Background: The mechanism of ligand mediated ERα N-terminal transactivation function (AF-1) regulation is unclear.
Results: Disruption of ERα C-terminal transactivation function (AF-2) resulted in reversal of antagonists to AF-1-dependent agonists.
Conclusions: ERα AF-2 contains AF-1 repression activity.
Significance: This function may explain partial agonist/antagonist activity of selected estrogen receptor modulators.
Keywords: estrogen, estrogen receptor, protein structure, steroid hormone, steroid hormone receptor, agonist, antagonist, selected estrogen receptor modulators
Abstract
ERα has a ligand-dependent transactivation function in the ligand binding domain of ERα C terminus (AF-2) and a ligand-independent activation function in the N terminus (AF-1). It is still not fully understood how AF-1 and AF-2 activities are regulated cooperatively by ligands. To evaluate the AF-1 involvement in the estrogenic activities of various compounds, we analyzed these transactivation functions using AF-1-truncated and AF-2-mutated ERα mutants. AF-2 is composed of two domains with flexible and static regions. We used an AF-2 flexible region mutant and an AF-2 static region mutant. Both mutants have been reported as non-E2 responsive due to disruption of E2-mediated coactivator recruitment to the AF-2. The AF-2 mutants were not activated by agonists, but surprisingly antagonists and selective estrogen receptor modulators (SERMs) activated the AF-2 mutants. This antagonist reversal activity was derived from AF-1. Furthermore, we demonstrated that the AF-2 contains an AF-1 suppression function using C-terminal-truncated ERα mutants. From these findings we hypothesized that the mutation of AF-2 disrupted its ability to suppress AF-1, causing the antagonist reversal. To assess the AF-2-mediated AF-1 suppression, we analyzed the transcription activity of physically separated AF-1 and AF-2 using a novel hybrid reporter assay. We observed that the AF-1 activity was not suppressed by the physically separated AF-2. Furthermore, SERMs did not induce the AF-1-mediated activity from the separated mutant AF-2, which differed from the intact protein. These results imply that SERM activity is dependent on a conformational change of the full-length ERα molecule, which allows for AF-1 activation.
Introduction
Estrogen has various physiological activities, and estrogen receptor (ER)2 is a key regulator for those actions (1). ERα is a ligand-dependent transcription factor that belongs to the nuclear receptor superfamily (2, 3). ERα possesses two transactivation function (AF) domains, AF-1 and AF-2. These are located in the N terminus and C terminus of the ER protein, respectively. AF-2 is a well characterized ligand-dependent transcriptional activation domain that is localized in the ligand binding domain (LBD) of ERα. Various man-made chemicals and natural compounds termed xenoestrogens or endocrine-disrupting chemicals (EDCs) have been screened as estrogenic compounds based on their binding affinity to the ERα LBD (4–6). Xenoestrogens have diverse chemical structures distinct from the steroid hormone structure (Fig. 1). On the other hand, various estrogen receptor antagonists and selective estrogen receptor modulators (SERMs) have been developed by analyzing the derivatives of certain estrogenic compounds (7, 8). Although the chemical structures are different from steroidal estrogen, crystallographic analysis indicated that these compounds bind to ERα LBD to regulate transcription activity. The LBD consists of 12 helices (H), and the H3, H4, and H12 are involved in the ligand-dependent transactivation domain, AF-2. Using the evidence from crystallographic analyses, the H3 and H4 are designated as the static region of AF-2 and H12 as the flexible region of AF-2 (9). The ligand binding is believed to change the conformation of the LBD and configuration of the flexible region, H12, to induce a transcriptionally active or inactive form of the receptor (3, 9). When agonists bind to the LBD, H12 in cooperation with H3 and H4 form a co-activator binding surface. When antagonists bind to the LBD, H12 is relocated, thereby preventing the co-activator binding and disrupting AF-2-mediated transcription activity. Several ligands induce different H12 positioning from agonist or antagonist to display partial agonist/antagonist activity of those compounds (10, 11). Even though this observation is regarded as a mechanism of partial agonist/antagonist activity, it is still unclear whether this differential H12 positioning in AF-2 cooperates with other domains such as AF-1 to produce the partial agonist/antagonist activity.
FIGURE 1.
Chemical structures. Chemical structures are illustrated. The chemicals are categorized in estrogen, mycotoxin, phytoestrogens, EDCs, SERMs, and antagonist. GSK1648229 and GSK1648230 are enantiomers with undetermined absolute configuration of trans-cyclopropanes.
The N-terminal activation function, AF-1, consists of a constitutive transactivation function that is based on the experimental evidence that truncation of the LBD from the ERα protein induces higher basal transcription activity compared with the full-length ERα without ligand (12). It is known that SERMs possess partial agonist/antagonist activity for ERα and that partial agonist activity is derived from AF-1 but not AF-2-mediated activity. This AF-1-derived transcription activity is dependent upon the gene promoter context and is cell type-specific (12, 13). It is believed that such AF-1 characteristics lead to the tissue-selective action of SERMs. However, there is minimal understanding about the molecular mechanisms of SERM-mediated AF-1 regulation to fully explain the partial agonist/antagonist activity that regulates ERα-mediated transcription and the spectrum of biological responsiveness.
We have previously reported the generation of an AF-2 flexible region mutant mouse model (AF2ERKI) consisting of alanine replacement of leucines at 543 and 544 in H12 of the LBD (14, 15). In the AF2ERKI mice, estradiol (E2) does not induce any estrogen responsive functions even though AF-2-mutated ERα protein is expressed in the tissues and resulted in an identical phenotype to ERα null mutant mice (αERKO), supporting a major role of AF-2 in E2-mediated responses in some tissues. One of the interesting characteristics of the AF2ER mutant receptor is an antagonist reversal activity, where antagonists such as 4-hydroxytamoxifen (4OHT) and fulvestrant/ICI182780 (ICI) activate the AF2ER mutant receptor through the activation of AF-1 in a similar manner to SERM-mediated ERα activation (14, 16). The antagonist reversal through AF-1 was tissue-selective as it was observed in certain tissues of AF2ERKI mice but not all tissues (14, 15), suggesting that the AF-1 activity is different in the various tissues. Recently, we determined that the antagonist reversal activity of AF2ER is caused by antagonist-dependent AF2ER-LBD dimerization associated with DNA binding activity (16). Nevertheless, it is still unclear what constitutes the molecular mechanism of the antagonist-mediated AF-1 activation through the AF-2-mutated ERα.
To further probe the molecular mechanism of AF-2-mediated AF-1 regulation, we first evaluated the AF-1 involvement in agonist and antagonist activities of estrogenic compounds through the full-length ERα as contrasted with the activities through AF-1-deleted ERα (121-ERα). Second, we examined the functional characteristics of AF-2 on the ligand-dependent AF-1 regulation. We used the AF2ER (mERα-L543A,L544A) as an AF-2 flexible region mutant and another mutant mERα-I362D, which contains a mutation of isoleucine 362 to aspartic acid, as an AF-2 static region mutant. The mERα-I362D has been reported as a non-E2-responsive mutant due to disruption of E2-mediated p160/SRC1 recruitment to AF-2 the same as AF2ER (17). Both AF-2 flexible and static region mutants induced antagonist reversal through AF-1-mediated activity. From these observations we hypothesized that the AF-2 domain may contain an AF-1 suppression activity. Third, to test our hypothesis that AF-2 mediates AF-1 regulation, we used various C-terminal truncated ERα mutants to identify the AF-1 controlling domain in LBD/AF-2. Furthermore, to analyze the functional connection between AF-1 and AF-2, we developed a novel hybrid reporter assay. From these analyses we concluded that AF-2 is composed of a bifunctional transcription regulation domain, which exhibits the property of transactivation and AF-1 suppression functions. Moreover, the activity of AF-2-dependent AF-1 suppression appears to require a conformational change of full-length ERα protein. These observations provide further insights into the molecular mechanisms of partial agonist/antagonist activity of SERMs and potentially EDCs to explain their unique tissue-selective activities.
Experimental Procedures
Chemicals and Plasmid Constructions
Chemicals used in this study are listed in Table 1. The following plasmids have been described previously: pcDNA3-mERa, pcDNA3 plasmid containing full-length mouse ERα (mERα1–599); pcDNA3-121-ERa, pcDNA3 plasmid containing N-terminal 120 amino acid-truncated mouse ERα (mERα121–599); pcDNA3-AF2ER, pcDNA3 plasmid containing L543A,L544A-mutated full-length mouse ERα (mERα1–599, L543A,L544A); pcDNA3-121-AF2ER, pcDNA3 plasmid containing N-terminal 120 amino acid-truncated L543A,L544A-mutated mouse ERα (mERα121-599, L543A,L544A); pcDNA3-mERa339, pcDNA3 plasmid containing 1–339 amino acids of mouse ERα with an extension of 10 extra amino acids (GPYSIVSPKC) in the C terminus derived from pcDNA3 sequence (14). To generate the plasmid pcDNA3-mERa384, pcDNA3-mERa was digested by XhoI and then pcDNA3-mERa_XhoI fragment was self-ligated. pcDNA3-mERa384 expressing 1–384 amino acids of mouse ERα with an extension of 14 extra amino acids (HASRGPYSIVSPKC) in the C terminus derived from pcDNA3 sequence. The plasmids pcDNA3-mERa-I362D and pcDNA3-AF2ER-I362D were generated by PCR-based site-directed mutagenesis, and the following oligo DNAs were used for the mutagenesis: I362D_S, 5′-AGA TAG GGA GCT GGT TCA TAT GGA CAA CTG GGC AAA GAG AG-3′; I362D_AS, 5′-CTC TCT TTG CCC AGT TGT CCA TAT GAA CCA GCT CCC TAT CT-3′. PCR was performed using the Pfu Turbo DNA polymerase, a pair of sense (S) and antisense (AS) oligo DNAs, and the plasmid pGEM3Zf-mERaWT_SmaI (the SmaI fragment from pcDNA3-mERa was subcloned into the SmaI site of pGEM3Zf+ vector) as a template following the manufacturer's instructions (Agilent Technologies). A mutated clone was confirmed by sequencing (NIEHS (NIH) Sequencing Laboratory). The NotI and XhoI fragment from pGEM3Zf-mERa-I362D_SmaI was subcloned into the NotI and XhoI sites of pcDNA3-mERa and generated pcDNA3-mERa-I362D_XhoI (it is identical to pcDNA3-mERa382-I362D). The XhoI fragments from pcDNA3-mERa or pcDNA3-AF2ER were subcloned into the XhoI site of pcDNA3-mERa-I362D_XhoI, and the direction of the inserted fragment was determined by NotI digestion. The structures of ERα WT and mutants are diagramed in Fig. 2A. The expression level of recombinant proteins is shown in Fig. 2B. The pGL3–3xERE-TATA-Int-Luc reporter plasmid contained three repeats of vitellogenin estrogen-responsive element (ERE); 3xERE-Luc (14) and the C3-T1-Luc reporter plasmid containing the luciferase reporter gene fused with the −1030 to +58 region of human complement 3 (C3) gene (18) were used for luciferase assay. The plasmid pRL-TK renilla luciferase expression plasmid (Promega) was used for the internal control. 2x(ERE-m17)-TATA-Luc reporter plasmid was generated by the following steps. The DNA fragment (ERE-m17_S, 5′-CAG GTC ACT GTG ACC TGC GGC CGC GGA GTA CAG TCC TCC GCC TTA CGC GTG-3′; ERE-m17_AS, 5′-CTA GCA CGC GTA AGG CGG AGG ACT GTA CTC CGC GGC CGC AGG TCA CAG TGA CCT GAG CT-3′) was inserted in the SacI and NehI sites of pGL3-TK plasmid (gifted from Dr. Sueyoshi at NIEHS) to generate pGL3-(ERE-m17)-tk-Luc plasmid. Next, the DNA fragment (X-m17-ERE-N_S, 5′-TCG AGC GGA GTA CAG TCC TCC GCG GCC GCA GGT CAC AGT GAC CTG-3′; X-m17-ERE-N_AS, 5′-CTA GCA GGT CAC TGT GAC CTG CGG CCG CGG AGG ACT GTA CTC CGC-3′) was inserted in the NehI and XhoI sites of pGL3-(ERE-m17)-tk-Luc to generate pGL3–2x(ERE-m17)-tk-Luc plasmid. Lastly, the BglII and KpnI fragment from pGL3–2x(ERE-m17)-tk-Luc was subcloned into the BglII and KpnI sites of pGL3-Basic-TATA-Int-Luc to create pGL3–2x(ERE-m17)-TATA-Int-Luc plasmid, 2x(ERE-m17)-TATA-Luc. Plasmids used for mammalian two-hybrid assay are as follows. The plasmid pACT (Promega) was used for the prey, the plasmid pBIND (Promega) was used for the bait, and the plasmid pG5-Luc (Promega) was used for GAL4 binding element reporter gene. The plasmids pACT-LBD/WT and pBIND-LBD/WT have been described previously (16). The plasmids pACT-LBD/AF2ER-I362D and pBIND-LBD/AF2ER-I362D were generated by PCR-based site-directed mutagenesis with the same set of oligo DNAs as described (I362D_S and I362D_AS), and PCR was performed using the plasmid pCR2.1-mE/F(AF2) (16) as a template. Mutated clones were confirmed by sequencing then subcloned into the pACT or pBIND plasmids.
TABLE 1.
The list of chemicals that used in this report
NTP, National Toxicology Program; GSK, GlaxoSmithKline; APAC, APAC Pharmaceutical, LLC; o,p′-DDT, o,p′-dichloro-diphenyl-trichloroethane.
| Chemicals | Category | Source |
|---|---|---|
| Apigenin | Phytoestrogen | NTP |
| Bazedoxifene | SERM | APAC |
| Bisphenol A | EDC | NTP |
| Bisphenol AF | EDC | NTP |
| 1-Bromopropane | EDC | NTP |
| Clomifene | SERM | GSK |
| Coumestrol | Phytoestrogen | NTP |
| Daidzein | Phytoestrogen | NTP |
| o,p'-DDT | EDC | NTP |
| p,p'-DDT | EDC | NTP |
| DBAC | SERM | GSK |
| DES | EDC | Sigma |
| Endosulfan | EDC | NTP |
| Endoxifen | SERM | Sigma |
| E2 | Estrogen | Sigma |
| ICI | Antagonist | Tocris Bioscience |
| Genistein | Phytoestrogen | NTP |
| GW-5638 | SERM | GSK |
| GW-7604 | SERM | GSK |
| 4OHT | SERM | Sigma |
| HPTE | EDC | NTP |
| Idoxifene | SERM | GSK |
| Kaemphenol | Phytoestrogen | NTP |
| Kepone | EDC | NTP |
| Lasofoxifene | SERM | GSK |
| 4-n-Nonylphenol | EDC | NTP |
| Ormeloxifene | SERM | GSK |
| Ospemifene | SERM | GSK |
| Raloxifene | SERM | Tocris bioscience |
| Toremifene | SERM | GSK |
| Triclosan | EDC | NTP |
| Zearalenone | Mycotoxin | Sigma |
| GSK205566 | Novel compound | GSK |
| GSK228794 | Novel compound | GSK |
| GSK269504 | Novel compound | GSK |
| GSK270999 | Novel compound | GSK |
| GSK1648229 | Novel compound | GSK |
| GSK1648230 | Novel compound | GSK |
FIGURE 2.
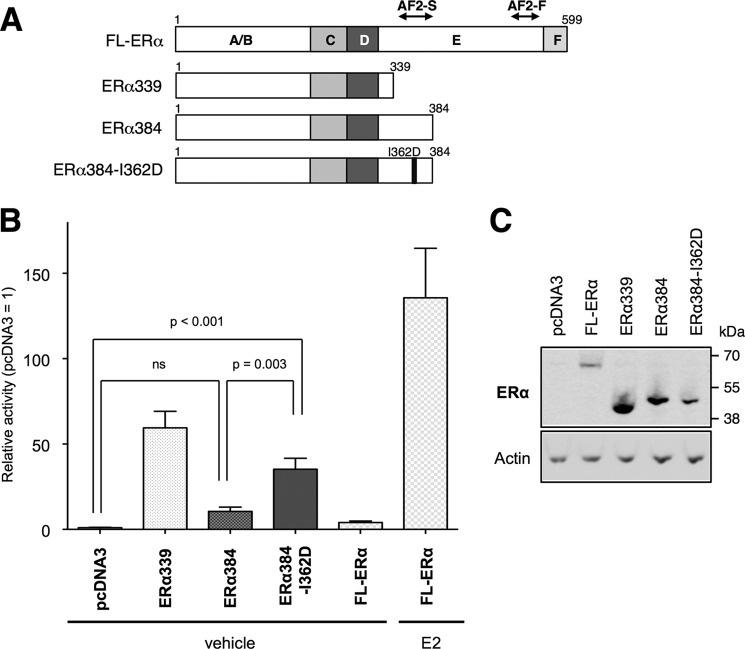
Schematic structure of mERα WT and mutants. A, ERα consists of six domains named A to F (FL-ERα). A/B-domain possesses AF-1 activity (AF1). E-domain possesses ligand-dependent transcription activation domain (AF-2). AF-2 is composed of static region (AF2-S) and flexible region (AF2-F). 121-ERα, the mutant with entire AF-1 domain deletion from FL-ERα; AF2ER, leucines 543 and 544 in AF2-F were mutated to alanines; mERα-I362D, isoleucine 362 in AF2-S was mutated to aspartic acid; AF2ER-I362D, the combined mutation of AF2ER and I362D; ERα339, the mutant with entire AF-2 truncation; ERα384, the mutant with AF2-F truncation but retains AF2-S; ERα384-I362D, ERα384 contains AF2-S mutation (I362D). B, whole cell lysates extracted from the plasmid-transfected HepG2 cells were analyzed by immunoblotting with anti-ERα antibody (MC-20 and H-184) to demonstrate expression levels of ERα WT and mutants. β-Actin was used as a loading control (Actin). * suggests nonspecific signals. A representative Western blot analysis is shown.
Cell Culture and Transfection Condition for Luciferase Assay
HepG2 cells (human hepatocellular carcinoma) were cultured in phenol red-free minimum essential media (Life Technologies) supplemented with 10% FBS (Gemini-Bio) and 1% penicillin-streptomycin (Sigma). For transient transfections, the cells were cultured in phenol red-free medium supplemented with 10% charcoal-stripped FBS (Gemini-Bio) and seeded in 48-well plates at a density of 1.2 × 105 cells/well. The cells were transfected with the following DNA mixture for 6 h using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. For reporter assays, a DNA mixture containing 50 ng of expression plasmids for WT or mutated ERα, 100 ng of reporter plasmids for 3xERE-Luc or C3-T1-Luc, and 100 ng of renilla luciferase expression plasmid pRL-TK was transfected in each well. For hybrid reporter assay, the DNA mixture contained 100 ng of expression plasmids for Gal4 DBD fusion proteins (pBIND) and 50 ng of expression plasmids for 121-ERα339 or ERα339, and 100 ng of 2x(ERE-m17)-TATA-Luc reporter plasmid was transfected in each well. For the mammalian two-hybrid assay, the DNA mixture contained 50 ng of expression plasmids for GAL4 DBD fusion proteins (pBIND) and 50 ng of expression plasmids for VP16 activation domain fusion proteins (pACT), and 100 ng of pG5-Luc reporter plasmid was transfected in each well. pBIND vector (Promega) contained renilla luciferase expression units for transfection normalization.
Luciferase Assay
The cells were cultured in fresh medium supplemented with the chemicals 6 h after transfections. Luciferase and renilla luciferase activities were assayed 18 h after treatments using the Dual-luciferase Reporter Assay System (Promega). Luciferase activity was normalized for transfection efficiency using renilla luciferase as an internal control. All results are representative of at least two independent experiments and represent the mean ± S.E. of triplicate samples.
Western Blot Analysis
The transfected cells on 24-well plates were washed with warm PBS, and 50 μl of 2× Laemmli sample buffer near 100 °C was then added to the wells. The cells were pipetted vigorously and then put into a 1.5-ml centrifuge tube in a heat block at 100 °C. The tubes were heated for 10 min, cooled on ice, and stored at −20 °C until samples were analyzed on SDS-PAGE. Proteins were resolved by SDS-PAGE and subsequently transferred to nitrocellulose membranes. Blots were incubated overnight in 4 °C with primary antibody for ERα (1:650; MC-20; Santa Cruz Biotechnology or 1:350; H-184; Santa Cruz Biotechnology) or β-actin (1:1500; AC-74; Sigma). The blots were washed then incubated with IRDye 800CW-conjugated anti-rabbit antibody (LI-COR Biosciences) for ERα or with IRDye 680RD-conjugated anti-mouse antibody (LI-COR Biosciences) for β-actin. The signals were visualized by the Odyssey infrared imaging system (LI-COR Biosciences).
Statistical Analysis
Statistical analyses were performed with one-way ANOVA or two-way ANOVA as described in each figure legend by GraphPad Prism software. p < 0.05 was considered statistically significant.
Results
Characterization of Estrogenic Xenochemical Activity on Full-length and AF-1-deleted ERα
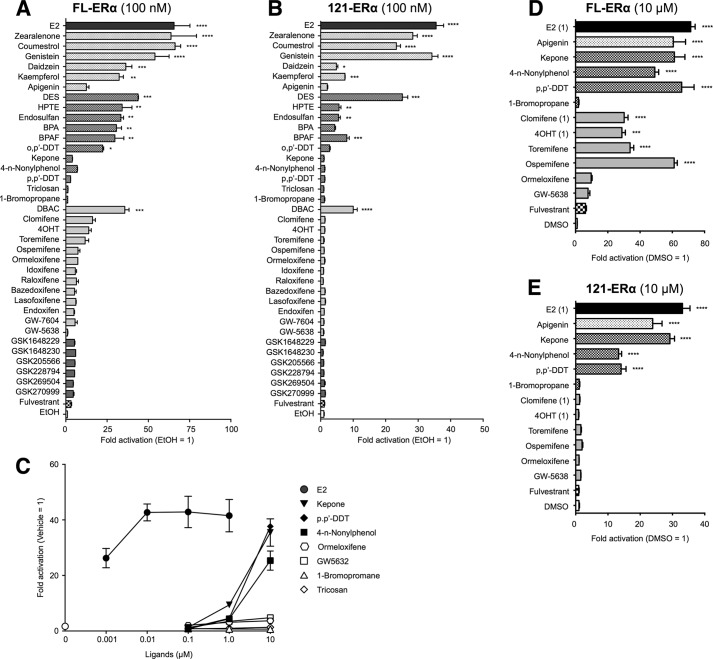
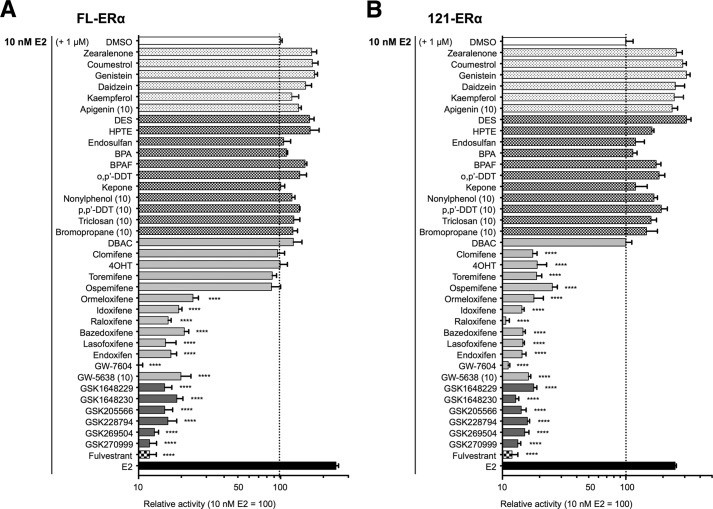
We examined the ERα-dependent ERE-mediated transcription activation function using a variety of known and unknown chemical compounds as probes (Table 1 and Fig. 1) that have been reported as EDCs and SERMs, including six novel compounds that were generated as SERM candidates (GSK1648229, GSK1648230, GSK205566, GSK228794, GSK269504, and GSK270999). To evaluate the ERα AF-1-dependent activities of these compounds, we compared the ERE-mediated transcription activities of full-length (FL) ERα and N-terminal-truncated ERα (AF-1-deleted-ERα; 121-ERα) using the classical 3× vitellogenin-ERE TATA box-fused luciferase (3xERE-Luc) or the estrogen-responsive promoter from the human complement 3 gene-fused luciferase (C3-T1-Luc) reporters. The reporter assays were performed using HepG2 cells, as HepG2 cells are ERα-negative. At first, we tested the effect of the chemicals at 100 nm on the estrogenic transactivation function. 20 of 38 compounds activated the FL-ERα-mediated ERE transcription (Fig. 3A and 4A). The trends of estrogenic activities of those compounds on 3xERE-Luc and C3-T1-Luc are similar; however, we observed that seven compounds (apigenin, kepone, clomifene, 4OHT, toremifene, ospemifene and ormeloxifene) have significant activities through C3-T1-Luc but not through the 3xERE-Luc-mediated transcription at a 100 nm concentration. 14 of 20 estrogenic compounds activated FL-ERα and 121-ERα-mediated transcription, whereas the other 6 compounds (kepone, clomifene, 4OHT, toremifene, ospemifene, and ormeloxifene) did not activate 121-ERα at a concentration of 100 nm (Figs. 3B and 4B). Some previous studies suggested that pharmacological/toxicological concentrations (10–100 μm) of EDCs activated ERE-mediated transcription (19, 20). Thus, we assessed the estrogenic transactivation function of the following 12 compounds at higher concentrations specifically, 5 chemicals that did not manifest any agonist activity in 100 nm (0.1 μm) treatment (4-n-nonylphenol, p,p′-DDT, triclosan, 1-bromopropane, and GW5638) and 7 chemicals that activated C3-T1-Luc but did not activate 3xERE-Luc at 100 nm (apigenin, kepone, ormeloxifene, ospemifene, toremifene, clomifene, and 4OHT). At first we analyzed the dose dependence of FL-ERα transactivation function of the selected chemicals (ormeloxifene, kepone, 4-n-nonylphenol, p,p'-DDT, triclosan, 1-bromopropane, and GW5638) (Figs. 3C and 4C). Because the activity of pRL-TK renilla luciferase, which was used for determining the transfection efficiency, was attenuated strongly by these chemicals at 100 μm, we could assess the activity only up to a 10 μm concentration. The FL-ERα-mediated transcription was activated by kepone, 4-n-nonylphenol, and p,p'-DDT at 10 μm but not ormeloxifene, triclosan, 1-bromopropane, and GW5638. Thus we used a 10 μm concentration for further analysis of the ERα AF-1 dependence of those 12 compounds. As shown in Fig. 3, D and E, the FL-ERα- and 121-ERα-mediated transcription was activated by apigenin, 4-n-nonylphenol, kepone, and p,p'-DDT. On the other hand, clomifene, 4OHT, toremifene, and ospemifene activated FL-ERα but not 121-ERα. Triclosan, 1-bromopropane, ormeloxifene, and GW5638 did not activate either FL-ERα or 121-ERα significantly. The estrogenic activities of tested compounds on 3xERE-Luc and C3-T1-Luc are summarized in Table 2. We categorized those compounds into two groups according to their activities for FL-ERα and 121-ERα. Namely, the compounds that activated both FL-ERα and 121-ERα were categorized into group A (17 compounds), the compounds that showed activity from FL-ERα but not 121-ERα were categorized into group B (4 compounds).
FIGURE 3.
Agonist activity of xenochemicals for 3xERE-TATA reporter. HepG2 cells were cotransfected with the reporter gene (3xERE-Luc), reference gene (pRL-TK), and expression vectors for full-length ERα (FL-ERα) (A) or N-terminal-truncated ERα (121-ERα) (B) and treated with either vehicle (EtOH) or 100 nm chemical. BPA, bisphenol A; BPAF, bisphenol AF. C, the cells were cotransfected with 3xERE-Luc, pRL-TK, and FL-ERα then treated with vehicle (0 μm; DMSO), 0.001–1.0 μm E2, or 0.1–10 μm indicated chemical. The cells were cotransfected with 3xERE-Luc, pRL-TK, and FL-ERα (D) or 121-ERα (E) and treated with either vehicle (DMSO) or 10 μm chemical (E2, 4OHT, and clomifene were 1 μm). The luciferase activities for each panel are represented as -fold change over vehicle. The activity is represented as the mean ± S.E. One-way ANOVA was performed to indicate significant differences against vehicle in each panel. ****, p < 0.0001; ***, p < 0.001; **, p < 0.01; *, p < 0.05.
FIGURE 4.
Agonist activity of xenochemicals for C3-T1 reporter. HepG2 cells were cotransfected with the reporter gene (C3-T1-Luc), reference gene (pRL-TK), and expression vectors for FL-ERα (A) or 121-ERα (B) and treated with either vehicle (EtOH) or 100 nm chemical. BPA, bisphenol A; BPAF, bisphenol AF. C, the cells were cotransfected with 3xERE-Luc, pRL-TK, and FL-ERα then treated with vehicle (0 μm; DMSO), 0.001–1.0 μm E2, or 0.1–10 μm indicated chemical. The cells were cotransfected with 3xERE-Luc, pRL-TK, and FL-ERα (D) or 121-ERα (E) and treated with either vehicle (DMSO) or 10 μm chemical (E2, 4OHT and clomifene were 1 μm). The luciferase activities for each panel are represented as -fold change over vehicle. The activity is represented as the mean ± S.E. One-way ANOVA was performed to indicate significant differences against vehicle in each panel. ****, p < 0.0001; ***, p < 0.001; **. p < 0.01; *, p < 0.05.
TABLE 2.
Summary of agonist and antagonist activities
The table presents the summary of results that are illustrated in Figs. 3A, 3B, 4A, 4B, 5A, 5B, 6A, 7A and 8A. * denotes the results that were illustrated in Figs. 3D, 3E, 4D, 4E, 6C, 7C and 8B. Agonist activities were analyzed by one-way ANOVA against vehicle (EtOH or DMSO (*)). Antagonist activities were analyzed by one-way ANOVA against 10 nm E2 with DMSO treatment. ++++, p < 0.0001; +++, p < 0.001; ++, p < 0.01; +, p < 0.05.

Next we examined the anti-estrogenic activities of those compounds. The chemicals were added in a reporter assay at 1 or 10 μm concentrations to evaluate the antagonist effect against 10 nm estradiol (E2)-activated FL-ERα or 121-ERα-dependent 3xERE-Luc transcription. The E2 concentration used was the minimum amount that led to the plateau level of activity of FL-ERα as suggested in Fig. 3C. FL-ERα- and 121-ERα-mediated transcription was not antagonized by any group A compounds. On the other hand, group B compounds (clomifene, 4OHT, toremifene, and ospemifene) antagonized 121-ERα-mediated transcription but not FL-ERα activity at the concentration tested (Fig. 5, A and B). Antagonist activities are summarized in Table 2.
FIGURE 5.
Antagonist activity of xenochemicals. HepG2 cells were cotransfected with the reporter gene (3xERE-Luc), reference gene (pRL-TK), and expression vectors for FL-ERα (A) or 121-ERα (B) then treated with 10 nm E2 and 1 μm chemical (apigenin, 4-nonylphenol, p,p'-DDT, triclosan, 1-bromopropane, and GW5638 were 10 μm). The luciferase activities for the each panel are represented as relative luciferase activity compared with the level of 10 nm E2-dependent activity (white column; DMSO), which is indicated as 100. Dotted lines denote the E2-mediated luciferase activity level. The activity is represented as the mean ± S.E. One-way ANOVA was performed to indicate significant reductions against E2-dependent activity in each panel. ****, p < 0.0001. BPA, bisphenol A; BPAF, bisphenol AF.
The Effect of AF-2 Flexible Region Mutation on Estrogenic Activity
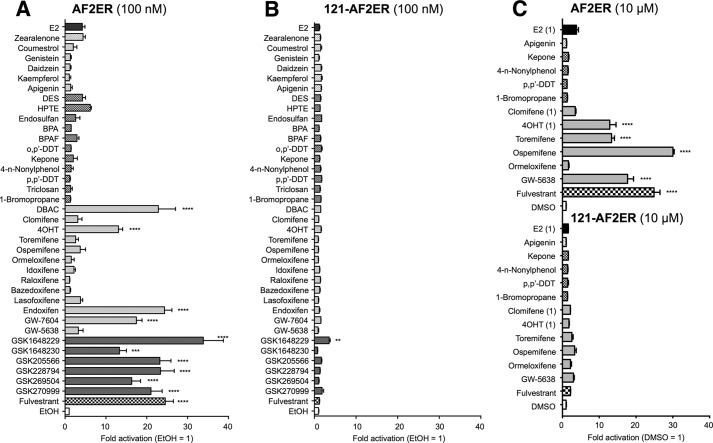
To understand the functional characteristics of AF-2 on the ligand-dependent AF-1 regulation, we analyzed the 3xERE-Luc activation function of the listed compounds using ERα AF-2 mutants. At first, we analyzed the activities of the chemicals through the AF-2 flexible region (H12)-mutated ERα (mERα-L543A,L544A: AF2ER). It has been reported that the AF2ER mutation disrupted E2-mediated transactivation and reversed several antagonists (ICI and 4OHT) to agonists (14, 16). The compounds that were classified as group A agonists did not activate AF2ER with the exception of DBAC (Fig. 6, A and C). In contrast, antagonists including the group B agonists activated AF2ER-mediated transcription, except for a subgroup of SERMs (clomifene, ormeloxifene, bazedoxifene, lasofoxifene, idoxifene, and raloxifene). We reported previously that AF-1 activity is necessary to demonstrate antagonist reversal activity of AF2ER (16). Thus, we analyzed the 121-AF2ER-mediated transcription activities of those chemicals. As shown in Fig. 6, B and C, ligand-dependent transactivation was dramatically reduced by AF-1 deletion, suggesting that the AF2ER activation function of those compounds is derived from AF-1. Activities for AF2ER are summarized in Table 2.
FIGURE 6.
The profile of xenochemical activity through an ERα AF-2 flexible region mutant (AF2ER). HepG2 cells were cotransfected with the reporter gene (3xERE-Luc), reference gene (pRL-TK), and expression vectors for the L543A,L544A-mutated ERα (AF2ER) (A) or N-terminal-truncated AF2ER (121-AF2ER) (B) and treated with either vehicle (EtOH) or 100 nm chemical. BPA, bisphenol A; BPAF, bisphenol AF. C, the cells were cotransfected with 3xERE-Luc, pRL-TK, and AF2ER or 121-AF2ER and treated with either vehicle (DMSO) or 10 μm chemical (E2, 4OHT, and clomifene were 1 μm). The luciferase activities for each panel are represented as -fold change over vehicle. The activity is represented as the mean ± S.E. One-way ANOVA was performed to indicate significant differences against vehicle in each panel. ****, p < 0.0001; ***, p < 0.001; **, p < 0.01.
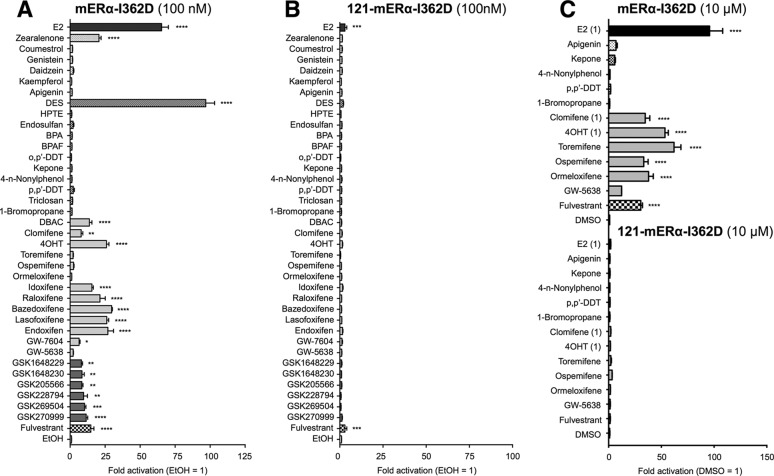
The Effect of AF-2 Static Region Mutation on Estrogenic Activity
Next, we analyzed the activities of the chemicals through the AF-2 static region (H3) mutant ERα (mERα-I362D). The mERα-I362D has been reported as a non E2-responsive mutant (17); however, we found that 100 nm or higher concentrations of E2 activated the mERα-I362D mutant. All group A agonists except E2, zearalenone, and diethylstilbestrol (DES) showed no mERα-I362D-mediated transcription (Fig. 7, A and C). On the other hand, the group B agonists and the antagonists including clomifene, ormeloxifene, bazedoxifene, lasofoxifene, idoxifene, and raloxifene, which did not activate AF2ER, showed activation of mERα-I362D (Fig. 7, A and C). In contrast, GW-5638, which activated AF2ER-mediated transcription at 10 μm, did not activate mERα-I362D (Fig. 7C). We also examined the transactivation functions for 121-mERα-I362D to evaluate the involvement of AF-1 activity. The deletion of AF-1 caused a dramatic reduction of ligand-dependent mERα-I362D mediated transcription (Fig. 7, B and C), suggesting that mERα-I362D-mediated transactivation by those compounds is derived from AF-1 but not AF-2. Activities for mERα-I362D are summarized in Table 2.
FIGURE 7.
The profile of xenochemical activity through an ERα AF-2 static region mutant (mERα-I362D). HepG2 cells were cotransfected with the reporter gene (3xERE-Luc), reference gene (pRL-TK), and expression vectors for the I362D-mutated ERα (mERα-I362D) (A) or N-terminal-truncated mERα-I362D (121-mERα-I362D) (B) and treated with either vehicle (EtOH) or 100 nm chemical. BPA, bisphenol A; BPAF, bisphenol AF. C, the cells were cotransfected with 3xERE-Luc, pRL-TK, and mERα-I362D or 121-mERα-I362D and treated with either vehicle (DMSO) or 10 μm chemical (E2, 4OHT, and clomifene were 1 μm). The luciferase activities for each panel are represented as -fold change over vehicle. The activity is represented as the mean ± S.E. One-way ANOVA was performed to indicate significant differences against vehicle in each panel. ****, p < 0.0001; ***, p < 0.001; **, p < 0.01; *, p < 0.05.
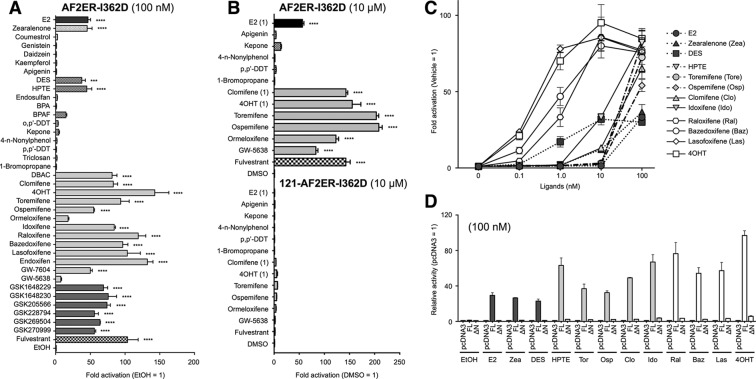
The Effect of Combined AF-2 Mutation on Estrogenic Activity
Furthermore, we analyzed the functional activities of chemicals on the combined disruption of the AF-2 flexible and static regions using the AF2ER-I362D mutant ERα. As shown in Fig. 8, A and B, with the exception of E2, DES, zearalenone, DBAC, and HPTE, the other group A agonists did not activate AF2ER-I362D similar to mERα-I362D. It was surprising that 100 nm HPTE activated AF2ER-I362D significantly, whereas HPTE did not activate either mERα-I362D or AF2ER. On the other hand, all the group B agonists and antagonists activated AF2ER-I362D. Activities for AF2ER-I362D are summarized in Table 2. To evaluate the potency of transactivation of the compounds, we analyzed the dose dependence of selected compounds that activate either the static region mutant (mERα-I362D) and/or the static and flexible combined mutant (AF2ER-I362D) at 100 nm; E2, DES, zearalenone, HPTE, toremifene, ospemifene, clomifene, idoxifene, raloxifene, bazedoxifene, and lasofoxifene. The activities were compared with 4OHT. E2, zearalenone, and DES activated AF2ER-I362D at 1–100 nm (dark gray symbols in Fig. 8C). The maximum activities of those three group A agonists were observed at 100 nm and that level was lower than other chemicals. HPTE, clomifene, toremifene, ospemifene, and idoxifene activated AF2ER-I362D at 10–100 nm (light gray symbols in Fig. 8C). The maximum activities were the same level as other ERα antagonists. Raloxifene, bazedoxifene, and lasofoxifene (antagonists) activated AF2ER-I362D at 0.1 nm, and the activities reached the maximum level at 10 nm (open symbols in Fig. 8B). As shown in Fig. 8, B and D, the activities of chemicals were significantly reduced by N-terminal truncation of AF2ER-I362D (ΔΝ; 121-AF2ER-I362D), suggesting that the transactivation function of those chemicals for AF2ER-I362D was through an AF-1-dependent manner.
FIGURE 8.
The profile of xenochemical activity through a compound AF-2 mutant (AF2ER-I362D). A, HepG2 cells were cotransfected with the reporter gene (3xERE-Luc), reference gene (pRL-TK), and the expression vector for I362D, L543A, and L544A mutated ERα (AF2ER-I362D) and treated with either vehicle (EtOH) or 100 nm chemical. BPA, bisphenol A; BPAF, bisphenol AF. B, the cells were cotransfected with 3xERE-Luc, pRL-TK, and AF2ER-I362D or 121-AF2ER-I362D and treated with either vehicle (DMSO) or 10 μm chemical (E2, 4OHT, and clomifene were 1 μm). The luciferase activity is represented as -fold change over vehicle. The activity is represented as the mean ± S.E. One-way ANOVA was performed to indicate significant differences against vehicle. ****, p < 0.0001; ***, p < 0.001. C, HepG2 cells were cotransfected with 3xERE-Luc, pRL-TK, and the expression vector for AF2ER-I362D and treated with either vehicle (0 nm; EtOH) or 0.1–100 nm indicated chemical. Luciferase activities are represented as -fold change over vehicle, and the activity is represented as the mean ± S.E. D, HepG2 cells were cotransfected with 3xERE-Luc, pRL-TK, and the expression plasmids for AF2ER-I362D (FL) or 121-AF2ER-I362D (ΔN) then treated with 100 nm chemical. Luciferase activities are represented as relative activity compared with the empty expression plasmid transfected cells for each chemical (pcDNA3). The activity is represented as the mean ± S.E.
AF-2 Harbors an AF-1 Repression Activity
As we demonstrated above, the disruption of AF-2 function reversed antagonists to agonists, and that activity was derived from AF-1. From these results we hypothesized that AF-2 possesses AF-1 repression activity, and the SERMs modulate that activity. We previously reported that the entire LBD-deleted ERα (ERα339) showed significantly higher basal transcription activity compared with FL-ERα without ligand (14), supporting our hypothesis, i.e. AF-2 represses AF-1 activity. To demonstrate the AF-1-associated AF-2 functionality, we generated other C-terminal-truncated mutants that included H3 and H4, components of the AF-2 static region (ERα384 and ERα384-I362D; Fig. 9A). As shown in Fig. 9B, the activity of ERα384 was significantly lower than ERα339, and that activity was comparable with FL-ERα without ligand (vehicle), suggesting that AF-1-derived basal transcription activity was suppressed by the region composed of the 45 amino acids between residues 340 and 384. Interestingly, the I362D mutation of ERα384 (ERα384-I362D) restored transcription activity. These results suggest that the AF-2 harbors AF-1 repression activity, and the mutation of I362D in the AF-2 static region reduces that activity.
FIGURE 9.
AF-2 static region harbors AF-1 repression activity. A, schematic diagram of C-terminal truncated ERα mutants. AF2-S and AF2-F denote the static and flexible regions of AF-2 constituent elements respectively. B, HepG2 cells were cotransfected with the 3xERE-Luc, pRL-TK, and the expression vector for ERα339, ERα384, ERα384-I362D, or FL-ERα treated with vehicle (EtOH) and 100 nm E2 for FL-ERα. The luciferase activities are represented as relative activity compared with the empty expression plasmid transfected cells (pcDNA3). The activity is represented as the mean ± S.E. One-way ANOVA was performed to indicate significant differences. ns, not significant. C, whole cell lysates extracted from the plasmid-transfected HepG2 cells were analyzed by immunoblotting with anti-ERα antibody (H-184) to demonstrate expression levels of ERα WT and mutants. β-Actin was used as a loading control (Actin). A representative Western blot analysis is shown.
Conformational Change of an ERα Molecule Is Necessary for the AF-2-mediated AF-1 Regulation
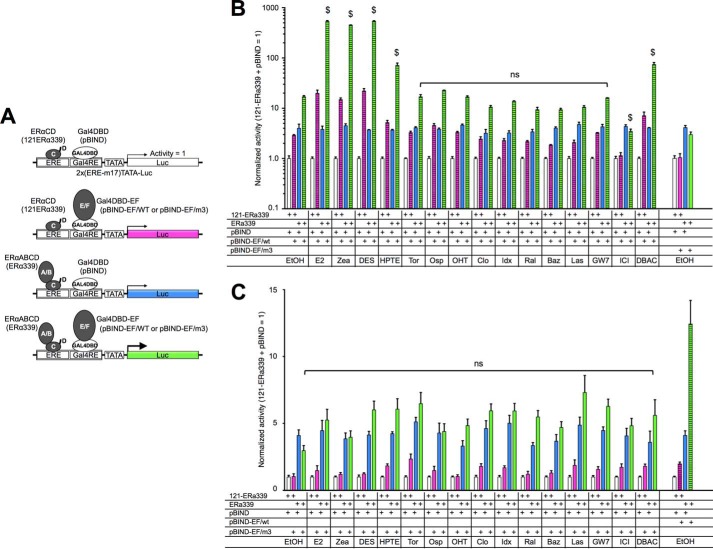
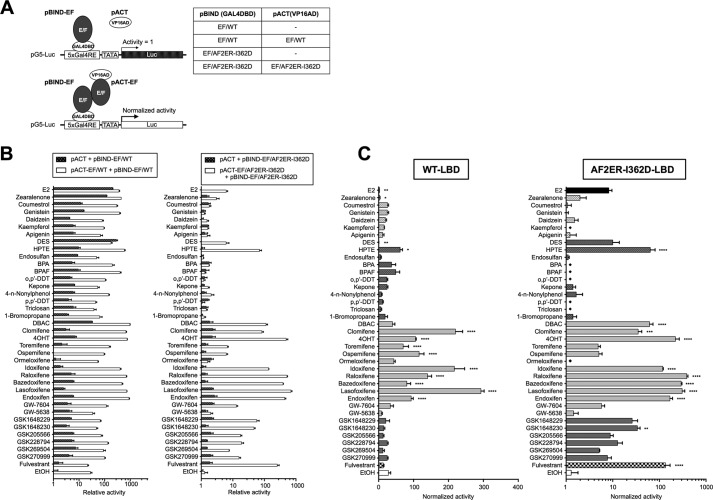
One possibility to explain the activity interplay between AF-1 and AF-2 is to consider that cellular factors present in some tissues may be capable of eliciting the AF-1 repression activity of AF-2. The other explanation is that a conformational change of the ERα protein molecule occurs to repress the AF-1 activity. To assess these possibilities, we set up a novel hybrid reporter assay, a modified method from Benecke et al. (21). This method was originally used for assessing the AF-1 and AF-2 bridging capability of transactivation coactivator, TIF2 (21). The scheme of this experiment is shown in Fig. 10A. The cells were transfected with a hybrid reporter (2x(ERE-m17)-TATA-Luc) containing a Gal4 binding element (m17) juxtaposed to an ERE. The expression plasmids for the ERα339, which contains ERα ABCD domains, and the Gal4 DBD fused with ERα EF domains (pBIND-EF/WT or pBIND-EF/AF2ER-I362D) were cotransfected with the reporter. The activities of each ligand (100 nm) were normalized by the 121-ERα339, which contains ERα CD domains, and pBIND (Gal4 DBD) cotransfected samples (white columns in Fig. 10). The hybrid reporter was activated by ERα339 (blue column) or pBIND-EF/WT (pink column) without ligand (Fig. 10B) but not pBIND-EF/AF2ER-I362D (pink column in Fig. 10C). The additive but not suppressive effect was observed in the cells that were cotransfected with ERα339 and pBIND-EF/WT (green column in Fig. 10B, EtOH). Moreover, neither additive nor repressive activities were observed in the ERα339 and pBIND-EF/AF2ER-I362D-cotransfected cells (green column in Fig. 10C, EtOH). These results suggested that the intrinsic AF-1 activity appeared constantly and that activity was not suppressed by the AF-2 when the AF-2 was physically disconnected from the AF-1. The SERMs did not induce or reduce the activity of ERα339 and pBIND-EF/WT-cotransfected cells, whereas agonists induced the activity strongly (green column in Fig. 10B). Furthermore, none of the SERMs or agonists (E2, zearalenone, DES, HPTE, and DBAC), which activated the intact AF2ER-I362D mutant, changed the reporter activity of ERα339 and pBIND-EF/AF2ER-I362D-cotransfected cells (green column in Fig. 10C). These results suggested that an intact ERα molecule is needed for SERM-dependent activation. It implies that a SERM-dependent conformational change of the ERα molecule is likely to be involved in mediating the SERM action.
FIGURE 10.
Conformational change of ERα molecule is necessary for SERM-mediated AF-1 activation. A, schematic diagram of a hybrid reporter assay. The reporter gene contains a Gal4-binding element (Gal4RE) juxtaposed to an ERE, coexpressed with ERα339 and pBIND-EF/WT or pBIND-EF/AF2ER-I362D (pBIND-EF/m3). The activities were normalized by the activity of 121-ERα339 and pBIND-cotransfected cells (white column). B, HepG2 cells were cotransfected with the hybrid reporter (2x(ERE-m17)-TATA-Luc), 121-ERα339, ERα339, pBIND, and pBIND-EF/WT as indicated in figure then treated with either vehicle (EtOH) or 100 nm chemical. The activity of pBIND-EF/AF2ER-I362D (same result as vehicle in C) is displayed in right column as a reference. C, HepG2 cells were cotransfected with the hybrid reporter, 121-ERα339, ERα339, pBIND, and pBIND-EF/AF2ER-I362D (pBIND-EF/m3) as indicated then treated with either vehicle (EtOH) or 100 nm chemical. The activity of pBIND-EF/WT (same result as vehicle in B) is displayed in the right column as a reference. Normalized activity is represented as the mean ± S.E. Two-way ANOVA was performed to indicate the significance of ligand-dependent activation of ERα339 and pBIND-EF/WT (green column in B) or ERα339 and pBIND-EF/AF2ER-I362D (green column in C) cotransfected cells comparing vehicle. $ suggests significant difference; ns denotes non significant difference. Zea, zearalenone; Tor, toremifene; Osp, ospemifene; Clo, clomifene; Idx, idoxifene; Ral, raloxifene; Baz, bazedoxifene; Las, lasofoxifene. DBAC, des-bis(acetoxy)cyclofenil.
LBD Dimerization Is Associated with AF-2 Mutant-mediated Transactivation of Estrogenic Xenochemicals
We previously reported that the LBD dimerization associated with ERE binding activity causes AF2ER-mediated antagonist reversal activity (16). Therefore, we analyzed the ligand-dependent LBD dimerization activity of AF2ER-I362D using the mammalian two-hybrid assay. The cells were cotransfected with a Gal4-responsive reporter (pG5-Luc) and the expression plasmids for the Gal4 DBD-fused ERα LBD (pBIND-EF/WT or pBIND-EF/AF2ER-I362D) in the presence of the expression plasmids for VP16AD alone (pACT) or VP16AD-fused ERα LBD (pACT-EF/WT or pACT-EF/AF2ER-I362D) as illustrated in the experimental scheme (Fig. 11A). Cells were treated with 100 nm compounds. At first, the ligand-dependent basal activities of pBIND-EF/WT with pACT and pBIND-EF/AF2ER-I362D with pACT were analyzed. Several agonists (E2, zearalenone, DES, and DBAC) activated the pBIND-EF/WT and pACT-cotransfected samples significantly (Fig. 11B, left panel, black column), thereby increasing the control values such that the mammalian two-hybrid assay was not capable of proper assessment of WT-LBD dimerization activity with some agonists. In contrast, no compounds induced a significant activation of pBIND-EF/AF2ER-I362D and pACT-cotransfected samples (Fig. 11B, right panel, black column). Therefore, the mammalian two-hybrid assay could be used to analyze the dimerization of the AF2ER-I362D LBD. The homodimerization activities of WT-LBD (Fig. 11C, left panel) and AF2ER-I362D-LBD (Fig. 11C, right panel) are displayed as the normalized activity against pBIND-EF/WT with pACT or pBIND-EF/AF2ER-I362D with pACT-transfected samples for each ligand. As shown in Fig. 11C, right panel, we found that the profile of 100 nm ligand-dependent LBD dimerization of AF2ER-I362D mutant was consistent with the profile of AF2ER-I362D-dependent ERE-mediated transactivation with 100 nm ligands (Fig. 8A).
FIGURE 11.
AF2ER-I362D LBD dimerization is associated with AF-1-mediated activation. A, schematic diagram of a mammalian two-hybrid assay. HepG2 cells were cotransfected with pG5-Luc and expression vector for Gal4 DBD-fused ERα LBD (pBIND-EF/WT or pBIND-EF/AF2ER-I362D) in the presence of expression vector for VP16AD (pACT) alone or VP16AD-fused ERα LBD (pACT-EF/WT or pACT-EF/AF2ER-I362D). B, left panel, the result of mammalian two-hybrid assay for pACT and pBIND-EF/WT-cotransfected samples (black column) and pACT-EF/WT and pBIND-EF/WT-cotransfected samples (white column). The cells were treated with 10 nm chemical. BPA, bisphenol A; BPAF, bisphenol AF. The luciferase activity is represented as -fold over vehicle (EtOH) in the pACT and pBIND-EF/WT-cotransfected cells. B, right panel, the result of mammalian two-hybrid assay for pACT and pBIND-EF/AF2ER-I362D cotransfected samples (black column) and pACT-EF/AF2ER-I362D and pBIND-EF/AF2ER-I362D-cotransfected samples (white column). The cells were treated with 100 nm chemical. The activity is represented as -fold over vehicle (EtOH) in the pACT and pBIND-EF/AF2ER-I362D-cotransfected cells. Luciferase activity is represented as the mean ± S.E. C, the normalized luciferase activities are represented. Left panel, the luciferase activity in the pACT-EF/WT and pBIND-EF/WT-cotransfected samples (white column in B) was normalized over the pACT and pBIND-EF/WT-cotransfected samples (black column in B) in each compound. Right panel, the luciferase activity in the pACT-EF/AF2ER-I362D and pBIND-EF/AF2ER-I362D-cotransfected samples (white column in B) was normalized over the pACT and pBIND-EF/AF2ER-I362D-cotransfected samples (black column in B) in each compound. Normalized activity is represented as the mean ± S.E. One-way ANOVA was performed to indicate significant differences against vehicle. ****, p < 0.0001; ***, p < 0.001; **, p < 0.01. The activities of samples denoted as ♦ are <1.
Discussion
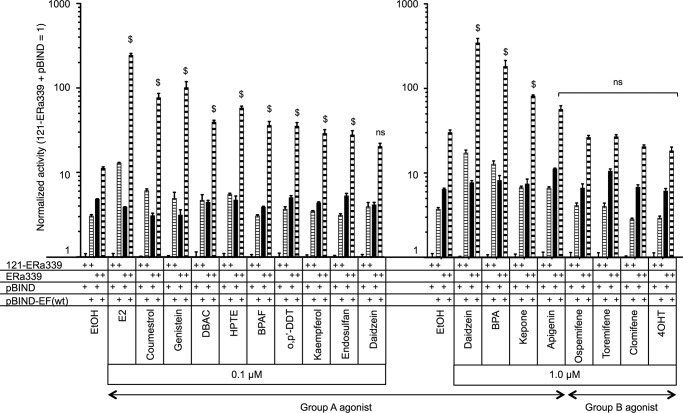
In this study we used compounds with a variety of structures to probe the differential functionality of AF-1 and AF-2 in the ERα transactivation. The compounds were classified in two groups according to the preferences of FL-ERα and AF-1-deleted ERα (121-ERα) activation (Table 2). The group A agonists activated FL-ERα and 121-ERα. The group B agonists activated FL-ERα but not 121-ERα. Hypothetically, the compounds belonging to group A activate ERα-mediated transcription through AF-2, whereas group B compounds activate through AF-1 rather than AF-2 for ERα-mediated transcription. Four SERMs were categorized into group B, and those compounds also possess antagonist activity, although most of the SERMs did not activate ERα-mediated transcription under these conditions. EDCs were categorized into group A, and those compounds did not display any antagonist activity under this condition. Furthermore, we analyzed the AF-1 activation function of group A and group B agonists using the 2x(ERE-m17)-TATA-Luc hybrid reporter cotransfected with ERα339 and pBIND-EF/WT (Fig. 12). The cooperative activity of ERα339 and pBIND-EF/WT was enhanced by most of group A agonists but not group B agonists. These results suggested that the group A agonists induced recruitment of cellular factors to AF-2 such as p160 coactivators that synergistically stimulate AF-1 and AF-2 activities. On the other hand, it is likely that the group B agonists did not recruit the ligand-dependent AF-2 coactivators strongly to enhance transactivation. It may suggest that group B agonists could induce only the exposure of AF-1 activity selectively from a ligand-induced conformational change of the ERα molecule.
FIGURE 12.
Group A agonists induce AF-1 and AF-2 cooperative activity. HepG2 cells were cotransfected with the hybrid reporter, 121-ERα339, ERα339, pBIND, and pBIND-EF/WT as displayed in the figure then treated with either vehicle (EtOH) or chemical (0.1 or 1.0 μm). The activities were normalized by the activity of 121-ERα339 and pBIND-cotransfected cells, which are represented as 1. Normalized activity is represented as the mean ± S.E. Two-way ANOVA was performed to indicate a significant difference of ligand-dependent enhancement of ERα339 and pBIND-EF/WT-cotransfected cells against vehicle. $ suggests significant difference; ns denotes non significant difference.
To evaluate the ligand-dependent AF-1 controlling activity of AF-2, we analyzed the transactivation activity of 38 compounds for AF-2 mutants (AF2ER, mERα-I362D, and AF2ER-I362D). We found that the antagonists for FL-ERα activated AF2ER-mediated transcription with the exception of clomifene, ormeloxifene, idoxifene, raloxifene, bazedoxifene, and lasofoxifene (Fig. 6, A and 6C). Interestingly, these six antagonists worked as potent agonists through the AF-2 static region mutant, mERα-I362D (Fig. 7, A and C). These results suggest that there are subcategories of SERM compounds that have differential effects on the AF-2 static region. Therefore, the varying biological activity of SERMs may relate to their chemical properties to alter the static region of AF-2 adjusting the AF-1 activity. Previously, Mak et al. (17) reported that the I362D mutation disrupted the recruitment of p160/SRC1 to the LBD; however, replacement to alanine (I362A) did not affect p160/SRC1 recruitment and transcription activity. There have been reports that altering the surface charge of H3 causes the antagonist reversal (22–24). The mutation of aspartic acid 351 on human ERα corresponding to mouse Asp-355 to tyrosine (hERα-D351Y) enhances agonist activity of 4OHT and alters raloxifene from an antagonist to a partial agonist (23). Replacement of Asp-351 to alanine (hERα-D351A) attenuated ligand (E2 and raloxifene)-mediated transactivation. In contrast, replacement to glutamic acid (hERα-D351E) attenuated the E2-mediated transactivation but did not affect raloxifene-mediated transcription (24). Importantly, the residues of mouse I362 corresponding to human Ile-358 and human Asp-351 are localized on the same surface of H3 forming a highly conserved region between mouse and human ERα. Our current analysis coupled with the previous reports would suggest that the surface charge of H3 plays a role in the AF-2 static region functionality. Furthermore, we found that the higher concentration of E2 (0.1 and 1 μm) activated the I362D mutant through AF-1 but not AF-2 (Fig. 7), suggesting that the possible conformational change of H3 may contribute to the E2-dependent AF-1 regulation. Interestingly, zearalenone, DES, and DBAC activated the mERα-I362D-mediated transcription the same as E2;, however, the other group A agonists (most of EDCs) did not activate the I362D mutant (Fig. 7). This observation may suggest that the mechanism of differential estrogenic functionality of EDCs compared with E2 is through the AF-1 regulation activity related to the conformational change of H3.
The profile of ligand-mediated AF2ER-I362D activation (Fig. 8, A and B) shows the combination of features of AF2ER (Fig. 6, A and C) and mERα-I362D (Fig. 7. A and C) activation profiles. For instance, 10 μm clomifene and ormeloxifene activated mERα-I362D, but not AF2ER; conversely, GW5638 activated AF2ER but not mERα-I362D (Figs. 6C and 7C). These three compounds worked as agonists through AF2ER-I362D (Fig. 8C). These results suggest that the SERMs act differently on the AF-2 flexible and static regions, and those functions appear to act in an additive manner. We demonstrated in this report that the AF-2 static region consists of an AF-1 blocking activity using the C-terminal truncated ERα mutants. This observation suggested that the residues between 340 and 384 are likely to be harboring AF-1 repression activity and that activity was disrupted thereby altering the surface charge of H3 (ERα384-I362D) (Fig. 9). In addition, we previously reported that the AF-2 flexible region mutation (AF2ER) prevents ICI-dependent ERα proteolysis (16). One possible explanation for our findings is that the disruption of both AF-2 flexible and static region functionalities gives more prominence to the defect of AF-2 static region functionality with prevention of antagonist-mediated proteolysis of ERα. These observations suggest that the disruption of AF-1 suppression activity in the AF-2 may enable the agonistic properties of SERMs, resulting in the antagonist reversal activity.
To understand the molecular mechanism of the tissue or hormonal response-selective activity of SERM-mediated AF-1 regulation, we assessed two possibilities. Namely, the first possibility is the existence of cellular factors associating between AF-1 and AF-2. The second possibility is the conformational change of the ERα molecule to expose AF-1. Métivier et al. (25) have reported that the A-domain (N terminus) of hERα physically interacts with LBD to silence the unliganded hERα transactivation function. To assess these hypotheses, we performed a hybrid reporter assay. We found that the basal activity of ERα339 (AF-1) was not repressed but rather increased additively by the pBIND-EF/WT (WT AF-2) without ligand, suggesting that the AF-1 activity was not suppressed by the AF-2 under the conditions in which the ERα molecule is physically separated into AF-1 and AF-2 domains. In addition, the agonists enhanced the cooperative activity of AF-1 and WT AF-2; however, SERMs neither enhanced nor repressed that activity (Fig. 10B). In contrast, the basal activity of AF-1 was not changed by the mutated AF-2 (pBIND-EF/AF2ER-I362D), and no ligands induced or reduced that activity (Fig. 10C). These results suggest that an intact ERα molecule is needed for controlling the AF-1 activity, and SERMs are likely to induce the conformational change of the ERα molecule, which results in exposing the N-terminal structure and recruiting cellular factors to the AF-1.
We previously reported that the LBD dimerization causes the AF2ER-mediated antagonist reversal activity (16). Here we suggest that the antagonist reversal activity of AF2ER-I362D also correlates with LBD dimerization (Figs. 8A and 11C, right panel). Even though the mammalian two-hybrid assay was not a suitable method to detect the WT-LBD dimerization activity with agonists (AF-2 activating chemicals), we tried to estimate SERM-mediated WT-LBD dimerization activity (Fig. 11C, left panel). Interestingly, the SERMs, which displayed higher dimerization activity of AF2ER-I362D LBD, also showed higher levels of WT-LBD dimerization activity. This result suggests that the mutation does not cause SERM-mediated LBD dimerization. Métivier et al. (25) reported a correlation between hERα dimerization and the efficacy of A-domain and the LBD interaction using the dimerization disrupting hERα mutants. Their observation suggested that the disruption of hERα dimerization increased the binding activity between A-domain and LBD. In other words the dimerized ERα releases the A-domain (N terminus) from the LBD. Our findings with the mouse ERα are consistent with their observation using the human ERα.
Results of WT-LBD dimerization made us consider whether the LBD dimerization is involved in the efficacy of AF-1 release; there was no correlation between the dimerization efficiency of WT-LBD based on the two-hybrid assay and SERM-mediated transactivation or antagonist activity for FL-ERα. These observations imply that there are more factors that are involved in SERM-dependent AF-1 regulation. Carascossa et al. (26) reported that the binding of CARM1 to the hERα LBD is necessary for the ligand-independent cAMP- or PKA-mediated ERα activation. The phosphorylation signals have been reported as modulators for AF-1 activity (27, 28). Interestingly, Carascossa et al. 26) suggested that CARM1 with 4OHT may bind to a newly formed surface of the dimerized LBD that is distinct from the known coactivator binding surface, allowing the subsequent binding of other AF-2 binding factors. Further investigation would be needed to identify whether cellular factors exist that would bind to a new surface of the SERM-dependent dimerized LBD similar to CARM1 regulating the AF-1 activity.
We previously reported that treatment with 4OHT and ICI activated uterine growth in the AF2ERKI mouse but not raloxifene (14). As shown in this report, 4OHT and ICI activated 3xERE-Luc reporter in AF2ER-transfected HepG2 cells, but raloxifene was inactive consistent with the AF2ERKI uterine response (Fig. 6A). The results from our in vitro experiments are comparable with estrogenic responses in the AF2ERKI mouse uterus. In the previous report we also suggested that the treatment of 4OHT and ICI did not regulate estrogenic hormonal pituitary gene responses in AF2ERKI mice in contrast to the uterine response (14). These findings illustrate the limitation of in vitro experiments for the assessment of tissue specific ERα functionality. It is known from in vitro cell culture studies that 4OHT does not activate the C3-T1-Luc reporter through FL-ERα in HeLa cells, in contrast to it being active in HepG2 cells (18). Those observations suggest that SERM-dependent ERα associating factors are most likely to be cell type- and promoter context-specific. In vitro experiments with different cell types will be needed to examine the molecular mechanism of tissue-specific functionalities of ERα AF-1 and AF-2.
Author Contributions
Y. A. and K. S. K. conceived and coordinated the study and wrote the paper. L. A. C. performed the experiments shown in Figs. 3A, 3B, 4A, 4B, 6A, 6B, 7A, 7B, and 8A. Y. A. performed and analyzed experiments. W. J. Z. provided the chemicals that used in experiments. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank Dr. Sueyoshi and Hamilton for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant Z01ES70065 (Division of Intramural Research of the NIEHS; to K. S. K.). The authors declare that they have no conflicts of interest with the contents of this article.
- ER
- estrogen receptor
- AF
- transactivation function
- LBD
- ligand binding domain
- DBD
- DNA binding domain
- EDC
- endocrine disrupting chemical
- SERM
- selected estrogen receptor modulator
- H
- helix
- AF2ER
- L543A,L544A mutated mouse ERα
- KI
- knock-in
- E2
- estradiol
- DES
- diethylstilbestrol
- 4OHT
- 4-hydroxytamoxifen
- ICI
- fulvestrant/ICI182780
- DBAC
- des-bis(acetoxy)cyclofenil
- S
- sense
- AS
- anti-sense
- FL
- full-length
- ERE
- estrogen responsive element
- C3
- complement 3
- ANOVA
- analysis of variance
- HPTE
- 2,2-bis(phydroxyphenyl)-1,1,1-trichloroethane
- p,p′-DDT
- p,p′-dichloro-diphenyl-trichloroethane.
References
- 1. Couse J. F., Korach K. S. (1999) Estrogen receptor null mice: what have we learned and where will they lead us? Endocr. Rev. 20, 358–417 [DOI] [PubMed] [Google Scholar]
- 2. Mangelsdorf D. J., Thummel C., Beato M., Herrlich P., Schütz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P., Evans R. M. (1995) The nuclear receptor superfamily: the second decade. Cell 83, 835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nettles K. W., Greene G. L. (2005) Ligand control of coregulator recruitment to nuclear receptors. Annu. Rev. Physiol. 67, 309–333 [DOI] [PubMed] [Google Scholar]
- 4. Blair R. M., Fang H., Branham W. S. (2000) The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol. Sci. 54, 138–153 [DOI] [PubMed] [Google Scholar]
- 5. Coldham N. G., Dave M., Sivapathasundaram S., McDonnell D. P., Connor C., Sauer M. J. (1997) Evaluation of a recombinant yeast cell estrogen screening assay. Environ. Health Perspect. 105, 734–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Waller C. L., Oprea T. I., Chae K., Park H. K., Korach K. S., Laws S. C., Wiese T. E., Kelce W. R., Gray L. E. (1996) Ligand-based identification of environmental estrogens. Chem. Res. Toxicol. 9, 1240–1248 [DOI] [PubMed] [Google Scholar]
- 7. Jordan V. C. (2003) Tamoxifen: a most unlikely pioneering medicine. Nat. Rev. Drug. Discov. 2, 205–213 [DOI] [PubMed] [Google Scholar]
- 8. Jordan V. C., Morrow M. (1999) Tamoxifen, raloxifene, and the prevention of breast cancer. Endocr. Rev. 20, 253–278 [DOI] [PubMed] [Google Scholar]
- 9. Bourguet W., Germain P., Gronemeyer H. (2000) Nuclear receptor ligand-binding domains: three-dimensional structures, molecular interactions and pharmacological implications. Trends Pharmacol. Sci. 21, 381–388 [DOI] [PubMed] [Google Scholar]
- 10. Pike A. C., Brzozowski A. M., Hubbard R. E., Bonn T., Thorsell A. G., Engström O., Ljunggren J., Gustafsson J. A., Carlquist M. (1999) Structure of the ligand-binding domain of oestrogen receptor β in the presence of a partial agonist and a full antagonist. EMBO J. 18, 4608–4618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu Y.-L., Yang X., Ren Z., McDonnell D. P., Norris J. D., Willson T. M., Greene G. L. (2005) Structural basis for an unexpected mode of SERM-mediated ER antagonism. Mol. Cell 18, 413–424 [DOI] [PubMed] [Google Scholar]
- 12. Berry M., Metzger D., Chambon P. (1990) Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 9, 2811–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McDonnell D. P., Clemm D. L., Hermann T., Goldman M. E., Pike J. W. (1995) Analysis of estrogen receptor function in vitro reveals three distinct classes of antiestrogens. Mol. Endocrinol. 9, 659–669 [DOI] [PubMed] [Google Scholar]
- 14. Arao Y., Hamilton K. J., Ray M. K., Scott G., Mishina Y., Korach K. S. (2011) Estrogen receptor α AF-2 mutation results in antagonist reversal and reveals tissue selective function of estrogen receptor modulators. Proc. Natl. Acad. Sci. U.S.A. 108, 14986–14991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arao Y., Hamilton K. J., Goulding E. H., Janardhan K. S., Eddy E. M., Korach K. S. (2012) Transactivating function (AF) 2-mediated AF-1 activity of estrogen receptor α is crucial to maintain male reproductive tract function. Proc. Natl. Acad. Sci. U.S.A. 109, 21140–21145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arao Y., Hamilton K. J., Coons L. A., Korach K. S. (2013) Estrogen receptor α L543A, L544A mutation changes antagonists to agonists, correlating with the ligand binding domain dimerization associated with DNA binding activity. J. Biol. Chem. 288, 21105–21116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mak H. Y., Hoare S., Henttu P. M., Parker M. G. (1999) Molecular determinants of the estrogen receptor-coactivator interface. Mol. Cell. Biol. 19, 3895–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fan J. D., Wagner B. L., McDonnell D. P. (1996) Identification of the sequences within the human complement 3 promoter required for estrogen responsiveness provides insight into the mechanism of tamoxifen mixed agonist activity. Mol. Endocrinol. 10, 1605–1616 [DOI] [PubMed] [Google Scholar]
- 19. Yoon K., Pellaroni L., Ramamoorthy K., Gaido K., Safe S. (2000) Ligand structure-dependent differences in activation of estrogen receptor α in human HepG2 liver and U2 osteogenic cancer cell lines. Mol. Cell Endocrinol. 162, 211–220 [DOI] [PubMed] [Google Scholar]
- 20. Yoon K., Pallaroni L., Stoner M., Gaido K., Safe S. (2001) Differential activation of wild-type and variant forms of estrogen receptor α by synthetic and natural estrogenic compounds using a promoter containing three estrogen-responsive elements. J. Steroid Biochem. Mol. Biol. 78, 25–32 [DOI] [PubMed] [Google Scholar]
- 21. Benecke A., Chambon P., Gronemeyer H. (2000) Synergy between estrogen receptor α activation functions AF1 and AF2 mediated by transcription intermediary factor TIF2. EMBO Rep. 1, 151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anghel S. I., Perly V., Melançon G., Barsalou A., Chagnon S., Rosenauer A., Miller W. H., Jr., Mader S. (2000) Aspartate 351 of estrogen receptor α is not crucial for the antagonist activity of antiestrogens. J. Biol. Chem. 275, 20867–20872 [DOI] [PubMed] [Google Scholar]
- 23. Liu H., Lee E. S., Deb Los Reyes A., Zapf J. W., Jordan V. C. (2001) Silencing and reactivation of the selective estrogen receptor modulator-estrogen receptor α complex. Cancer Research 61, 3632–3639 [PubMed] [Google Scholar]
- 24. Dayan G., Lupien M., Auger A., Anghel S. I., Rocha W., Croisetière S., Katzenellenbogen J. A., Mader S. (2006) Tamoxifen and raloxifene differ in their functional interactions with aspartate 351 of estrogen receptor α. Mol. Pharmacol. 70, 579–588 [DOI] [PubMed] [Google Scholar]
- 25. Métivier R., Stark A., Flouriot G., Hübner M. R., Brand H., Penot G., Manu D., Denger S., Reid G., Kos M., Russell R. B., Kah O., Pakdel F., Gannon F. (2002) A dynamic structural model for estrogen receptor-α activation by ligands, emphasizing the role of interactions between distant A and E domains. Mol. Cell 10, 1019–1032 [DOI] [PubMed] [Google Scholar]
- 26. Carascossa S., Dudek P., Cenni B., Briand P.-A., Picard D. (2010) CARM1 mediates the ligand-independent and tamoxifen-resistant activation of the estrogen receptor α by cAMP. Genes Dev. 24, 708–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lannigan D. A. (2003) Estrogen receptor phosphorylation. Steroids 68, 1–9 [DOI] [PubMed] [Google Scholar]
- 28. Zwart W., Griekspoor A., Berno V., Lakeman K., Jalink K., Mancini M., Neefjes J., Michalides R. (2007) PKA-induced resistance to tamoxifen is associated with an altered orientation of ERα towards co-activator SRC-1. EMBO J. 26, 3534–3544 [DOI] [PMC free article] [PubMed] [Google Scholar]