Background: FLU negatively regulates glutamyl-tRNA reductase (GluTR) during chlorophyll biosynthesis.
Results: The structures of the TPR domain of FLU in its uncomplexed form and the GluTR dimeric domain-bound form were solved.
Conclusion: The non-canonical TPR domain of FLU mediates complex formation with GluTR.
Significance: The work enables a delineation of the spatial regulation of GluTR.
Keywords: Arabidopsis thaliana, biosynthesis, chlorophyll, crystal structure, protein-protein interaction, Fluorescent (FLU), chlorophyll biosynthesis, tetratricopeptide repeat (TPR)
Abstract
The tetratricopeptide repeat (TPR)-containing protein FLU is a negative regulator of chlorophyll biosynthesis in plants. It directly interacts through its TPR domain with glutamyl-tRNA reductase (GluTR), the rate-limiting enzyme in the formation of δ-aminolevulinic acid (ALA). Delineation of how FLU binds to GluTR is important for understanding the molecular basis for FLU-mediated repression of synthesis of ALA, the universal tetrapyrrole precursor. Here, we characterize the FLU-GluTR interaction by solving the crystal structures of the uncomplexed TPR domain of FLU (FLUTPR) at 1.45-Å resolution and the complex of the dimeric domain of GluTR bound to FLUTPR at 2.4-Å resolution. Three non-canonical TPR motifs of each FLUTPR form a concave surface and clamp the helix bundle in the C-terminal dimeric domain of GluTR. We demonstrate that a 2:2 FLUTPR-GluTR complex is the functional unit for FLU-mediated GluTR regulation and suggest that the formation of the FLU-GluTR complex prevents glutamyl-tRNA, the GluTR substrate, from binding with this enzyme. These results also provide insights into the spatial regulation of ALA synthesis by the membrane-located FLU protein.
Introduction
Fluorescent (FLU),3 a plastid membrane protein, directly interacts with glutamyl-tRNA reductase (GluTR), the initial enzyme in plant tetrapyrrole biosynthetic pathway (1, 2). The mature FLU protein is about 27 kDa and contains an N-terminal hydrophobic region that may form a transmembrane structure, a coiled-coil domain, and a C-terminal tetratricopeptide repeat (TPR) domain. Amino acid sequence analysis predicted that the C-terminal TPR domains of Arabidopsis FLU and Chlamydomonas FLU-like protein consist of two TPR motifs (1, 3), whereas barley Tigrina d protein, an ortholog of Arabidopsis FLU, consists of three TPR motifs (4). Mutations in the TPR domain, such as A262V in Arabidopsis FLU (flu1-1) and frameshift at Ala249 in barley Tigrina d (tigrina d), cause defect in the regulation of chlorophyll biosynthesis (1, 4, 5). The flu and tigrina d mutants lose their ability to suppress protochlorophyllide (PChlide) accumulation upon a dark/light shift and resemble phenotypes similar to wild-type plants fed with exogenous δ-aminolevulinic acid (ALA). GluTR is the rate-limiting enzyme for endogenous ALA production (6), and hence the physical interaction between FLU and GluTR suggests an inhibitory role of FLU on GluTR activity (2). Indeed, an up-regulation of ALA synthesis was observed for the flu1-1 mutant under continuous light (7). In a yeast two-hybrid system, it was shown that the C-terminal 43 residues of GluTR were required for interacting with the TPR domain of FLU (7). However, in the crystal structures of GluTR, instead of being exposed, this C-terminal region packs inside the cleft of the V-shaped GluTR dimer (8, 9). Therefore, the FLU-GluTR interaction needs to be characterized at the atomic level.
As a hub for the post-transcriptional regulation of tetrapyrrole biosynthesis, GluTR is controlled by multiple mechanisms besides the above mentioned FLU-mediated down-regulation (10, 11). Metabolic feedback inhibition is a common strategy to repress GluTR activity, although how the tetrapyrrole metabolites, including heme and, particularly interestingly, PChlide (12, 13), affect GluTR awaits to be elucidated. An immunoprecipitation/mass spectrometry study has identified a chloroplast membrane complex containing FLU and several key enzymes devoted to chlorophyll synthesis (14). This complex is proposed to interact with GluTR upon accumulation of fluorescent PChlide. Although the above model explains how FLU mediates GluTR activity, the spatial organization of such a membrane complex is unclear. A delineation of the FLU-GluTR interaction should provide a structural insight into how FLU exerts its regulatory role in this complex.
Here we describe the 1.45 Å structure of TPR domain of FLU (FLUTPR) consisting of three non-canonical TPR motifs. To crystalize a complex containing the components for direct FLU-GluTR interaction, we constructed GluTR C-terminal truncations that preserve dimerization ability but with different polypeptide size and successfully obtained a 2.4 Å structure of the dimeric domain of GluTR (GluTRDD) in complex with FLUTPR. The FLUTPR-GluTRDD complex has a dissociation constant similar to that of FLUTPR with the full-length GluTR, which indicates that this complex structure can represent the FLU-GluTR interaction adequately. Together with an enzymatic ALA synthesis assay, these findings allow an elucidation of the molecular basis for FLU-mediated GluTR regulation.
Experimental Procedures
Protein Expression and Purification
The PCR-amplified cDNA fragments containing the TPR domain of Arabidopsis FLU (At3g14110) and the dimeric domain of Arabidopsis GluTR (At1g58290) were inserted into expression vector pET-22b(+) (Novagen). Recombinant N-His6-tagged FLUTPR and GluTRDD were overexpressed in Escherichia coli BL21(DE3) and purified to homogeneity by nickel affinity column (Ni2+-nitrilotriacetic acid, Qiagen) and size-exclusion chromatography (GE Healthcare). For preparation of the FLUTPR-GluTRDD complex, pET-22b(+) vectors containing N-His6-tagged FLUTPR and the non-tagged form of GluTRDD were co-transferred into E. coli BL21(DE3) cells. The purification procedure was the same as described above. Protein purity was monitored by SDS-PAGE. Proteins for crystallization were concentrated to ∼12 mg ml−1 in buffer containing 20 mm Tris-HCl, 100 mm NaCl, 5% glycerol, and 1 mm DTT (pH 7.5) and then stored at −80 °C.
Isothermal Titration Calorimetry
Experiments were performed on a MicroCal iTC200 calorimeter (GE Healthcare). The purified proteins for ITC measurement were in buffer containing 200 mm NaCl, 20 mm Tris-HCl, 5% glycerol, and 1 mm DTT (pH 7.5) and were degassed before titration. Each titration series consisted of 20 injections of 2 μl of FLUTPR into 200 μl of GluTR or GluTRDD. Control experiments were carried out by injecting FLUTPR into the buffer, and the resulting heat of dilution was subtracted from the binding isotherm data. The data were analyzed with the Origin software (OriginLab) using a single-site binding model.
Crystallization and Structure Determination
Crystals of FLUTPR and FLUTPR-GluTRDD were grown by the hanging-drop vapor diffusion method. Crystals of FLUTPR were formed within 1 week in 0.2 m NaCl, 0.1 m Bis-Tris (pH 6.5), and 25% (w/v) polyethylene glycol 3350. Crystals of FLUTPR-GluTRDD were formed over 3 weeks in 0.15 m KBr and 30% (w/v) polyethylene glycol monomethyl ether 2000. Prior to data collection, crystals were cryo-protected in the above crystallization solution supplemented with 20% (v/v) glycerol and flash-frozen in liquid nitrogen.
X-ray diffraction data were collected at beamline BL17U of the Shanghai Synchrotron Radiation Facility (SSRF) at a wavelength of 0.9793 Å at 100 K and then processed using HKL2000 (HKL Research). The model of FLUTPR was determined by molecular replacement using the program Phaser (15), and the search model was the TPR domain of the mouse Leu-Gly-Asn repeat-enriched protein (16) (Protein Data Bank (PDB) code 3RO3). The resulting model was rebuilt using ARP/wARP (17). Manual correction was performed in Coot (18) according to the 2Fo − Fc and Fo − Fc electron density maps, and further refinement was done with phenix.refine (19). The structure of the FLUTPR-GluTRDD complex was determined by molecular replacement using the FLUTPR structure as the search model, and the dimeric domain of GluTR was fitted into the density manually and then refined as described above. The overall quality of the final structural models was assessed by MolProbity (20) with 99.2 and 0.8% in favored and additional allowed regions for the FLUTPR structure, and 99.5 and 0.5% in favored and disallowed regions for the FLUTPR-GluTRDD complex structure (see Table 1). The protein structure figures were prepared using the program PyMOL (31).
TABLE 1.
Data collection and structure refinement statistics
Values in parentheses are for the data in the highest-resolution shell.
| FLUTPR | FLUTPR-GluTRDD | |
|---|---|---|
| Data collection | ||
| Wavelength (Å) | 0.9793 | 0.9793 |
| Space group | P3121 | P6522 |
| Cell dimensions | ||
| a, b, c (Å) | 60.2, 60.2, 67.5 | 74.7, 74.7, 161.7 |
| α, β, γ (°) | 90, 90, 120 | 90, 90, 120 |
| Resolution (Å) | 50 – 1.45 (1.50 – 1.45) | 50 – 2.4 (2.49 – 2.40) |
| Rsym or Rmerge | 0.091 (0.292) | 0.106 (0.756) |
| I/σI | 24.9 (5.5) | 49.8 (5.4) |
| Completeness (%) | 98.0 (89.2) | 100 (100) |
| Redundancy | 10.7 (5.8) | 33.7 (34.9) |
| Unique reflections | 25,072 (2247) | 11,105 (1063) |
| Refinement | ||
| Resolution (Å) | 26.1 – 1.45 (1.51 – 1.45) | 34.28 – 2.40 (2.64 – 2.10) |
| No. of reflections | 24,973 | 11,051 |
| Rwork/Rfree | 0.138/0.178 | 0.208/0.243 |
| No. of atoms | ||
| Protein | 987 | 1624 |
| Water | 152 | 66 |
| Mean B-factors (Å2) | 13.5 | 47.8 |
| r.m.s.d.a | ||
| Bond lengths (Å) | 0.008 | 0.004 |
| Bond angles (°) | 1.097 | 0.675 |
| PDB code | 4YVO | 4YVQ |
a r.m.s.d., root mean square deviation.
ALA Synthesis Assay
The assay was performed as described previously (9), except that an equal volume of buffer was replaced by FLUTPR for measurement with FLUTPR. The molar ratio of FLUTPR:GluTR was kept as 1.2:1. The reaction time was 60 min.
Results
Crystal Structure of FLUTPR
The final model of the recombinant FLUTPR contains 119 residues (Pro198–Asp316) and is refined to 1.45 Å resolution (Table 1). FLUTPR consists of three TPR motifs (TPR1-TPR3) and forms dimer through TPR3 (Fig. 1A). When compared with the prototype of TPR motif (21), these three FLU TPR motifs are composed of 40 residues rather than the canonical 34 residues (Fig. 1B). The three non-canonical TPR motifs are structurally conserved and can be well superimposed (Fig. 1C). The flu1-1 mutation (A262V) occurs on the absolutely conserved alanine of TPR2, and the tigrina d mutation, a frameshift at Ala249 (corresponding to Ala246 of FLU), results in loss of TPR2 and TPR3. The structure of FLUTPR clarifies the discrepancy among the predicted TPR domains from different species (1, 3, 4).
FIGURE 1.
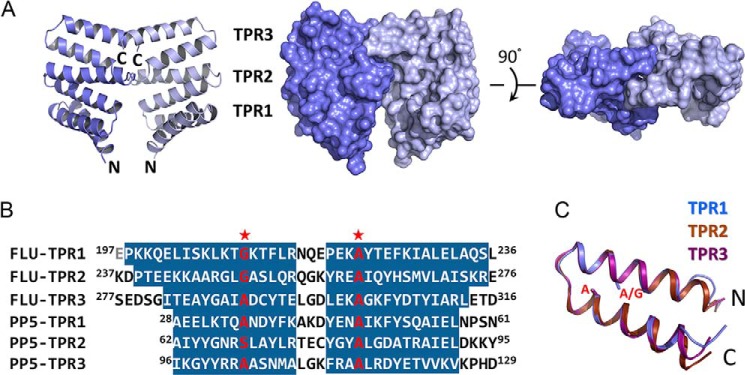
Structure of FLUTPR at 1. 45 Å. A, left, a ribbon representation showing overall structure of FLUTPR dimer. One protomer is colored indigo, and the other is light blue. Middle and right, two perpendicular views in surface representation. B, alignment of TPR motifs from FLU and protein phosphatase 5 (PP5). Residues in α-helix are highlighted. Glu197 is colored gray because it is not observed in the FLUTPR structure. Red stars indicate the strictly conserved residues in TPR motif. C, superimposition of the three TPR motifs. The individual motifs are color-coded. Side chains of residues at the two strictly conserved positions are shown as sticks.
The FLUTPR-GluTRDD Complex
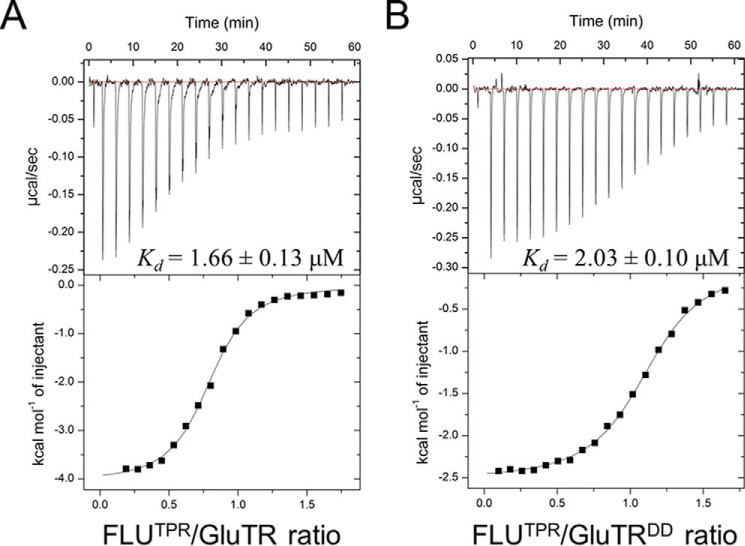
FLU directly interacts through its TPR domain with GluTR (2). To gain a structural insight into the FLU-GluTR interaction, we purified the full-length GluTR in complex with FLUTPR (Fig. 2A). However, the FLUTPR-GluTR complex failed to crystallize. We speculated that the two relatively flexible arms of the V-shaped GluTR dimer could prevent crystal formation. Thus, GluTR truncations that constitute only the stem of the V shape were constructed, and a fragment corresponding to the C-terminal 104 residues (Leu440–Lys543) was able to form stable complex with FLUTPR (Fig. 2B). The functional integrity of GluTRDD was tested by comparison of its binding affinity to FLUTPR with that of the full-length GluTR (Fig. 3). The Kd value, as measured by isothermal titration calorimetry, is 2.03 ± 0.10 μm for FLUTPR and GluTRDD, similar to that for FLUTPR and GluTR (Kd = 1.66 ± 0.13 μm), indicating that GluTRDD maintains binding affinity to FLUTPR.
FIGURE 2.
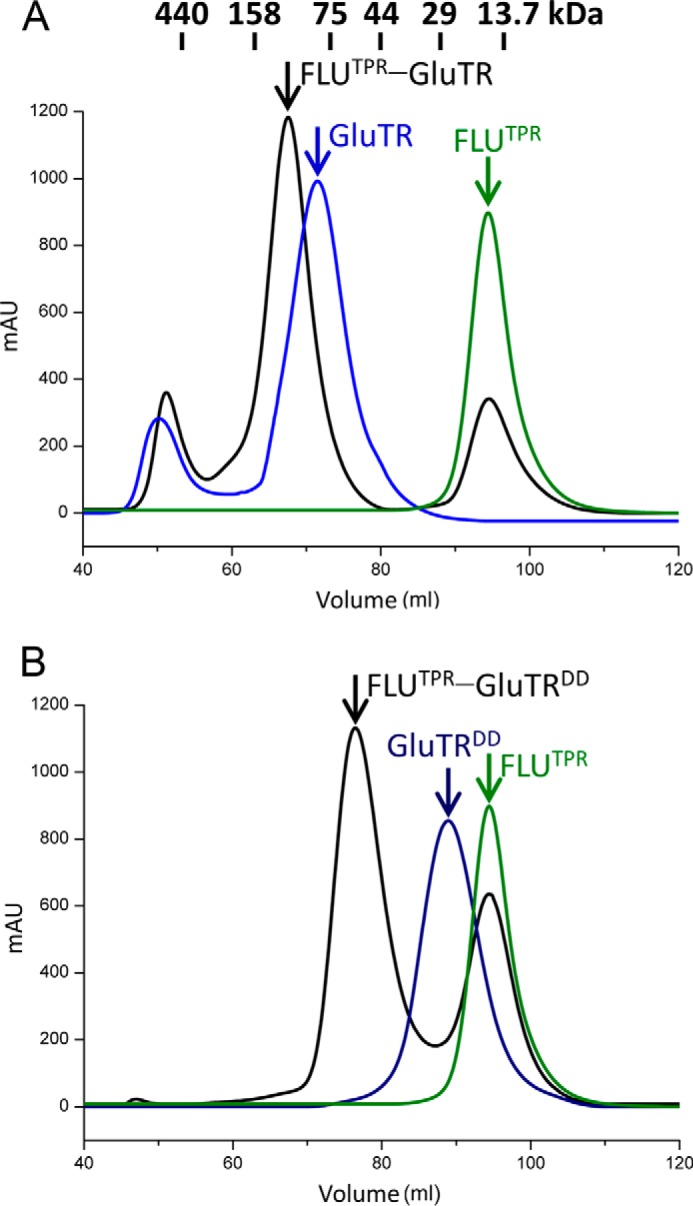
Size-exclusion chromatography profiles on a HiLoad 16/60 Superdex 200 column. A, elution profiles of FLUTPR, GluTR, and mixture of FLUTPR and GluTR. B, elution profiles of FLUTPR, GluTRDD, and mixture of FLUTPR and GluTRDD. mAU, milli-absorbance units.
FIGURE 3.
ITC analysis of the interaction between FLUTPR and GluTR. A, titration of full-length GluTR with FLUTPR. The top panel shows the heat response to injections, and the bottom panel shows the integrated heats of each injection (■) and the fit (−) to a single-site binding model. B, titration of GluTRDD with FLUTPR.
The crystal structure of the FLUTPR-GluTRDD complex was solved at 2.4 Å resolution (Table 1). The 2:2 complex resembles two hands holding up a cup (Fig. 4), with two FLUTPR molecules clamping the GluTRDD dimer through their concave face. Because the concave face is a conventional site for TPR-mediated protein-protein interaction (22), the complex structure shows that despite its non-canonical TPR motifs, FLU uses a canonical binding mode for GluTR recognition. Because the GluTRDD structure starts from the middle of a long α-helix (Fig. 5), we attempted to crystalize GluTR truncations including this whole α-helix but did not succeed. Notably, this long α-helix could undergo a wobbling motion (8, 9), which suggests that the compact GluTRDD structure reported here is likely the stable core for the GluTR dimer.
FIGURE 4.
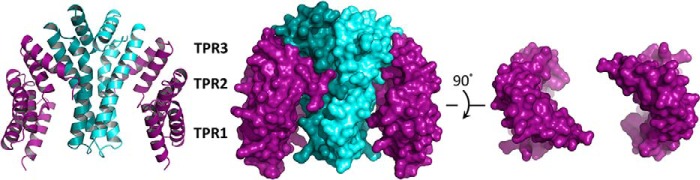
Structure of the FLUTPR-GluTRDD complex at 2.4 Å. Left, overall structure in ribbon representation; Middle, overall structure in surface representation. Two FLUTPR molecules are colored purple; one GluTRDD protomer is colored teal, and the other is in cyan. Right, FLUTPR structure in surface representation with GluTRDD not shown.
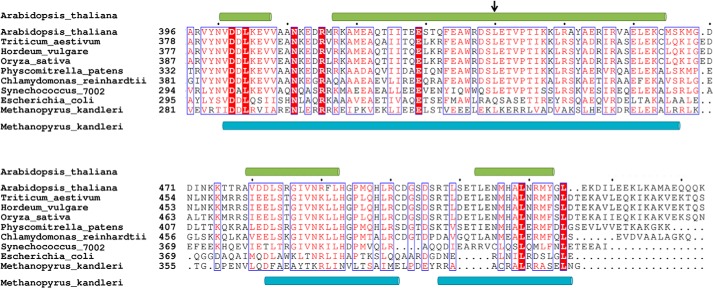
FIGURE 5.
Multiple sequence alignment of the C-terminal regions of GluTRs. Identical amino acid residues are boxed in red, and similar residues are printed in red in a blue box. Dots indicate gaps introduced during alignment. The secondary structure elements, as observed in the GluTR structures from Arabidopsis thaliana and Methanopyrus kandleri, are shown on the top and the bottom of the aligned sequences, respectively. The N-terminal end of GluTRDD in the FLUTPR-GluTRDD structure is indicated by an arrow.
The FLUTPR-GluTRDD Interaction
The FLUTPR-GluTRDD complex structure reveals how FLU recognizes GluTR without significant conformational changes. When the structures of FLUTPR in its free and GluTRDD-bound forms are superimposed (Fig. 6A), the root mean square deviation of backbone Cα atoms is only 0.79 Å. Rigidity is a common property for repeat proteins including TPR proteins (23). Interestingly, detailed analysis of the FLUTPR-GluTRDD structure demonstrates that the TPR2 of FLUTPR does not participate in direct protein-protein interaction (Fig. 6B). This differs from the usual concave binding pocket formed by TPR proteins (24). Only TPR1 and TPR3 are involved in GluTR binding, and the FLUTPR-GluTRDD interactions include five salt bridges: Arg215–Glu462, Glu284–Arg485, Asp291–Arg450, Glu295–Lys447, and Asp307–Arg500, and a hydrogen bond between Tyr309 and Arg450 (Fig. 6B). In a yeast two-hybrid assay, deletion of the C-terminal 43 residues of GluTR (Cys501–Lys543) abolished interaction with FLU (7). The FLU-GluTR interaction, as represented by the FLUTPR-GluTRDD structure, does not require this C-terminal region, which is instead involved in GluTR dimerization. Deletion of this region would disrupt the GluTR dimer, suggesting that a 2:2 complex is the functional unit for FLU-mediated GluTR regulation.
FIGURE 6.
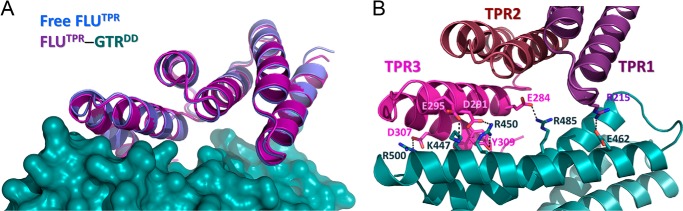
The FLUTPR-GluTRDD interaction. A, superimposition of FLUTPR in its free and GluTRDD-bound forms. GluTRDD is in surface representation. B, detailed FLUTPR-GluTRDD interactions. The individual TPR motifs are color-coded. Side chains of residues forming intermolecular salt bridge and hydrogen bond are shown as sticks. Salt bridge and hydrogen bond are shown as dashed lines.
FLUTPR Down-regulates GluTR Activity
GluTR is the rate-limiting enzyme for ALA formation (6). The inhibitory role of FLU on GluTR activity has been suggested physiologically in the flu mutant (1, 7). To experimentally address the effect of FLU on GluTR activity, we measured ALA synthesis by an in vitro coupled enzyme assay (Fig. 7A). The rate of ALA formation decreased significantly in the presence of FLUTPR when compared with the rate in the absence of FLUTPR. FLUTPR also repressed ALA formation when GluTR-binding protein (GluBP), a positive regulator of GluTR, was added to the assay. These results show that FLU and GluBP have antagonizing effects on GluTR activity and function independently from each other. Indeed, FLU exclusively binds to the C-terminal dimeric domain, whereas GluBP binds to the N-terminal catalytic domain of GluTR (9). Notably, the surface area of GluTR that is shielded by FLUTPR is mainly composed of positively charged residues (Fig. 6B). Therefore, it is likely that FLU may prevent the negatively charged tRNA from binding to GluTR due to stereo clash (Fig. 7B).
FIGURE 7.
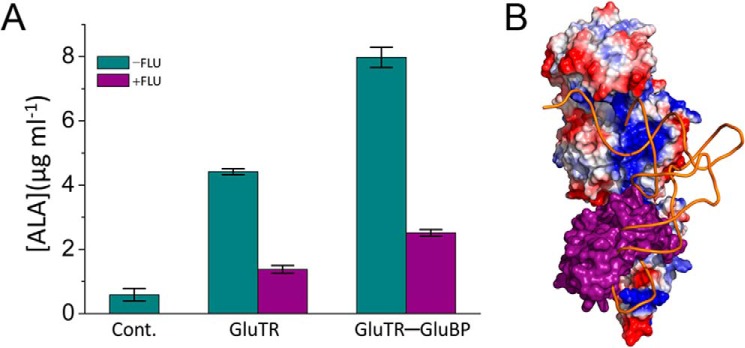
FLUTPR inhibits GluTR activity. A, in vitro ALA synthesis assay. Data are presented as the mean ± S.E. of three independent experiments. B, predicted clash between tRNA and FLUTPR. GluTR is represented as electrostatic potential surface, FLUTPR is represented as purple surface, and tRNA is represented as an orange tube. Cont., control.
Discussion
The biosynthesis of tetrapyrroles is vital for plant growth and development (10, 11). Its biosynthetic pathway starts with a universal tetrapyrrole precursor, ALA, and branches into heme and chlorophyll pathways after the formation of protoporphyrin IX. The heme pathway requires only one further step, chelation with a ferrous iron, whereas the chlorophyll pathway needs several hallmark steps: chelation with a magnesium ion, formation of the fifth ring, light-dependent reduction of the fourth ring, and attachment of a phytol tail. FLU has been implicated in the formation of the universal precursor ALA and of PChlide, the product of the only light-dependent reaction in the chlorophyll pathway (1–5).
The non-canonical TPR motifs of FLU make them difficult to predict in previous studies (1, 3). The 1.45 Å FLUTPR and 2.4 Å FLUTPR-GluTRDD structures reported here demonstrate how this three-repeat TPR domain recognizes the dimeric domain of the V-shaped GluTR dimer. The FLU-GluTR interaction, as represented by the FLUTPR-GluTRDD complex structure, indicates that the 2:2 complex is the functional unit for FLU-mediated GluTR regulation. In addition, the inhibitory effect of FLU on GluTR activity is confirmed by the in vitro ALA synthesis assay.
FLU has been identified as a negative regulator of chlorophyll biosynthesis over the past decades (1, 5). Furthermore, Arabidopsis flu mutant (or tigrina d of barley) has become a strategy to study the singlet oxygen-mediated signaling pathway in plants (25, 26). This strategy utilizes accumulation of the photosensitizer PChlide in the flu plants upon a dark/light shift. In the wild-type plants, PChlide does not accumulate because of FLU repression of GluTR. However, the link between PChlide accumulation and FLU-mediated GluTR inhibition remains unclear despite extensive research on PChlide-generated singlet oxygen responses (27). Based on an immunoprecipitation/mass spectrometry study, a model incorporating enzymes in the chlorophyll biosynthetic pathway was proposed in which magnesium protoporphyrin IX monomethyl ester cyclase and PChlide oxidoreductase form a complex with FLU. This FLU-containing complex would interact with GluTR upon accumulation of fluorescent PChlide, and thus inhibit ALA synthesis (14). Given the small size of FLU (27 kDa for mature protein) and its membrane-anchoring ability, to unravel its bridging role between membrane and GluTR regulation requires detailed information. The 2:2 FLU-GluTR complex model (Fig. 8) should provide a structural clue of how FLU acts as a bridge between GluTR and other chlorophyll synthesis enzymes. It is possible that the coiled-coil domain of FLU participates in binding of the other enzymes because the coiled coils are a protein motif involved in recognition between proteins (28).
FIGURE 8.
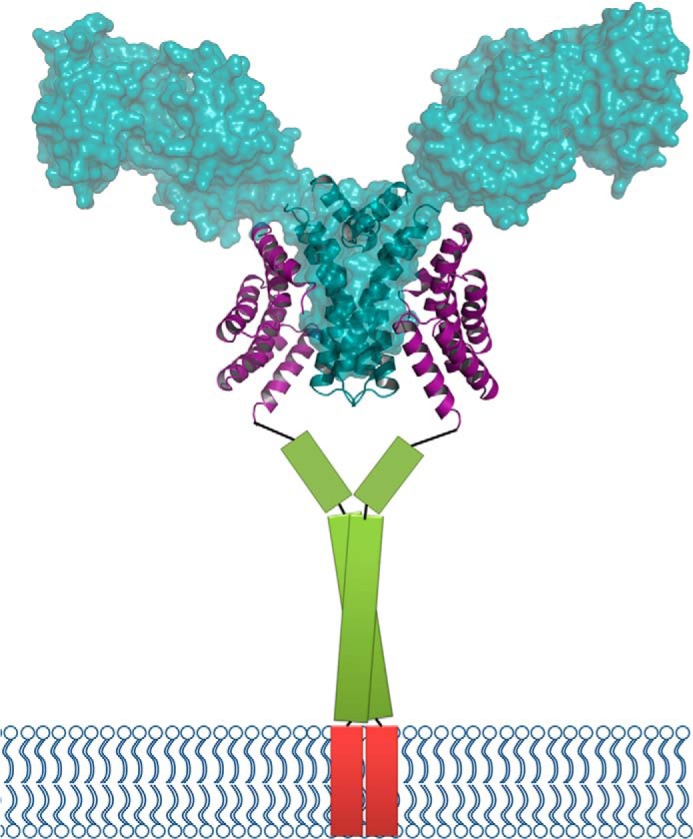
Model of the FLU-GluTR complex. The FLUTPR-GluTRDD complex in ribbon representation is overlaid with the transparent surface of GluTR (PDB code 4N7R, chains A and B). The predicted coiled-coil domain of FLU is shown as a green column, and the transmembrane domain is shown as a red column.
Spatial regulation is a recently recognized strategy for GluTR (29). The way GluTR is anchored to the plastid membrane through FLU has been predicted (30). Based on the two states of FLUTPR (Figs. 1A and Fig. 4) and the inhibitory effect of FLUTPR on GluTR activity (Fig. 7A), the working model proposed by Kauss et al. (14) is further developed here. In this scenario, the FLU-containing complex senses PChlide accumulation and switches FLU from a resting (Fig. 1A) to an active (Fig. 4) state. The active FLU pulls GluTR from the stroma to the proximity of plastid membrane, where binding of the substrate glutamyl-tRNA is inhibited.
Author Contributions
M. Z. and L. L. designed the research; M. Z., F. Z., Y. F., X. C., Y. C., and W. Z. performed experiments; M. Z., F. Z., and L. L. analyzed the data; H. D., R. L, and L. L. wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank Ming-Zhu Wang at the Institute of Biophysics, Chinese Academy of Sciences, and the Shanghai Synchrotron Radiation Facility beamline scientists for technical support during data collection.
This work was supported by the National Natural Science Foundation of China Grant 31370759, the National Basic Research Program of China Grant 2011CBA00901, the Key Research Program (KGZD-EW-T05), and the Hundred Talents Program of the Chinese Academy of Sciences. The authors declare that they have no conflicts of interest with the contents of this article.
The atomic coordinates and structure factors (codes 4YVO and 4YVQ) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- FLU
- Fluorescent
- GluTR
- glutamyl-tRNA reductase
- TPR
- tetratricopeptide repeat
- ALA
- δ-aminolevulinic acid
- PChlide
- protochlorophyllide
- GluBP
- GluTR-binding protein
- Bis-Tris
- 2-(bis(2-hydroxyethyl)amino)-2-(hydroxymethyl)propane-1,3-diol
- DD
- dimeric domain.
References
- 1. Meskauskiene R., Nater M., Goslings D., Kessler F., op den Camp R., Apel K. (2001) FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 98, 12826–12831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meskauskiene R., Apel K. (2002) Interaction of FLU, a negative regulator of tetrapyrrole biosynthesis, with the glutamyl-tRNA reductase requires the tetratricopeptide repeat domain of FLU. FEBS Lett. 532, 27–30 [DOI] [PubMed] [Google Scholar]
- 3. Falciatore A., Merendino L., Barneche F., Ceol M., Meskauskiene R., Apel K., Rochaix J. D. (2005) The FLP proteins act as regulators of chlorophyll synthesis in response to light and plastid signals in Chlamydomonas. Genes Dev. 19, 176–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee K. P., Kim C., Lee D. W., Apel K. (2003) TIGRINA d, required for regulating the biosynthesis of tetrapyrroles in barley, is an ortholog of the FLU gene of Arabidopsis thaliana. FEBS Lett. 553, 119–124 [DOI] [PubMed] [Google Scholar]
- 5. von Wettstein D. V., Kahn A., Nielsen O. F., Gough S. (1974) Genetic regulation of chlorophyll synthesis analyzed with mutants in barley. Science 184, 800–802 [DOI] [PubMed] [Google Scholar]
- 6. Kumar A. M., Söll D. (2000) Antisense HEMA1 RNA expression inhibits heme and chlorophyll biosynthesis in Arabidopsis. Plant Physiol. 122, 49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goslings D., Meskauskiene R., Kim C., Lee K. P., Nater M., Apel K. (2004) Concurrent interactions of heme and FLU with Glu tRNA reductase (HEMA1), the target of metabolic feedback inhibition of tetrapyrrole biosynthesis, in dark- and light-grown Arabidopsis plants. Plant J. 40, 957–967 [DOI] [PubMed] [Google Scholar]
- 8. Moser J., Schubert W. D., Beier V., Bringemeier I., Jahn D., Heinz D. W. (2001) V-shaped structure of glutamyl-tRNA reductase, the first enzyme of tRNA-dependent tetrapyrrole biosynthesis. EMBO J. 20, 6583–6590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao A., Fang Y., Chen X., Zhao S., Dong W., Lin Y., Gong W., Liu L. (2014) Crystal structure of Arabidopsis glutamyl-tRNA reductase in complex with its stimulator protein. Proc. Natl. Acad. Sci. U.S.A. 111, 6630–6635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanaka R., Tanaka A. (2007) Tetrapyrrole biosynthesis in higher plants. Annu. Rev. Plant Biol. 58, 321–346 [DOI] [PubMed] [Google Scholar]
- 11. Mochizuki N., Tanaka R., Grimm B., Masuda T., Moulin M., Smith A. G., Tanaka A., Terry M. J. (2010) The cell biology of tetrapyrroles: a life and death struggle. Trends Plant Sci. 15, 488–498 [DOI] [PubMed] [Google Scholar]
- 12. Vothknecht U. C., Kannangara C. G., von Wettstein D. (1998) Barley glutamyl tRNAGlu reductase: mutations affecting haem inhibition and enzyme activity. Phytochemistry 47, 513–519 [DOI] [PubMed] [Google Scholar]
- 13. Richter A., Peter E., Pörs Y., Lorenzen S., Grimm B., Czarnecki O. (2010) Rapid dark repression of 5-aminolevulinic acid synthesis in green barley leaves. Plant Cell Physiol. 51, 670–681 [DOI] [PubMed] [Google Scholar]
- 14. Kauss D., Bischof S., Steiner S., Apel K., Meskauskiene R. (2012) FLU, a negative feedback regulator of tetrapyrrole biosynthesis, is physically linked to the final steps of the Mg++-branch of this pathway. FEBS Lett. 586, 211–216 [DOI] [PubMed] [Google Scholar]
- 15. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhu J., Wen W., Zheng Z., Shang Y., Wei Z., Xiao Z., Pan Z., Du Q., Wang W., Zhang M. (2011) LGN/mInsc and LGN/NuMA complex structures suggest distinct functions in asymmetric cell division for the Par3/mInsc/LGN and Gαi/LGN/NuMA pathways. Mol. Cell 43, 418–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perrakis A., Harkiolaki M., Wilson K. S., Lamzin V. S. (2001) ARP/wARP and molecular replacement. Acta Crystallogr. D 57, 1445–1450 [DOI] [PubMed] [Google Scholar]
- 18. Emsley P., Lohkamp B., Scott W. G., Cowtan K. (2010) Features and development of Coot. Acta Crystallogr. D 66, 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Afonine P. V., Grosse-Kunstleve R. W., Echols N., Headd J. J., Moriarty N. W., Mustyakimov M., Terwilliger T. C., Urzhumtsev A., Zwart P. H., Adams P. D. (2012) Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D 68, 352–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen V. B., Arendall W. B., 3rd, Headd J. J., Keedy D. A., Immormino R. M., Kapral G. J., Murray L. W., Richardson J. S., Richardson D. C. (2010) MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Das A. K., Cohen P. W., Barford D. (1998) The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein-protein interactions. EMBO J. 17, 1192–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D'Andrea L. D., Regan L. (2003) TPR proteins: the versatile helix. Trends Biochem. Sci. 28, 655–662 [DOI] [PubMed] [Google Scholar]
- 23. Grove T. Z., Cortajarena A. L., Regan L. (2008) Ligand binding by repeat proteins: natural and designed. Curr. Opin. Struct. Biol. 18, 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zeytuni N., Zarivach R. (2012) Structural and functional discussion of the tetra-trico-peptide repeat, a protein interaction module. Structure 20, 397–405 [DOI] [PubMed] [Google Scholar]
- 25. op den Camp R. G., Przybyla D., Ochsenbein C., Laloi C., Kim C., Danon A., Wagner D., Hideg E., Göbel C., Feussner I., Nater M., Apel K. (2003) Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15, 2320–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khandal D., Samol I., Buhr F., Pollmann S., Schmidt H., Clemens S., Reinbothe S., Reinbothe C. (2009) Singlet oxygen-dependent translational control in the tigrina-d.12 mutant of barley. Proc. Natl. Acad. Sci. U.S.A. 106, 13112–13117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim C., Apel K. (2013) Singlet oxygen-mediated signaling in plants: moving from flu to wild type reveals an increasing complexity. Photosynth. Res. 116, 455–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burkhard P., Stetefeld J., Strelkov S. V. (2001) Coiled coils: a highly versatile protein folding motif. Trends Cell Biol. 11, 82–88 [DOI] [PubMed] [Google Scholar]
- 29. Czarnecki O., Hedtke B., Melzer M., Rothbart M., Richter A., Schröter Y., Pfannschmidt T., Grimm B. (2011) An Arabidopsis GluTR binding protein mediates spatial separation of 5-aminolevulinic acid synthesis in chloroplasts. Plant Cell 23, 4476–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Czarnecki O., Grimm B. (2013) New insights in the topology of the biosynthesis of 5-aminolevulinic acid. Plant Signal. Behav. 8, e23124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DeLano W. L. (2010) The PyMOL Molecular Graphics System, version 1.3r1, Schrödinger, LLC, New York [Google Scholar]