Abstract
The tumor microenvironment is a complex, heterogeneous, and dominant component of solid tumors. Cancer imaging strategies of a subset of characteristics of the tumor microenvironment are under active development and currently used modalities and novel approaches are summarized here. Understanding the dynamic and evolving functions of the tumor microenvironment is critical to accurately inform imaging and clinical care of cancer. Novel insights into distinct roles of the tumor microenvironment in cancer progression urge careful interpretation of imaging data and impel the development of novel modalities.
Keywords: Tumor microenvironment, Cancer associated fibroblasts, angiogenesis, extracellular matrix, imaging
The tumor microenvironment: definition and heterogeneity
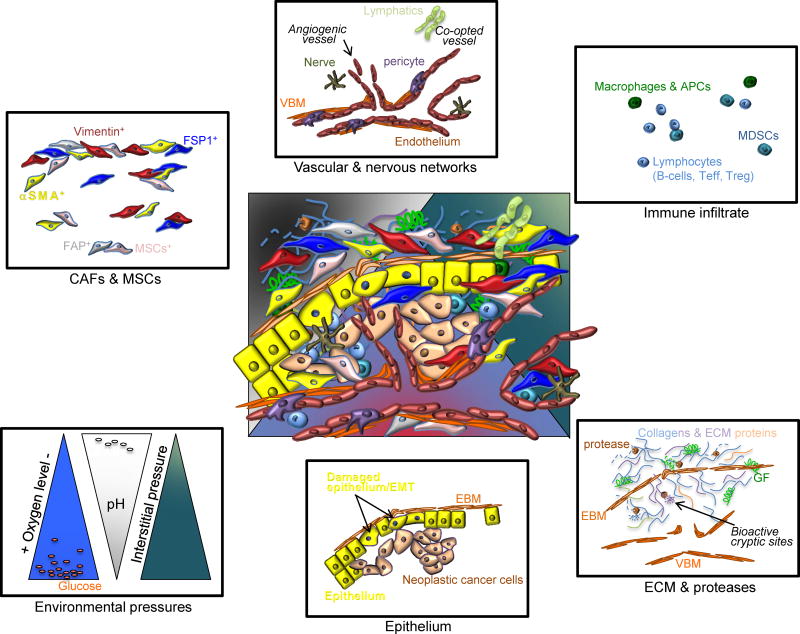
The tumor microenvironment (TME) represents a dynamic milieu of complex cellular and acellular composites, with evolving functions and synergistic reactions in cancer progression. In essence, the tumor microenvironment represents all components of a solid tumor that are not cancer cells. A significant proportion of the cellular TME includes co-opted and angiogenic tumor vessels and perivascular cells, cancer-associated fibroblasts (CAFs) and mesenchymal cells, nervous network, and immune infiltrate (Figure 1). The extracellular matrix (ECM), composed of fibrillar collagens, hyaluronan, fibronectin and other matrix proteins associated with the vascular and epithelial basement membranes, constitute the exquisitely diverse acellular component of the TME1–3. The TME ECM is under continuous remodeling by various proteases that are not only postulated to impact ECM structural stiffness and permeability, as well as release and presentation of embedded growth factors, but also to uncover ECM cryptic sites with significant biological functions implicated in tumor growth and vascular remodeling4–8. The oxygen tension, pH/redox potential and interstitial pressures also constitute principal components of the TME and tumor physiology (Figure 1), with important implication on tumor growth and invasion. In some regards, the non-neoplastic epithelium, often times damaged epithelium within a growing tumor, is also a component of the TME, and these cells are dynamically influenced by the proximate cancer cells and the dynamic cytokine and growth factor milieu.
Figure 1. Heterogeneity of the TME.
At the center, an overlay of defined TME components depicts the complexity and heterogeneity of the TME. Cancer associated fibroblasts (CAFs) and mesenchymal stem cells (MSCs) express various proteins often times used for classification; some of which are alpha-smooth muscle actin (_SMA), vimentin, fibroblast specific protein 1 (FSP1, also known as S100A4) and fibroblast activation protein (FAP). The vascular and nervous networks are depicted together with the lymphatic system. The vascular network schematic shows both co-opted vasculature and angiogenic vessels, composed of a vascular basement membrane (VBM), perivascular cells or pericytes and endothelium. The diversity of the immune infiltrate is vast and broad categories are listed, including macrophages and antigen presenting cells (APCs), myeloid-derived suppressor cells (MDSCs) and lymphocytes. The ECM is composed of collagens and various matrix proteins, which are remodeled by proteases to release growth factors (GF) and to reveal bioactive cryptic sites. The VBM and epithelial basement membrane (EBM) also make up the ECM of the TME. Physiological components of the TME include oxygen levels, pH, metabolites (glucose and lactate are depicted), and interstitial pressure, depicted here in relative gradients. Finally, damaged epithelial cells, epithelial cells with epithelial to mesenchymal (EMT) program, often adjacent to cancer cells, are also dynamic components of the TME.
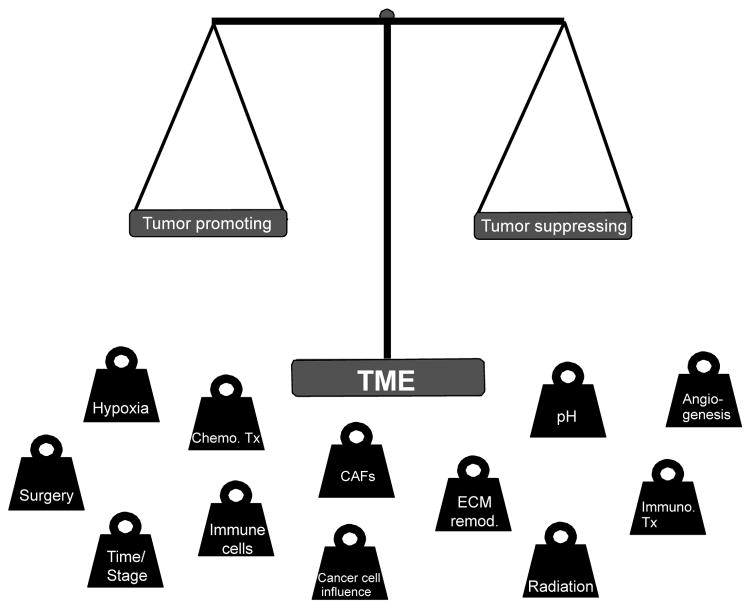
A critically, yet still underappreciated aspect of the TME, is its relative abundance in comparison to cancer cells, with a proportional ratio nearly always in favor of the TME. In specific solid tumors, such as the breast and pancreas, the TME may compose up 90% of the tumor lesion9–11. TME compositional and spatial heterogeneity varies greatly across tumor types, amongst patients with a given cancer types (defined by tissue of origin and molecular characterization), and across distinct lesions in a given patient. The compositional diversity of the TME is only matched by its functional diversity, and composition and functions are not always linearly connected in the evolving TME. Furthermore, chemotherapy, radiotherapy, and even surgical resection, may further expand TME heterogeneity as all of these interventions will influence, directly and indirectly, the survival and functional contribution of the cellular components of the TME. For example, it has only recently been better appreciated that chemotherapeutic drugs aimed to suppress proliferation of (cancer) cells will have a significant impact on the stroma12; and radiotherapy may alter TME composition and impact response to therapeutic irradiation13. In these settings, the functional diversity of the TME may be further enhanced by bystander effects that may tip the dynamic balance of the tumor suppressing vs. tumor promoting roles of TME components (Figure 2). Such balance is complex, dynamic, and interdependent on the consequential influences of TME components with each other and with cancer cells. Finally, the cancer cells’ heterogeneity and responses to TME influences or therapy may act to re-program the TME towards tumor promoting functions.
Figure 2. The tumor promoting and tumor suppressing functions of the TME.
The duality in the role of the TME toward tumor suppressing or tumor promoting functions is balanced by various factors, ranging from evolving TME components (hypoxia, pH, angiogenesis, etc) in time (tumor stage), which are influenced by one another and by cancer cells, to therapeutic interventions (chemotherapy, immunotherapy, etc). ECM remod,: ECM remodeling. Chemo. Tx: chemotherapy. Immuno. Tx: Immunotherapy.
The (historical) concept and functional importance of the microenvironment in cancer progression emerged with pioneering studies that defined the ‘seed’ (cancer cell) relationship to its ‘soil’ (TME)(original work of Dr. Stephen Paget14, see also 15). The field of TME was subsequently enriched by the work of many who recognized and aimed to harness therapeutic utility of the TME. Appreciation for the functional heterogeneity of the TME and response of tumors to TME targeting has challenged time and time again our understanding of tumor biology and understanding of therapeutic response (or lack thereof). While the importance of TME in cancer evolution and progression has permeated the cancer biology field, yielding therapeutic advances with encouraging outcomes for patients16, understanding dynamic changes in the composition of TME in 3D, in vivo, site specific tumors will offer new valuable clinical insights. Beyond ascertaining the functional contribution of the TME in response to therapy, new, more specific and sensitive imaging modalities to visualize the dynamic changes of the TME would likely aid cancer screening, diagnosis and monitoring. Nonetheless challenging, in vivo imaging the TME in longitudinal studies in cancer emergence, progression, and response to therapy thus represent the next frontier in TME research17.
Imaging TME: next generation TME studies
Considering the breadth in complexity and heterogeneity of TME in cancer progression, we aimed to offer below a comprehensive, while not exhaustive, list of imaging modalities developed to offer insight on specific components of the TME. These developments, including applications, strengths, and limitations, have been reviewed elsewhere18 in details and we offer a below a summary of the studies to date. While most of these recent developments are largely at the pre-clinical phase of testing, they have already offered new insights in tumor response to therapies.
Angiogenesis and blood flow18,19
Angiogenesis is often seen as a controlling event in the multi-step metastatic cascade and may constitute a rate-limiting step in solid tumor growth. The angiogenic response and blood flow remodeling in solid tumors may precede clinical symptoms and inform on response and progression on treatment. Several magnetic resonance modalities have thus been developed to offer high spatial resolution of tumor vasculature organization, perfusion, and permeability, and include dynamic contrast-enhanced (DCE) MRI using gadolinium-diethylenetriaminepentacetate (DTPA) or dynamic susceptibility contrast (DSC) MRI using gadolinium–DTPA or (ultrasmall) superparamagnetic particles of iron oxide (USPIO/SPIO). Contrast agents have also been developed using the vitronectin receptor integrin αvβ3 to detect and monitor tumor angiogenesis. While this specific type of integrin is expressed on platelets, macrophages, and dendritic cells, as well as endothelial cells engaged in angiogenesis; its application in imaging is often confined to its use in probing angiogenic vessels, on the basis of its high level of expression and macroscopic resolution of tumor vascular beds. The arginine-glycine-aspartic acid (RGD) peptide, which shows high affinity for integrin αvβ3 expressing cells, has been modified to label and target tumor angiogenesis, and RDG-labeled agents have been developed for MRI (RDG-targeting of USPIOs), single-photon emission computed tomography (SPECT), positron emission tomorgraphy (PET), and optical imaging modalities. Contrast agent free techniques, including arterial spin-labeling (ASL) MRI and diffusion-weighted (DW) MRI have also emerged to probe tumor blood flow and edema, respectively.
Hypoxia19–23
Several methods have been developed to image the characteristic changes in oxygen levels in solid tumors. The angiogenic program launched by solid tumors initiates as a response to cellular sensing of oxygen availability. In growing tumors, the inadequate oxygen availability to cells –in part as a result of an abnormal or inefficient angiogenic response that fails to meet the demand of rapidly proliferating and accumulating cells– result in a sustained hypoxic milieu that may endow cancer cells with invasive properties. Hypoxia can be imaged using nitroimidazole probes for PET imaging (18F-FMISO) in the clinic and in pre-clinical studies, and MR-based imaging include electron paramagnetic resonance imaging using oxygen-sensitive paramagnetic spin probes, 19F-MRI and DCE-MRI, blood oxygen levels-dependent (BOLD) contrast MRI. Oxygen-sensitive and bioreductive fluorescent probes have also been developed for optical imaging of hypoxia and are being developed in preclinical models.
pH and metabolism19,24–26
The inadequate vascular supply and lymphatic drainage in growing solid tumors, combined with the glycolytically favored metabolism of the majority of proliferating cancer cells, contribute to the acidic tumor pH level (6.2–6.9 compared to 7.4 in normal tissue). The proliferative stroma also likely contributes significantly to the relatively lower intratumoral pH level. Fluorescent probes in development for optical imaging of tissue pH levels include pH-sensitive fluorescence probes (boron-dipyrromethene (BOPIDY)) and near-infrared fluorescent dye cyanine (Cy). 1H (2-imidazole-1-yl-3-ethoxycarboneylpropionic acid/IEPA), 19F (e.g. vitamin B6 derivative), 31P (e.g. 3-aminopropylphosphonate or 3-APP), and 13C (hyperpolarized 13C-labeled bicarbonate) labeled probes have been developed for Magnetic Resonance Spectroscopy imaging (MRSI). Although suspected to promote ECM remodeling and mutagenesis, it remains unclear whether pH levels correlate with tumor aggressivity and metastasis. Lactate production by highly glycolytic cells also contributes to relatively more acidic pH in solid tumor. The Warburg effect noted in cancer cells was readily applied to tumor imaging, with glucose uptake measurement primarily detected using 18F-FDG-PET. 18F-FDG-PET/CT imaging offers valuable clinical insights to cancer staging and detection of recurrence. Additional developments for metabolic imaging of tumors include NADH/flavoprotein fluorescence imaging (redox scanning) and a series of labeled probes for MRSI to inform on glucose, lipid, and nucleoside metabolism. Of note, these modalities likely inform on the metabolic status of the TME rather than the cancer cells specifically, and thus will likely offer future knowledge on the role of TME metabolism in cancer progression.
ECM19,27
The structural characteristics, porosity or permeability, and complex composition of the tumor ECM have been the recent target for novel therapies/combined therapies, as well as for the development of new imaging strategies for the detection and dynamic progression of solid tumors. To this end, protease activated fluorescent probes for optical imaging of proteases themselves, or reaction with known substrates, have retooled our comprehensions of ECM dynamics and complexity. Such efforts also include MRI measure of transglutaminase and hyaluronidase activities using specific peptide substrate conjugated to gadolinium-DTPA. The permeability (degradation) of the ECM can also be assayed by MRI, via the measure of extravascularization of albumin-gadolinium-DTPA and spatial reconstruction of imaging draining and pooling. While often referred to as “degradation” of the ECM, this measure may more accurately probe the dynamic changes in the structural characteristics of the ECM and vascular basement membranes at a given tumor stage.
Stromal cells18,27–31
The cellular components of the TME are diverse and highly dynamic in both functions and cellular signaling, rendering minimally invasive live imaging of distinct populations challenging. Micron-sized microparticles of iron oxide in vitro labeling of cells and DSC-MRI may be used for cell tracking in vivo, while USPIO in vivo labeling may be used for discerning normal from metastatic lymph nodes via differential phagocytic uptake. Iron oxide particles have also been used in molecular imaging via conjugation to monoclonal antibodies (such as vascular cell adhesion molecule-1 or VCAM-1) and may enhance sensitivity and early detection of tumor masses. Biotin-bovine serum albumin-gadolinium-DTPA labeled fibroblasts were successfully imaged by MRI in preclinical tumor studies. While these modalities offer a general picture of abundance and tumor spatial organization of large pools of (presumably) similar cell types, a cellular resolution of stromal cell activities, motilities, and reactions is needed to inform on the functional, dynamic interplay of these cells in tumors. Intravital imaging and multiphoton microscopy in pre-clinical models have paved new understanding on individual stromal cells, whether using fluorescent labeling of specific cell population prior to administering them to mice, or using animals engineered with genetically-tagged fluorescent proteins to define specific stromal cells. Studies by the Condeelis and Egeblad laboratories29,32,33 have led the field in intravital imaging of the TME, and these studies have offered new insights on the functional contributions of the TME. Imaging of cancer cell mobility in the MMP guided remodeling of the ECM offered an appreciation of the spatial heterogeneity of the invasive front of solid tumors. In the study led by Nakasone et al.33, live imaging of TME components of mammary tumors in response to doxorubicin revealed that sensitivity to the chemotherapeutic compound did not linearly follow tumor classification stage but rather associated with MMP mediated remodeling of the ECM and vascular leakage, as well as CCR2+ monocyte tumor infiltration. This study33 illuminated and challenged concepts of vascular permeability and chemotherapeutic drug delivery.
Most of the clinically translatable imaging modalities of the TME suffer from the difficult reconciliation of macro- and micro-resolution of sensitivity and diversity. While multimodality imaging combining functional and anatomical measurements34 are beneficial for imaging of tumor masses and generalized functional parameters (e.g. angiogenesis and hypoxia), imaging of both macro- and microscopic changes to study TME is necessary to inform on the functional output of a diverse group of cells with dynamic interplay, and their impact on successful treatment of cancer. In this regards, the advent of theranostics35 (non-invasive imaging of a target combined with therapeutic targeting) in TME studies may offer the functional targeting necessary to understand such dynamic interplay between cancer cells and stromal component of the TME.
Imaging TME: perspective on interpretation
Early studies on TME have often associated many of its components with an overall tumor promoting, pro-metastatic, and cancer cell shielding function. While the TME is a dynamic ‘organ’ of the tumor which could be co-opted to favor tumor growth and invasion, the initial remodeling of the microenvironment in tumors -or ‘wounds that do not heal’36 - rather constitutes a scar forming, host response against neoplastic events. In this view, the TME acts rather as a tumor suppressor than a tumor promoter. This perspective was highlighted in recent studies from our group37 and other’s38, when functional characterization of the role of myofibroblasts in pancreas tumors yield to the discovery of these cells presenting anti-tumor properties, and targeting them resulted in a more aggressive disease progression. While myofibroblasts in organ fibrosis are drivers of the ECM and tissue remodeling39,40, their role in cancer may be more complex and differentially influenced by cancer cells. As our pre-clinical cancer models and tools to define and target specific components of the TME expanded, so did our appreciation for its functional, dynamic role in disease progression. Whereas increased vascular permeability may be more permissive to chemotherapeutic drug delivery33, the accumulating intratumoral hypoxia, despite regression of the tumor volume, can promote invasion and metastasis39,40. The duality in functionality of the TME (Figure 2), thus calls for caution in imaging data interpretation. The fibrotic scar, that has yet to resolve in shrinking tumors, may play a tumor suppressive role, but, for lack of more specific assessment of functional contribution of the TME, may be interpreted as lack of response to treatment and may contribute to false positive cancer diagnosis. Our advances in understanding the TME continue to challenge old dogmas, and these conceptual advances are of critical importance in interpretation of imaging data. While extracellular matrix material in the TME is often thought as a physical barrier to drug penetrance, the concept is still under investigation41 and the outcome unclear and likely tumor type and stage specific. Indeed, therapies aimed to target the ECM remodeling enzymes will not only impact the structural stability of the ECM as defined by diffusion rate of specific molecules, but will also have critical impact on vascular basement membrane integrity and intratumoral hypoxia, as well as cell behavior via release of growth factors and biologically active cryptic peptides. The interpretation of imaging findings, irrespective of modalities, must therefore take in consideration the biological significance of the results, in particular when targeting TME components, to ascertain the overall benefit or detriment on tumor burden. The imaging consideration for the revised Response Evaluation Criteria in Solid Tumors (RECIST) guidelines relies on anatomical assessments of lesions as criteria to assess tumor burden. Gaining a more robust understanding on the proportional and functional changes of the TME in cancer treatment may thus also offer important consideration for ongoing clinical imaging.
Acknowledgments
V.S.L. is supported by the NIH/NCI CCSG New Faculty Award P30CA016672 and the UT MDACC Khalifa Bin Zayed Al Nahya Foundation. I wish to thank Judith Kaye and Jiha Kim for critical reading of the manuscript, and Raghu Kalluri for his valuable guidance.
References
- 1.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hynes RO, Naba A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harbor perspectives in biology. 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oskarsson T. Extracellular matrix components in breast cancer progression and metastasis. Breast. 2013;22(Suppl 2):S66–72. doi: 10.1016/j.breast.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Cretu A, Brooks PC. Impact of the non-cellular tumor microenvironment on metastasis: potential therapeutic and imaging opportunities. Journal of cellular physiology. 2007;213:391–402. doi: 10.1002/jcp.21222. [DOI] [PubMed] [Google Scholar]
- 5.Maeshima Y, et al. Distinct antitumor properties of a type IV collagen domain derived from basement membrane. The Journal of biological chemistry. 2000;275:21340–21348. doi: 10.1074/jbc.M001956200. [DOI] [PubMed] [Google Scholar]
- 6.Kamphaus GD, et al. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. The Journal of biological chemistry. 2000;275:1209–1215. doi: 10.1074/jbc.275.2.1209. [DOI] [PubMed] [Google Scholar]
- 7.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason SD, Joyce JA. Proteolytic networks in cancer. Trends in cell biology. 2011;21:228–237. doi: 10.1016/j.tcb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neesse A, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–868. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 10.Polyak K, Kalluri R. The role of the microenvironment in mammary gland development and cancer. Cold Spring Harbor perspectives in biology. 2010;2:a003244. doi: 10.1101/cshperspect.a003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conklin MW, Keely PJ. Why the stroma matters in breast cancer: insights into breast cancer patient outcomes through the examination of stromal biomarkers. Cell adhesion & migration. 2012;6:249–260. doi: 10.4161/cam.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dittmer J, Leyh B. The impact of tumor stroma on drug response in breast cancer. Seminars in cancer biology. 2015;31C:3–15. doi: 10.1016/j.semcancer.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Thompson RF, Maity A. Radiotherapy and the tumor microenvironment: mutual influence and clinical implications. Advances in experimental medicine and biology. 2014;772:147–165. doi: 10.1007/978-1-4614-5915-6_7. [DOI] [PubMed] [Google Scholar]
- 14.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer metastasis reviews. 1989;8:98–101. [PubMed] [Google Scholar]
- 15.Langley RR, Fidler IJ. The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. International journal of cancer. Journal international du cancer. 2011;128:2527–2535. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 17.Paulmurugan R, et al. Real time dynamic imaging and current targeted therapies in the war on cancer: a new paradigm. Theranostics. 2013;3:437–447. doi: 10.7150/thno.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serres S, O’Brien ER, Sibson NR. Imaging angiogenesis, inflammation, and metastasis in the tumor microenvironment with magnetic resonance imaging. Advances in experimental medicine and biology. 2014;772:263–283. doi: 10.1007/978-1-4614-5915-6_12. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, Zhang W, Li J, Zhang Y. Optical imaging of tumor microenvironment. American journal of nuclear medicine and molecular imaging. 2013;3:1–15. [PMC free article] [PubMed] [Google Scholar]
- 20.Avni R, Cohen B, Neeman M. Hypoxic stress and cancer: imaging the axis of evil in tumor metastasis. NMR in biomedicine. 2011;24:569–581. doi: 10.1002/nbm.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krohn KA, Link JM, Mason RP. Molecular imaging of hypoxia. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2008;49(Suppl 2):129S–148S. doi: 10.2967/jnumed.107.045914. [DOI] [PubMed] [Google Scholar]
- 22.Matsuo M, Matsumoto S, Mitchell JB, Krishna MC, Camphausen K. Magnetic resonance imaging of the tumor microenvironment in radiotherapy: perfusion, hypoxia, and metabolism. Seminars in radiation oncology. 2014;24:210–217. doi: 10.1016/j.semradonc.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajendran JG, Krohn KA. F-18 Fluoromisonidazole for Imaging Tumor Hypoxia: Imaging the Microenvironment for Personalized Cancer Therapy. Seminars in nuclear medicine. 2015;45:151–162. doi: 10.1053/j.semnuclmed.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashim AI, Zhang X, Wojtkowiak JW, Martinez GV, Gillies RJ. Imaging pH and metastasis. NMR in biomedicine. 2011;24:582–591. doi: 10.1002/nbm.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penet MF, Chen Z, Bhujwalla ZM. MRI of metastasis-permissive microenvironments. Future Oncol. 2011;7:1269–1284. doi: 10.2217/fon.11.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glunde K, Bhujwalla ZM. Metabolic tumor imaging using magnetic resonance spectroscopy. Seminars in oncology. 2011;38:26–41. doi: 10.1053/j.seminoncol.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penet MF, Glunde K, Jacobs MA, Pathak AP, Bhujwalla ZM. Molecular and functional MRI of the tumor microenvironment. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2008;49:687–690. doi: 10.2967/jnumed.107.043349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suetsugu A, et al. Multi-color palette of fluorescent proteins for imaging the tumor microenvironment of orthotopic tumorgraft mouse models of clinical pancreatic cancer specimens. Journal of cellular biochemistry. 2012;113:2290–2295. doi: 10.1002/jcb.24099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sidani M, Wyckoff J, Xue C, Segall JE, Condeelis J. Probing the microenvironment of mammary tumors using multiphoton microscopy. Journal of mammary gland biology and neoplasia. 2006;11:151–163. doi: 10.1007/s10911-006-9021-5. [DOI] [PubMed] [Google Scholar]
- 30.Zal T, Chodaczek G. Intravital imaging of anti-tumor immune response and the tumor microenvironment. Seminars in immunopathology. 2010;32:305–317. doi: 10.1007/s00281-010-0217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakasone ES, Askautrud HA, Egeblad M. Live imaging of drug responses in the tumor microenvironment in mouse models of breast cancer. Journal of visualized experiments : JoVE. 2013:e50088. doi: 10.3791/50088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gligorijevic B, Bergman A, Condeelis J. Multiparametric classification links tumor microenvironments with tumor cell phenotype. PLoS biology. 2014;12:e1001995. doi: 10.1371/journal.pbio.1001995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakasone ES, et al. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer cell. 2012;21:488–503. doi: 10.1016/j.ccr.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Histed SN, et al. Review of functional/anatomical imaging in oncology. Nuclear medicine communications. 2012;33:349–361. doi: 10.1097/MNM.0b013e32834ec8a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stasinopoulos I, et al. Exploiting the tumor microenvironment for theranostic imaging. NMR in biomedicine. 2011;24:636–647. doi: 10.1002/nbm.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dvorak HF. Tumors: wounds that do not heal, Similarities between tumor stroma generation and wound healing. The New England journal of medicine. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 37.Ozdemir BC, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rhim AD, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooke VG, et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer cell. 2012;21:66–81. doi: 10.1016/j.ccr.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keskin D, et al. Targeting Vascular Pericytes in Hypoxic Tumors Increases Lung Metastasis via Angiopoietin-2. Cell reports. 2015;10:1066–1081. doi: 10.1016/j.celrep.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi IK, Strauss R, Richter M, Yun CO, Lieber A. Strategies to increase drug penetration in solid tumors. Frontiers in oncology. 2013;3:193. doi: 10.3389/fonc.2013.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]