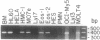Abstract
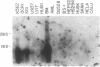
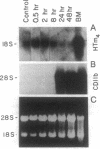
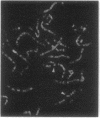
We report the cloning of the cDNA for a human gene whose mRNA is expressed specifically in hematopoietic cells. A long open reading frame in the 1.7-kb mRNA encodes a 214-aa protein of 25 kDa with four hydrophobic regions consistent with a protein that traverses the membrane four times. To reflect the structure and expression of this gene in diverse hematopoietic lineages of lymphoid and myeloid origin, we named the gene HTm4. The protein is about 20% homologous to two other "four-transmembrane" proteins; the B-cell-specific antigen CD20 and the beta subunit of the high-affinity receptor for IgE, Fc epsilon RI beta. The highest homologies among the three proteins are found in the transmembrane domains, but conserved residues are also recognized in the inter-transmembrane domains and in the N and C termini. Using fluorescence in situ hybridization, we localized HTm4 to human chromosome 11q12-13.1, where the CD20 and Fc epsilon RI beta genes are also located. Both the murine homologue for CD20, Ly-44, and the murine Fc epsilon RI beta gene map to the same region in murine chromosome 19. We propose that the HTm4, CD20, and Fc epsilon RI beta genes evolved from the same ancestral gene to form a family of four-transmembrane proteins. It is possible that other related members exist. Similar to CD20 and Fc epsilon RI beta, it is likely that HTm4 has a role in signal transduction and, like Fc epsilon RI beta, might be a subunit associated with receptor complexes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaout M. A. Leukocyte adhesion molecules deficiency: its structural basis, pathophysiology and implications for modulating the inflammatory response. Immunol Rev. 1990 Apr;114:145–180. doi: 10.1111/j.1600-065x.1990.tb00564.x. [DOI] [PubMed] [Google Scholar]
- Butterfield J. H., Weiler D., Dewald G., Gleich G. J. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12(4):345–355. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Lane P. J. Regulation of human B-cell activation and adhesion. Annu Rev Immunol. 1991;9:97–127. doi: 10.1146/annurev.iy.09.040191.000525. [DOI] [PubMed] [Google Scholar]
- Eiseman E., Bolen J. B. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992 Jan 2;355(6355):78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- Ernst L. K., Duchemin A. M., Anderson C. L. Association of the high-affinity receptor for IgG (Fc gamma RI) with the gamma subunit of the IgE receptor. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6023–6027. doi: 10.1073/pnas.90.13.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. The CD19-CR2-TAPA-1 complex, CD45 and signaling by the antigen receptor of B lymphocytes. Curr Opin Immunol. 1993 Jun;5(3):341–348. doi: 10.1016/0952-7915(93)90051-s. [DOI] [PubMed] [Google Scholar]
- Hupp K., Siwarski D., Mock B. A., Kinet J. P. Gene mapping of the three subunits of the high affinity FcR for IgE to mouse chromosomes 1 and 19. J Immunol. 1989 Dec 1;143(11):3787–3791. [PubMed] [Google Scholar]
- Kinet J. P., Blank U., Ra C., White K., Metzger H., Kochan J. Isolation and characterization of cDNAs coding for the beta subunit of the high-affinity receptor for immunoglobulin E. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6483–6487. doi: 10.1073/pnas.85.17.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Espinosa R., 3rd, Thirman M. J., Davis E. M., Diaz M. O., Le Beau M. M., Rowley J. D. Variability of 11q23 rearrangements in hematopoietic cell lines identified with fluorescence in situ hybridization. Blood. 1993 Jun 1;81(11):3027–3033. [PubMed] [Google Scholar]
- Kurosaki T., Gander I., Wirthmueller U., Ravetch J. V. The beta subunit of the Fc epsilon RI is associated with the Fc gamma RIII on mast cells. J Exp Med. 1992 Feb 1;175(2):447–451. doi: 10.1084/jem.175.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lelias J. M., Adra C. N., Wulf G. M., Guillemot J. C., Khagad M., Caput D., Lim B. cDNA cloning of a human mRNA preferentially expressed in hematopoietic cells and with homology to a GDP-dissociation inhibitor for the rho GTP-binding proteins. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1479–1483. doi: 10.1073/pnas.90.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur O., Kennedy I. C., Brini A. T., Ortaldo J. R., O'Shea J. J., Kinet J. P. Characterization of the family of dimers associated with Fc receptors (Fc epsilon RI and Fc gamma RIII). J Immunol. 1991 Oct 15;147(8):2652–2656. [PubMed] [Google Scholar]
- Maurer D., Fiebiger E., Reininger B., Wolff-Winiski B., Jouvin M. H., Kilgus O., Kinet J. P., Stingl G. Expression of functional high affinity immunoglobulin E receptors (Fc epsilon RI) on monocytes of atopic individuals. J Exp Med. 1994 Feb 1;179(2):745–750. doi: 10.1084/jem.179.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L., Blank U., Metzger H., Kinet J. P. Expression of high-affinity binding of human immunoglobulin E by transfected cells. Science. 1989 Apr 21;244(4902):334–337. doi: 10.1126/science.2523561. [DOI] [PubMed] [Google Scholar]
- Orloff D. G., Ra C. S., Frank S. J., Klausner R. D., Kinet J. P. Family of disulphide-linked dimers containing the zeta and eta chains of the T-cell receptor and the gamma chain of Fc receptors. Nature. 1990 Sep 13;347(6289):189–191. doi: 10.1038/347189a0. [DOI] [PubMed] [Google Scholar]
- Paolini R., Jouvin M. H., Kinet J. P. Phosphorylation and dephosphorylation of the high-affinity receptor for immunoglobulin E immediately after receptor engagement and disengagement. Nature. 1991 Oct 31;353(6347):855–858. doi: 10.1038/353855a0. [DOI] [PubMed] [Google Scholar]
- Pinna L. A. Casein kinase 2: an 'eminence grise' in cellular regulation? Biochim Biophys Acta. 1990 Sep 24;1054(3):267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- Ra C., Jouvin M. H., Blank U., Kinet J. P. A macrophage Fc gamma receptor and the mast cell receptor for IgE share an identical subunit. Nature. 1989 Oct 26;341(6244):752–754. doi: 10.1038/341752a0. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Kinet J. P. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- Reth M. Antigen receptor tail clue. Nature. 1989 Mar 30;338(6214):383–384. doi: 10.1038/338383b0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl P. R., Geha R. S. Physical association between the high-affinity IgG receptor (Fc gamma RI) and the gamma subunit of the high-affinity IgE receptor (Fc epsilon RI gamma). Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8847–8850. doi: 10.1073/pnas.90.19.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa T., Li A., Dubowitz M., Dekker J. W., Shaw A. E., Faux J. A., Ra C., Cookson W. O., Hopkin J. M. Association between atopy and variants of the beta subunit of the high-affinity immunoglobulin E receptor. Nat Genet. 1994 Jun;7(2):125–129. doi: 10.1038/ng0694-125. [DOI] [PubMed] [Google Scholar]
- Sutton B. J., Gould H. J. The human IgE network. Nature. 1993 Dec 2;366(6454):421–428. doi: 10.1038/366421a0. [DOI] [PubMed] [Google Scholar]
- Tedder T. F., Klejman G., Disteche C. M., Adler D. A., Schlossman S. F., Saito H. Cloning of a complementary DNA encoding a new mouse B lymphocyte differentiation antigen, homologous to the human B1 (CD20) antigen, and localization of the gene to chromosome 19. J Immunol. 1988 Dec 15;141(12):4388–4394. [PubMed] [Google Scholar]
- Tedder T. F., Streuli M., Schlossman S. F., Saito H. Isolation and structure of a cDNA encoding the B1 (CD20) cell-surface antigen of human B lymphocytes. Proc Natl Acad Sci U S A. 1988 Jan;85(1):208–212. doi: 10.1073/pnas.85.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Littman D. R. Signal transduction by lymphocyte antigen receptors. Cell. 1994 Jan 28;76(2):263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Wulf G. M., Adra C. N., Lim B. Inhibition of hematopoietic development from embryonic stem cells by antisense vav RNA. EMBO J. 1993 Dec 15;12(13):5065–5074. doi: 10.1002/j.1460-2075.1993.tb06200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]