Abstract
Background
Duchenne muscular dystrophy (DMD), an inherited recessive X chromosome-linked disease, is the most severe childhood form of muscular dystrophy. Boys with DMD experience muscle loss, with infiltration of intramuscular fat into muscles.
Objectives
This case series describes the progression of DMD in boys using magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS). Magnetic resonance results are then compared with an established functional timed test.
Methods
Four boys with DMD and 4 healthy age-matched controls were chosen from a larger cohort. Boys with DMD were assessed at 4 time points over 2 years, with controls assessed at baseline only. Progression of the disease was documented by assessing the plantar flexors using MRI and MRS techniques and by assessing ambulation using the 30-Foot Fast Walk Test.
Results
Transverse relaxation time (T2) values were elevated in all boys with DMD at baseline. The lipid ratio increased rapidly as the disease progressed in 2 boys. Discrete changes in T2 in the other 2 boys with DMD indicated a slower disease progression. Magnetic resonance imaging and MRS allowed monitoring of the disease over all time periods regardless of ambulation status.
Limitations
The magnetic resonance data were collected with 2 different magnets at 2 different field strengths (1.5 and 3.0 T). Although we corrected for this difference, care must be taken in interpreting data when different image collection systems are used. This was a case series of 4 boys with DMD taken from a larger cohort study.
Conclusions
Magnetic resonance imaging and MRS are objective, noninvasive techniques for measuring muscle pathology and can be used to detect discrete changes in both people who are ambulatory and those who are nonambulatory. These techniques should be considered when monitoring DMD progression and assessing efficacy of therapeutic interventions.
Duchenne muscular dystrophy (DMD) is the most common and most severe childhood form of muscular dystrophy.1 It is an inherited recessive X chromosome-linked disease that occurs primarily in boys. In this disease, the absence of dystrophin, a large sarcolemmal protein, leads to an increased susceptibility to contraction-induced muscle damage. As boys with DMD become older, they experience progressive muscle loss, and muscles becomes progressively infiltrated with intramuscular fat.2 The clinical manifestation of DMD includes muscular weakness, activity restrictions, and loss of ambulation between the ages of 10 and 15 years. Males with DMD typically die in their early twenties as a result of cardiopulmonary complications. There is currently no known cure for DMD. However, many clinical trials to modify disease progression are under way.3–5
As new therapeutic strategies for DMD are developed, there is a need for new objective, sensitive measurement techniques that can be used to monitor disease progression and to assess the efficacy of these treatments. Although various types of clinical measurements are currently used, there are some drawbacks associated with each of them. Historically, clinical observations, timed functional measures, and manual muscle testing have been used to monitor DMD. However, these measures provide little information about the underlying muscle pathophysiology.6–11 Although serum creatine kinase, a marker of muscle damage, has been used as an endpoint measure in some clinical trials, this method lacks sensitivity, especially when there is a significant decrease in muscle mass.12–14 Muscle biopsies are commonly used to confirm a diagnosis of DMD and to provide information during clinical trials. However, this is an invasive technique that allows for the examination of only relatively small amounts of muscle tissue from selected portions of a muscle and may not be representative of the entire muscle.15 Therefore, finding a reliable, objective noninvasive measure to document disease progression has become essential as clinical trials move forward.
A recent development is the use of magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) techniques to examine skeletal muscles during disease progression in DMD. Magnetic resonance imaging creates images using a series of successive pulse sequences with varying lengths of echo time (TE) and repetition time (TR), resulting in changes of contrast and brightness of the acquired images. Longitudinal relaxation time (T1)-weighted MRI, which uses a short TE and short TR, has been used to monitor changes in muscle cross-sectional area. Transverse relaxation time (T2)-weighted MRI, which uses a longer TE and TR, has been implemented as a marker of muscle damage and inflammation. Although MRI creates images, MRS yields an assay of the elements that make up a sample by placing the sample in a strong, very uniform magnetic field and exposing it to electromagnetic energy that is in the radio frequency range. The different elements in the sample each resonate at a particular known frequency. In DMD research, MRS is used to measure intramuscular fat, a key pathophysiological feature of disease progression.16–19 Another advantage of MRI and MRS is that these techniques are noninvasive and can be used to examine multiple muscle groups simultaneously.
Together, MRI and MRS provide detailed information relevant to the examination of skeletal muscle in DMD. However, few studies have been published in which these techniques have been used in concert to examine disease progression in boys with DMD.12
The objective of this investigation was to describe the progression of DMD in humans using a combination of MRI and MRS measurement techniques. A secondary goal was to compare the results from MRI and MRS of the participants' performance of the 30-Foot Fast Walk Test, a standardized timed test of functional ambulation. To this end, 4 boys with DMD were assessed over a 2-year period using T1- and T2-weighted MRI and 1H-spectroscopy MRS.
Method
Consent for participation in the study was given by a parent or guardian of each boy at the initial visit. Participants were chosen from a larger cohort study illustrating the heterogeneity of this population and varying degrees of progression in similar age groups. Each boy with DMD was age matched with a healthy control. Magnetic resonance screening was performed to ensure safety prior to entering the magnet. The total time to conduct a combined MRI and MRS was 1.5 to 2 hours. Our examination specifically targeted the ankle plantar flexors, as they play a primary role in ambulation. After the MRI and MRS were completed, participants were assessed using the 30-Foot Fast Walk Test. In addition, qualitative muscle analyses of lower leg compartments were described.
Participants
The study participants were 4 boys with DMD and 4 healthy age-matched controls. The boys with DMD were tested at baseline and at 6, 12, and 24 months. The controls were tested once at baseline. Each participant was given an identifier, which denoted either DMD (Duchenne muscular dystrophy) or C (control) and the boy's age. Because 2 boys with DMD and their controls were the same age, a letter (A or B) was appended to the participant identifier.
All 4 boys with DMD were taking corticosteroids as part of their medical care at the time of the study; however, the dose and age of initiation of steroids varied across participants. Demographic information for the 4 boys with DMD and their aged-matched controls is presented in the eTable.
Participant DMD 8A was 8.1 years old at baseline. He was diagnosed with DMD at 6 years of age. He ambulated with increased lumbar lordosis, toe walking, and waddling side to side with a wide base of support to shift his weight over his feet. He frequently stopped to rest due to fatigue, holding on to the wall for balance when ambulating short distances. The Brooke Lower Extremity Functional Rating Scale (Brooke score) scores functional ability in the lower extremities (LEs) in people with neuromuscular disease.20 The participant's Brooke score was 4, representing that he could walk unassisted and rise from a chair but could not climb stairs. The combination of gait deviations seen in this individual is characteristic of this disease.21–23 Participant DMD 8A was the only participant who had muscle contractures of the heel cords, hamstrings, hip abductors, and hip flexors at the start of the study.
Participant DMD 8B was 8.9 years old at baseline, ambulated without difficulty, and was capable of completing the timed 30-Foot Fast Walk Test. His walking pattern did not demonstrate any apparent gait deviations at the initial time point, and his Brooke score was 1, indicating he could walk and climb stairs without assistance. He was diagnosed at age 4 years after the family noted he was having difficulty keeping up with his peers. His range of motion for the lower extremities was within normal limits.
Participant DMD 11 was 11. 9 years of age at baseline. He was diagnosed at 6 years of age after the family noticed he had difficulty keeping up with his peers. On the Brooke scale, he had a score of 1, indicating his ability to walk and ascend 4 stairs without assistance. His gait pattern by observation demonstrated a normal heel-toe pattern of progression across the floor without any gait deviations. He had no contractures at the time of entry into the study.
Participant DMD 13 was 13.9 years of age at baseline and ambulating, although slowly and cautiously, without an assistive device. He was diagnosed at 8 years of age after several years of treatment for coordination problems. His gait pattern demonstrated increased lumbar lordosis, in-toeing, and side-to-side waddling; however, no toe walking was observed. On the Brooke scale, participant DMD 13 had an initial score of 4, indicating he could walk unassisted and rise from a chair but could not climb stairs. He was without muscle contractures at the beginning of the study.
MRI and MRS
Magnetic resonance imaging of the lower leg muscles was performed on the right lower extremity with a Signa 1.5-T scanner (GE Healthcare, Waukesha, Wisconsin) for participants DMD 13 and DMD 8B. Participants DMD 8A and DMD 11 and all 4 control participants were tested with a 3-T Achieva Quasar dual imaging unit (Philips, Best, the Netherlands). All participants were placed supine in the scanner with their lower leg positioned in a lower extremity quadrature coil (1.5 T) or an 8-channel SENSE receive-only knee coil (3.0 T) (Invivo, Gainesville, Florida) during collection of data. For the imaging scans, the fields of view were optimized (calf: 12–14 cm2), and 3-dimensional gradient-echo imaging was performed to obtain transaxial, fat-suppressed, T1-weighted images (1.5-T system: TR=18 milliseconds, TE=3.5 milliseconds, flip angle=10°; 3T system: TR=24 milliseconds, TE=1.8 milliseconds, and flip angle=20°). Spin echo images were acquired to measure T2 (11–18 axial slices, slice thickness=7 mm; 1.5-T system: TR=2 seconds, 4 echoes, TE=26, 52, 78, and 104 milliseconds; 3-T system: TR=3 seconds, 5 echoes, TE=20, 40, 60, 80, and 100 milliseconds). For 1H-MRS measurements, a voxel (volume of ∼5,800 mm3) was selected using the transaxial, fat-suppressed T1-weighted images of the lower leg. The voxel was placed inside the soleus muscle, with care to avoid visible vasculature, subcutaneous fat, and myofascial divisions. Spectra were acquired using MRS with the following parameters: TR=3,000 milliseconds, TE=108 milliseconds, 64 scans, 2,084 data points, and spectral width=2,500 Hz.
MRI/MRS Data Analysis
The MRI and MRS analyses were carried out using methods and measures, described below, that have been shown to have excellent interrater reliability and day-to-day reproducibility.24 Individuals carrying out the analyses were appropriately trained and experienced. Furthermore, similar methods have been used by other groups.12,18
First, individual muscles (soleus, medial, and lateral gastrocnemius) were manually participant's outlined on all of each participant's fat-suppressed T1-weighted images using OsiriX software (version 3.8.1, http://www.osirix-viewer.com). The slice with the greatest cross-sectional area (CSA) was then identified for the soleus and medial and lateral gastrocnemius muscles.
Maximum CSA (CSAmax) was calculated for each muscle as the mean CSA from 3 consecutive slices (the greatest CSA, the slice proximal to the greatest CSA, and the slice distal to the greatest CSA). The CSAmax of the triceps surae muscle group in the posterior compartment of the lower leg was calculated by summing the CSAmax values of the soleus and medial and lateral gastrocnemius muscles. In addition to measuring muscle size, T2 has been shown to be sensitive to changes in muscle composition and involvement in people with DMD.25 Mean T2 values of the triceps surae muscles were calculated. Three axial slices of the lower leg were chosen in the region in which the most proximal slice of the flexor digitorum longus was visually present. T2 MRI maps were then developed using echoes 2 to 4 (1.5 T) or 2 to 5 (3.0 T) and fitting the signal intensity with a mono-exponential equation. Due to differences in T2 field strengths, a correction factor was applied. For this purpose, T2 measurements were acquired from a volunteer using the 1.5- and 3.0-T systems. The correction factor allowed for direct comparisons of both systems. Relative concentrations of water and lipid were determined from fitting the 1H-MRS data in the time domain using jMRUI software (developed by European Communities Project IMR/Networks ERB-FMRX-CT970160) and a lipid fraction (lipid/[lipid+water]) was then calculated. Water and lipid signals were corrected for partial saturation using T2 of 1H2O measured for each participant using stimulated echo acquisition mode and literature values for T1 and T2 of lipid and T1 of 1H2O.26,27
Timed Functional Tests
Boys with DMD and control participants were asked to perform the 30-Foot Fast Walk Test, which is a standard clinical measure of functional ability.8 The participants were asked to perform the task 3 times, as fast as they could without running. Participants were timed using a stopwatch. The best time from the 3 trials was used for analysis and comparison purposes.
Results
Participant DMD 8A
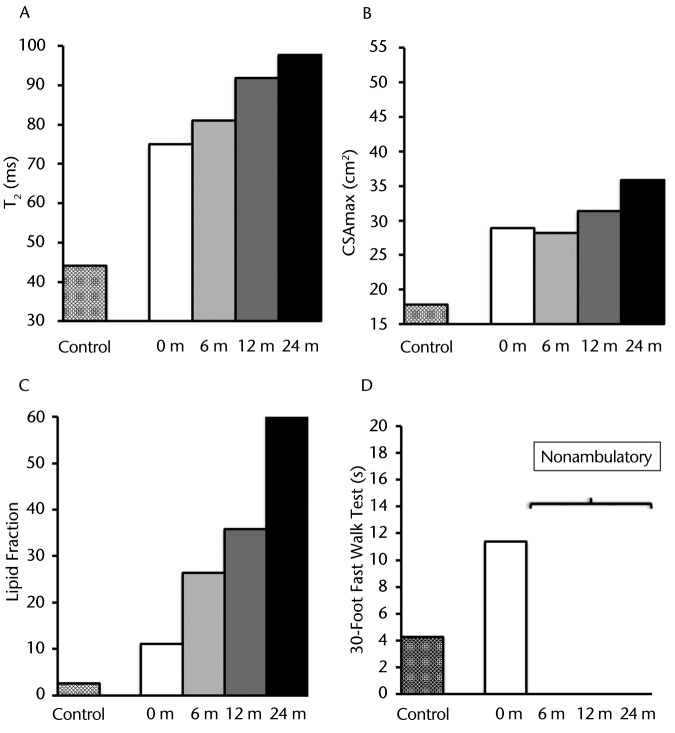
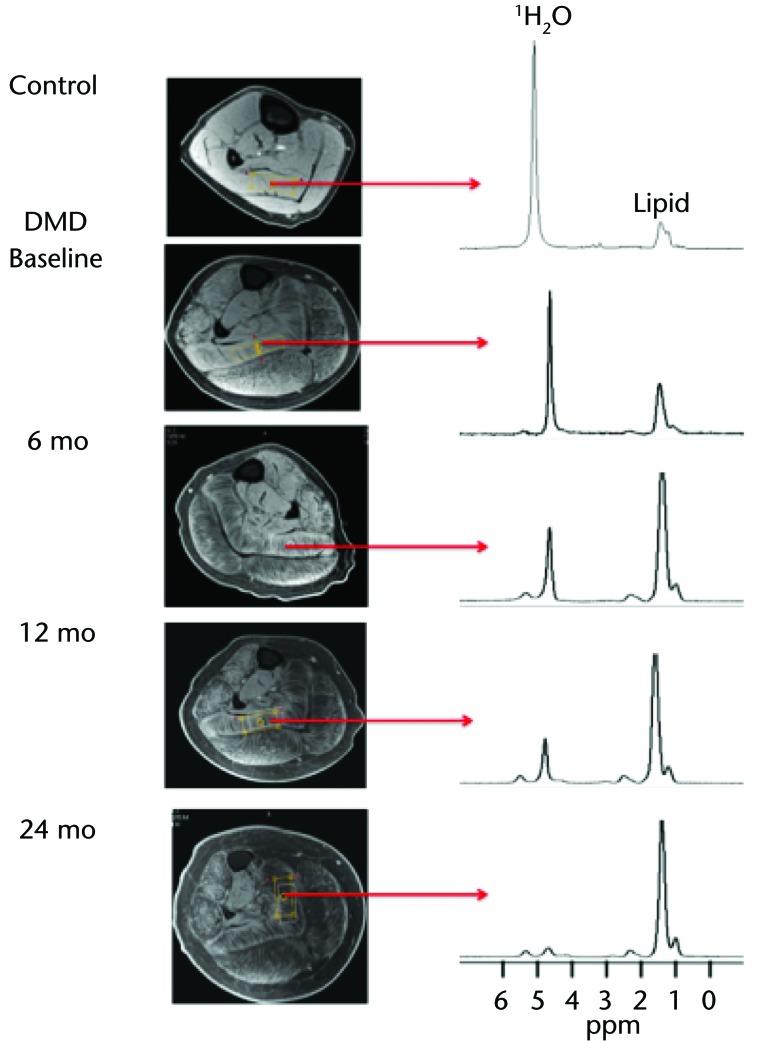
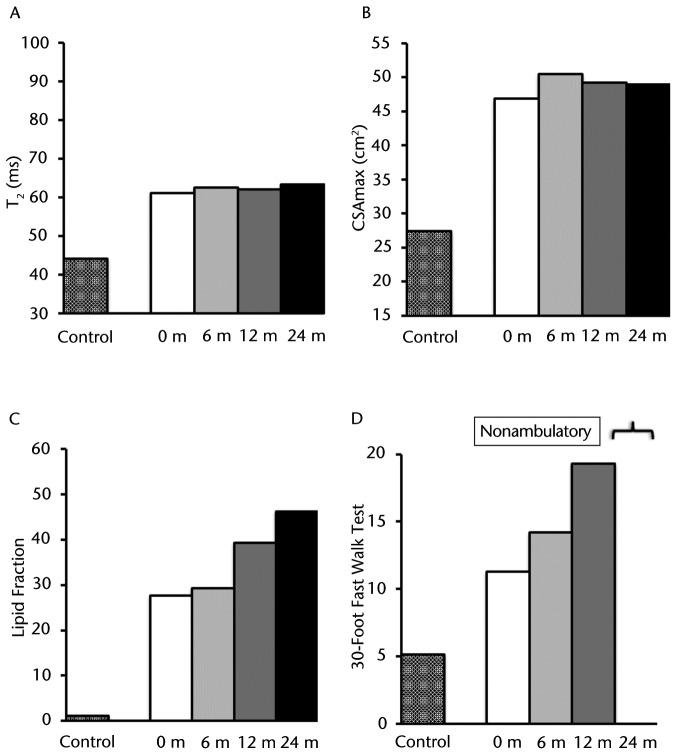
As illustrated in Figure 1A at baseline, the MRI T2 value in the plantar flexors for participant DMD 8A was greater than that of his age-matched control (participant C 8A), and these T2 values progressively increased over the 2-year period. Participant DMD 8A's CSAmax, as measured on T1-weighted images, was greater than that of participant C 8A at all time points. This value progressively increased approximately 1.25-fold between baseline and 24 months (Fig. 1B). At baseline, in the soleus muscle, intramuscular fat infiltration was greater than that of participant C 8A. Furthermore, the lipid fraction increased progressively and dramatically over the course of the study. As shown in Figure 1C, there was a 6.4-fold increase of total muscle infiltrate (70% at 24 months versus 11% at baseline) over 2 years. Figure 2 demonstrates the muscle composition of the lipid fraction using MR spectroscopy. Note the replacement of muscle by fat in the soleus muscle. This participant was able to complete the 30-Foot Fast Walk Test initially at a time that was considerably slower than that of the age-matched control (Fig. 1D). Shortly after the baseline assessment, this individual became nonambulatory and was unable to complete the walk on any of the subsequent assessments.
Figure 1.
Participant DMD 8A: (A) T2 (transverse relaxation time), a marker of muscle damage and inflammation; (B) CSAmax (maximal cross-sectional area); (C) lipid fraction presented as total percentage of muscle infiltrated with lipid; and (D) 30-Foot Fast Walk Test. These measurements are represented with different patterned columns for the baseline measure of the healthy age-matched control and participant with Duchenne muscular dystrophy (DMD) for baseline, 6, 12, and 24 months (except for the 30-Foot Fast Walk Test, which had only the baseline measure prior to becoming nonambulatory). This participant demonstrated a rapid disease progression over time across all measurements.
Figure 2.
Example of lipid changes over 2 years in the soleus muscle of participant DMD 8A compared with age-matched control at baseline. T1 fat-suppressed images with voxel placement and associated magnetic resonance spectroscopy for corresponding time points were used to demonstrate changes. Note the water peak decreased and the lipid peak increased over time in this individual, demonstrating the replacement of muscle by fat. The y-axis units of the spectra represent arbitrary units, and the values were quantified as a ratio lipid/(lipid+water) for comparisons. DMD=Duchenne muscular dystrophy.
Participant DMD 8B
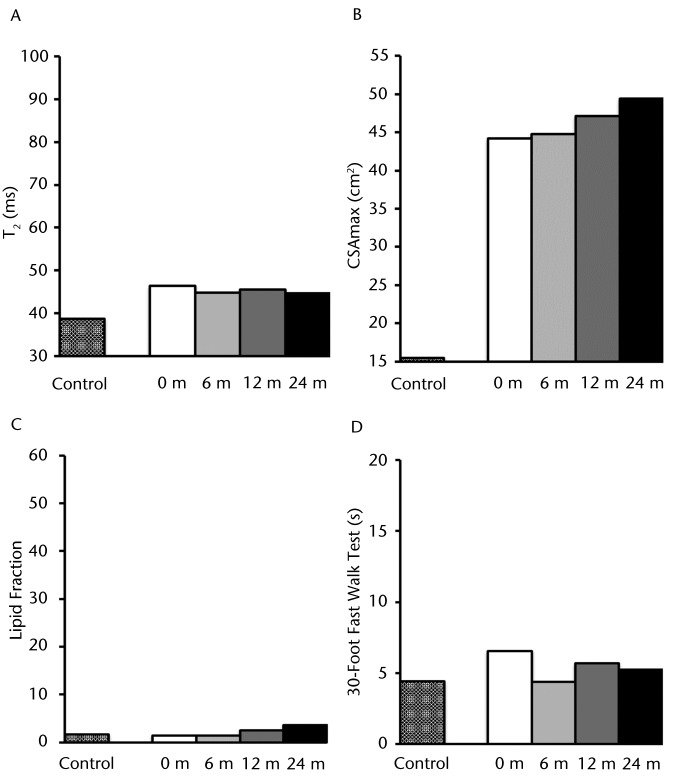
As illustrated in Figure 3A, the baseline T2 values from MRI of the plantar flexors for participant DMD 8B were modestly different from those of participant C 9B. However, the T2 values remained relatively stable over the 2-year period. The CSAmax of the plantar flexors initially was 2.85-fold higher than that of the control participant (44 cm2 compared with 16 cm2) and gradually increased during the subsequent 2 years (Fig. 3B). The baseline lipid fraction of the soleus muscle for this participant was essentially the same as that of participant C 9B, as demonstrated in Figure 3C. Although the lipid fraction increased over the 2-year period, the final level only slightly exceeded that of the control participant (4% of total muscle infiltrate). The time required by participant DMD 8B initially to complete the 30-Foot Fast Walk Test was slightly longer than that for the age-matched control participant (6.5 seconds versus 4.8 seconds); however, this time remained relatively constant over the 2-year period (Fig. 3D). In general, with the exception of an increase in CSAmax, these results indicate a rather mild progression of DMD in this participant relative to the control over 24 months (8–10 years old).
Figure 3.
Participant DMD 8B: (A) T2 (transverse relaxation time), a marker of muscle damage and inflammation; (B) CSAmax (maximal cross-sectional area); (C) lipid fraction presented as total percentage of muscle infiltrated with lipid; and (D) 30-Foot Fast Walk Test. These measurements are represented with different patterned columns for the baseline measure of the healthy matched control and participant with Duchenne muscular dystrophy (DMD) for baseline, 6, 12, and 24 months. Note that the lipid fraction and CSAmax demonstrated a gradual increase over the 2-year time period. However, the T2 values and 30-Foot Fast Walk Test remain fairly stable over this same time period.
Participant DMD 11
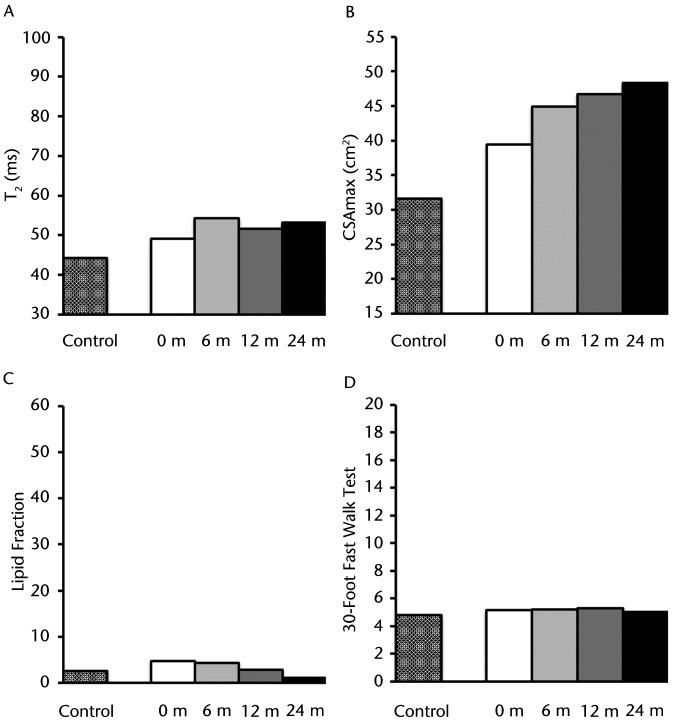
As illustrated in Figure 4A, baseline T2 values for the plantar flexors of participant DMD 11 were elevated over those of participant C 11 (49.1 milliseconds versus 44.2 milliseconds), with minimal change in this level during the following 24 months. The CSAmax for the plantar flexors was initially greater than that of the age-matched control participant, and muscle size continued to increase progressively over the subsequent 2 years (Fig. 4B). The lipid values of the soleus muscle at baseline were similar to those of the control participant (5% versus 3% of total muscle infiltrate), with values essentially unchanged over the 2-year period (Fig. 4C). Participant DMD 11 initially was able to complete the 30-Foot Fast Walk Test, comparable to participant C 11 (5.2 seconds versus 4.8 seconds); this time remained relatively constant over the 24 months (Fig. 4D). These results indicate that, except for the increase in muscle size, the disease in this participant remained reasonably stable.
Figure 4.
Participant DMD 11: (A) T2 (transverse relaxation time), a marker of muscle damage and inflammation; (B) CSAmax (maximal cross-sectional area), (C) lipid fraction presented as total percentage of muscle infiltrated with lipid; and (D) 30-Foot Fast Walk Test. These measurements are represented with different patterned columns for the baseline measure of the healthy matched control and participant with Duchenne muscular dystrophy (DMD) for baseline, 6, 12, and 24 months. Note that this individual remained stable across time periods except for an increase in CSAmax, demonstrating a slower progression of this disease.
Participant DMD 13
As illustrated in Figure 5A, the baseline T2 values of the plantar flexors for participant DMD 13 was greater than that of participant C 12 by 38% (61.1 milliseconds versus 44.2 milliseconds). These values did not change significantly over 24 months. The CSAmax of the plantar flexors for participant DMD 13 initially was 1.7-fold greater than that of the age-matched control participant, and muscle size remained relatively constant over the next 2 years (Fig. 5B). At baseline, in the soleus muscle, intramuscular fat infiltration was greater than that of participant C 12. As shown in Figure 5C, the baseline lipid fraction for the soleus muscle of this boy was greater than that of the control participant (28% versus 1% of total muscle infiltrate). These values continued to progressively increase in participant DMD 13 over the 2-year test period (46% versus 28% of total muscle infiltrate), as demonstrated in Figure 5C. The baseline time for this boy to complete the 30-Foot Fast Walk Test was 2.23-fold longer than that of the control participant (11.3 seconds versus 5.1 seconds). Furthermore, this time continued to steadily increase over the first year until the participant became nonambulatory and was unable to participate at the 2-year time point. These results suggest that there was a moderate amount of progression in participant DMD 13 over the 2-year period (13–15 years of age) compared with the other participants with DMD in this study.
Figure 5.
Participant DMD 13: (A) T2 (transverse relaxation time), a marker of muscle damage and inflammation; (B) CSAmax (maximal cross-sectional area); (C) lipid fraction presented as total percentage of muscle infiltrated with lipid; and (D) 30-Foot Fast Walk Test. These measurements are represented with different patterned columns for the baseline measure of the healthy matched control and participant with Duchenne muscular dystrophy (DMD) for baseline, 6, 12, and 24 months (except for the 30-Foot Fast Walk Test; 24 months, the participant was nonambulatory). Note the progressive nature of the lipid fraction and increase in time to perform the 30-Foot Fast Walk Test over the 2-year period. T2 and CSAmax remained relatively stable across time periods.
Qualitative Analysis of Lower Leg MR Images
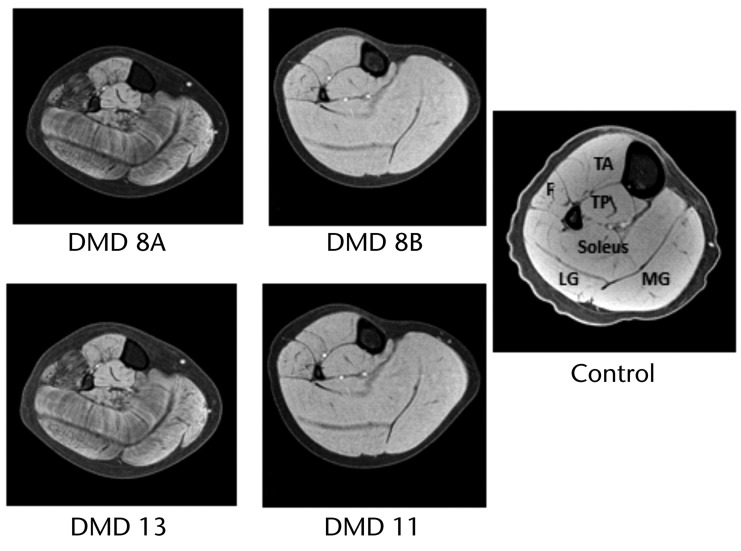
The T1-weighted MR images obtained at the 24-month assessment, showing fat suppression of all muscles of the lower leg, are presented for each boy with DMD in Figure 6. The image in Figure 6 for the control participant was obtained at baseline. In the figure, fat appears as an area of hypointensity, represented by dark streaks within the soleus muscle and dark speckled marbling in the gastrocnemius and fibularis muscle bellies (fibularis longus and brevis), with the exception of the fascial divides between individual muscles in these images. This hypointensity was noted in all images analyzed regardless of the field strength of the magnet used to acquire the images. This finding is indicative of disease progression as fat enters the muscle in this population.
Figure 6.
T1–weighted magnetic resonance images with fat suppression at the 2-year time point for all participants with Duchenne muscular dystrophy (DMD) were used for visual inspection, and the control image was taken at baseline. The individual muscles of the lower leg are indicated in the control image: F=fibularis muscles (fibularis longus and brevis), TA=tibialis anterior muscle, TP=tibialis posterior muscle, LG=lateral gastrocnemius muscle, and MG=medial gastrocnemius muscle. Note the general pattern of hypointensity with fibularis muscles most affected, soleus and gastrocnemius muscles intermediate, and tibialis posterior and tibialis anterior muscles least affected. These findings are indicative of the disease progression.
Visual inspection indicated that there was more fat infiltration in the lower leg muscles of participants DMD 8A and DMD 13 relative to the muscles of participants DMD 8B and DMD 11. This qualitative finding is confirmed by the quantitative results from MRS and presented above. More specifically, lipid fractions (percentage of lipid in the muscle) in the soleus muscle were 70% for participant DMD 8A, 46% for participant DMD 13, 4% for participant DMD 8B, and 1% for participant DMD 11. Although we observed qualitative differences between some muscles, we did not observe a difference between the soleus and gastrocnemius muscles in quantitative T2 (eg, at the 2-year time point in DMD, soleus muscle: X̅=63.6 milliseconds, SD=11.8; medial gastrocnemius muscle: X̅=62.5 milliseconds, SD=12.3; and lateral gastrocnemius muscle: X̅=66.9 milliseconds, SD=11.3). Therefore, we combined these muscles and reported the combined triceps surae muscle values for the T2 analysis.
Discussion
Previous studies have used MRI to investigate fat infiltration and patterns of muscle involvement across dystrophies and myopathies.28–31 Recent DMD studies have investigated the utility of MRI measures, such as fat fraction of the thigh and pelvic muscles, as markers of disease severity and as predictors of future loss of function.32–34 Magnetic resonance imaging has not been used routinely as a clinical measure in this population due, in part, to the high cost of imaging, the lack of data documenting longitudinal disease progression, and data showing a strong correlation of MRI and MRS measures with concurrent functional measures and future disease progression. In this study, we addressed this gap in the literature by describing the progression of DMD over a 24-month period in 4 boys who had different presenting DMD phenotypes and different ages. Assessments were performed over a 24-month period using noninvasive MRI and MRS techniques to gather data.
The results support our hypothesis that imaging is a useful tool for assessing the current DMD disease status and progression of the DMD disease process over time. The T2 values were elevated in all of the boys with DMD at baseline. The lipid ratio increased rapidly as the disease progressed in participants DMD 8A and DMD 13. In addition, the lipid ratio measure was able to detect discrete changes in participants DMD 8B and DMD 11, although the disease remained relatively stable in these 2 boys. When qualitative analyses of fat infiltration from MR images of the lower leg were performed, it appeared as though muscles lying close to the tibia and interosseous membrane were less affected in the boys with DMD. Taken together, these results suggest that MRI and MRS techniques may be useful in following the progression of DMD and that further studies are warranted.
Participants DMD 8A and DMD 13 demonstrated a progressive increase in lipid values, peaking at 70% and 46% of the total muscle, respectively, at the last time point, and both boys were nonambulatory at that time. Increasing fat infiltration likely contributed to the loss of ambulation in these boys. Akima et al35 reported a strong inverse relationship between noncontractile area and ambulatory function in boys with DMD. In contrast, in participants DMD 8B and DMD 11, the maximal lipid values were 1% and 4% of the total muscle, respectively, demonstrating a more stable disease process. Detecting variations in the pathophysiology of the muscles in this disease process affords a unique view of the disease progression that has not been available by other techniques.36,37 Overall, these results indicate that intramuscular fat deposition assessed using MRS is a highly sensitive way to follow the progression of DMD, even when changes are relatively small, and may provide an important predictive biomarker.16,36,37
In addition, as expected, higher T2 MRI values were seen in boys with DMD compared with matched controls. All boys with DMD demonstrated elevated T2 values at baseline, including those with a slower progression, compared with the controls. T2 maps, which are sensitive to muscle inflammation, damage, and intramuscular fat, varied among boys with differing rates of DMD progression.38 In boys with more rapidly developing disease, the T2 levels reached maximal values at the 2-year period of 60 to 98 milliseconds. This finding represents a 2-fold increase in participant DMD 8A over that of his age-matched control. Conversely, the T2 values in boys with more slowly progressing disease demonstrated a relatively constant T2, although elevated in comparison with controls at all time points.12,34
All boys with DMD were compared with age-matched controls and found to have higher CSAmax values independent of disease progression. One of the well-known clinical hallmarks of DMD is enlarged calf muscles. These results suggest that although calf muscle size increases with age in individuals with DMD as expected from growth, measurements of CSAmax may not be one of the more sensitive methods to track the advancement of DMD. Furthermore, it is important to note that this increase in CSAmax does not routinely translate to infiltration of fat in the muscle, as evidenced by the 2 slower progressing participants (DMD 8B and DMD 11) shown in Figures 1 and 3.
Another factor to consider when examining the results is the use of steroids. Participants DMD 8A and DMD 13 both began steroids at age 7 years, which was later than the other boys in the study (eTable). Although there is not a clear consensus on the age of initiation of corticosteroids or on the frequency of dosing, we note that changes in dosing and variations in frequency, which may have been related to side effects, were not documented in this case series. A recommended dose for prednisone of 0.75 mg per kilogram of body weight per day, or 0.9 mg per kilogram per day for deflazacort, has been published by Manzur et al39 and subsequently supported in recent literature.5,40,41 The boys in this case series were generally within those parameters, as noted in the eTable.
Qualitative analysis using visual inspection revealed that there was individual variability in the degree of fat deposition across all lower leg muscles and among boys. However, general patterns were observed in all boys. In lower leg muscles, the fibularis muscles (fibularis longus and fibularis brevis) appeared to be most affected. The soleus and intermediate gastrocnemius muscles and muscles lying close to the tibia and interosseous membrane (ie, tibialis posterior, long toe flexors, extensors, and tibialis anterior) appeared to be least affected. Torriani et al,18 using MRI and MRS, found that boys with DMD had the highest fat infiltration in the fibularis muscles. More recently, Lott et al42 demonstrated lipid to be greatest in the fibularis muscles for the lower leg using MRS. Further substantiating this finding, Willcocks et al38 demonstrated that T2 values were elevated significantly in the fibularis and soleus muscles.
A measured, timed walk test is typically used as an outcome measure in the DMD population. In this study, the 30-Foot Fast Walk Test was used for all boys. Two participants (DMD 8A and DMD 13) lost ambulation by the 6- and 24-month time points. With the loss of ambulation, the 30-Foot Fast Walk Test can no longer be utilized to follow disease progression or be predictive of pathology in these individuals. Magnetic resonance imaging and MRS allowed for the disease to be monitored over all time periods and for all participants (Figs. 1, 3, 4, and 5). Our results indicate that, regardless of the rate of disease progression, MRS and MRI may be especially valuable to follow progression of DMD in these instances, documenting subtle, discrete changes in the muscle pathophysiology.18,43,44
There were at least 3 limitations to our study. First, this was a case series with a limited number of participants taken from a larger cohort study. Second, the magnetic resonance data were collected with 2 different magnets at 2 different field strengths (1.5 and 3.0 T). Although we corrected for this difference, care should be taken in the interpretation of data when different image collection systems are used. Third, the controls in this study were imaged only at baseline. However, Mathur et al45 demonstrated there was a steady increase in CSAmax of the lower leg muscles across controls aged 5 to 14 years compared with individuals with DMD. Recently, Forbes et al24 demonstrated no change in lipid fraction or T2 in controls among age groups 5 to 7 years up to 11 to 14 years.24 Both studies support the use of a single baseline measure in controls. Despite these limitations, the results of this study provide unique longitudinal findings in children with DMD and should be of utility for future investigations.
Magnetic resonance imaging and spectroscopy may play a critical role in future clinical trials in their capability to assess muscular contractile tissue as an inclusion criterion for drug trials. Clinically, these measures may assist in determining the appropriate medication paired with the stage of disease progression established from imaging. Data gleaned from these measures may prove to be predictive of loss of ambulation and other functional skills. This information may benefit health care providers in preparing the family for loss of function and subsequent assessment of medical and equipment needs for the care of young men with this disease.
Taken together, our results show that MRS and MRI techniques may be beneficial in the study of DMD. In particular, measurements of lipid fractions with MRS and T2-weighted images from MRI may provide sensitive ways to monitor progression of the disease in all boys with DMD at all time points regardless of the stage of ambulation. Both techniques are capable of assessing multiple muscles at one time and can provide information about the progression of muscle pathophysiology when standard clinical assessments, such as the 30-Foot Fast Walk Test, are self-limited by narrow age ranges due to loss of function. Magnetic resonance measures were able to detect the diffuse and discrete disease progression of boys that lost ambulation early versus late. Although further studies are needed, these results suggest that MRS and MRI are objective, noninvasive measures of muscle pathology that should be considered for monitoring progression of DMD, treatment planning, and assessing the efficacy of any therapeutic intervention.
Supplementary Material
Footnotes
Dr Claudia Senesac, Dr Mathur, Dr Walter, and Dr Vandenborne provided concept/idea/research design. Dr Claudia Senesac, Dr Lott, Dr Forbes, Dr Mathur, Dr Walter, and Dr Vandenborne provided writing. All authors provided data collection. Dr Lott, Dr Forbes, Dr Arpan, Ms Emily Senesac, and Dr Walter provided data analysis. Dr Claudia Senesac and Dr Walter provided project management. Dr Vandenborne provided fund procurement and facilities/equipment. Dr Mathur, Dr Arpan, and Dr Vandenborne provided consultation (including review of manuscript before submission). The authors thank Dr Phil Miles for assistance in the preparation of the manuscript.
The study was approved by the University of Florida Institutional Review Board.
This study was funded by the Muscular Dystrophy Association (MDA4170), Parent Project Muscular Dystrophy, and the National Institutes of Health (R01AR056973).
References
- 1. Petrof BJ, Shrager JB, Stedman HH, et al. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A. 1993;90:3710–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deconinck N, Dan B. Pathophysiology of duchenne muscular dystrophy: current hypotheses. Pediatr Neurol. 2007;36:1–7. [DOI] [PubMed] [Google Scholar]
- 3. Kohler M, Clarenbach CF, Bahler C, et al. Disability and survival in Duchenne muscular dystrophy. J Neurol Neurosurg Psychiatry. 2009;80:320–325. [DOI] [PubMed] [Google Scholar]
- 4. Strober JB. Therapeutics in duchenne muscular dystrophy. NeuroRx. 2006;3:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bushby K, Finkel R, Birnkrant DJ, et al. ; for the DMD Care Considerations Working Group. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol. 2010;9:177–189. [DOI] [PubMed] [Google Scholar]
- 6. Beenakker EA, Maurits NM, Fock JM, et al. Functional ability and muscle force in healthy children and ambulant Duchenne muscular dystrophy patients. Eur J Paediatr Neurol. 2005;9:387–393. [DOI] [PubMed] [Google Scholar]
- 7. Lue YJ, Jong YJ, Lin YT, Chen SS. The strength and functional performance of patients with Duchenne muscular dystrophy based on natural history. Kaohsiung J Med Sci. 1992;8:597–604. [PubMed] [Google Scholar]
- 8. McDonald CM, Abresch RT, Carter GT, et al. Profiles of neuromuscular diseases: Becker's muscular dystrophy. Am J Phys Med Rehabil. 1995;74:S93–S103. [DOI] [PubMed] [Google Scholar]
- 9. Scott E, Mawson SJ. Measurement in Duchenne muscular dystrophy: considerations in the development of a neuromuscular assessment tool. Dev Med Child Neurol. 2006;48:540–544. [DOI] [PubMed] [Google Scholar]
- 10. Mazzone E, Martinelli D, Berardinelli A, et al. North Star Ambulatory Assessment, 6-minute walk test and timed items in ambulant boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2010;20:712–716. [DOI] [PubMed] [Google Scholar]
- 11. McDonald CM, Henricson EK, Abresch RT, et al. The 6-minute walk test and other endpoints in Duchenne muscular dystrophy: longitudinal natural history observations over 48 weeks from a multicenter study. Muscle Nerve. 2013;48:343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim HK, Laor T, Horn PS, et al. T2 mapping in Duchenne muscular dystrophy: distribution of disease activity and correlation with clinical assessments. Radiology. 2010;255:899–908. [DOI] [PubMed] [Google Scholar]
- 13. Mendell JR, Shilling C, Leslie ND, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 2012;71:304–313. [DOI] [PubMed] [Google Scholar]
- 14. Sun SC, Peng YS, He JB. Changes of serum creatine kinase levels in children with Duchenne muscular dystrophy [in Chinese]. Zhongguo Dang Dai Er Ke Za Zhi. 2008;10:35–37. [PubMed] [Google Scholar]
- 15. Kinali M, Arechavala-Gomeza V, Cirak S, et al. Muscle histology vs MRI in Duchenne muscular dystrophy. Neurology. 2011;76:346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang Y, Majumdar S, Genant HK, et al. Quantitative MR relaxometry study of muscle composition and function in Duchenne muscular dystrophy. J Magn Reson Imaging. 1994;4:59–64. [DOI] [PubMed] [Google Scholar]
- 17. Marden FA, Connolly AM, Siegel MJ, Rubin DA. Compositional analysis of muscle in boys with Duchenne muscular dystrophy using MR imaging. Skeletal Radiol. 2005;34:140–148. [DOI] [PubMed] [Google Scholar]
- 18. Torriani M, Townsend E, Thomas BJ, et al. Lower leg muscle involvement in Duchenne muscular dystrophy: an MR imaging and spectroscopy study. Skeletal Radiol. 2012;41:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zoabli G, Mathieu PA, Aubin CE. Magnetic resonance imaging of the erector spinae muscles in Duchenne muscular dystrophy: implication for scoliotic deformities. Scoliosis. 2008;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brooke MH, Griggs RC, Mendell JR, et al. Clinical trial in Duchenne dystrophy, I: the design of the protocol. Muscle Nerve. 1981;4:186–197. [DOI] [PubMed] [Google Scholar]
- 21. Ando N, Fujimoto Y, Ando M, et al. A new method of gait analysis in Duchenne muscular dystrophy in Japanese. Rinsho Shinkeigaku. 1992;32:962–968. [PubMed] [Google Scholar]
- 22. D'Angelo MG, Berti M, Piccinini L, et al. Gait pattern in Duchenne muscular dystrophy. Gait Posture. 2009;29:36–41. [DOI] [PubMed] [Google Scholar]
- 23. Doglio L, Pavan E, Pernigotti I, et al. Early signs of gait deviation in Duchenne muscular dystrophy. Eur J Phys Rehabil Med. 2011;47:587–594. [PubMed] [Google Scholar]
- 24. Forbes SC, Willcocks RJ, Triplett WT, et al. Magnetic resonance imaging and spectroscopy assessment of lower extremity skeletal muscles in boys with Duchenne muscular dystrophy: a multicenter cross sectional study. PloS One. 2014;9:e106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arpan I, Forbes SC, Lott DJ, et al. T2 mapping provides multiple approaches for the characterization of muscle involvement in neuromuscular diseases: a cross-sectional study of lower leg muscles in 5–15-year-old boys with Duchenne muscular dystrophy. NMR Biomed. 2013;26:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gold GE, Han E, Stainsby J, et al. Musculoskeletal MRI at 3.0 T: relaxation times and image contrast. AJR Am J Roentgenol. 2004;183:343–351. [DOI] [PubMed] [Google Scholar]
- 27. Krssák M, Mlynárik V, Meyerspeer M, et al. 1H NMR relaxation times of skeletal muscle metabolites at 3 T. MAGMA. 2004;16:155–159. [DOI] [PubMed] [Google Scholar]
- 28. Jungbluth H, Davis MR, Müller C, et al. Magnetic resonance imaging of muscle in congenital myopathies associated with RYR1 mutations. Neuromuscul Disord. 2004;14:785–790. [DOI] [PubMed] [Google Scholar]
- 29. Kornblum C, Lutterbey G, Bogdanow M, et al. Distinct neuromuscular phenotypes in myotonic dystrophy types 1 and 2: a whole body highfield MRI study. J Neurol. 2006;253:753–761. [DOI] [PubMed] [Google Scholar]
- 30. Mercuri E, Lampe A, Allsop J, et al. Muscle MRI in Ullrich congenital muscular dystrophy and Bethlem myopathy. Neuromuscul Disord. 2005;15:303–310. [DOI] [PubMed] [Google Scholar]
- 31. Susman RD, Quijano-Roy S, Yang N, et al. Expanding the clinical, pathological and MRI phenotype of DNM2-related centronuclear myopathy. Neuromuscul Disord. 2010;20:229–237. [DOI] [PubMed] [Google Scholar]
- 32. Fischmann A, Hafner P, Gloor M, et al. Quantitative MRI and loss of free ambulation in Duchenne muscular dystrophy. J Neurol. 2013;260:969–974. [DOI] [PubMed] [Google Scholar]
- 33. Gaeta M, Messina S, Mileto A, et al. Muscle fat-fraction and mapping in Duchenne muscular dystrophy: evaluation of disease distribution and correlation with clinical assessments—preliminary experience. Skeletal Radiol. 2012;41:955–961. [DOI] [PubMed] [Google Scholar]
- 34. Kim HK, Merrow AC, Shiraj S, et al. Analysis of fatty infiltration and inflammation of the pelvic and thigh muscles in boys with Duchenne muscular dystrophy (DMD): grading of disease involvement on MR imaging and correlation with clinical assessments. Pediatr Radiol. 2013;43:1327–1335. [DOI] [PubMed] [Google Scholar]
- 35. Akima H, Lott D, Senesac C, et al. Relationships of thigh muscle contractile and non-contractile tissue with function, strength, and age in boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2012;22:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wokke BH, Bos C, Reijnierse M, et al. Comparison of dixon and T1-weighted MR methods to assess the degree of fat infiltration in Duchenne muscular dystrophy patients. J Magn Resonan Imaging. 2013;38:619–624. [DOI] [PubMed] [Google Scholar]
- 37. Triplett WT, Baligand C, Forbes SC, et al. Chemical shift-based MRI to measure fat fractions in dystrophic skeletal muscle. Magn Resonan Med. 2014;72:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Willcocks RJ, Arpan IA, Forbes SC, et al. Longitudinal measurements of MRI-T in boys with Duchenne muscular dystrophy: effects of age and disease progression. Neuromuscul Disord. 2014:24:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2008;1:CD003725. [DOI] [PubMed] [Google Scholar]
- 40. Gold BT, Balota DA, Cortese MJ, et al. Differing neuropsychological and neuroanatomical correlates of abnormal reading in early-stage semantic dementia and dementia of the Alzheimer type. Neuropsychologia. 2005;43:833–846. [DOI] [PubMed] [Google Scholar]
- 41. McDonald CM, Henricson EK, Abresch RT, et al. ; for the Cooperative International Neuromuscular Research Group Investigators. The Cooperative International Neuromuscular Research Group Duchenne natural history study—a longitudinal investigation in the era of glucocorticoid therapy: design of protocol and the methods used. Muscle Nerve. 2013;48:32–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lott DJ, Forbes SC, Mathur S, et al. Assessment of intramuscular lipid and metabolites of the lower leg using magnetic resonance spectroscopy in boys with Duchenne muscular dystorphy. Neuromuscul Disord. 2014;24:574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hollingsworth KG, Garrood P, Eagle M, et al. Magnetic resonance imaging in Duchenne muscular dystrophy: longitudinal assessment of natural history over 18 months. Muscle Nerve. 2013;48:586–588. [DOI] [PubMed] [Google Scholar]
- 44. Hsieh TJ, Jaw TS, Chuang HY, et al. Muscle metabolism in Duchenne muscular dystrophy assessed by in vivo proton magnetic resonance spectroscopy. J Comput Assist Tomogr. 2009;33:150–154. [DOI] [PubMed] [Google Scholar]
- 45. Mathur S, Lott DJ, Senesac C, et al. Age-related differences in lower-limb muscle cross-sectional area and torque production in boys with Duchenne muscular dystrophy. Arch Phys Med Rehabil. 2010;91:1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.