Abstract
Fluoride has long been known to inhibit bacterial and fungal cell growth most likely by blocking the functions of key metabolic enzymes. In this study, we demonstrate that antifungal compounds that disrupt cell membrane integrity exhibit improved ability to inhibit cell growth when used with millimolar concentrations of fluoride. Specifically, antifungal compounds of the polyene class and an antifungal peptide exhibit synergy with fluoride to inhibit the growth of various fungal species, including Candida albicans. Our results demonstrate that certain compounds can be found that increase the cellular uptake of fluoride, and provide new opportunities for creating antimicrobial compounds whose functions are enhanced when combined with otherwise sub-inhibitory concentrations of small ions.
Keywords: antifungal, amphotericin B, Candida albican, riboswitch, yeast
Humans can be infected with a diversity of fungal species and the outcomes of these diseases can range from minor discomfort and disfiguration to death. Numerous antifungal therapies have been developed over the last several decades that have been very effective,1,2 but many challenges still exist when treating fungal infections, including the emergence of drug resistance.3 Fungal infections on the surface of the body are among the most common4 and usually can be overcome by topical treatment with antifungal agents, although poor efficacy can sometimes limit the utility of existing compounds.
In this study, we assessed the hypothesis that fluoride can be used to enhance the in vitro activity of certain classes of antifungal agents. This hypothesis has emerged from our recent discovery of a fluoride-responsive riboswitch class5,6 in many bacterial and archaeal species. Riboswitches are metabolite- or ion-sensing domains found within the noncoding portions of certain messenger RNAs where they control the expression of adjoining protein coding regions.7-9 Members of the fluoride riboswitch class bind fluoride anions and regulate numerous genes whose protein products appear to overcome the inherent toxicity of this anion.6
Although fungi lack representatives of the known fluoride riboswitch class, many fungal species carry a homolog of the gene most commonly associated with fluoride riboswitches in bacteria. This gene (called crcB) codes for a member of a family of proteins predicted to be membrane-associated transporters.10,11 A genetic knock-out of crcB in the bacterium Escherichia coli results in a strain that is approximately 200-fold more sensitive to fluoride, and these cells accumulate higher cytoplasmic concentrations of fluoride compared to wild-type cells when grown in identical fluoride-supplemented growth media.6
We speculate that CrcB proteins are most likely fluoride transporters and, if true, fungal expression of this protein and the subsequent ejection of this anion from cells may be a major mechanism for how these organisms overcome fluoride toxicity. To assess whether fluoride simply halts fungal growth (fungistatic) or kills fungal cells (fungicidal), we prepared a culture of Saccharomyces cerevisiae and added 300 mM fluoride to the medium (see Supplementary data for all materials and methods). Samples of this mixture were taken at various times, plated, and the number of resulting colonies were recorded (Fig. 1). Cells experience a rapid loss of viability, and a 160 minute incubation results in near complete killing of the cells in the culture. Therefore, compounds that facilitate the uptake and/or retention of fluoride, or otherwise inhibit the fluoride toxicity mitigation responses of fungi, should also function as fungicidal compounds when used in combination with this anion.
Figure 1.
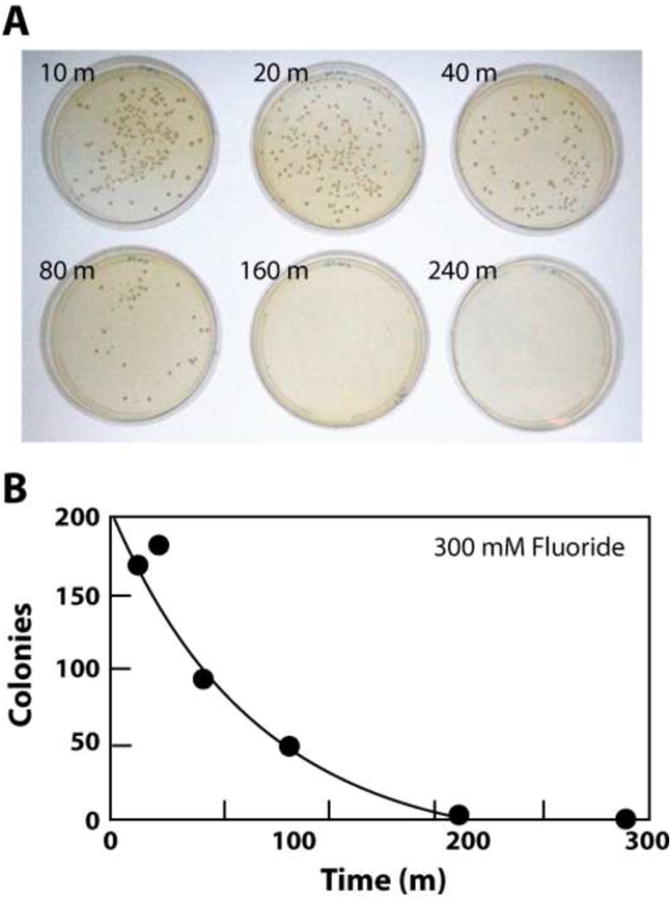
(A) Demonstration that high fluoride concentration in S. cerevisiae culture causes loss of cell viability. Cells were cultured in liquid medium in the presence of 300 mM fluoride for the times (in minutes) indicated and then plated on solid medium in the absence of added fluoride. Plate images were recorded after extended incubation and colonies were counted. See Supplementary data for all methods details. (B) Plots of the numbers of colonies present versus incubation time as noted in A.
Among the existing antifungal compound classes are examples that are known or predicted to selectively disrupt the integrity of fungal membranes. In particular, members of the polyene class are believed to form multimer complexes in fungal membranes12,13 resulting in pores that permit leakage of cytoplasmic constituents.14 Since it is unlikely that this flow of small molecules and ions is unidirectional, chemicals from the growth medium also should begin to equilibrate with the interiors of cells whose membranes have been compromised by the fungicide.
Given that fluoride is an exceedingly small chemical entity, and given the fungicidal activity of this anion when present at high concentrations in cells (Fig. 1), we reasoned that antifungal compounds that disrupt the integrity of cell membranes could interact synergistically with fluoride to more effectively inhibit fungal growth. Specifically, the addition of fluoride to a fungal growth medium containing a concentration of such an antifungal agent below its typical MIC (minimum inhibitory concentration) should improve its MIC value. Likewise, these antifungal compounds should lower the concentration of fluoride needed to inhibit fungal growth.
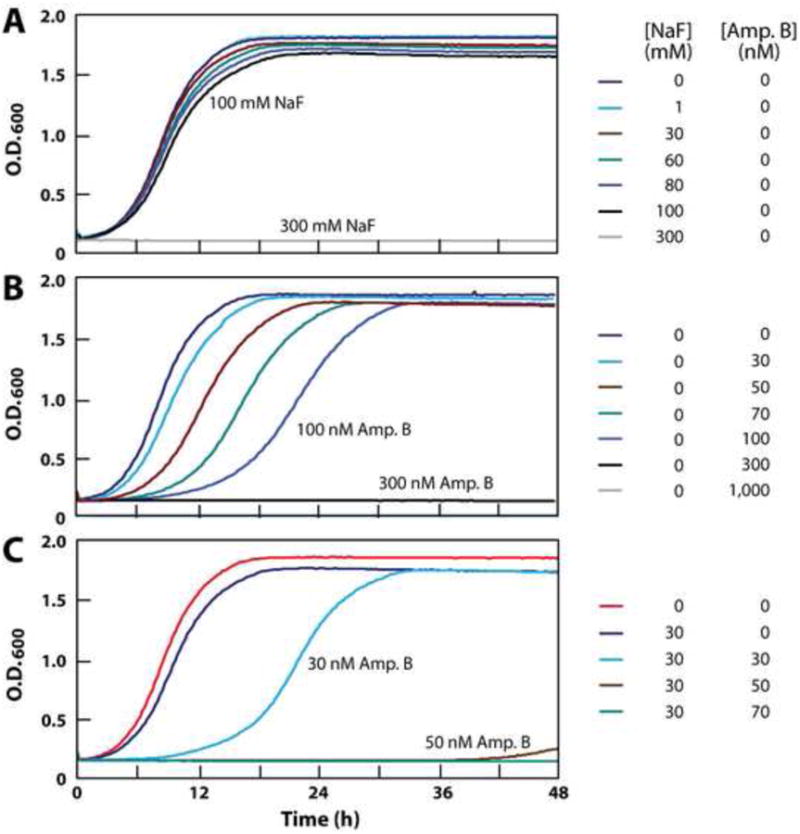
This hypothesis was initially tested by conducting growth curve analyses of S. cerevisiae in the presence or absence of various concentrations of sodium fluoride and amphotericin B (Fig. 2). Amphotericin B is a prominent member of the polyene class of antifungal agents and therefore should allow fluoride to more readily gain entry into cells. We found that fluoride alone only marginally affects S. cerevisiae growth when present at 30 mM in liquid medium under the growth conditions used in this study (Fig. 2A). The addition of more than 100 mM fluoride is required to completely inhibit fungal growth over a 48-hour culture. In the absence of added fluoride, concentrations of amphotericin B ranging from 30 to 70 nM only marginally delay near full growth of the S. cerevisiae culture, while 300 nM of this antifungal compound is required to almost completely inhibit growth over 48 hours (Fig. 2B).
Figure 2.
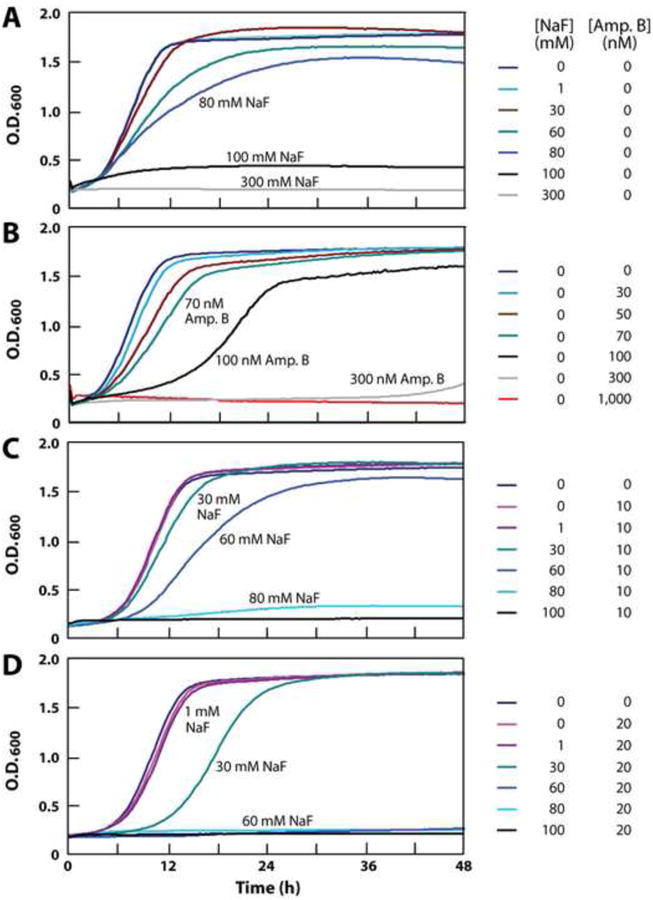
(A) Fluoride inhibition of S. cerevisiae growth in liquid culture over 48 hours. (B) Amphotericin B inhibition of S. cerevisiae growth. (C), (D) Synergistic inhibition of S. cerevisiae growth by a combination of fluoride and 10 or 20 nM amphotericin B, respectively.
To assess the effects of the combination of fluoride and amphotericin B, we exposed S. cereviciae cultures containing 10 nM of the antifungal compound to a range of fluoride concentrations. Although 10 nM amphotericin B has almost no effect on cell growth when used alone, its presence causes a dramatic increase in the growth-inhibition effects of fluoride (Fig. 2C). The toxicity of fluoride is further enhanced by doubling the concentration of amphotericin B to 20 nM (Fig. 2D). For example, 60 mM fluoride has only a modest effect on fungal growth when tested alone, but causes complete inhibition of growth when combined with a concentration of amphotericin B (20 nM) that otherwise has almost no growth inhibition effect on S. cerevisiae.
This synergistic activity between fluoride and polyene class compounds is made further evident when fluoride concentrations are held constant and antifungal compound concentrations are varied (Fig. 3). For example, amphotericin B undergoes an improvement in MIC for S. cerevisiae of ∼10 fold when 30 mM fluoride is added to liquid medium (Fig. 3A). Moreover, 80 mM fluoride alone only lengthens the time to near full culture growth by two fold, but improves the MIC of amphotericin B by ∼30 fold (Fig. 3B). We observed that KCl and NaCl also improve the MIC values of this antifungal compound when tested at 30 mM and 80 mM, suggesting that they too may exploit the membrane-destabilization mechanism to adversely affect fungal growth. However, KCl and NaCl are consistently less effective than NaF at causing fungal growth inhibition. Similar anion enhancement effects are observed for 30 mM (Fig. 3C) and 80 mM (Fig. 3D) fluoride when combined with nystatin, a related polyene class member.
Figure 3.
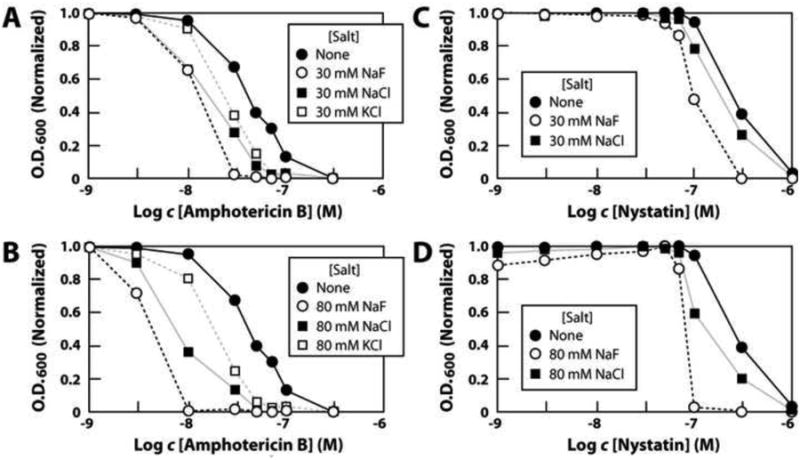
(A) Effect of 30 mM fluoride or other ions on amphotericin B inhibition of S. cereveciae growth. Experiments were conducted as described for the data in Fig. 2, and data points (normalized to the maximum O.D.600 value recorded for each culture) reflect 9 h incubations at 30°C with shaking. (B) Effect of 80 mM fluoride or other ions on amphotericin B inhibition of S. cerevesiae growth. (C), (D) Effects of 30 mM or 80 mM fluoride or other ions on nystatin inhibition of S. cerevisiae growth.
Fluoride did not improve the function of compounds from most other antifungal agent classes examined. For example, aculeacin A, a member of an antifungal class that inhibits cell wall biosynthesis,14 is not affected at all by the presence of 30 mM fluoride (Fig. 4). Likewise, representatives of other classes either failed to be improved by the addition of fluoride (itraconazole) or did not significantly inhibit S. cerevisiae growth (tolnaftate, terbinafine) under our assay conditions (data not shown). These findings suggest that the synergistic effects we observe for fluoride (and to a lesser extent other ions) are likely to broadly improve the function of only those antifungal compounds that compromise the ability of cell membranes to act as a barrier to ions.
Figure 4.
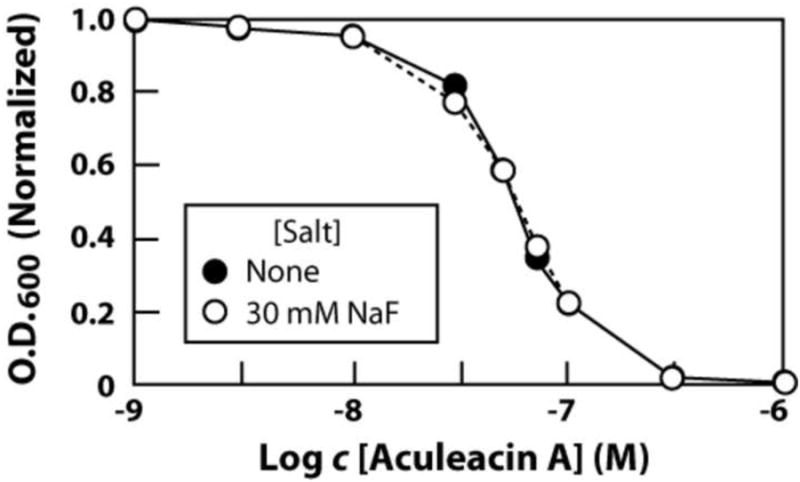
Lack of an effect of 30 mM fluoride on aculeacin A inhibition of S. cerevesiae growth. Experiments were conducted as described for the data in Fig. 3.
Since polyene antifungal compounds have broad efficacy against numerous species, we sought to determine whether the synergy between fluoride and certain antifungal compounds observed with S. cerevisiae also occurs with other species of fungi. Neurospora crassa is a filamentous fungus and therefore we conducted antifungal activity assays by visually examining liquid media cultures incubated with either fluoride, amphotericin B, or both (Fig 5). When tested independently, fluoride concentrations of greater than 80 mM were required to completely prevent N. crassa growth (Fig. 5A), and a concentration of greater than 300 nM amphotericin B was required to prevent growth (Fig. 5B). In contrast, combining 10 mM or 30 mM fluoride with amphotericin B reduces the MIC for the antifungal compound by at least 4 fold (Fig. 5C) and 10 fold (Fig. 5D), respectively. Similar synergy in the actions of fluoride and amphotericin B was also evident with another filamentous fungal species Aspergillus nidulans (see Supplementary data).
Figure 5.
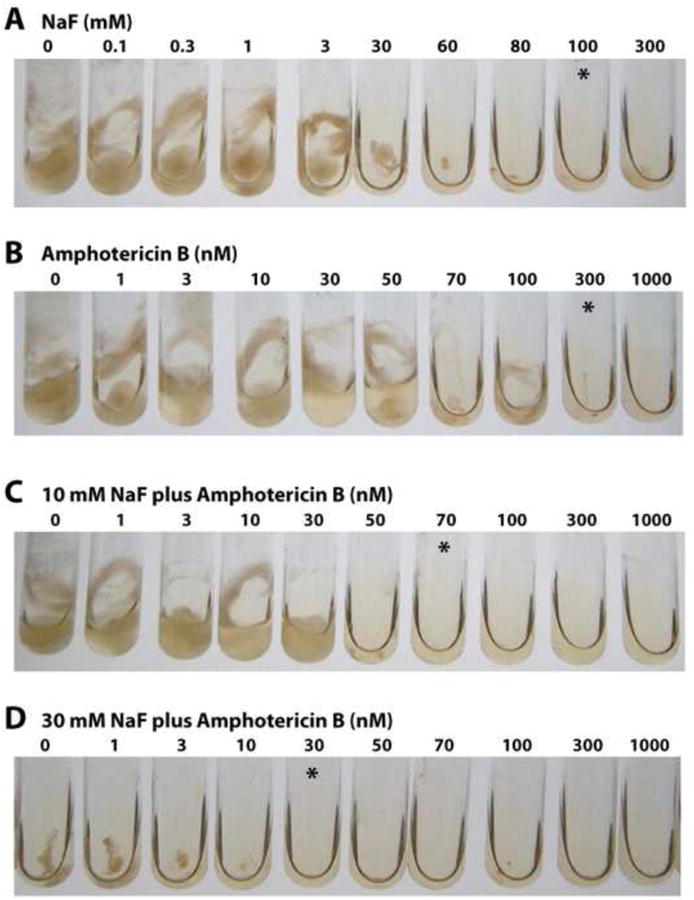
(A) Fluoride inhibition of N. crassa growth in liquid culture after incubation for 24 h. The asterisks denote tubes with the lowest concentration of fluoride or amphotericin B where little or no cell growth is visible. (B) Amphotericin B inhibition of N. crassa growth. (C), (D) Synergistic inhibition of N. crassa growth by a combination of amphotericin B and 10 or 30 mM fluoride, respectively.
Fluoride also improves the MIC values for amphotericin B with the fungal pathogen Candida albicans (Fig. 6). Among the four fungal species to be examined in this study, C. albicans exhibited the greatest resistance to fluoride-mediated growth inhibition. When tested alone, 300 mM fluoride completely prevented growth in liquid medium after 48 hours, whereas 100 mM fluoride had only a modest inhibitory effect (Fig. 6A). Increasing concentrations of amphotericin B ranging from 30 to300 nM progressively inhibited cell growth, with the MIC for the compound falling somewhere between 100 and 300 nM (Fig. 6B). However, this MIC is improved to ∼50 nM when the medium is supplemented with only 30 mM fluoride (Fig. 6C).
Figure 6.
(A) Fluoride inhibition of C. albicans growth in liquid culture over 48 hours. (B) Amphotericin B inhibition of C. albicans growth. (C) Synergistic inhibition of C. albicans growth by a combination of 30 mM fluoride and amphotericin B.
Another antifungal compound that has been proposed to function by destabilizing membranes is a circular hepta-arginine peptide with the commercial name Novexatin® (NP213; NovaBiotics, Aberdeen, UK).15-17 Growth curves conducted with S. cereviciae (see Supplemental data) confirm that Novexatin has MIC values for fungi that are high relative to most commercial antifungal compounds. Under our culture conditions, 3 mM Novexatin was needed to almost completely prevent fungal growth over 48 hours. Solubility of Novexatin at millimolar concentrations becomes an issue, which made precise determination of MIC values problematic. Regardless, a greater than 3-fold improvement in MIC was obtained for Novexatin when combined with 30 mM fluoride. A similar result was obtained when evaluating cultures that were allowed to incubate for approximately 24 hours (Fig. 7). These results are consistent with the proposed mechanism of membrane destabilization by this new antifungal compound.
Figure 7.
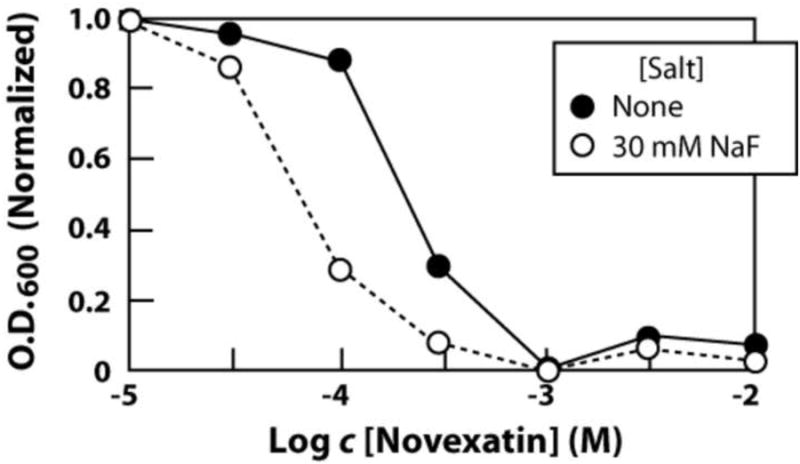
Improvement in MIC of S. cerevisiae with Novexatin at 26.25 h upon the addition of 30 mM NaF.
In summary, our findings are consistent with the hypothesis that the toxic effects of fluoride on fungi can be augmented by the use of compounds that facilitate the uptake of fluoride from growth media. Although we observe positive results with antifungal compounds known to destabilize membranes, we propose that compounds affecting other processes involved in fluoride toxicity resistance would similarly increase the antifungal effects of fluoride. Likewise, compounds that affect bacterial membrane integrity or fluoride toxicity resistance systems also should enhance the antibacterial effects of this anion. Indeed, evidence exists for the synergistic function of 50 mM fluoride with two amphiphilic peptides that form ion channels in bacterial membranes.18 Fluoride has been known for many decades to broadly inhibit the growth of fungi,19-21 albeit the concentrations of this anion required to achieve this effect are very high. Our findings indicate that compounds can be identified that reduce the requirement for high fluoride or of its more potent salts22 to achieve fungicidal activity.
Membrane destabilization by various compounds should permit increased uptake of other antimicrobial chemical entities, and therefore a broader utility for this approach can be envisioned. However, agents that are small and have little inherent toxicity to humans might be most advantageously applied in combination with membrane destabilizing antimicrobial compounds. Fluoride is routinely and daily applied to human tissues in the form of oral healthcare products such as over-the-counter (∼70 mM fluoride) or prescription (∼250 mM fluoride) toothpastes and mouthwashes. Furthermore, the relative selectivity of compounds such as amphotericin B and nystatin for fungal cells suggests that antifungal formulations containing both a polyene drug and low millimolar amounts of fluoride may offer a safe and effective topical treatment for certain fungal infections.
Supplementary Material
Acknowledgments
We thank members of the Breaker laboratory for helpful discussions. This work was supported by NIH (GM022778) and by the Howard Hughes Medical Institute.
Footnotes
Supplementary data: Supplementary data and methods associated with this article are available.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Dismukes WE. Clin Infect Disease. 2006;42:1289. doi: 10.1086/503043. [DOI] [PubMed] [Google Scholar]
- 2.Pitman S, Drew RH, Perfect JR. Expert Opin Emerg Drugs. 2011;16:559. doi: 10.1517/14728214.2011.607811. [DOI] [PubMed] [Google Scholar]
- 3.Ghannoum MA, Rice LB. Clin Microbiol Rev. 1999:501. doi: 10.1128/cmr.12.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur IP, Kakkar S. Expert Opin Drug Deliv. 2010;7:1303. doi: 10.1517/17425247.2010.525230. [DOI] [PubMed] [Google Scholar]
- 5.Weinberg Z, Wang JX, Bogue J, Yang J, Corbino K, Moy RH, Breaker RR. Genome Biol. 2010;11:R31. doi: 10.1186/gb-2010-11-3-r31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker JL, Sudarsan N, Weinberg Z, Roth A, Breaker RR. Science. 2011 Epub ahead of print. [Google Scholar]
- 7.Mandal M, Breaker RR. Nature Rev Mol Cell Biol. 2004;5:451. doi: 10.1038/nrm1403. [DOI] [PubMed] [Google Scholar]
- 8.Roth A, Breaker RR. Annu Rev Biochem. 2009;78:305. doi: 10.1146/annurev.biochem.78.070507.135656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith AM, Fuchs RT, Grundy FJ, Henkin TM. RNA Biol. 2010;7:104–110. doi: 10.4161/rna.7.1.10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rapp M, Granseth E, Seppälä S, von Heijne G. Nat Struct Mol Biol. 2006;13:112. doi: 10.1038/nsmb1057. [DOI] [PubMed] [Google Scholar]
- 11.Finn RD, et al. Nucleic Acids Res. 2010;38:D211. doi: 10.1093/nar/gkp985. [DOI] [PMC free article] [PubMed] [Google Scholar]; Holt RJ. Ann N Y Acad Sci. 1974;235:469. doi: 10.1111/j.1749-6632.1974.tb43284.x. [DOI] [PubMed] [Google Scholar]
- 12.De Kruijff B, Demel RA. Biochim Biophys Acta. 1974;339:57. doi: 10.1016/0005-2736(74)90332-0. [DOI] [PubMed] [Google Scholar]
- 13.Kerridge D. In: The Eukaryotic Cell. Gooday GW, Lloyd D, Trinci APJ, editors. Cambridge University Press; 1980. p. 103. [Google Scholar]
- 14.Hector RF. Clin Rev Microbiol. 1993;6:1. doi: 10.1128/cmr.6.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Neil D. US Patent No. 7,847,059 B2. 2010
- 16.Duncan VMS, Robertson J, Turvey L, Miller L, Charrier C, Bamford CA, Stewart CS, Mercer DK, O'Neil DA. ICAAC 49th Conference Proceedings. 2009 Poster Abstract F1-852. [Google Scholar]
- 17.Bamford CA, Galloway DB, O'Neil DA. ICAAC 49th Conference Proceedings. 2009 Poster Abstract F1-854. [Google Scholar]
- 18.Zasloff M, Steinberg WH. U.S. Patent 5,217,956 1993
- 19.Nickerson WJ, Chung CW. Am J Botany. 1952;39:669–679. [Google Scholar]
- 20.Treshow M. Mycologia. 1965;57:216. [PubMed] [Google Scholar]
- 21.Leslie R, Parbery DJ. Trans British Mycol Soc. 1972;58:351. [Google Scholar]
- 22.Flisfisch S, Meyer J, Meurman JH, Waltimo T. Oral Diseases. 2008;14:296. doi: 10.1111/j.1601-0825.2007.01385.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


