Abstract
Previous electrophysiological investigation shows that combinations of compounds classified by humans as umami-tasting, such as glutamate salts and 5′-ribonucleotides, elicit synergistic responses in neurons throughout the rodent taste system and produce a pattern that resembles responses to sweet compounds. The current study tested the hypothesis that a synergistic mixture of monopotassium glutamate (MPG) and inositol monophosphate (IMP) possesses perceptual similarity to sucrose in mice. We estimated behavioral similarity among these tastants and the individual umami compounds using a series of conditioned taste aversion (CTA) tests, a procedure that measures whether a CTA formed to one stimulus generalizes to another. Our primary finding was that a CTA to a synergistic mixture of MPG + IMP generalizes to sucrose, and vice-versa. This indicates umami synergistic mixtures are perceived as having a sweet, or at least sucrose-like, taste to mice. Considering other recent studies, our data argue strongly in favor of multiple receptor mechanisms for umami detection, and complexity in taste perception models for rodents.
Key words: brief-access test, distributive model, labeled line theory, monosodium glutamate, perceptual discrimination, taste cell receptors
Introduction
The ability of humans to perceive a savory taste known as umami was first described over a century ago (Ikeda 1909). Since then, tastes of compounds described as umami by humans have been well characterized in both humans and rodents by way of molecular, physiological, and behavioral experimentation (e.g., Yasuo et al. 2008; Delay et al. 2009; Kinnamon and Vandenbeuch 2009). A class of G-protein-coupled heterodimeric taste receptors called T1Rs have been found to transduce compounds characterized as tasting sweet or umami to humans (Li et al. 2002; Nelson et al. 2002; Sako et al. 2003; Zhao et al. 2003; Zhang et al. 2008). Sweet-tasting compounds, including sugars and artificial sweeteners (e.g., saccharine, aspartame), activate a T1R2/T1R3 heterodimer whereas umami-tasting compounds, including several l-type amino acids as well as certain 5′-ribonucleotides, activate a T1R1/T1R3 heterodimer. Moreover, evidence indicates T1R1/T1R3 has the ability to cooperatively bind l-glutamate and 5′-ribonucleotides, likely underlying the synergism observed when these 2 types of compounds are mixed (Zhang et al. 2008). In addition to these canonical mechanisms, there is increasing evidence for non-T1R mechanisms for both classes of tastants (Chaudhari et al. 1996, 2000, 2009; Yee et al. 2011; Nakashima et al. 2012).
Humans can clearly discriminate at least 5 different taste qualities, including sweet and umami (Yamaguchi 1991), but studies with rodents paint a more complex picture regarding the uniqueness of umami taste (note that the terms “sweet” and “umami” are used throughout this report to describe taste qualities of these compounds, although these terms are necessarily based on human, rather than animal, perception). The prototypical umami taste compound monosodium l-glutamate (MSG) was initially shown both behaviorally and physiologically to have similarities to NaCl (Yamamoto et al. 1985; Ninomiya and Funakoshi 1989). However, when the sodium component of MSG is blocked with the Na+ channel inhibitor amiloride, rats find this compound and sucrose perceptually similar (Stapleton et al. 1999). Although both rats and mice can discriminate between MSG and sucrose in the presence of amiloride, they have some difficulty doing so (Stapleton et al. 2002; Heyer et al. 2004; Delay et al. 2006). In addition to l-glutamate, Dotson and Spector (2007) found that mice could discriminate sucrose from 2 other l-type amino acids, l-serine and l-threonine. Thus, evidence from studies of rodent taste perception using behavioral paradigms has not discretely categorized compounds such as glutamate salts and putatively sweet- and salty-tasting compounds.
In previous studies, it has been shown that stimulating the oral cavity with a mixture of a glutamate salt and a 5′-ribonucleotide elicits a synergistic response in the taste epithelium, afferent nerve, and taste neurons found in brainstem gustatory areas (Sato et al. 1970; Adachi and Aoyama 1991; Nishijo et al. 1991; Ninomiya et al. 1992; Sako and Yamamoto 1999; Yasumatsu et al. 2012; Zhang et al. 2013). In our previous studies (Tokita et al. 2012; Tokita and Boughter 2012), we measured responses to sucrose and umami compounds in single taste neurons isolated in the mouse parabrachial nucleus (PBN). Strikingly, a mixture of 0.1M monopotassium glutamate (MPG) and 0.01M inositol monophosphate (IMP) elicited a response across all taste neurons that closely mirrored that elicited by 0.3M sucrose, similar to effects found in earlier studies (Adachi and Aoyama 1991). In contrast, the individual umami compounds at these concentrations evoked a weaker response that did not correlate with sucrose.
Behaviorally, whether synergistic mixtures and sucrose may possess a similar taste has been directly studied in rats (Yamamoto et al. 1991) but not mice. Yamamoto et al. (1991) found that aversion conditioned to a mixture of MSG + IMP (with amiloride added) generalized to sucrose, as well as the individual umami compounds. The current study therefore tested the hypothesis that synergistic mixtures of MPG + IMP possessed perceptual similarity to sucrose in mice. We first evaluated innate orosensory responses for sucrose, MPG + IMP, and the individual compounds of the mixture, using brief-access tests. Because water-restricted mice lick water at a near maximal rate, it is straightforward to measure an aversive, but not appetitive, response (i.e., there is a ceiling effect). Therefore, in addition to using a standard 23-h deprivation schedule, we also tested mice with a partial food and water restriction schedule designed to measure appetitive responses (Glendinning et al. 2002). Next, we estimated behavioral similarity among compounds using a series of conditioned taste aversion (CTA) tests, procedures used to evaluate whether an aversion formed to one stimulus generalizes to another (e.g., Ninomiya and Funakoshi 1989; St. John and Boughter 2009; Yamamoto et al. 2009).
Materials and methods
Subjects
Adult male and female C57BL/6J (B6) mice (N = 112) were obtained either directly from Jackson Laboratory or were the offspring of such mice bred in the University of Tennessee Health Science Center’s animal facility. Roughly equal numbers of males and females were used, and all mice were 54–179 days of age (mean = 82.7; SD = 23.6) at the start of training. Mice were housed in shoebox cages on a 12/12-light/dark cycle. Chow (22/5 rodent diet; Harlan Teklad) was provided freely before, during, and after testing (except for the partial restriction brief-access tests; see below). The University of Tennessee Health Science Center Animal Care and Use Committee approved this study, and all mice were handled in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 80-23 revised 1996).
Taste solutions
All tastants (sucrose, MPG, IMP, the MPG + IMP mixture, and potassium chloride [KCl]) were prepared fresh daily using reagent grade chemicals (Sigma-Aldrich) dissolved in distilled water. MPG was used instead of MSG to preclude the taste of the sodium ion. For each compound tested in the brief-access experiments, 4-concentration series were constructed using half-log molar steps, including MPG + IMP, where the concentration of MPG used was always 1 log step higher than that of IMP.
Apparatus and training
Licking was measured using Davis MS-160 contact lickometers (DiLog Instruments). The basic design and use of these devices in gustatory behavioral tests in our lab has been described in detail (Boughter et al. 2002; St. John and Boughter 2009). All mice were initially given 5 days of training in the lickometer. Body weight was measured daily, and on average mice remained at or above 80% their initial weight throughout the experiments. On day 1, water was removed from the home cage, and mice were placed in the test chamber for 5min with no access to water (chamber habituation). On day 2, mice were given a single 20-min access period to a single bottle containing water (sipper tube training). On days 3–5, mice licked water in 5-s trials from 4 randomly presented bottles (trial training); trials were spaced by 7.5 s. The time limit for a mouse to initiate licking in a given trial was 2min; if no lick was made, the shutter closed and the next intertrial interval began, followed by the next trial in sequence (i.e., the trial was not repeated). The sessions (consisting of these short trials) lasted at least 5min and ended after either 24 trials were initiated or 30min, whichever came first. Throughout the first week of training, mice only received water during daily sessions. Mice and water bottles were restored to the home cage after training on day 5. Mice were then given a 2-day rest period and tested either with brief-access or CTA paradigms (see below), where a similar 5-s trial design (total 24 trials/30-min time limit) was used.
Brief-access test
Using this protocol, 9 naïve mice were tested to determine taste-guided sensitivity to 4 concentrations (half-log molar steps) of 5 compounds (sucrose [0.03–1.0 M], MPG [0.03–1.0 M], IMP [0.01–0.3 M], MPG + IMP [0.03–1.0M + 0.003–0.1M; concentrations of both compounds increased a half-log step in each mixture], and KCl [0.03–1.0 M]; see Figure 1 for groups, except KCl) under water restriction. Mice were tested with these stimuli over a 2-week period, one compound per day (3 consecutive days per week), with rest days (no restriction) between weeks and water training days before the tests. For each test, 4 concentrations of compound plus water were delivered using a randomized block design. Twenty-four total 5-s trials were divided into 4 blocks of 6; within each block, each concentration of compound plus 2 water trials was presented in a random order.
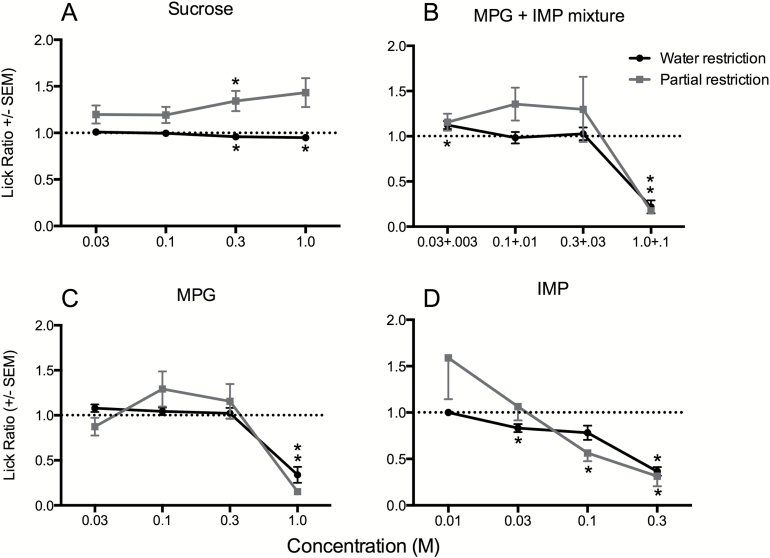
Figure 1.
Concentration response functions (mean LR ± SEM) for brief-access responses to sucrose (A), MPG + IMP mixture (B), MPG (C), and IMP (D) using either a water (black circles) or partial (gray squares) restriction procedure. The dotted line on each graph represents a ratio score of 1.0, which indicates a lick rate equal to that of water. Concentrations for the mixture (B) are the concentration of MPG + the concentration of IMP. Asterisks indicate statistically significant deviations from the water lick rate.
Following this testing, mice were allowed to recover for 2 days, and then were placed on a partial water and food restriction schedule: 2ml of water and 1g of chow per day. While on this schedule, mice were tested for 4 consecutive days with taste stimuli, 1 compound per day. KCl was not tested, as we did not anticipate the mice would lick it avidly. All partial water and food restriction brief-access tests were otherwise similar to those for the water restriction tests described above.
CTA tests
In this paradigm, 95 naïve mice were conditioned to avoid a compound by pairing its taste with a malaise-producing intraperitoneal (i.p.) injection of 0.24M lithium chloride (LiCl). Following this conditioning, mice were tested with a panel of 4 compounds in order to assess whether the aversion generalized to other compounds. As above, bottles were removed from the home cage on day 1, and the animals were given a sipper tube training session on day 2. On day 3, mice were given a single 20-min trial with the conditioned stimulus (CS) (0.3M sucrose, 0.1 + 0.01M MPG + IMP, 0.3 + 0.03M MPG + IMP, 0.3M MPG, 1.0M MPG, 0.03M IMP, 0.1M IMP, or 0.3M KCl; see Figure 2 for groups, except KCl). Immediately following this trial, they received a single i.p. injection of 0.24M LiCl (dose = 204mg/kg). A single control group (CS = 0.3M sucrose) was given an equimolar dose of NaCl in place of the LiCl. Mice were subsequently monitored to ensure signs of sickness (laying on their belly, huddling in the corner) were displayed (Stafstrom-Davis et al. 2001). One hour after the injection, a water bottle was placed in the cage, and mice were allowed to drink for 5min. On day 4, mice were given a trial training session with water (see above). On day 5, mice were tested with single concentrations of 4 compounds (sucrose, MPG, IMP, MPG + IMP mixture) plus water. The “standard” panel was 0.3M sucrose, 0.3M MPG, 0.03M IMP, and 0.1 + 0.01M MPG + IMP; in 4 of these tests (see Figures 4 and 5), 1 or more of these concentrations was increased. As in the brief-access test, this was accomplished with a 24-trial, randomized block design with 2 water trials per block.
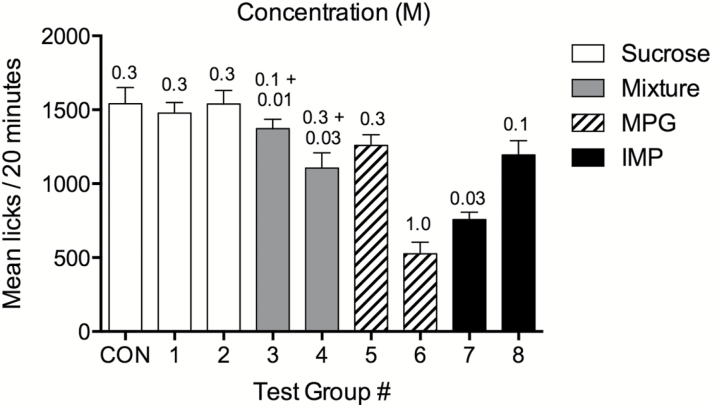
Figure 2.
Mean licks ± SEM for mice in response to sucrose, MPG + IMP mixtures, MPG, and IMP in the 20-min conditioning procedure. There were 9 groups of mice in the experiment, listed along the abscissa. The legend and bar pattern indicates which tastant mice licked and the concentrations are indicated above each bar.
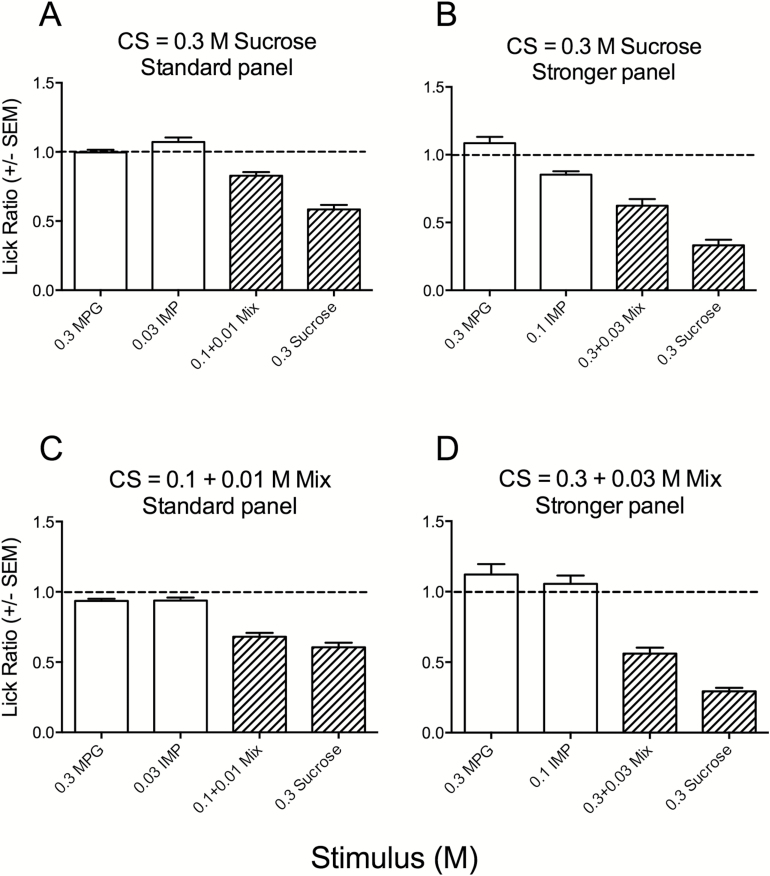
Figure 4.
Mean LR (±SEM) to taste stimuli in mice conditioned to avoid 0.03M sucrose (A, B), 0.1 + 0.01M mixture (C), or 0.3 + 0.01M mixture (D). Mice were conditioned under a water restriction regimen and correspond to groups 1–4 of Figure 2. Mice were tested with a standard panel, or a stronger panel that included higher concentrations of IMP (0.1M) and the mixture (0.3 + 0.03M). The dotted line represents a ratio score of 1.0, which indicates a lick rate equal to water. Crosshatched bars indicate a significant deviation (P < 0.0125) from the water lick rate for each group of mice.
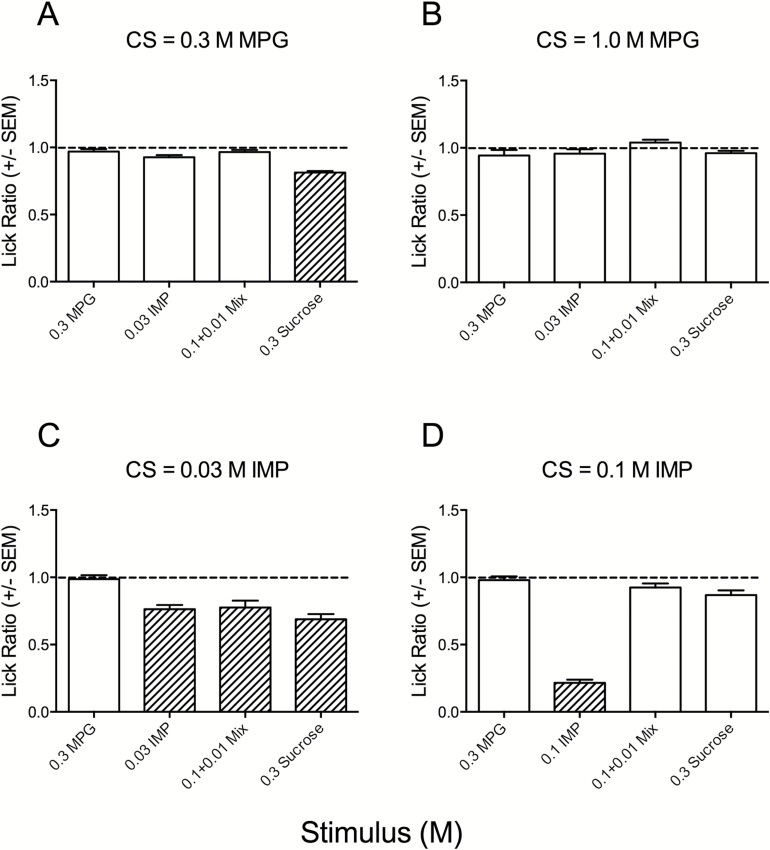
Figure 5.
Mean LR (±SEM) to taste stimuli in mice conditioned to avoid 0.3M MPG (A), 1.0M MPG (B), 0.03M IMP (C), and 0.1M IMP (D). Mice were conditioned under a water restriction regimen and correspond to groups 5–8 of Figure 2. Mice were tested with a standard panel, except for CS = 0.1M IMP (D) which were tested with 0.1M IMP. No CTA formed to MPG. The dotted line represents a ratio score of 1.0, which indicates a lick rate equal to water. Crosshatched bars indicate a significant deviation (P < 0.0125) from the water lick rate for each group of mice.
Each mouse was only conditioned to avoid a single compound; a total of 10 groups of mice were tested. The N per group ranged from 6 to 14 mice. The average group size was 9.1 mice (SD = 2.6). Group size varied due to 1 mouse failing to learn to lick in the lickometer, 4 deaths, and 3 being excluded after testing (see below).
Data analysis
Intake data from the aversion conditioning session is presented as mean licks. For the generalization tests, as well as for the brief-access tests, data are presented as lick ratios (LR) for each stimulus (average licks to stimulus/average number of licks during water trials). For the generalization experiment, LR data was examined for each of 4 compounds used as a CS (sucrose, MPG + IMP, MPG, IMP) with a general linear model, including a within subjects (repeated measures) factor for compound and a between subjects factor for panel. This analysis was conducted in order to investigate whether higher concentrations of the CS and/or test panel produced stronger conditioned aversions. A drawback to this approach was that for MPG + IMP and IMP, both the CS concentration and the concentration of some of the compounds used in the test session varied between panels, making the source of potential effects hard to pinpoint. For both the generalization and brief-access tests, we also assessed significance of aversion (or preference) for individual stimuli using a series of paired t-tests, licks to stimulus versus licks to water. The α level was set at 0.0125 to correct for multiple comparisons using Bonferroni’s method (assumes 4 interdependent means per test). These t-tests yielded essentially identical results to one-sample t-tests (LR vs. hypothetical mean of 1.0); only the former method was reported.
Generally speaking, in CTA approaches with mice involving i.p. injections, not all animals develop strong aversions to an orosensory-salient CS (Murata et al. 2009), perhaps due to variation in procedure, physiological response, or perceived weakness of the CS. As we used a single pairing paradigm, we had to balance consideration of such factors with the possibility that some concentrations of compounds used as the CS were not particularly salient to mice, and therefore did not produce strong aversions. This was the case for MPG and KCl. We therefore developed an exclusion criterion: Individual mice that licked the CS in the test session more than 2 SD above the mean (mouse licks > mean licks + 2 SD) were excluded. Overall, 3 mice in the CTA experiments were excluded; this included 1 mouse out of 19 when the CS was sucrose, a stimulus that we expected to be significantly avoided following pairing with LiCl injection.
Results
Brief-access testing
LRs for mice in response to concentration series of sucrose, the MPG + IMP mixture, MPG, and IMP using either a water or partial restriction procedure are shown in Figure 1. When water-restricted mice were tested with a concentration series of sucrose, they tended to lick each concentration at about the same rate as water, although lick rates to 0.3 and 1.0M sucrose were slightly, but significantly, lower than the water lick rate (Figure 1A; P values < 0.01). Under partial food and water restriction, mice displayed a trend for concentration-dependent avidity to sucrose, with elevated LRs at the 2 higher concentrations, although only 0.3M was significantly different from water (P > 0.012). Clear appetitive responses (i.e., LR > 1.0) were not evident for the MPG + IMP mixture across different concentrations, or for the individual compounds in these tests (Figure 1B–D). However, regardless of deprivation procedure, mice significantly avoided the strongest concentration of both MPG and MPG + IMP (P values < 0.001). In addition, mice avoided several concentrations of IMP, again regardless of procedure (P values < 0.001). In the case of MPG or the mixture, we hypothesized that the aversion was due to a high concentration of potassium ions. In support of this, mice under water restriction significantly avoided both 0.3 and 1.0M KCl (data not shown; P values < 0.001).
Acquisition and expression of CTA
Consumption of tastant (number of licks per 20min) during the conditioning session for all CTA mice varied significantly according to compound (Figure 2; F [8,80] = 18.96; P < 0.0001). Mean intake of 0.3M sucrose was similar between control and CTA groups. Mice displayed reduced consumption of 1.0M MPG and 0.03M IMP as compared to other compounds. MPG is aversive to mice at 1.0M as shown in the brief-access tests (Figure 1C). It is not readily apparent why consumption of 0.03M IMP was reduced relative to 0.1M IMP.
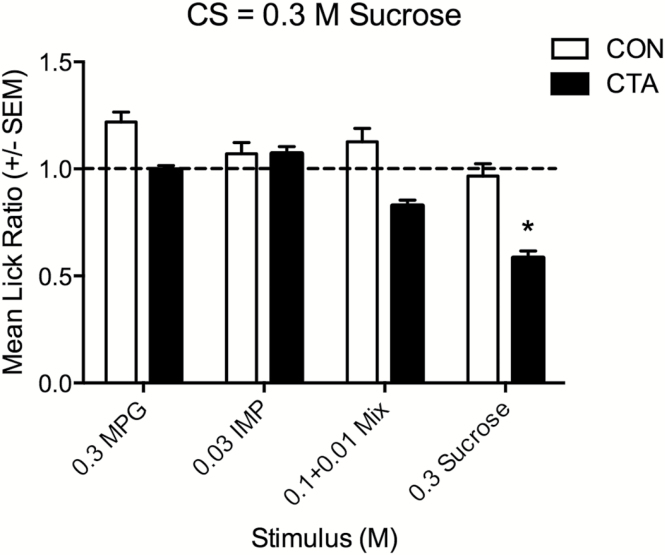
The test responses to CTA (N = 11) and control mice (N = 7) conditioned with 0.3M sucrose are shown in Figure 3. These mice were tested under water-deprived conditions and correspond to the groups CON (control) and 1 in Figure 2. Control mice were tested the same way with the exception that the LiCl injection was replaced with equimolar NaCl during the conditioning session. Control mice were not expected to express a CTA; these mice licked all tastants during the test session at rates equal to or above those for water (LR ≥ 1.0). CTA and control data were compared with a general linear model; there was a significant main effect of group (F [1,16] = 5.979; P = 0.026) and a significant group × compound interaction (F [3,48] = 5.052; P = 0.007). Comparisons (Bonferroni-corrected t-tests) between groups for each compound indicated a significant difference for sucrose (P = 0.007) but not quite for the mixture or the other 2 compounds (P values ≥ 0.02). These data demonstrate acquisition and expression of a CTA relative to controls.
Figure 3.
Mean LR (±SEM) to the test panel for mice conditioned to avoid sucrose (hatched bars; n = 11) versus NaCl-injected control mice (open bars; n = 7). Mice were conditioned under a water restriction regimen and correspond to groups CON and 1 of Figure 2. The dotted line represents a ratio score of 1.0, which indicates a lick rate equal to water. An asterisk indicates a significant group difference (CON vs. CTA) for 0.3M sucrose (P < 0.0125).
To examine whether mice might use olfactory cues to guide ingestive decisions in this paradigm, we analyzed latency to first lick in both the CTA and CON groups (e.g., Rhinehart-Doty et al. 1994). There was not a significant effect of either group or compound, suggesting mice did not use olfactory cues to anticipate or discriminate among particular stimuli.
Conditioned aversions to sucrose and MPG + IMP mixtures cross-generalize
Mice were conditioned to avoid 0.3M sucrose and then tested with either the standard panel (Figure 4A; same data as CTA group in Figure 3) of taste stimuli (0.3M MPG, 0.03M IMP, 0.1 + 0.01M MPG + IMP, and 0.3M sucrose) or a panel (Figure 4B) featuring the same sucrose concentration but higher concentrations of IMP (0.1M) and MPG + IMP (0.3 + 0.03M). A higher MPG concentration was not used as mice displayed native aversion to 1.0M MPG (e.g., Figure 1). Following conditioning, mice displayed significant aversion to the CS; this aversion generalized to the MPG + IMP mixture, but not to MPG or IMP when presented alone (P values < 0.006). In a comparison between panels (standard vs. high), there was both a significant effect of panel (F [1,16] = 4.82, P < 0.05), and a significant stimulus × panel interaction (F [3,48] = 4.47, P < 0.05), reflecting that mice showed greater aversion to sucrose and MPG + IMP with the stronger panel. Although it may be expected that mice would display more robust avoidance of the higher concentration of MPG + IMP, it is not clear why aversion was stronger to sucrose in the stronger panel, as the concentration of this stimulus did not change.
We next tested mice that were conditioned to avoid MPG + IMP at 2 concentrations (Figure 4C,D). Mice given a CS concentration of 0.1 + 0.01M were tested with the standard panel; these mice displayed avoidance of both sucrose and the mixture (P values < 0.0001). Mice that received a higher concentration of mixture as the CS (0.3 + 0.03M) similarly avoided sucrose and mixture in the test session (P values < 0.001) but not 0.3M MPG or 0.1M IMP. ANOVA indicated there was no significant overall effect of CS concentration/panel concentration (both were elevated in the second experiment; Figure 4D), although there was a significant stimulus × panel interaction (F [3,54] = 3.88; P < 0.05). This likely reflects the greater aversion of mice to sucrose, but not the mixture, in the stronger panel.
Conditioned aversions to MPG and IMP
Additional groups of mice were conditioned to avoid either 0.3M or 1.0M MPG. They were tested with the same, standard panel of compound concentrations. As mentioned above, 1.0M MPG was not used as a test stimulus because mice avoided this concentration in the brief-access tests. Somewhat surprisingly, the MPG-conditioned mice displayed no significant aversion to most of the compounds, including 0.3M MPG itself (Figure 5A,B); this indicates no CTA formed to MPG. The exception was significant aversion shown by the 0.3M conditioned mice to sucrose (P < 0.002). Additional attempts to explore this lack of CTA were made by evaluating generalizations after conditioning with 0.3M KCl (to target potassium; data not shown), but these mice displayed no significant aversions to any of the stimuli of the standard panel (P values > 0.1).
We also used 2 concentrations of IMP (0.03, 0.1M) as the CS. In the 0.03M group, mice displayed significant aversions of all compounds except MPG (Figure 5C) (P values < 0.007). As mentioned above, conditioning to sucrose and MPG + IMP did not generalize to IMP (Figure 4); thus, this generalization is asymmetrical. Interestingly, 0.1M IMP mice did not generalize aversions to sucrose or MPG + IMP (Figure 5D), indicating a concentration dependence of IMP generalizations with sweet-tasting compounds.
Latency to first lick data from all CTA panels, for each stimulus plus water, were examined via repeated measures ANOVA with a between subjects factor for group. Overall, there was not a significant effect of compound, indicating that mice did not hesitate more or less before licking from a given stimulus bottle. There was both a significant effect of group (F [8,74] = 4.25; P < 0.001), and group × compound interaction (F [32,296] = 2.14, P < 0.001). When latencies within each group were examined individually, a significant effect of compound was found only for mice that received the higher concentration of MPG + IMP (i.e., one of nine groups). The lack of consistent significant differences in latency among compounds suggests that mice did not use olfactory or other potential extraneous (e.g., motor noise) cues to anticipate stimuli, including the (usually avoided) CS.
Discussion
Synergistic umami mixtures and sucrose evoke similar perceptions in mice
In this study, we show that a CTA to a synergistic mixture of MPG + IMP generalizes to sucrose, and vice-versa. This is evidence that synergistic mixtures are perceived as having a sweet or at least sucrose-like taste to mice. (Note that our results also support the statement that sucrose is perceived as “MPG + IMP-like” to mice, but we choose to use the former to more conveniently explain our results.) A similar cross-generalization between these compounds using the CTA paradigm was previously described for rats (Yamamoto et al. 1991). However, although a CTA to IMP generalized to both the mixture and sucrose, CTAs conditioned to the mixture or to sucrose did not generalize to MPG or IMP alone in our study. The lack of cross-generalization between sucrose and glutamate salts (either MSG + amiloride or MPG) has been described in previous studies with mice (Ninomiya and Funakoshi 1989; Murata et al. 2009; Yamamoto et al. 2009; Kusuhara et al. 2013), but not rats (Yamamoto et al. 1991; Chaudhari et al. 1996; Stapleton et al. 1999; Heyer et al. 2003), pointing to a likely species difference.
Current models of taste cell function posit that umami and sweet compounds activate different T1R heterodimers, which are located in separate subpopulations of “Type II” taste receptor cells (TRCs) (Chandrashekar et al. 2006; Liman et al. 2014). This peripheral segregation includes synergistic umami mixtures, which activate the T1R1 + T1R3 but not T1R2 + T1R3 heterodimer (Li et al. 2002; Nelson et al. 2002; Zhao et al. 2003). In fact, the synergistic response may be related to the ability of the T1R1 subunit to cooperatively bind l-glutamate and 5′-ribonucleotides (Zhang et al. 2008). Careful behavioral studies with mice possessing genetic deletions of T1R receptors confirm the dependence of the synergistic response on T1R1 + T1R3 (Smith and Spector 2014).
Despite apparent receptor specificity, emerging evidence suggests the link between synergistic umami mixtures and sweet taste begins at the periphery and is maintained throughout the central nervous system, resulting in behavioral similarity in mice. Kusuhara et al. (2013) found that single TRCs often responded to both sweet- and umami-tasting compounds. Additionally, in the same study, T1R1 knockout mice had a compromised response to both sweet tastants and umami mixtures. This common response may be due to the presence of all 3 T1R subunits in individual taste cells (Kim et al. 2003; Dando et al. 2012; Kusuhara et al. 2013; but see Zhao et al. 2003). On the other hand, the behavioral data of Smith and Spector (2014) argue strongly against the involvement of T1R2 in the synergistic response. In any case, it is clear that at the level of the afferent taste nerve, certain chorda tympani (CT) fibers are activated by both umami mixtures and sweet-tasting compounds (Yasumatsu et al. 2012). Unsurprisingly, the sweet taste inhibitor gurmarin has been found to block the mixture response in the CT nerve (Sako et al. 2003). As mentioned earlier, there is a strong correlation between mixture and sucrose responses in the PBN of the mouse, with an MPG + IMP mixture predominantly activating neurons classified as sucrose- or sweet-best (Tokita et al. 2012; Tokita and Boughter 2012).
The above and further evidence may explain in the current experiment why a CTA conditioned to sucrose generalized to the mixture, but not to MPG or IMP. In the Kusuhara et al. (2013) study, genetic deletion of T1R1 did not affect the response to individually presented umami compounds, including MSG, MPG, and IMP, in either the CT or glossopharyngeal (GL) nerve. The GL nerve is known in mice to lack a synergistic response to umami mixtures (Damak et al. 2003). These and other data (Maruyama et al. 2006) argue in favor of alternate mechanisms for umami detection. There is evidence for contributions from both taste-specific and brain-expressed forms of metabotropic glutamate receptors (mGluR1 and mGluR4) (Chaudhari et al. 2000; San Gabriel et al. 2005; Nakashima et al. 2012). It is also possible, however, that the lack of generalization from sucrose or the mixture to MPG and IMP found in our experiment has more to do with concentration, and the weakness of MPG as a taste stimulus.
Although CTAs formed to sucrose or the synergistic mixture did not generalize to IMP in our study, a CTA to IMP generalized to both sucrose and the mixture. Cross-generalization of CTAs to IMP and glutamate salts in mice has been previously established, although neither was shown to cross-generalize with sucrose (Ninomiya and Funakoshi 1989; Murata et al. 2009; Yamamoto et al. 2009). Murata et al. (2009) did find that a CTA to 0.01M IMP generalized to 20mM Saccharin in 129/J (but not B6) mice. We suggest that if IMP or MPG (presented individually, not in mixture) has sweet attributes in mice, as they apparently do in rats (Yamamoto et al. 1991), any palatable response is weak and concentration dependent. Indeed, mice did not generalize IMP to sucrose and the mixture when the CS concentration was raised to 0.1M. In our brief-access tests, B6 mice were not generally responsive to these stimuli at lower concentrations but displayed strong aversion to IMP at 0.3M and to MPG at 1.0M. In 2-bottle tests, wild-type mice prefer 0.01–0.1M IMP but display complete avoidance by 0.3M (He et al. 2004). MSG is preferred at 0.001–0.3M but avoided at higher concentrations (Bachmanov et al. 2000). Either compound tends to provoke relatively weak taste nerve responses at concentrations below 0.3M (He et al. 2004; Inoue et al. 2004; Kusuhara et al. 2013), although mice can detect IMP at a concentration as low as 0.0025M (Smith and Spector 2014). Aversion to these compounds at high concentrations is most likely driven by cations (K+ for MPG, Na+ for MSG and IMP).
The absence of a strong taste response was most pronounced for MPG, where we were not able to condition a significant aversion with a single pairing in most mice, even when 1.0M was used as the CS. In this case, mice still failed to generalize the CTA to 0.3M, seemingly confirming that this concentration is only weakly perceived. These data support the recent findings by Smith and Spector (2014), who show that most mice (wild-type or T1R KO) cannot be trained to detect 0.6M MSG when amiloride was added. It is also notable that Murata et al. (2009) needed to repeat the CS-US pairing (using 0.1M MSG and 0.24M i.p. LiCl) in 75% of B6 mice in order to condition an aversion. On the other hand, no retesting was apparently necessary for mice to form a CTA to 0.1M MPG in Yamamoto et al. (2009). It is unclear if this is due to procedural differences.
Methodological considerations
In this study, we used a brief-access design to minimize non-orosensory cues (St. John and Spector 2008). However, it is possible that such factors impacted our findings. For example, studies using lickometers have shown that rodents, including mice, are able to use olfactory cues to identify compounds thought to be odorless such as sucrose (Rhinehart-Doty et al. 1994). Although we took no extra steps to prevent the possibility of mice sensing taste compounds via olfactory cues, results from an analysis of the latency to first lick in trials from our study indicates that mice did not hesitate more to make an initial lick of the CS during testing than they did for other tastants. Another factor to be considered is the possibility of postingestive effects modulating behavioral responses. Mice consume appetitive compounds such as sucrose throughout the experimental session, and postingestive effects for certain compounds have been shown to be detectable within a short period (i.e., ≥6–10min) after consumption (Baird et al. 2005; Glendinning et al. 2008; Zukerman et al. 2011). The test sessions in our generalization experiments varied quite a bit in length, but on average lasted for less than 10min (mean ± SD = 9.1±2.5min; range = 6.0–18.9min). In humans, umami compounds appear to have a prolonged aftertaste (i.e., Imamura and Matsushima 2013), although it is unclear if this effect extends to mice. Future studies may use longer intertrial intervals or water rinses between trials to prevent potential carryover effects. Finally, neophobia has also been shown to influence results in CTA studies (Domjan 1976; De La Casa and Lubow 1995). At least for particular concentrations of each compound tested, effects of neophobia may be negligible, as indicated by lack of aversion to any compound in CTA control mice (Figure 3).
Although it is clear that synergistic umami mixtures are sensed as sweet- or sucrose-like by inbred B6 mice, it is important to draw a distinction between taste similarity and identity. This is an inherent limitation of the CTA paradigm (St. John and Spector 2008). Indeed, previous investigations have shown that rats can often discriminate (assessed using operant procedures) between compounds that have been shown to cross-generalize such as the disaccharides sucrose and maltose (Nissenbaum and Sclafani 1987; Spector et al. 1997). Similarly, although MSG and sucrose have some degree of behavioral similarity to rats as revealed by CTA (Yamamoto et al. 1991; Stapleton et al. 1999), they can be discriminated from one another even when concentration is accounted for and the sodium ion is blocked (Stapleton et al. 2002; Heyer et al. 2004).
Additionally, concentration choice can be key, as generalization can take place along an intensity dimension (St. John and Spector 2008). Concentrations in the current study used for CTA were selected on the basis of both prior studies (Yamamoto et al. 1991, 2009; Tokita et al. 2012) and LR functions shown in Figure 1. Note that if IMP at concentrations less than 0.1M is perceived as only weakly or mildly sweet by the mice, it may explain why aversions to this stimulus (but not 0.1M) asymmetrically generalized to sucrose and MPG + IMP and may generalize more strongly to weaker concentrations of putatively “sweet” compounds and synergistic mixtures.
Conclusion
To our knowledge, this study is the first to report that aversions conditioned to sucrose and a synergistic umami mixture (MPG + IMP) cross-generalize in mice, supporting the assertion that this mixture possesses a sweet- or sucrose-like taste to B6 mice. The asymmetry of generalizations between sucrose and IMP suggests IMP may possess a less intense sweet- or sucrose-like taste and demonstrates the concentration dependence of perceptual similarity. Although sucrose and the mixture are selectively transduced by different T1R heterodimers, the finding of behavioral similarity fits in with an emerging body of evidence supporting the substantial overlap in both the peripheral and central representation of these compounds. Further studies will elucidate the patterns in the mechanisms by which such taste similarities emerge.
Funding
This work was supported by the National Institutes of Health [grant number NIH DC000353 to J.D.B.].
Acknowledgment
We would like to thank Dr Lianyi Lu for technical assistance in the collection of data in the behavioral experiments.
References
- Adachi A, Aoyama M. 1991. Neuronal responses of the nucleus tractus solitarius to oral stimulation with umami substances. Physiol Behav. 49(5):935–941. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. 2000. Intake of umami-tasting solutions by mice: a genetic analysis. J Nutr. 130(4S Suppl):935S–941S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JP, St John SJ, Nguyen EA. 2005. Temporal and qualitative dynamics of conditioned taste aversion processing: combined generalization testing and licking microstructure analysis. Behav Neurosci. 119(4):983–1003. [DOI] [PubMed] [Google Scholar]
- Boughter JD, Jr, St John SJ, Noel DT, Ndubuizu O, Smith DV. 2002. A brief-access test for bitter taste in mice. Chem Senses. 27(2):133–142. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. 2006. The receptors and cells for mammalian taste. Nature. 444(7117):288–294. [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Landin AM, Roper SD. 2000. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci. 3(2):113–119. [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Pereira E, Roper SD. 2009. Taste receptors for umami: the case for multiple receptors. Am J Clin Nutr. 90(3):738S–742S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Yang H, Lamp C, Delay E, Cartford C, Than T, Roper S. 1996. The taste of monosodium glutamate: membrane receptors in taste buds. J Neurosci. 16(12):3817–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. 2003. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 301(5634):850–853. [DOI] [PubMed] [Google Scholar]
- Dando R, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. 2012. Adenosine enhances sweet taste through A2B receptors in the taste bud. J Neurosci. 32(1):322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Casa G, Lubow RE. 1995. Latent inhibition in conditioned taste aversion: the roles of stimulus frequency and duration and the amount of fluid ingested during preexposure. Neurobiol Learn Mem. 64:125–132. [DOI] [PubMed] [Google Scholar]
- Delay ER, Eddy MC, Eschle BK. 2009. Behavioral studies of umami: tales told by mice and rats. Ann N Y Acad Sci. 1170:41–45. [DOI] [PubMed] [Google Scholar]
- Delay ER, Hernandez NP, Bromley K, Margolskee RF. 2006. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chem Senses. 31(4):351–357. [DOI] [PubMed] [Google Scholar]
- Domjan M. 1976. Determinants of the enhancement of flavored-water intake by prior exposure. J Exp Psychol Anim Behav Process. 2(1):17–27. [DOI] [PubMed] [Google Scholar]
- Dotson CD, Spector AC. 2007. Behavioral discrimination between sucrose and other natural sweeteners in mice: implications for the neural coding of T1R ligands. J Neurosci. 27(42):11242–11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning JI, Gresack J, Spector AC. 2002. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem Senses. 27(5):461–474. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Yiin YM, Ackroff K, Sclafani A. 2008. Intragastric infusion of denatonium conditions flavor aversions and delays gastric emptying in rodents. Physiol Behav. 93(4-5):757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Yasumatsu K, Varadarajan V, Yamada A, Lem J, Ninomiya Y, Margolskee RF, Damak S. 2004. Umami taste responses are mediated by alpha-transducin and alpha-gustducin. J Neurosci. 24(35):7674–7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer BR, Taylor-Burds CC, Mitzelfelt JD, Delay ER. 2004. Monosodium glutamate and sweet taste: discrimination between the tastes of sweet stimuli and glutamate in rats. Chem Senses. 29(8):721–729. [DOI] [PubMed] [Google Scholar]
- Heyer BR, Taylor-Burds CC, Tran LH, Delay ER. 2003. Monosodium glutamate and sweet taste: generalization of conditioned taste aversion between glutamate and sweet stimuli in rats. Chem Senses. 28(7):631–641. [DOI] [PubMed] [Google Scholar]
- Ikeda K. 1909. On a new seasoning. J Tokyo Chem Soc Jpn. 27:279–284. [Google Scholar]
- Imamura M, Matsushima K. 2013. Suppression of umami aftertaste by polysaccharides in soy sauce. J Food Sci. 78(8):C1136–C1143. [DOI] [PubMed] [Google Scholar]
- Inoue M, Beauchamp GK, Bachmanov AA. 2004. Gustatory neural responses to umami taste stimuli in C57BL/6ByJ and 129P3/J mice. Chem Senses. 29(9):789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MR, Kusakabe Y, Miura H, Shindo Y, Ninomiya Y, Hino A. 2003. Regional expression patterns of taste receptors and gustducin in the mouse tongue. Biochem Biophys Res Commun. 312(2):500–506. [DOI] [PubMed] [Google Scholar]
- Kinnamon SC, Vandenbeuch A. 2009. Receptors and transduction of umami taste stimuli. Ann N Y Acad Sci. 1170:55–59. [DOI] [PubMed] [Google Scholar]
- Kusuhara Y, Yoshida R, Ohkuri T, Yasumatsu K, Voigt A, Hübner S, Maeda K, Boehm U, Meyerhof W, Ninomiya Y. 2013. Taste responses in mice lacking taste receptor subunit T1R1. J Physiol. 591(Pt 7):1967–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. 2002. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 99(7):4692–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Zhang YV, Montell C. 2014. Peripheral coding of taste. Neuron. 81(5):984–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y, Pereira E, Margolskee RF, Chaudhari N, Roper SD. 2006. Umami responses in mouse taste cells indicate more than one receptor. J Neurosci. 26(8):2227–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Beauchamp GK, Bachmanov AA. 2009. Taste perception of monosodium glutamate and inosine monophosphate by 129P3/J and C57BL/6ByJ mice. Physiol Behav. 98(4):481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Eddy MC, Katsukawa H, Delay ER, Ninomiya Y. 2012. Behavioral responses to glutamate receptor agonists and antagonists implicate the involvement of brain-expressed mGluR4 and mGluR1 in taste transduction for umami in mice. Physiol Behav. 105(3):709–719. [DOI] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. 2002. An amino-acid taste receptor. Nature. 416(6877):199–202. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M. 1989. Behavioral discrimination between glutamate and the four basic taste substances in mice. Comp Biochem Physiol A Comp Physiol. 92(3):365–370. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Kurenuma S, Nomura T, Uebayashi H, Kawamura H. 1992. Taste synergism between monosodium glutamate and 5′-ribonucleotide in mice. Comp Biochem Physiol A Comp Physiol. 101(1):97–102. [DOI] [PubMed] [Google Scholar]
- Nishijo H, Ono T, Norgren R. 1991. Parabrachial gustatory neural responses to monosodium glutamate ingested by awake rats. J Neurophysiol. 78:2254–2268. [DOI] [PubMed] [Google Scholar]
- Nissenbaum JW, Sclafani A. 1987. Qualitative differences in polysaccharide and sugar tastes in the rat: a two-carbohydrate taste model. Neurosci Biobehav Rev. 11(2):187–196. [DOI] [PubMed] [Google Scholar]
- Rhinehart-Doty JA, Schumm J, Smith JC, Smith GP. 1994. A non-taste cue of sucrose in short-term taste tests in rats. Chem Senses. 19(5):425–431. [DOI] [PubMed] [Google Scholar]
- Sako N, Tokita K, Sugimura T, Yamamoto T. 2003. Synergistic responses of the chorda tympani to mixtures of umami and sweet substances in rats. Chem Senses. 28(3):261–266. [DOI] [PubMed] [Google Scholar]
- Sako N, Yamamoto T. 1999. Analyses of taste nerve responses with special reference to possible receptor mechanisms of umami taste in the rat. Neurosci Lett. 261(1-2):109–112. [DOI] [PubMed] [Google Scholar]
- San Gabriel A, Uneyama H, Yoshie S, Torii K. 2005. Cloning and characterization of a novel mGluR1 variant from vallate papillae that functions as a receptor for L-glutamate stimuli. Chem Senses. 1:25–26. [DOI] [PubMed] [Google Scholar]
- Sato M, Yamashita S, Ogawa H. 1970. Potentiation of gustatory response to monosodium glutamate in rat chorda tympani fibers by addition of 5′-ribonucleotides. Jpn J Physiol. 20(4):444–464. [DOI] [PubMed] [Google Scholar]
- Smith KR, Spector AC. 2014. The importance of the presence of a 5′-ribonucleotide and the contribution of the T1R1 + T1R3 heterodimer and an additional low-affinity receptor in the taste detection of l-glutamate as assessed psychophysically. J Neurosci. 34(39):13234–13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC, Markison S, St John SJ, Garcea M. 1997. Sucrose vs. maltose taste discrimination by rats depends on the input of the seventh cranial nerve. Am J Physiol. 272(4 Pt 2):R1210–R1218. [DOI] [PubMed] [Google Scholar]
- St. John SJ, Boughter JD., Jr 2009. Orosensory responsiveness to and preference for hydroxide-containing salts in mice. Chem Senses. 34(6):487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John SJ, Spector AC. 2008. Behavioral analysis of taste function in rodent models. In: Firestein S, Beauchamp GK, editors. The senses: a comprehensive reference, vol 4, olfaction & taste. San Diego, CA: Academic Press; p. 409–428. [Google Scholar]
- Stafstrom-Davis CA, Ouimet CC, Feng J, Allen PB, Greengard P, Houpt TA. 2001. Impaired conditioned taste aversion learning in spinophilin knockout mice. Learn Mem. 8(5):272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton JR, Luellig M, Roper SD, Delay ER. 2002. Discrimination between the tastes of sucrose and monosodium glutamate in rats. Chem Senses. 27(4):375–382. [DOI] [PubMed] [Google Scholar]
- Stapleton JR, Roper SD, Delay ER. 1999. The taste of monosodium glutamate (MSG), L-aspartic acid, and N-methyl-D-aspartate (NMDA) in rats: are NMDA receptors involved in MSG taste? Chem Senses. 24(4):449–457. [DOI] [PubMed] [Google Scholar]
- Tokita K, Boughter JD., Jr 2012. Sweet-bitter and umami-bitter taste interactions in single parabrachial neurons in C57BL/6J mice. J Neurophysiol. 108(8):2179–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokita K, Yamamoto T, Boughter JD., Jr 2012. Gustatory neural responses to umami stimuli in the parabrachial nucleus of C57BL/6J mice. J Neurophysiol. 107(6):1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S. 1991. Basic properties of umami and effects on humans. Physiol Behav. 49(5):833–841. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Matsuo R, Fujimoto Y, Fukunaga I, Miyasaka A, Imoto T. 1991. Electrophysiological and behavioral studies on the taste of umami substances in the rat. Physiol Behav. 49(5):919–925. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Watanabe U, Fujimoto M, Sako N. 2009. Taste preference and nerve response to 5’-inosine monophosphate are enhanced by glutathione in mice. Chem Senses. 34(9):809–818. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yuyama N, Kato T, Kawamura Y. 1985. Gustatory responses of cortical neurons in rats. III. Neural and behavioral measures compared. J Neurophysiol. 53(6):1370–1386. [DOI] [PubMed] [Google Scholar]
- Yasumatsu K, Ogiwara Y, Takai S, Yoshida R, Iwatsuki K, Torii K, Margolskee RF, Ninomiya Y. 2012. Umami taste in mice uses multiple receptors and transduction pathways. J Physiol. 590(Pt 5):1155–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuo T, Kusuhara Y, Yasumatsu K, Ninomiya Y. 2008. Multiple receptor systems for glutamate detection in the taste organ. Biol Pharm Bull. 31(10):1833–1837. [DOI] [PubMed] [Google Scholar]
- Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. 2011. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci U S A. 108(13):5431–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhang F, Zhang Q, Lu Y, Liu Q, Wang P. 2013. Umami evaluation in taste epithelium on microelectrode array by extracellular electrophysiological recording. Biochem Biophys Res Commun. 438(2):334–339. [DOI] [PubMed] [Google Scholar]
- Zhang F, Klebansky B, Fine RM, Xu H, Pronin A, Liu H, Tachdjian C, Li X. 2008. Molecular mechanism for the umami taste synergism. Proc Natl Acad Sci U S A. 105(52):20930–20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. 2003. The receptors for mammalian sweet and umami taste. Cell. 115(3):255–266. [DOI] [PubMed] [Google Scholar]
- Zukerman S, Ackroff K, Sclafani A. 2011. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol Regul Integr Comp Physiol. 301(6):R1635–R1647. [DOI] [PMC free article] [PubMed] [Google Scholar]