Abstract
Background
Although studies have established the safety and feasibility of physical therapy in the critical care setting, minimal information about physical therapist practice in the neurological intensive care unit (NICU) is available.
Objective
This study describes physical therapists' treatment of people admitted to a NICU.
Design
People admitted to the NICU with a diagnosis of subarachnoid hemorrhage, subdural hematoma, intracranial hemorrhage, or trauma were retrospectively studied.
Methods
Data on patient demographics, use of mechanical ventilation, and intracranial pressure (ICP) monitoring were collected. For each physical therapy session, the length of the session, the location (NICU or post-NICU setting), and the presence of mechanical ventilation or ICP monitoring were recorded. Data on safety parameters, including vital sign response, falls, and dislodgement of lines, were collected.
Results
Over 1 year, 180 people were admitted to the NICU; 86 were evaluated by a physical therapist, for a total of 293 physical therapy sessions in the NICU (n=132) or post-NICU setting (n=161). Only one session (0.3%) was stopped, secondary to an increase in ICP. The first physical therapy session occurred on NICU day 3.0 (25%–75% interquartile range=2.0–6.0). Patients received a median of 3.4 sessions per week (25%–75% interquartile range=1.8–5.9). Patients with mechanical ventilation received less frequent physical therapy sessions than those without mechanical ventilation. Patients with ICP monitoring received less frequent sessions than those without ICP monitoring. However, after multivariate analysis, only the admission Glasgow Coma Score was independently associated with physical therapy frequency in the NICU. Patients were more likely to stand, transfer, and walk in the post-NICU setting than in the NICU.
Limitations
The results are limited by the retrospective, single-center nature of the study. There is inherent bias of evaluating only those patients who had physical therapy, and therapists were unable to completely adjust for the severity of illness of a given patient.
Conclusions
Physical therapy was performed safely in the NICU. Patients who required invasive support received less frequent physical therapy.
Growing evidence supports early mobilization and physical therapy to improve both short and long-term physical function in patients who are critically ill. Muscle strength decreases 1% to 2% daily in patients who are critically ill and confined to bed, and early interventions appear to limit the long-term effects of neurological intensive care unit (NICU)–associated weakness.1,2 In a recent systematic review, early mobilization of patients who required mechanical ventilation improved muscle strength, increased ventilator-free days, and decreased length of hospital stay.3 Patients who received physical therapy while critically ill had improved long-term outcomes, including the ability to perform independent activities of daily living after hospital discharge.2 Importantly, physical therapy can be performed safely for most patients who are critically ill.1,3
Despite this growing body of evidence supporting early physical therapy for patients who are critically ill, the efficacy and safety of early physical therapy remain unclear with respect to people who have acute nervous system injury. People are often admitted to the NICU with the diagnosis of acute ischemic stroke, subdural hematoma (SDH), subarachnoid hemorrhage (SAH), intracranial hemorrhage (ICH), hydrocephalus, or traumatic brain injury. Patients who require neurocritical care differ from patients who have other critical illnesses in specific and important ways. For example, patients in the neurocritical care setting often require specialized and invasive monitoring, such as intracranial pressure (ICP) monitoring. Such monitoring can limit physical therapy interventions. Indeed, physical therapy may increase the ICP and be contraindicated for some patients in the neurocritical care setting. Additionally, people with acute nervous system injury often have altered motor control, which can have immediate and profound effects on balance, mobility, and the ability to perform skilled movements. Alterations in muscle tone may impair range of motion (ROM) more rapidly in people with central nervous system impairments. Moreover, such people frequently have specific and debilitating impairments of perception and cognition that may affect the implementation of early physical therapy.4
The focus of neurocritical care is to attempt to reduce the chronic dysfunction and debilitation of acute neurological diseases, and some evidence supports the use of intensive physical therapy in the neurocritical care setting.5 For example, a randomized controlled trial of early physical therapy within 24 hours of admission in people who experienced a stroke suggested improved functional recovery. However, that study excluded people who required admission to the NICU.6,7 In a carefully selected cohort of people with SAH and normal ICP, early physical therapy was performed safely and did not significantly change the ICP with ROM or limb exercises.8,9 Despite these studies, data about the implementation, effects, and safety of physical therapy for patients in the NICU are scarce.
The purpose of this report is to describe the current physical therapist interventions for people with SAH, SDH, ICH, or trauma in a NICU in a large university teaching hospital. We hypothesized that people admitted to the NICU with invasive monitoring or mechanical ventilation would receive physical therapy safely but might receive less frequent and less intensive physical therapy than people without such invasive support.
Method
Design
We conducted a retrospective cohort study to describe the current physical therapist interventions received by people admitted to a NICU and through their acute care hospitalization. Adults initially admitted to the NICU at a university hospital from January 1, 2012, until December 31, 2012, with a primary diagnosis of SAH, SDH, ICH, or trauma were identified from hospital admission records. Physical therapy department administrative records identifying people who received physical therapy orders during their hospitalization were cross-referenced with NICU admission records. From this group of people, those with active physical therapist consultations were included for further review. People younger than 18 years of age were excluded.
The NICU consists of 10 beds primarily used for patients who need neurosurgical and neurological care. It is staffed by one physical therapist Monday through Friday, with weekend coverage determined by the primary physical therapist. Additional physical therapists can be added to the unit as needed.
Data Collection
Administrative data, physician notes, physical therapist notes, nursing flow sheets, and respiratory therapist flow sheets were extracted from electronic medical records. Data were extracted by a single person (P.D.S.). We did not perform a prospective audit of data entry to evaluate the accuracy of the retrospective data. Basic characteristics—including age, sex, race, admission diagnosis, admission Glasgow Coma Score (GCS), tracheostomy, craniotomy, date of physical therapist consultation, post–acute care discharge location (home, skilled nursing facility, long-term acute care setting, inpatient rehabilitation setting, or another hospital), and mortality—were extracted. The duration of mechanical ventilation, the type and duration of ICP monitoring, and the lengths of NICU and hospital stays also were collected. Ventilator and NICU days were calculated.
For each physical therapy session, data on safety parameters and their effects on the physical therapy session were collected; these data included changes in vital signs, arrhythmia, changes in the ICP, falls, and dislodgement of lines. Physical therapists and bedside nurses generally decided the safety of initiating a given therapy session. Additionally, date and location of therapy (NICU or post-NICU setting [ie, after NICU discharge to the floor or ward but before discharge from the acute care setting]), mean GCS on the day of each session, duration of each session, and the presence of mechanical ventilation or ICP monitoring were obtained from physical therapist notes. The types of therapy performed were categorized as ROM, bed-based interventions (sitting at the edge of the bed, posture activities, or bed weights), transferring (sitting or standing), standing, and ambulating.
Data Analysis
Characteristics are reported as mean and standard deviation or as median and 25% to 75% interquartile range (IQR). Outcome variables included the following: time to first physical therapy session, frequency of physical therapy sessions per week (in the NICU and over the course of the hospitalization), median duration of sessions, and types of interventions at each session. Data were stratified between NICU sessions and post-NICU sessions, sessions with ventilation and sessions without ventilation in the NICU, and sessions with ICP monitoring and sessions without ICP monitoring in the NICU. All outcomes were compared with Wilcoxon rank sum tests. To adjust for potential confounders, we performed linear regression analysis, while adjusting for age, admission GCS, craniotomy, presence of mechanical ventilation, and presence of ICP monitoring, to compare the frequency of physical therapy in the NICU with that during the hospitalization. Similarly, we performed logistic regression analysis, while adjusting for age, craniotomy, presence of mechanical ventilation, and presence of ICP monitoring, to determine the odds of particular physical therapist interventions occurring in the NICU. Data were analyzed with JMP 10.0 (SAS Institute Inc, Cary, North Carolina).
Role of the Funding Source
Funding was provided by National Institutes of Health grants R01 NR011051 and K24 HL089223.
Results
Characteristics of Patients
Over the course of 1 year, 180 people were admitted to the NICU with SAH, SDH, ICH, or trauma; 87 (48%) received a physical therapist consultation, and 86 were formally evaluated by a physical therapist. The primary diagnoses in these 86 patients were SAH (n=42), SDH (n=17), ICH (n=19), and trauma (n=8) (Tab. 1). The average age was 60 years (SD=17), 45 patients (52%) were men, and 68 patients (79%) were white. The admission GCS was 14.1 (25%–75% IQR=9.7–15) and did not vary significantly by age (P=.48) or sex (P=.20). Overall, 50 patients (58%) required mechanical ventilation for a median of 3.0 days (25%–75% IQR=1.0–12.0), and 35 patients (41%) required ICP monitoring or treatment. Of these, 21 had external ventricular drains, 4 had ICP monitoring devices, 9 had subdural evacuating port systems, and 1 had a lumbar drain. Twenty-three patients (27%) required a craniotomy. The median NICU length of stay was 9.0 days (25%–75% IQR=3.0–14.0), and the median hospital length of stay was 12.0 days (25%–75% IQR=6.0–20.3). Eight patients (9%) died, 46 (53%) were discharged to home, 7 (8%) were discharged to a skilled nursing facility, 12 (14%) were discharged to a long-term acute care setting, 11 (13%) were discharged to an acute care rehabilitation setting, and 3 (3%) were discharged to another acute care hospital.
Table 1.
Characteristics of Patients by Diagnosisa
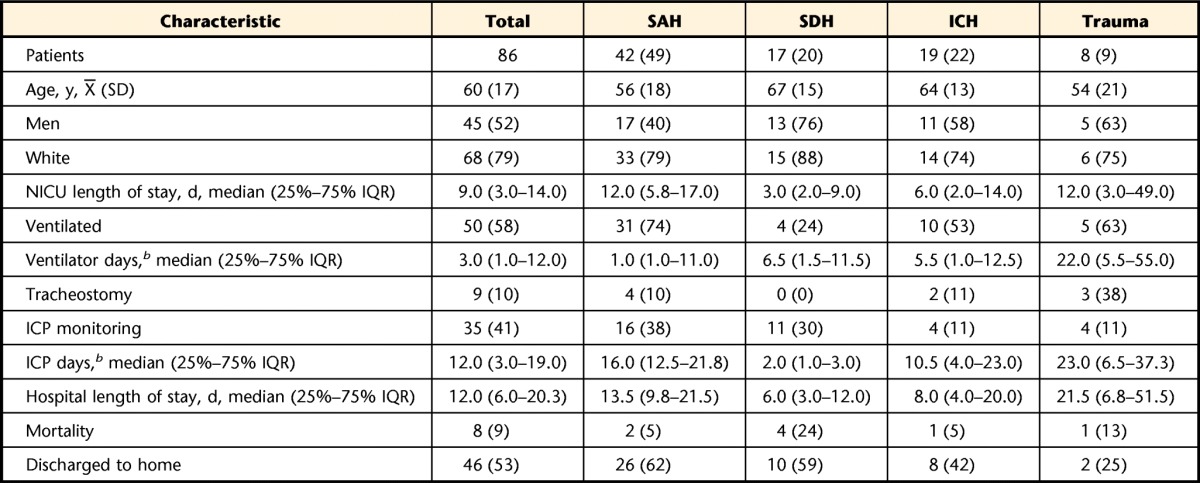
Data are numbers (percentages) of patients unless otherwise indicated. SAH=subarachnoid hemorrhage, SDH=subdural hematoma, ICH=intracranial hemorrhage, NICU=neurological intensive care unit, IQR=interquartile range, ICP=intracranial pressure.
b Ventilator days and ICP days were calculated for patients who needed mechanical ventilation or ICP monitoring only.
Safety
Eighty-six patients received 293 physical therapy sessions. In only 5 of these sessions (2.0%) was a change in the patient's condition or vital signs noted. Only 1 of these 5 sessions (0.3% of total sessions) was stopped because of a change in the patient's status—in this case, an elevation of the ICP in the NICU. In 3 of these 5 sessions (1.0% of total sessions), the patient became hypoxic and needed increased oxygen delivery via a nasal cannula; 1 of these episodes occurred in the NICU. In 1 of these 5 sessions (0.3% of total sessions), the patient became hypertensive in a non-NICU setting; the hypertension was treated with oral medications. No invasive monitors, airways, or central venous access lines were dislodged during physical therapy. No falls occurred during physical therapy. In 2 additional sessions (0.7%), the patient's ability to ambulate was limited by equipment (ICP monitor and arterial line). One additional session was limited by the patient's discomfort. Consequently, 8 sessions were noted as being limited.
NICU Versus Post-NICU Setting
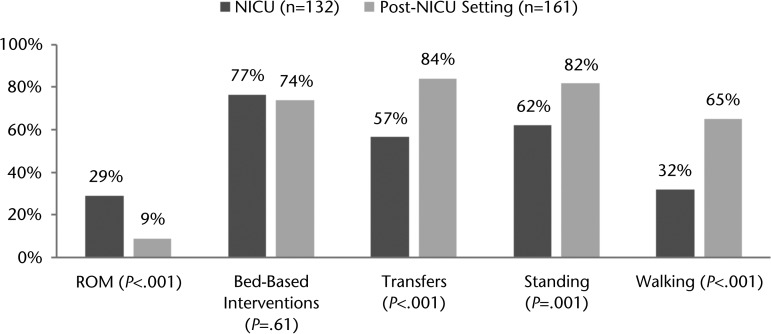
Of the 86 patients who were evaluated by a physical therapist, 75 had physical therapist consultations and 60 received physical therapy while in the NICU. The remaining patients had physical therapist consultations and evaluations after transfer to the post-NICU setting but before hospital discharge. The median time to ordering of a physical therapist consultation was 1.0 day (25%–75% IQR=0.0–3.0) after NICU admission, and physical therapy sessions started at hospital day 3.0 (25%–75% IQR=2.0–6.0). Patients with a GCS of ≤8 started physical therapy later than those whose GCS was greater than 8 (10 days [25%–75% IQR=3–19.5] versus 3 days [25%–75% IQR=1–5], P<.001). In total, there were 293 physical therapy sessions; 132 occurred in the NICU and 161 occurred in the post-NICU setting. Patients received a median of 3.4 sessions per week (25%–75% IQR=1.8–5.9) once physical therapy was initiated. Patients in the NICU received less frequent physical therapy sessions per week than did those in the post-NICU setting (2.1 [25%–75% IQR=1.2–5.1] versus 5.3 [25%–75% IQR=3.5–7.0], P<.0001). Overall, physical therapy sessions lasted a median of 25.0 minutes (25%–75% IQR=15.5–30.0) and did not differ significantly in length between the NICU and the post-NICU setting (P=.12). Physical therapists in the NICU were more likely to perform ROM interventions than those in the post-NICU setting (29% versus 9% of sessions, P<.0001). Standing, transferring, and gait training were less likely to be performed in the NICU than in the post-NICU setting (Fig. 1).
Figure 1.
Percentage of physical therapy sessions with specific interventions in the neurological intensive care unit (NICU) and in the post–neurological intensive care unit (Post-NICU) setting. ROM=range of motion.
Ventilated Versus Not Ventilated
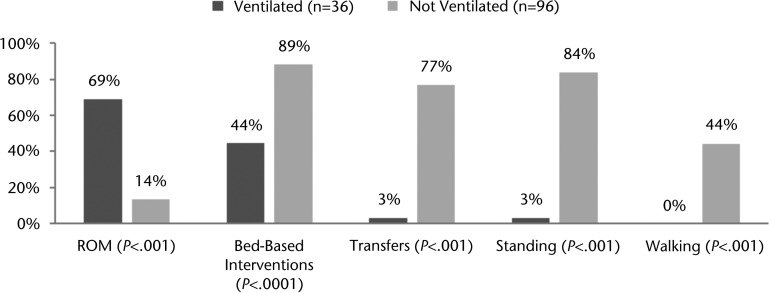
Of the 50 patients who required mechanical ventilation, 20 (40%) received physical therapy while still ventilated, for a total of 36 physical therapy sessions. The median time to ordering of a physical therapist consultation was similar for patients requiring mechanical ventilation in the NICU and those who did not require mechanical ventilation in the NICU (1.0 day [25%–75% IQR=0.0–4.0] versus 1.0 day [25%–75% IQR=0.0–2.8], P=.35), but patients needing mechanical ventilation started physical therapy later (5.0 days [25%–75% IQR=2.0–13.5] versus 2.0 days [25%–75% IQR=1.0–3.8], P=.0001). Patients who received mechanical ventilation and physical therapy had a lower median GCS at each physical therapy session than those who did not receive mechanical ventilation (9.3 [25%–75% IQR=6.9–10.3] versus 14.8 [25%–75% IQR=14–15], P<.001). Physical therapy sessions for patients needing mechanical ventilation were not statistically shorter than those for patients not needing mechanical ventilation in the NICU (20.0 minutes [25%–75% IQR=15.0–25.0] versus 25.0 minutes [25%–75% IQR=15.0–30.0], P=.06). Once physical therapy was initiated, patients who needed mechanical ventilation received less frequent physical therapy sessions per week in the NICU (1.9 [25%–75% IQR=1.2–3.5] versus 4.7 [25%–75% IQR=1.4–7.0], P=.01) and over the entire course of the hospitalization (2.8 [25%–75% IQR=1.4–4.5] versus 4.4 [25%–75% IQR=2.2–7.0], P=.01) than those who never required mechanical ventilation (Tab. 2). Physical therapy sessions in the NICU for patients requiring mechanical ventilation were more likely to include ROM interventions than physical therapy sessions for patients who did not need mechanical ventilation (68% versus 13% of sessions, P<.0001). All other interventions, including bed-based interventions, transferring, standing, and ambulating, were more likely to occur with patients who did not require mechanical ventilation (Fig. 2).
Table 2.
Characteristics of Patients in the NICU Stratified by Ventilator Status and ICP Monitoringa
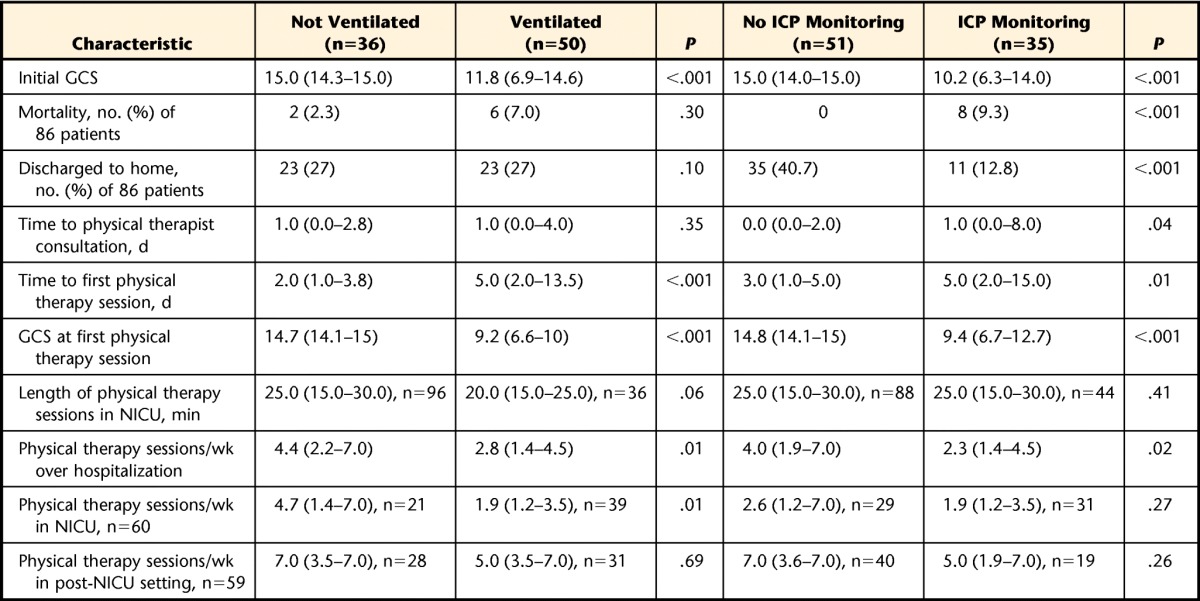
Data are reported as median (25%–75% interquartile range) unless otherwise indicated. NICU=neurological intensive care unit, ICP=intracranial pressure, GCS=Glasgow Coma Score.
Figure 2.
Percentage of physical therapy sessions with specific interventions in the neurological intensive care unit by mechanical ventilation. ROM=range of motion.
ICP Monitoring Versus No ICP Monitoring
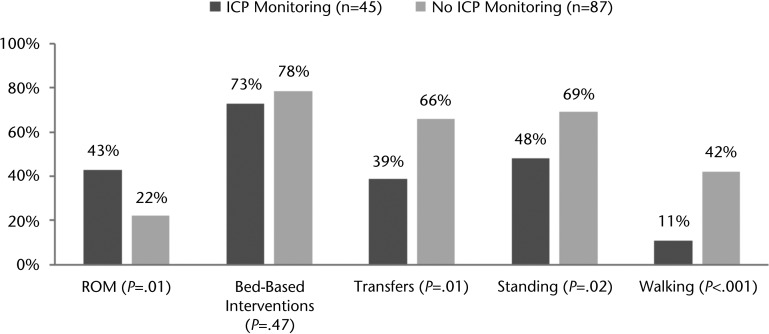
Of the 35 patients who required ICP monitoring, 23 (66%) received physical therapy while the ICP monitor was still in place, for a total of 44 physical therapy sessions in the NICU and 1 session with a lumbar drain in the post-NICU setting. The median time to ordering of a physical therapist consultation was delayed in patients receiving ICP monitoring relative to those not receiving ICP monitoring in the NICU (1.0 day [25%–75% IQR=0.0–8.0] versus 0.0 day [25%–75% IQR=0.0–2.0] after NICU admission, P=.04). Patients who required ICP monitoring started physical therapy later in their NICU course than those who did not need ICP monitoring in the NICU (5.0 days [25%–75% IQR=2.0–15.0] versus 3.0 days [25%–75% IQR=1.0–5.0] after NICU admission, P=.007). Individuals who required ICP monitoring during physical therapy sessions had a lower median GCS for each physical therapy session than those who did not require ICP monitoring (12.7 [25%–75% IQR=8.4–14.9] versus 14.7 [25%–75% IQR=13.2–15.0], P <.001). The durations of physical therapy sessions were similar for patients who required ICP monitoring and those who did not. Once physical therapy was initiated, patients who received ICP monitoring had rates of physical therapy in the NICU that were similar to those for patients who did not receive monitoring (1.9 [25%–75% IQR=1.2–3.5] versus 2.6 [25%–75% IQR=1.2–7.0], P=.27) but had less frequent physical therapy over the course of the hospitalization (2.3 [25%–75% IQR=1.4–4.5] versus 4.0 [25%–75% IQR=1.9–7.0], P=.02) (Tab. 2). Physical therapy sessions with ICP monitoring were more likely to include ROM interventions than those without ICP monitoring (43% versus 22% of sessions, P=.0135). Bed-based interventions were equally likely in physical therapy sessions with ICP monitoring and those without ICP monitoring. Other interventions, including transferring, standing, and walking, were less likely in physical therapy sessions with ICP monitoring than in those without ICP monitoring (Fig. 3).
Figure 3.
Percentage of physical therapy sessions with specific interventions in the neurological intensive care unit by intracranial pressure (ICP) monitoring. ROM=range of motion.
Multivariate Analysis
We performed multivariate analysis to assess the frequency of physical therapy both in the NICU and over the entire course of the hospitalization, while controlling for age, admission GCS, craniotomy, use of mechanical ventilation, use of ICP monitoring, and ability of the patient to follow instructions in the NICU. Only the admission GCS remained independently associated with less frequent physical therapy in the NICU (0.24 [25%–75% IQR=0.03–0.45], P=.02). None of the variables remained independently associated with overall physical therapy frequency over the course of the hospitalization.
For the physical therapy sessions in the NICU, we performed a similar analysis, while controlling for age, craniotomy, use of mechanical ventilation, and use of ICP monitoring, to analyze the odds of various interventions being performed. Only the 102 physical therapy sessions in which patients could follow instructions in the NICU were included because patients who could not follow instructions received only passive ROM and bed-based interventions. In this analysis, use of mechanical ventilation remained independently associated with more ROM interventions (odds ratio=20.85; 95% confidence interval=3.89, 144.50; P=.01) and fewer standing interventions (odds ratio=0.008; 95% confidence interval=0.0003, 0.07; P<.001) or transferring interventions (odds ratio=0.02; 95% confidence interval=0.001, 0.16; P<.001). For patients requiring mechanical ventilation, no physical therapy sessions included ambulation (Tab. 3). Intracranial pressure monitoring was associated with less ambulation (odds ratio=0.18; 95% confidence interval=0.05, 0.51; P=.01).
Table 3.
Logistic Regression of Physical Therapy Interventions in the NICU at a Given Sessiona
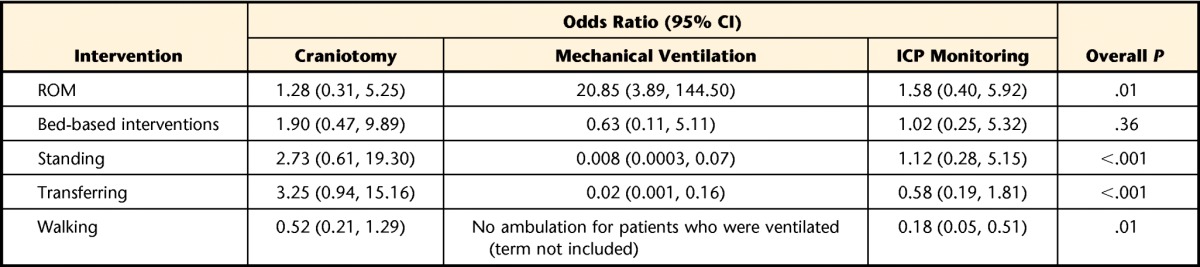
Age was not significantly associated with any intervention. NICU=neurological intensive care unit, CI=confidence interval, ICP=intracranial pressure, ROM=range of motion.
Discussion
We analyzed physical therapist treatment of 86 patients admitted to a university NICU with SAH, SDH, ICH, or trauma with regard to safety, NICU or post-NICU status, and invasive support and monitoring. First, we found that physical therapy can be safely performed in the NICU. Only a single treatment session (out of 293 reviewed) was discontinued, secondary to an increase in the ICP. There were no reports of adverse events associated with a physical therapy session. Second, physical therapy was performed less frequently and with a lower intensity in the NICU than in the post-NICU setting. Finally, patients requiring mechanical ventilation or ICP monitoring received less frequent and less intensive physical therapy than those who did not require mechanical ventilation or ICP monitoring.
Growing evidence supports early physical therapy in other populations of patients in the NICU, most notably in medical and surgical ICUs. Multiple retrospective and prospective studies have demonstrated the feasibility and safety of early, intensive physical therapy in this setting. A recent systemic review of 17 previous studies concluded that early physical therapy in this setting could be performed safely and likely resulted in improved outcomes.4,10 To this end, authors have argued for daily physical therapy in the NICU for patients who are critically ill.11 Currently, 2 randomized controlled trials are actively enrolling patients in medical NICUs to assess the benefits of early physical therapy.9 The participants in these trials are randomized to receive daily physical therapy focusing on breathing exercises, strength exercises, mobility activities, and ROM once they are able to follow instructions in the NICU.
However, data about physical therapy in the NICU are scarce. The present, descriptive study, in which the characteristics of physical therapist treatment and safety were examined, furthers the understanding of the current practice in a large medical center NICU. The present study adds to the work of Brimioulle et al,9 who demonstrated, for 65 people with and without normal ICP, that most physical therapy exercises—excluding isometric hip flexion—could be performed without significant changes in the ICP. Similarly, Olkowski et al8 demonstrated, for 25 people with SAH and a low risk for ischemic complications, that physical therapy could safely be started at hospital day 3. In contrast to Olkowski et al,8 who developed an early mobility program and prospectively examined safety and feasibility for patients who had SAH, we retrospectively examined physical therapist practice for a more diverse population of patients (with SAH, SDH, and ICH), who required mechanical ventilation, in the NICU. Additionally, we describe the effects of mechanical ventilation and ICP monitoring on physical therapist practice in the NICU. In general, early physical therapy in the NICU is associated with adverse events in 1% to 16% of physical therapy sessions.12 In the present study, only a single session of physical therapy was associated with a change in the ICP, and, in 4 other sessions, patients had easily corrected changes in vital signs.
Importantly, despite experiences with early and intensive physical therapy in surgical and medical NICUs, the present study demonstrated that earlier and potentially more intensive physical therapy is not being performed for patients in the NICU. We found that physical therapy started at a median of hospital day 3 and significantly later in patients requiring mechanical ventilation or ICP monitoring. In comparison, in the Very Early Rehabilitation Trial (AVERT) for stroke study, patients with stroke were randomized to receive mobilization within 24 hours of admission rather than the standard of care.6,7 Similarly, in studies of early physical therapy in the medical NICU with positive outcomes, physical therapy was started as early as 24 to 48 hours after intubation and focused on respiratory muscle strength, ROM, functional mobility training, and exercise training.13,14
Interestingly, although an admission GCS of 8 or less was associated with starting physical therapy later in the hospital course, the admission GCS and the GCS at each physical therapy session were lower in patients needing mechanical ventilation or ICP monitoring, suggesting that GCS alone was not a barrier to performing physical therapy. However, the admission GCS was independently associated with less frequent physical therapy in the NICU. Together, these data potentially indicate that although the GCS is not a barrier to initiating an individual physical therapy session, it does influence the timing of physical therapy and the frequency at which physical therapy can be performed. A low GCS, together with requirements for mechanical ventilation and ICP monitoring, is a marker of disease severity and suggests that a patient may not be stable enough for early physical therapy. Additionally, although physical therapy can be performed on a patient with a low GCS, we can hypothesize that it is performed less frequently because of the increased nurse and physical therapist time needed to perform physical therapy with such a patient and insufficient staff to spend that time.
The present study demonstrated that most of the physical therapy performed in the NICU consisted of ROM or bed-based interventions. Functional activities, including gait training, were performed less frequently in the NICU than in the post-NICU setting. Our results stand in contrast to the recent trend toward early mobilization for patients who are critically ill; it has been shown that physical therapy—specifically, ambulation—can be more intensive in the NICU than in the post-NICU setting.15 Moreover, the present study demonstrated that gait training and functional activities occurred less frequently in patients requiring invasive support in the NICU, including mechanical ventilation or ICP monitoring. Although these findings were likely due, in part, to the severity of illness of a given patient in the NICU, we were unable to evaluate the specific reasoning behind an individual therapist's decision to perform certain interventions. Thus, patients in the NICU with, perhaps, the greatest risk for debilitation received less intensive physical therapy interventions than their counterparts.
Our study had multiple limitations. First, an inherent bias was introduced by the inclusion in our analysis of only 86 of 180 patients who received physical therapy instead of all those admitted to the NICU. Data were not obtained for patients who did not have a physical therapist consultation, and we are unable to speculate on the appropriateness of physical therapy for those patients. Second, our study was a retrospective chart review; therefore, we can report only association and not causation. Importantly, our data were limited by the information recorded in the medical records. Specifically, physical therapist interventions were broadly described in the medical records, limiting our ability to describe completely the differences in interventions between groups of patients. Third, we could not completely adjust for the impact of the severity of illness on the ability to initiate physical therapy. The physical therapists did not consistently document specific reasons for deeming patients too medically unstable to participate in physical therapy. Presumably, patients needing ICP monitoring or mechanical ventilation had more severe illness and may have been less ideal candidates for physical therapy. Instead, we adjusted for age, craniotomy, and GCS as indicators of the severity of illness. However, there still may have been additional, unknown confounders in our multivariate analysis. Finally, our data described physical therapist practice at a single academic institution and may not be applicable to other institutions.
We described the current physical therapist practice in the NICU at a single academic center and demonstrated that physical therapy can be safely performed in the NICU. Additionally, we demonstrated that physical therapy was performed less frequently and with less intensity in the NICU than in the post-NICU setting and was performed less frequently and with less intensity in patients requiring mechanical ventilation or ICP monitoring in the NICU than in those who did not require such support. Despite these findings, a better understanding about the safety of starting physical therapy earlier in the NICU is necessary. Although we demonstrated that the current intensity of physical therapy was safe and feasible, further study is needed to investigate whether the current intensity of therapy is sufficient to improve outcomes. Similarly, the necessary elements of physical therapist interventions have not been established. A better understanding of cognitive and perceptual deficits as well as the therapies for treating these deficits is needed. A randomized controlled trial is needed to evaluate these issues.
Footnotes
Dr Sottile, Dr Nordon-Craft, Dr Malone, Dr Schenkman, and Dr Moss provided concept/idea/research design. Dr Sottile, Dr Nordon-Craft, Dr Schenkman, and Dr Moss provided writing. Dr Sottile and Dr Moss provided data collection and analysis. Dr Moss provided project management, fund procurement, facilities/equipment, institutional liaisons, and administrative support. Dr Malone, Dr Luby, and Dr Moss provided consultation (including review of manuscript before submission).
This study was approved by the Colorado Multiple Institutional Review Board (COMIRB).
A poster discussion of this research was presented at the American Thoracic Society International Conference; May 16–21, 2014; San Diego, California.
Funding was provided by National Institutes of Health grants R01 NR011051 and K24 HL089223.
References
- 1. Morris PE, Herridge MS. Early intensive care unit mobility: future directions. Crit Care Clin. 2007;23:97–110. [DOI] [PubMed] [Google Scholar]
- 2. Garzon-Serrano J, Ryan C, Waak K, et al. Early mobilization in critically ill patients: patients' mobilization level depends on health care provider's profession. PM&R. 2011;3:307–313. [DOI] [PubMed] [Google Scholar]
- 3. Li Z, Peng X, Zhu B, et al. Active mobilization for mechanically ventilated patients: a systematic review. Arch Phys Med Rehabil. 2013;94:551–561. [DOI] [PubMed] [Google Scholar]
- 4. van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–318. [DOI] [PubMed] [Google Scholar]
- 5. Kitchener N, Hashem S, Wahba M, et al. Critical Care in Neurology. Flying Publisher & Kamps; 2012. [Google Scholar]
- 6. Cumming TB, Thrift AG, Collier JM, et al. Very early mobilization after stroke fast-tracks return to walking: further results from the phase II AVERT randomized controlled trial. Stroke. 2011;42:153–158. [DOI] [PubMed] [Google Scholar]
- 7. Bernhardt J, Dewey H, Thrift A, et al. A very early rehabilitation trial for stroke (AVERT): phase II safety and feasibility. Stroke. 2008;39:390–396. [DOI] [PubMed] [Google Scholar]
- 8. Olkowski BF, Devine MA, Slotnick LE, et al. Safety and feasibility of an early mobilization program for patients with aneurysmal subarachnoid hemorrhage. Phys Ther. 2013;93:208–215. [DOI] [PubMed] [Google Scholar]
- 9. Brimioulle S, Moraine JJ, Norrenberg D, Kahn RJ. Effects of positioning and exercise on intracranial pressure in a neurosurgical intensive care unit. Phys Ther. 1997;77:1682–1689. [DOI] [PubMed] [Google Scholar]
- 10. Perme C, Chandrashekar R. Early mobility and walking program for patients in intensive care units: creating a standard of care. Am J Crit Care. 2009;18:212–221. [DOI] [PubMed] [Google Scholar]
- 11. Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nordon-Craft A, Moss M, Quan D, Schenkman M. Intensive care unit–acquired weakness: implications for physical therapist management. Phys Ther. 2012;92:1494–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243. [DOI] [PubMed] [Google Scholar]
- 14. Pohlman MC, Schweickert WD, Pohlman AS, et al. Feasibility of physical and occupational therapy beginning from initiation of mechanical ventilation. Crit Care Med. 2010;38:2089–2094. [DOI] [PubMed] [Google Scholar]
- 15. Hopkins RO, Miller RR, Rodriguez L, et al. Physical therapy on the wards after early physical activity and mobility in the intensive care unit. Phys Ther. 2012;92:1518–1523. [DOI] [PubMed] [Google Scholar]