee M. (2005) The pathobiology of diabetic complications a unifying mechanism. Diabetes 54, 1615–1625 [DOI] [PubMed] [Google Scholar]
- 15. Chung S. S., Ho E. C., Lam K. S., Chung S. K. (2003) Contribution of polyol pathway to diabetes-induced oxidative stress. J. Am. Soc. Nephrol. 14, S233–S236 [DOI] [PubMed] [Google Scholar]
- 16. Kikkawa R., Umemura K., Haneda M., Arimura T., Ebata K., Shigeta Y. (1987) Evidence for existence of polyol pathway in cultured rat mesangial cells. Diabetes 36, 240–243 [DOI] [PubMed] [Google Scholar]
- 17. Berrone E., Beltramo E., Solimine C., Ape A. U., Porta M. (2006) Regulation of intracellular glucose and polyol pathway by thiamine and benfotiamine in vascular cells cultured in high glucose. J. Biol. Chem. 281, 9307–9313 [DOI] [PubMed] [Google Scholar]
- 18. Sango K., Suzuki T., Yanagisawa H., Takaku S., Hirooka H., Tamura M., Watabe K. (2006) High glucose-induced activation of the polyol pathway and changes of gene expression profiles in immortalized adult mouse Schwann cells IMS32. J. Neurochem. 98, 446–458 [DOI] [PubMed] [Google Scholar]
- 19. Christakos S., Dhawan P., Porta A., Mady L. J., Seth T. (2011) Vitamin D and intestinal calcium absorption. Mol. Cell. Endocrinol. 347, 25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dawson-Hughes B., Harris S. S., Finneran S. (1995) Calcium absorption on high and low calcium intakes in relation to vitamin D receptor genotype. J. Clin. Endocrinol. Metab. 80, 3657–3661 [DOI] [PubMed] [Google Scholar]
- 21. Sakuma M., Endo N., Hagino H., Harada A., Matsui Y., Nakano T., Nakamura K. (2011) Serum 25-hydroxyvitamin D status in hip and spine-fracture patients in Japan. J. Orthop. Sci. 16, 418–423 [DOI] [PubMed] [Google Scholar]
- 22. Bischoff-Ferrari H. A., Dawson-Hughes B., Willett W. C., Staehelin H. B., Bazemore M. G., Zee R. Y., Wong J. B. (2004) Effect of vitamin D on falls. JAMA 291, 1999–2006 [DOI] [PubMed] [Google Scholar]
- 23. Le Roith D. (1997) Insulin-like growth factors. N. Engl. J. Med. 336, 633–640 [DOI] [PubMed] [Google Scholar]
- 24. Ameri P., Giusti A., Boschetti M., Bovio M., Teti C., Leoncini G., Ferone D., Murialdo G., Minuto F. (2013) Vitamin D increases circulating IGF1 in adults: potential implication for the treatment of GH deficiency. Eur. J. Endocrinol. 169, 767–772 [DOI] [PubMed] [Google Scholar]
- 25. Tashiro S., Shinozaki M., Mukaino M., Renault-Mihara F., Toyama Y., Liu M., Nakamura M., Okano H. (2014) BDNF induced by treadmill training contributes to the suppression of spasticity and allodynia after spinal cord injury via up-regulation of KCC2. Neurorehabil. Neural Repair 1–13 [DOI] [PubMed] [Google Scholar]
- 26. Porrello E., Rivellini C., Dina G., Triolo D., Del Carro U., Ungaro D., Panattoni M., Feltri M. L., Wrabetz L., Pardi R., Quattrini A., Previtali S. C. (2014) Jab1 regulates Schwann cell proliferation and axonal sorting through p27. J. Exp. Med. 211, 29–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. da Silva T. F., Eira J., Lopes A. T., Malheiro A. R., Sousa V., Luoma A., Avila R. L., Wanders R. J., Just W. W., Kirschner D. A., Sousa M. M., Brites P. (2014) Peripheral nervous system plasmalogens regulate Schwann cell differentiation and myelination. J. Clin. Invest. 124, 2560–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu G. S., Shi J. Y., Lai C. L., Hong Y. R., Shin S. J., Huang H. T., Lam H. C., Wen Z. H., Hsu K. S., Chen C. H., Howng S. L., Tai M. H. (2009) Peripheral gene transfer of glial cell-derived neurotrophic factor ameliorates neuropathic deficits in diabetic rats. Hum. Gene Ther. 20, 715–727 [DOI] [PubMed] [Google Scholar]
- 29. Kachroo S., Kawabata H., Colilla S., Shi L., Zhao Y., Mukherjee J., Iloeje U., Fonseca V. (2015) Association between hypoglycemia and fall-related events in type 2 diabetes mellitus: analysis of a United States Commercial Database. J. Manag. Care Spec. Pharm. 21, 243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sharp L. L., Jameson J. M., Cauvi G., Havran W. L. (2005) Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat. Immunol. 6, 73–79 [DOI] [PubMed] [Google Scholar]
- 31. Galuppo M., Giacoppo S., Bramanti P., Mazzon E. (2014) Use of natural compounds in the management of diabetic peripheral neuropathy. Molecules 19, 2877–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vinik A. I., Holland M. T., Le Beau J. M., Liuzzi F. J., Stansberry K. B., Colen L. B. (1992) Diabetic neuropathies. Diabetes Care 15, 1926–1975 [DOI] [PubMed] [Google Scholar]
- 33. Clements R. S. (1979) Diabetic neuropathy—new concepts of its etiology. Diabetes 28, 604–611 [DOI] [PubMed] [Google Scholar]
- 34. Frostick S. P., Yin Q., Kemp G. J. (1998) Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery 18, 397–405 [DOI] [PubMed] [Google Scholar]
- 35. Krajewski K. M., Lewis R. A., Fuerst D. R., Turansky C., Hinderer S. R., Garbern J., Kamholz J., Shy M. E. (2000) Neurological dysfunction and axonal degeneration in Charcot-Marie-Tooth disease type 1A. Brain 123, 1516–1527 [DOI] [PubMed] [Google Scholar]
- 36. Ho E. C., Lam K. S., Chen Y. S., Yip J. C., Arvindakshan M., Yamagishi S., Yagihashi S., Oates P. J., Ellery C. A., Chung S. S., Chung S. K. (2006) Aldose reductase-deficient mice are protected from delayed motor nerve conduction velocity, increased c-Jun NH2-terminal kinase activation, depletion of reduced glutathione, increased superoxide accumulation, and DNA damage. Diabetes 55, 1946–1953 [DOI] [PubMed] [Google Scholar]
- 37. Takamura Y., Tomomatsu T., Kubo E., Tsuzuki S., Akagi Y. (2008) Role of the polyol pathway in high glucose-induced apoptosis of retinal pericytes and proliferation of endothelial cells. Invest. Ophthalmol. Vis. Sci. 49, 3216–3223 [DOI] [PubMed] [Google Scholar]
- 38. Chapuy M. C., Arlot M. E., Duboeuf F., Brun J., Crouzet B., Arnaud S., Delmas P. D., Meunier P. J. (1992) Vitamin D3 and calcium to prevent hip fractures in elderly women. N. Engl. J. Med. 327, 1637–1642 [DOI] [PubMed] [Google Scholar]
- 39. Christiansen C., Christensen M. S., Rødbro P., Hagen C., Transbøl I. (1981) Effect of 1.25-dihydroxyvitamin D3 in itself or combined with hormone treatment in preventing postmenopausal osteoporosis. Eur. J. Clin. Invest. 11, 305–309 [DOI] [PubMed] [Google Scholar]