Abstract
The 8q24 polymorphisms have been implicated in various cancers. Three 8q24 polymorphisms (rs1447295 C>A, rs16901979 C>A, and rs6983267 T>G) have been extensively investigated for their association with prostate cancer (PCa) susceptibility, yet conclusions are contradictory. We conducted a comprehensive meta-analysis to reevaluate the associations between those polymorphisms and PCa susceptibility, according to the latest meta-analysis guidelines (PRISMA). Eligible publications were searched from MEDLINE, EMBASE and CBM. False positive report possibility analysis was performed. We totally collected 20184 cases and 20439 controls from 20 studies for the rs1447295 C>A, 1850 cases and 2090 controls from 7 studies for the rs16901979 C>A, and 12233 cases and 7582 controls from 17 studies for the rs6983267 T>G. Overall, each of studied 8q24 polymorphisms was significantly associated with PCa risk individually. Significant associations were also observed in stratified analysis by ethnicity, source of control, and quality score. Interestingly, the effect of rs1447295 on PCa risk was observed among Caucasians and Asians, but not Africa-Americans. The effect of rs16901979 was more prominent among Africa-Americans than Asians. Likewise, rs6983267 conferred a higher Pca risk among Caucasians than Asians. Collectively, these 8q24 variant(s) may modulate PCa risk in an ethnic-specific manner.
Prostate cancer (PCa) is one of the most common non-cutaneous malignancies among men in the US, with an estimated 240,890 new cases and 33,720 deaths in 20101. Although little is known about the etiology of the disease, accumulating evidence has demonstrated that genetic variations may play a crucial role in the carcinogenesis of PCa. For example, genome-wide association studies (GWASs) have identified more than 40 human PCa-predisposing variants, among which single nucleotide polymorphisms (SNPs) located in the 8q24 region were considered as promising biomarkers for PCa2,3,4,5,6. However, not all of those significant findings can be validated by the subsequent candidate-based studies.
Chromosomal region 8q24 has emerged recently as bona fide risk locus for multiple cancers7,8,9. Fine mapping and additional genome scans have identified three 8q24 regions (region 1: 128.54–128.62 Mb; region 2: 128.12–128.28 Mb; region 3: 128.47–128.54 Mb) that contain variants independently associated with PCa risk2,3,10,11. Since the 8q24 region was originally shown to confer a PCa risk in a genome wide linkage scan of 871 Icelandic men in 20068, numerous association studies have been performed to extensively explore the roles of 8q24 single nucleotide polymorphisms (SNPs) in the etiology of PCa. To date, there are about 64 variants in 8q24 investigated for the association with PCa risk, and only 20 of those variants were confirmed to be PCa risk-associated SNPs. Of those PCa risk SNP, rs1447295 C>A in region 1, rs16901979 C>A in region 2, and rs6983267 T>G in region 3, have shown strong association with PCa, with respective adjusted P value of 4 × 10−29 , 1 × 10−19, and 1 × 10−11 12. Similarly, significant associations with 8q24 polymorphisms were also identified for a wide spectrum of cancers, including cancers of the breast13,14, prostate2,4, bladder15, colon16, lung17, ovaries18, pancreas19, and brain20 among different ethnicities (Asian, Caucasian, and African of Americans). Taken together, these findings have made SNPs on 8q24 of particular interest because of their potential roles in screening strategies for high-risk individuals and discovering new therapeutic targets.
The mechanisms by which 8q24 influences the course of PCa are not yet fully understood. The 8q24 region has been described as a “gene desert” since the 600-kbp gene-poor region appears to have little or no transcriptional activity. Nevertheless, several lines of evidence has suggested that 8q24 may play an active role in PCa carcinogenesis. First, 8q24 is a highly conserved genomic region. Second, POU5F1P1, an important gene on 8q24, encodes a weak transcriptional activator that may contribute to carcinogenesis21. Third, recent studies have shown that the activity of the nearby oncogene MYC is associated with 8q244,22,23,24.
Although PCa carcinogenesis has been found to be associated with hereditary background, increasing molecular epidemiology studies have presented conflicting results on the association between 8q24 SNPs and PCa risk, which may be partially attributable to various sample sizes, different genetic backgrounds, and heterogeneous inclusion criteria among studies. With this in mind, we carried out the current meta-analysis to provide a quality assessment of the association of the most frequently analyzed 8q24 SNPs (i.e., rs1447295 C>A in region1, rs16901979 C>A in region2, and rs6983267 T>G in region3) with PCa risk.
Results
Eligible studies
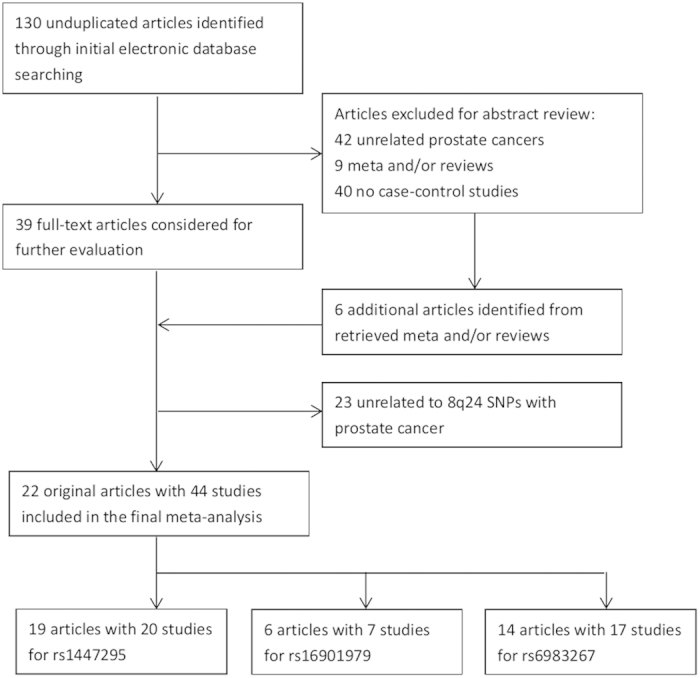
Based on the inclusion criteria, 22 eligible articles consisting of 44 studies were included in this meta-analysis1,12,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40. The sample sizes of those studies ranged from 103 to 24454. Of those articles, 9 and 13 were categorized as high and low quality, respectively, using methods described in the Methods section. Literature search and study selection yielded 20 studies for rs1447295 C>A analysis, which were performed among Caucasians (10 studies), Asians (7 studies), and Africa-Americans (3 studies). A total of 7 eligible studies were retrieved for rs16901979 C>A analysis with 3, 3, and 1 studies conducted in these three ethnic groups, respectively. Moreover, of 17 studies eligible for rs6983267 T>G analysis, there were 9, 6, and 2 carried out in these three ethnic groups, respectively. Other details were shown in Table 1. Genotype frequency distributions of studied SNPs in all of the control populations were agreed with HWE.
Table 1. Characteristics of studies included in the current meta-analysis.
| Surname | Year | Country | Ethnicity | Source | Genotype method | Case |
control |
MAF | HWE | Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WW | VW | VV | ALL | WW | VW | VV | ALL | |||||||||
| For rs1447295 C>A (region1) | ||||||||||||||||
| Chan | 2013 | Singapore | Asian | HB | Illumina 1M chip | 180 | 92 | 17 | 289 | 94 | 44 | 5 | 143 | 0.19 | 0.957 | 7 |
| Schumacher | 2007 | USA | Caucasians | PB | TaqMan | 8462 | 2736 | 268 | 11466 | 10344 | 2472 | 172 | 12988 | 0.11 | 0.079 | 14 |
| Zheng | 2007 | USA | Caucasians | HB | iPLEX | 1169 | 346 | 31 | 1546 | 485 | 82 | 4 | 571 | 0.08 | 0.794 | 11 |
| Joung | 2012 | Korea | Asian | HB | iPLEX | 114 | 67 | 12 | 193 | 127 | 38 | 3 | 168 | 0.13 | 0.936 | 7 |
| Francisco | 2013 | Chile | Caucasians | HB | TaqMan | 56 | 23 | 4 | 83 | 16 | 4 | 1 | 21 | 0.14 | 0.309 | 4 |
| Terada | 2008 | Japan | Asian | HB | PCR-RFLP | 310 | 172 | 25 | 507 | 335 | 165 | 11 | 511 | 0.18 | 0.071 | 9 |
| Okobia | 2011 | USA | Africa-American | HB | TaqMan | 156 | 162 | 36 | 354 | 173 | 207 | 58 | 438 | 0.37 | 0.291 | 11 |
| Brankovie | 2013 | Sebia | Caucasians | HB | PCR-RFLP | 86 | 61 | 3 | 150 | 11 | 82 | 7 | 100 | 0.48 | 0.000 | 7 |
| Suurinirmi | 2007 | USA | Caucasians | PB | TaqMan | 435 | 136 | 11 | 582 | 427 | 107 | 4 | 538 | 0.11 | 0.390 | 15 |
| Zeegers | 2011 | USA | Caucasians | PB | TaqMan | 224 | 53 | 4 | 281 | 196 | 64 | 7 | 267 | 0.15 | 0.523 | 10 |
| Salinas | 2008 | USA | Caucasians | PB | TaqMan | 937 | 288 | 27 | 1252 | 994 | 225 | 14 | 1233 | 0.10 | 0.753 | 15 |
| Benford | 2010 | USA | Africa-American | HB | TaqMan | 86 | 77 | 26 | 189 | 237 | 221 | 65 | 523 | 0.34 | 0.000 | 8 |
| Liu | 2012 | China | Asian | PB | PCR-RFLP | 150 | 102 | 8 | 260 | 197 | 86 | 4 | 287 | 0.16 | 0.111 | 11 |
| Liu | 2009 | Japan | Asian | HB | TaqMan | 217 | 149 | 25 | 391 | 218 | 89 | 16 | 323 | 0.19 | 0.088 | 8 |
| Severi | 2007 | Australian | Caucasians | PB | TaqMan | 595 | 212 | 14 | 821 | 586 | 135 | 11 | 732 | 0.11 | 0.319 | 13 |
| Wokolorczyk | 2010 | Poland | Caucasians | HB | PCR-RFLP | 515 | 156 | 19 | 690 | 484 | 115 | 3 | 602 | 0.10 | 0.165 | 8 |
| Chen | 2009 | Taiwan | Asian | PB | TaqMan | 215 | 119 | 6 | 340 | 253 | 75 | 9 | 337 | 0.14 | 0.237 | 10 |
| Cheng | 2008 | USA | Caucasians | HB | TaqMan | 318 | 97 | 2 | 417 | 344 | 69 | 4 | 417 | 0.09 | 0.795 | 11 |
| Cheng | 2008 | USA | Africa-American | HB | TaqMan | 39 | 44 | 6 | 89 | 43 | 35 | 11 | 89 | 0.32 | 0.919 | 11 |
| Zheng | 2010 | China | Asian | PB | iPLEX | 173 | 96 | 15 | 284 | 110 | 35 | 6 | 151 | 0.16 | 0.147 | 12 |
| For rs16901979 C>A (region2) | ||||||||||||||||
| Chan | 2013 | Singapore | Asian | HB | Illumina 1M chip | 139 | 119 | 31 | 289 | 64 | 68 | 12 | 144 | 0.32 | 0.302 | 7 |
| Joung | 2012 | Korea | Asian | HB | iPLEX | 99 | 81 | 14 | 194 | 100 | 57 | 12 | 169 | 0.24 | 0.333 | 7 |
| Okobia | 2011 | USA | Africa-American | HB | TaqMan | 81 | 158 | 99 | 338 | 131 | 193 | 102 | 426 | 0.47 | 0.064 | 11 |
| Chen | 2010 | Taiwan | Asian | HB | TaqMan | 148 | 148 | 35 | 331 | 173 | 138 | 24 | 335 | 0.28 | 0.62 | 11 |
| Benford | 2010 | USA | Africa-American | HB | TaqMan | 45 | 97 | 50 | 192 | 188 | 237 | 87 | 512 | 0.40 | 0.406 | 8 |
| Cheng | 2008 | USA | Caucasians | HB | TaqMan | 375 | 41 | 1 | 417 | 393 | 22 | 1 | 416 | 0.03 | 0.252 | 11 |
| Cheng | 2008 | USA | Africa-American | HB | TaqMan | 23 | 43 | 23 | 89 | 27 | 50 | 11 | 88 | 0.41 | 0.1 | 11 |
| For rs6983267 T>G (region3) | ||||||||||||||||
| Chan | 2013 | Singapore | Asian | HB | Illumina 1M chip | 89 | 136 | 63 | 288 | 47 | 74 | 23 | 144 | 0.42 | 0.493 | 7 |
| Zheng | 2007 | USA | Caucasians | HB | iPLEX | 495 | 771 | 285 | 1551 | 142 | 299 | 132 | 573 | 0.49 | 0.293 | 11 |
| Joung | 2012 | Korea | Asian | HB | iPLEX | 56 | 92 | 46 | 194 | 51 | 86 | 31 | 168 | 0.44 | 0.618 | 7 |
| Francisco | 2013 | Chile | Caucasians | HB | TaqMan | 19 | 33 | 30 | 82 | 5 | 13 | 3 | 21 | 0.45 | 0.253 | 4 |
| Terada | 2008 | Japan | Asian | HB | PCR-RFLP | 211 | 219 | 77 | 507 | 206 | 225 | 80 | 511 | 0.38 | 0.94 | 9 |
| Okobia | 2011 | USA | Africa-American | HB | TaqMan | 307 | 34 | 2 | 343 | 373 | 52 | 1 | 426 | 0.06 | 0.562 | 11 |
| Brankovie | 2013 | Sebia | Caucasians | HB | PCR-RFLP | 53 | 80 | 17 | 150 | 69 | 129 | 52 | 250 | 0.47 | 0.561 | 7 |
| Salinas | 2008 | USA | Caucasians | PB | PCR-RFLP | 364 | 652 | 242 | 1258 | 313 | 617 | 308 | 1238 | 0.50 | 0.91 | 15 |
| Liu | 2012 | China | Asian | PB | PCR-RFLP | 70 | 137 | 53 | 260 | 94 | 137 | 51 | 282 | 0.42 | 0.93 | 11 |
| Liu | 2009 | Japan | Asian | HB | TaqMan | 151 | 181 | 59 | 391 | 147 | 151 | 25 | 323 | 0.31 | 0.104 | 8 |
| Papanikolopoulou | 2011 | Greece | Caucasians | HB | TaqMan | 16 | 46 | 24 | 86 | 39 | 47 | 13 | 99 | 0.37 | 0.844 | 6 |
| Cheng | 2008 | USA | Caucasians | HB | TaqMan | 76 | 215 | 126 | 417 | 106 | 206 | 105 | 417 | 0.50 | 0.807 | 11 |
| Cheng | 2008 | USA | Africa-American | HB | TaqMan | 74 | 14 | 1 | 89 | 74 | 11 | 4 | 89 | 0.11 | 0.001 | 11 |
| Zheng | 2010 | China | Asian | PB | iPLEX | 86 | 134 | 62 | 282 | 51 | 72 | 29 | 152 | 0.43 | 0.69 | 12 |
| Penney | 2009 | USA | Caucasians | PB | iPLEX | 400 | 644 | 261 | 1305 | 372 | 707 | 323 | 1402 | 0.48 | 0.714 | 15 |
| Penney | 2009 | USA | Caucasians | PB | iPLEX | 1184 | 1776 | 812 | 3772 | 69 | 134 | 46 | 249 | 0.45 | 0.177 | 15 |
HB, Hospital based; PB, Population based; PCR-RFLP, Polymorphism chain reaction-restriction fragment length polymorphism; MAF, Minor allele frequency; HWE, Hardy-Weinberg equilibrium. WW, wild homozygous; WV, heterozygous; VV, variant homozygous.
Quantitative synthesis
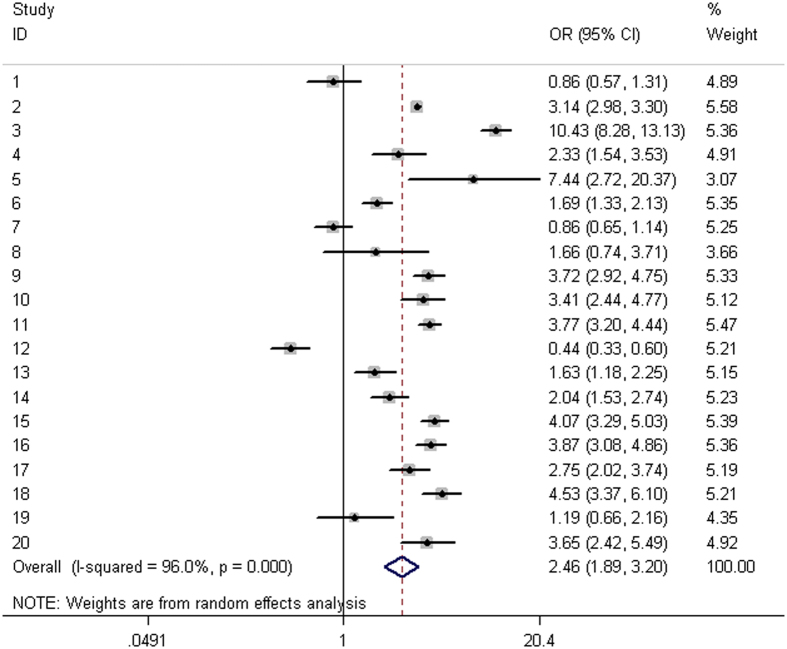
A total 20 eligible studies were pooled together to evaluate the association between 8q24 rs1447295 C>A and PCa risk, with 20184 cases and 20439 controls. Pooled risk estimates indicated the significant associations of rs1447295 C>A with increased PCa risk under homozygous (OR= 1.39; 95% CI= 1.01–1.91, P = 0.042), heterozygous (OR= 1.26, 95% CI= 1.09–1.45, P = 0.002), dominant models (OR= 2.46, 95% CI= 1.89–3.20, P = 0.000), and alleles comparison (OR= 1.23, 95% CI= 1.09–1.40, P = 0.001); intriguingly, the direction of the association was reversed under recessive genetic models (OR= 0.51, 95% CI= 0.39–0.67, P = 0.001) as shown in Table 2. Moreover, there were 7 eligible studies available for 8q24 rs16901979 C>A analysis, consisting of 1850 cases and 2090 controls. We found that carrier of AA, AC, or combined carriers of AA/AC risk genotypes had an OR of 1.71 (homozygous: 95% CI= 1.36–2.16, P = 0.000), 1.31 (heterozygous 95% CI= 1.12–1.53, P = 0.001), or 1.39 (dominant: 95% CI= 1.20–1.61, P = 0.000) for developing prostate cancer, respectively, compared with those with CC genotype. And OR of alleles comparison (C vs. A) for the association was 1.31 (95% CI= 1.18–1.46, P = 0.000). For 8q24 rs6983267 T>G analysis, a total of 17 studies comprising 12233 cases and 7582 controls were incorporated into the meta-analysis. Pooled analysis observed significant associations between increased PCa risk and the variant of interest under homozygous (OR= 1.44; 95% CI= 1.31–1.58, P = 0.000), heterozygous (OR= 1.19, 95% CI= 1.10–1.29, P = 0.000), recessive (OR= 1.26, 95% CI= 1.18–1.36, P = 0.000), and alleles comparison (OR= 1.19, 95% CI= 1.14–1.25, P = 0.000).
Table 2. Meta-analysis of the association between 8q24 rs1447295, rs16901979 and rs6983267 polymorphisms and prostate cancer risk.
| Variables | No. of individuals | No. of studies | Homozygous | Heterozygous |
Recessive |
Dominant |
Allele |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Phet | OR (95% CI) | Phet | OR (95% CI) | Phet | OR (95% CI) | Phet | OR (95% CI) | Phet | ||||
| rs1447295 C>A (region1) | |||||||||||||
| All | 20184/20439 | 20 | 1.39 (1.01–1.91) | 0.000 | 1.26 (1.09–1.45) | 0.000 | 0.51 (0.39–0.67) | 0.001 | 2.46 (1.89–3.20) | 0.000 | 1.23 (1.09–1.40) | 0.000 | |
| publication bias (Begg’s test) | P = 0.296 | P = 0.382 | P = 0.713 | P = 0.277 | P = 0.272 | ||||||||
| Ethnicity | |||||||||||||
| Caucasion | 17288/17469 | 10 | 1.35 (0.78–2.35) | 0.000 | 1.15 (0.92–1.44) | 0.000 | 0.48 (0.31–0.74) | 0.014 | 4.15 (3.26–5.28) | 0.000 | 1.18(0.97–1.43) | 0.000 | |
| Asian | 2264/1920 | 7 | 1.87 (1.33–2.62) | 0.469 | 1.49 (1.30–1.70) | 0.112 | 0.38 (0.27–0.53) | 0.438 | 1.97 (1.47–2.62) | 0.000 | 1.44 (1.29–1.60) | 0.425 | |
| Africa-American | 632/1050 | 3 | 0.82 (0.59–1.14) | 0.349 | 0.95 (0.77–1.18) | 0.411 | 0.91 (0.66–1.26) | 0.992 | 0.74 (0.43–1.29) | 0.001 | 0.92 (0.79–1.07) | 0.487 | |
| Source of control | |||||||||||||
| HB | 4898/3906 | 12 | 1.28 (0.75–2.17) | 0.000 | 1.12 (0.84–1.49) | 0.000 | 0.52 (0.33–0.82) | 0.001 | 2.07 (1.16–3.67) | 0.000 | 1.16 (0.92–1.47) | 0.000 | |
| PB | 15286/16533 | 8 | 1.80 (1.52–2.13) | 0.278 | 1.38 (1.22–1.57) | 0.000 | 0.44 (0.37–0.52) | 0.329 | 3.22 (2.78–3.74) | 0.000 | 1.34 (1.21–1.50) | 0.049 | |
| Score | |||||||||||||
| Low | 2492/2391 | 8 | 1.49 (0.74–2.99) | 0.000 | 1.17 (0.92–1.51) | 0.000 | 0.45 (0.25–0.81) | 0.003 | 1.79 (1.01–3.16) | 0.000 | 1.14 (0.82–1.58) | 0.000 | |
| High | 17692/18048 | 12 | 1.32 (0.92–1.91) | 0.002 | 1.19 (0.82–1.71) | 0.003 | 0.54 (0.39–0.74) | 0.016 | 3.05 (2.31–4.02) | 0.000 | 1.29 (1.14–1.46) | 0.000 | |
| rs16901979 C>A (region2) | |||||||||||||
| All | 1850/2090 | 7 | 1.71 (1.36–2.16) | 0.595 | 1.31 (1.12–1.53) | 0.136 | 1.22 (0.80–1.87) | 0.002 | 1.39 (1.20–1.61) | 0.144 | 1.31 (1.18–1.46) | 0.232 | |
| publication bias (Begg’s test) | P = 0.609 | P = 0.948 | P = 0.995 | P = 0.854 | P = 0.689 | ||||||||
| Ethnicity | |||||||||||||
| Asian | 714/648 | 3 | 1.41 (0.95–2.09) | 0.661 | 1.15 (0.92–1.43) | 0.134 | 1.65 (1.13–2.41) | 0.293 | 1.19 (0.97–1.47) | 0.179 | 1.17 (1.00–1.38) | 0.350 | |
| Africa-American | 619/1026 | 3 | 1.91 (1.44–2.55) | 0.342 | 1.41 (1.10–1.80) | 0.385 | 0.96 (0.54–1.71) | 0.007 | 1.56 (1.24–1.96) | 0.413 | 1.40 (1.21–1.62) | 0.415 | |
| Source of control | |||||||||||||
| HB | 1850/2090 | 7 | 1.71 (1.36–2.16) | 0.595 | 1.31 (1.12–1.53) | 0.136 | 1.22 (0.80–1.87) | 0.002 | 1.39 (1.20–1.61) | 0.144 | 1.31 (1.18–1.46) | 0.232 | |
| Score | |||||||||||||
| Low | 675/825 | 3 | 1.75 (1.22–2.51) | 0.160 | 1.26 (0.8–1.97) | 0.032 | 1.49 (1.08–2.05) | 0.475 | 1.32 (0.84–2.08) | 0.020 | 1.29 (1.10–1.52) | 0.04 | |
| High | 1175/1265 | 4 | 1.68 (1.25–2.27) | 0.826 | 1.34 (1.09–1.65) | 0.438 | 1.46 (1.13–1.89) | 0.548 | 1.42 (1.17–1.73) | 0.668 | 1.33 (1.16–1.53) | 0.585 | |
| rs6983267 T>G (region3) | |||||||||||||
| All | 12233/7582 | 17 | 1.44 (1.31–1.58) | 0.065 | 1.19 (1.10–1.29) | 0.065 | 1.26 (1.18–1.36) | 0.473 | 1.54 (0.93–2.57) | 0.000 | 1.19 (1.14–1.25) | 0.187 | |
| publication bias (Begg’s test) | P = 0.308 | P = 0.959 | P = 0.072 | P = 0.749 | P = 0.303 | ||||||||
| Ethnicity | |||||||||||||
| Caucasion | 9879/5487 | 9 | 1.47 (1.33–1.64) | 0.065 | 1.25 (1.07–1.46) | 0.027 | 1.27 (1.17–1.38) | 0.427 | 1.77 (0.81–3.87) | 0.000 | 1.21 (1.15–1.27) | 0.102 | |
| Asian | 1922/1580 | 6 | 1.33 (1.08–1.62) | 0.166 | 1.07 (0.92–1.24) | 0.746 | 1.27 (1.06–1.52) | 0.201 | 1.35 (1.04–1.75) | 0.005 | 1.14 (1.03–1.25) | 0.283 | |
| Source of control | |||||||||||||
| HB | 4098/3021 | 11 | 1.68 (1.30–2.16) | 0.038 | 1.17 (1.03–1.33) | 0.141 | 1.38 (1.22–1.55) | 0.223 | 1.43 (1.00–2.04) | 0.000 | 1.24 (1.15–1.33) | 0.092 | |
| PB | 8135/4561 | 6 | 1.37 (1.22–1.54) | 0.532 | 1.20 (1.09–1.33) | 0.065 | 1.21 (1.10–1.32) | 1.000 | 1.80 (0.63–5.11) | 0.000 | 1.17 (1.10–1.24) | 0.686 | |
| Score | |||||||||||||
| Low | 1698/1516 | 7 | 1.79 (1.19–2.67) | 0.009 | 1.11 (0.94–1.30) | 0.120 | 1.43 (1.19–1.73) | 0.069 | 1.34 (0.98–1.84) | 0.002 | 1.28 (1.07–1.54) | 0.015 | |
| High | 10535/6066 | 10 | 1.42 (1.31–1.58) | 0.547 | 1.22 (1.11–1.33) | 0.121 | 1.24 (1.14–1.34) | 0.986 | 1.66 (0.79–3.51) | 0.000 | 1.19 (1.13–1.25) | 0.839 | |
HB, Hospital based; PB, Population based. OR, Odds ratio; CI, confidence interval.
Stratification analysis
In the stratification analyses, subgroups with less than three studies were excluded from further analysis because of small number of subgroups would likely lead to a false association. In the current meta-analysis, positive associations were found among most of the remaining subgroups for each of the three 8q24 SNPs. Briefly, the associations of rs1447295 C>A polymorphism and PCa risk were observed among Asians populations under the all assumed genetic comparisons, among Caucasians under recessive and dominant models, but not among Africa-Americans. Similarly, stratified analysis by source of control found that rs1447295 C>A showed significant association with PCa risk for PB subgroup under all genetic models, while the association was only detected under recessive, dominant model, and allele comparison for HB subgroup. Moreover, the associations were observed for both low and high quality subgroups.
As shown in Table 2, due to the limited number of study, stratification analysis for rs1601979 C>A polymorphism was not conducted among Caucasians and PB studies. The significant risk effects of rs1601979 C>A polymorphism on PCa risk were consistently observed under the most of assumed genetic comparisons for Africa-Americans, hospital-based studies, and studies with high quality score. The effects were also existed among Asians and studies with low quality score, with fewer genetic models detecting the significance. We then compared the strength of the associations within the subgroups, the association with larger OR and smaller P value was considered as more prominent than others. Interestingly, the association between PCa and rs16901979 was more prominent among Africa-Americans than Asians under most of genetic models (e.g. Africa-Americans with homozygous OR= 1.91, 95% CI= 1.44–2.55, P = 0.000; heterozygous OR= 1.41 (1.10–1.80), P = 0.000; dominant OR= 1.56, 95% CI= 1.24–1.96, P = 0.000; and allele comparison OR= 1.40, 95% CI= 1.21–1.62, P = 0.000. In contrast, Asians with recessive OR= 1.65, 95% CI= 1.13–2.41), P = 0.000; and allele comparison OR= 1.17, 95% CI= 1.00–1.38, P = 0.053) . Moreover, stratified analyses observed significantly increased PCa risk associated with rs6983267 T>G polymorphism among all subgroups defined by ethnicity, source of control, and quality score. Likewise, rs6983267 T>G seemed to confer a higher Pca risk in Caucasians than Asians (homozygous OR= 1.47, 95% CI= 1.33–1.64, P = 0.000 vs. OR= 1.33, 95% CI= 1.08–1.62, P = 0.006; Allele comparison OR= 1.21, 95% CI= 1.15–1.27, P = 0.000 vs. Allele comparison OR= 1.14, 95% CI= 1.03–1.25, P = 0.01).
In the current meta-analysis, a high level of heterogeneity was detected in the pooled analysis for rs1447295 C>A. However, the heterogeneity decreased in the stratified analysis by ethnicity, indicating that ethnicity might be a source of heterogeneity. Moreover, the between-study heterogeneity was detected among several subgroups for each of studied SNPs. However, leave-one-out sensitivity analyses suggested that no single study substantially altered the pooled ORs of any SNP of interest (data not shown), suggesting the stability of this meta-analysis. Finally, the FPRP values for all significant findings at different prior probability levels were summarized in Table 3. With the assumption of prior probability of 0.01, the association with rs1447295 C>A was noteworthy for Asians, population-based studies and high quality studies (FPRP range: 0.010–0.144, 0.010–0.022, 0.010–0.014, respectively) under the assumed comparisons. Moreover, the association between PCa and rs16901979 C>A among Africa-American (FPRP: 0.026) under dominant and allele comparison was considered noteworthy. In term of rs6983267 T>G, the association was noteworthy for Caucasians, population-based studies, high quality studies, low quality studies. In contrast, based on FPRP values, the rest of significant associations between 8q24 variants and PCa risk might not be deserving of attention, suggesting some possible bias or chance finding in this meta-analysis. Therefore, our findings need to be further validated in large and well-designed studies, involving different ethnicities (Fig. 1).
Table 3. False-positive report probability values for associations between the risk of cancer and the frequency of genotypes of 8q24 variants.
| 8q24 SNP genotype | Crude OR (95%CI) | Pa | statistical powerb | Prior probability |
||||
|---|---|---|---|---|---|---|---|---|
| 0.250 | 0.100 | 0.010 | 0.001 | 0.000 | ||||
| 8q24-rs1447295 C>A | ||||||||
| All patients | ||||||||
| Homozygous | 1.39 (1.01–1.91) | <0.0001 | 0.993 | 0.000 | 0.001 | 0.010 | 0.091 | 0.502 |
| Heterozygous | 1.26 (1.09–1.45) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Recessive | 0.51 (0.39–0.67) | <0.0001 | 0.896 | 0.000 | 0.001 | 0.011 | 0.100 | 0.527 |
| Dominant | 2.46 (1.89–3.20) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Allele comparision | 1.23 (1.09–1.40) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Ethnicity-Caucasian | ||||||||
| Recessive | 0.48 (0.31–0.74) | <0.0001 | 0.472 | 0.001 | 0.002 | 0.021 | 0.175 | 0.680 |
| Dominant | 4.15 (3.26–5.28) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Ethnicity-Asian | ||||||||
| Homozygous | 1.87 (1.33–2.62) | <0.0001 | 0.059 | 0.005 | 0.015 | 0.144 | 0.630 | 0.944 |
| Heterozygous | 1.49 (1.30–1.70) | <0.0001 | 0.986 | 0.000 | 0.001 | 0.010 | 0.092 | 0.504 |
| Recessive | 0.38 (0.27–0.53) | <0.0001 | 0.028 | 0.011 | 0.031 | 0.262 | 0.781 | 0.973 |
| Dominant | 1.97 (1.47–2.62) | <0.0001 | 0.968 | 0.000 | 0.001 | 0.010 | 0.094 | 0.508 |
| Allele comparision | 1.44 (1.29–1.60) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Source of control-HB | ||||||||
| Recessive | 0.52 (0.33–0.82) | 0.0436 | 0.948 | 0.121 | 0.293 | 0.820 | 0.979 | 0.998 |
| Dominant | 2.07 (1.16–3.67) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Source of control-PB | ||||||||
| Homozygous | 1.80 (1.52–2.13) | <0.0001 | 0.772 | 0.000 | 0.001 | 0.013 | 0.115 | 0.564 |
| Heterozygous | 1.38 (1.22–1.57) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Recessive | 0.44 (0.37–0.52) | <0.0001 | 0.440 | 0.001 | 0.002 | 0.022 | 0.185 | 0.694 |
| Dominant | 3.22 (2.78–3.74) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Allele comparision | 1.34 (1.21–1.50) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Score-Low | ||||||||
| Recessive | 0.45 (0.25–0.81) | <0.0001 | 0.114 | 0.003 | 0.008 | 0.080 | 0.467 | 0.898 |
| Dominant | 1.79 (1.01–3.16) | <0.0001 | 0.995 | 0.000 | 0.001 | 0.010 | 0.091 | 0.501 |
| Score-High | ||||||||
| Recessive | 0.54 (0.39–0.74) | <0.0001 | 0.696 | 0.000 | 0.001 | 0.014 | 0.126 | 0.590 |
| Dominant | 3.05 (2.31–4.02) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Allele comparision | 1.29 (1.14–1.46) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| 8q24-rs16901979 C>A | ||||||||
| All patients | ||||||||
| Homozygous | 1.71 (1.36–2.16) | 0.0663 | 0.995 | 0.167 | 0.375 | 0.868 | 0.985 | 0.999 |
| Heterozygous | 1.31 (1.12–1.53) | 0.3859 | 1.000 | 0.537 | 0.776 | 0.974 | 0.997 | 1.000 |
| Dominant | 1.39 (1.20–1.61) | 0.1506 | 0.999 | 0.312 | 0.576 | 0.937 | 0.993 | 0.999 |
| Allele comparision | 1.31 (1.18–1.46) | 0.0524 | 1.000 | 0.136 | 0.320 | 0.838 | 0.981 | 0.998 |
| Ethnicity-Asian | ||||||||
| Recessive | 1.65 (1.13–2.41) | 0.3859 | 0.908 | 0.560 | 0.793 | 0.977 | 0.998 | 1.000 |
| Allele comparision | 1.17 (1.00–1.38) | 0.0394 | 0.999 | 0.106 | 0.262 | 0.796 | 0.975 | 0.997 |
| Ethnicity-Africa-American | ||||||||
| Homozygous | 1.91 (1.44–2.55) | <0.0001 | 0.902 | 0.116 | 0.282 | 0.812 | 0.978 | 0.998 |
| Heterozygous | 1.41 (1.10–1.80) | 0.0028 | 0.837 | 0.010 | 0.029 | 0.249 | 0.770 | 0.971 |
| Dominant | 1.56 (1.24–1.96) | <0.0001 | 0.375 | 0.001 | 0.002 | 0.026 | 0.210 | 0.727 |
| Allele comparision | 1.40 (1.21–1.62) | <0.0001 | 0.371 | 0.001 | 0.002 | 0.026 | 0.212 | 0.730 |
| Source of control-HB | ||||||||
| All patients | ||||||||
| Homozygous | 1.71 (1.36–2.16) | 0.0663 | 0.995 | 0.167 | 0.375 | 0.868 | 0.985 | 0.999 |
| Heterozygous | 1.31 (1.12–1.53) | 0.3859 | 1.000 | 0.537 | 0.776 | 0.974 | 0.997 | 1.000 |
| Dominant | 1.39 (1.20–1.61) | 0.1506 | 1.000 | 0.311 | 0.575 | 0.937 | 0.993 | 0.999 |
| Allele comparision | 1.31 (1.18–1.46) | 0.0524 | 1.000 | 0.136 | 0.320 | 0.838 | 0.981 | 0.998 |
| Score-Low | ||||||||
| Homozygous | 1.75 (1.22–2.51) | 0.6976 | 0.993 | 0.678 | 0.863 | 0.986 | 0.999 | 1.000 |
| Recessive | 1.49 (1.08–2.05) | 0.7287 | 0.994 | 0.687 | 0.868 | 0.986 | 0.999 | 1.000 |
| Allele comparision | 1.29 (1.10–1.52) | 0.6989 | 1.000 | 0.677 | 0.863 | 0.986 | 0.999 | 1.000 |
| Score-High | ||||||||
| Homozygous | 1.68 (1.25–2.27) | 0.0297 | 0.883 | 0.092 | 0.232 | 0.769 | 0.971 | 0.997 |
| Heterozygous | 1.34 (1.09–1.65) | 0.2149 | 1.000 | 0.392 | 0.659 | 0.955 | 0.995 | 1.000 |
| Recessive | 1.46 (1.13–1.89) | 0.0551 | 0.926 | 0.151 | 0.349 | 0.855 | 0.983 | 0.998 |
| Dominant | 1.42 (1.17–1.73) | 0.0546 | 0.999 | 0.141 | 0.330 | 0.844 | 0.982 | 0.998 |
| Allele comparision | 1.33 (1.16–1.53) | 0.0131 | 1.000 | 0.038 | 0.105 | 0.565 | 0.929 | 0.992 |
| 8q24-rs6983267 T>G | ||||||||
| All patients | ||||||||
| Homozygous | 1.44 (1.31–1.58) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Heterozygous | 1.19 (1.10–1.29) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Recessive | 1.26 (1.18–1.36) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Allele comparision | 1.19 (1.14–1.25) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Ethnicity-Caucasian | ||||||||
| Homozygous | 1.47 (1.33–1.64) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Heterozygous | 1.25 (1.07–1.46) | 0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Recessive | 1.27 (1.17–1.38) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Allele comparision | 1.21 (1.15–1.27) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Ethnicity-Aasian | ||||||||
| Homozygous | 1.33 (1.08–1.62) | 0.0026 | 0.939 | 0.008 | 0.024 | 0.215 | 0.734 | 0.965 |
| Recessive | 1.27 (1.06–1.52) | 0.0048 | 0.959 | 0.015 | 0.043 | 0.331 | 0.833 | 0.980 |
| Dominant | 1.35 (1.04–1.75) | 0.0477 | 1.000 | 0.125 | 0.300 | 0.825 | 0.979 | 0.998 |
| Allele comparision | 1.14 (1.03–1.25) | 0.0038 | 1.000 | 0.011 | 0.033 | 0.273 | 0.792 | 0.974 |
| Source of control-HB | ||||||||
| Homozygous | 1.68 (1.30–2.16) | 0.001 | 1.000 | 0.003 | 0.009 | 0.090 | 0.500 | 0.909 |
| Heterozygous | 1.17 (1.03–1.33) | 0.002 | 1.000 | 0.006 | 0.018 | 0.165 | 0.666 | 0.952 |
| Recessive | 1.38 (1.22–1.55) | 0.0755 | 1.000 | 0.185 | 0.405 | 0.882 | 0.987 | 0.999 |
| Dominant | 1.43 (1.00–2.04) | 0.0004 | 1.000 | 0.001 | 0.004 | 0.038 | 0.286 | 0.800 |
| Allele comparision | 1.24 (1.15–1.33) | 0.0009 | 1.000 | 0.003 | 0.008 | 0.082 | 0.473 | 0.900 |
| Source of control-PB | ||||||||
| Homozygous | 1.37 (1.22–1.54) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Heterozygous | 1.20 (1.09–1.33) | 0.0021 | 1.000 | 0.006 | 0.019 | 0.172 | 0.677 | 0.955 |
| Recessive | 1.21 (1.10–1.32) | <0.0001 | 1.000 | 0.006 | 0.019 | 0.172 | 0.677 | 0.955 |
| Allele comparision | 1.17 (1.10–1.24) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Score-Low | ||||||||
| Homozygous | 1.79 (1.19–2.67) | 0.0008 | 0.871 | 0.003 | 0.008 | 0.083 | 0.478 | 0.902 |
| Recessive | 1.43 (1.19–1.73) | 0.0007 | 0.863 | 0.002 | 0.007 | 0.074 | 0.448 | 0.890 |
| Allele comparision | 1.28 (1.07–1.54) | 0.0016 | 1.000 | 0.005 | 0.014 | 0.137 | 0.615 | 0.941 |
| Score-High | ||||||||
| Homozygous | 1.42 (1.31–1.58) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
| Heterozygous | 1.22 (1.11–1.33) | 0.0003 | 1.000 | 0.001 | 0.003 | 0.029 | 0.231 | 0.710 |
| Recessive | 1.24 (1.14–1.34) | 0.0034 | 1.000 | 0.010 | 0.030 | 0.252 | 0.773 | 0.971 |
| Allele comparision | 1.19 (1.13–1.25) | <0.0001 | 1.000 | 0.000 | 0.001 | 0.010 | 0.091 | 0.500 |
OR, Odds ratio; CI, Confidence interval. The results in false-positive report probability analysis were in bold, if the prior probability <0.2.
aChi-square test was used to calculate the genotype frequency distributions.
bStatistical power was calculated using the number of observations in the subgroup and the OR and P values in this table.
Figure 1. Forest plots of effect estimates for 8q24-rs1447295 polymorphism and prostate cancer risk (AA+AC vs. CC).
The size of the squares indicates the relative weight of each study. Weights were derived from random-effects analysis. Bars, 95% confidence interval (CI).
Publication Bias Analysis
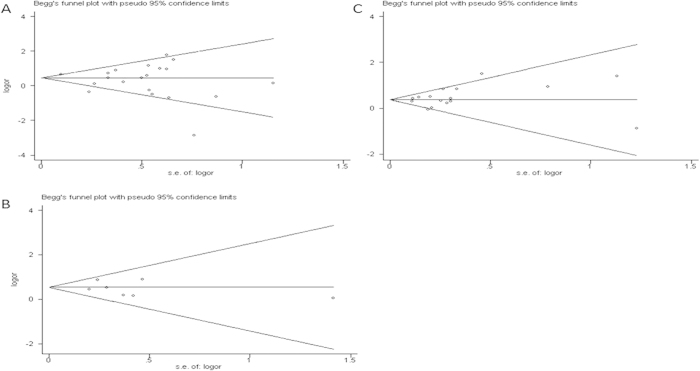
Publication bias was examined by Begg’s funnel plots and Egger’s tests across all genetic models for each of the three polymorphisms in the 8q24 region. The shape of funnel plot (Fig. 2) was symmetrical, suggesting that there was no evidence of publication bias in the meta-analysis.
Figure 2. Funnel plot analysis to detect publication bias for each of the 8q24 polymorphism by homozygous model.
(A) For rs1447295 polymorphism, (B) For rs16901979 polymorphism, and (C) For rs6983267 polymorphism. Each point represented an individual study for the indicated association.
Discussion
To the best of our knowledge, this is the largest meta-analysis to investigate the association between the selected 8q24 SNPs and PCa risk based on the candidate association studies. Up to now, more than 64 8q24SNPs have been explored for their role in PCa carcinogenesis, among which about 20 SNPs are regarded as PCa-related SNPs. However, those results were often controversial. In this meta-analysis, we evaluated the association of PCa risk with three most frequently investigated SNPs that scatter through the three regions of 8q24, respectively. We found significant associations between each of these 8q24 SNPs and PCa risk under most of the assumed comparisons, either in overall or in stratified analyses by ethnicity, source of control, and quality score. Intriguingly, stratified analyses by ethnicity revealed significant association between rs1447295 C>A and PCa risk among Caucasians and Asians, but not Africa-Americans. The effect of polymorphism rs16901979 C>A on PCa risk was more apparent in Africa-Americans populations when compared with Asian populations. Similarly, rs6983267 conferred a higher Pca risk among Caucasians than Asians. Although it is difficult to explain the ethnic-specific findings, the disparity in different ethnic populations is not completely surprising. Incidence rates of PCa vary internationally by over 25-fold. Developed countries in Oceania, Europe, and North American have the highest rates of PCa, while incidence in Asia is relatively low41. On the contrary, the highest prostate cancer mortality rates are recorded in African descent in the Caribbean region, which is partly explained by genetic predisposition42,43. Taken together, these epidemiological findings have suggested that both environmental and genetic factors may have profound impacts on PCa. Therefore, we speculated that differences in environments, genetic backgrounds, and gene-environment interaction may help to interpret the different results in the different ethnic groups. Future studies may provide some insights into the ethnic-specific findings, focusing on a difference in LD between these variants and the causal variants in each ethnic group, epistasis with other variants found in these ethnicities, or gene-environment interaction.
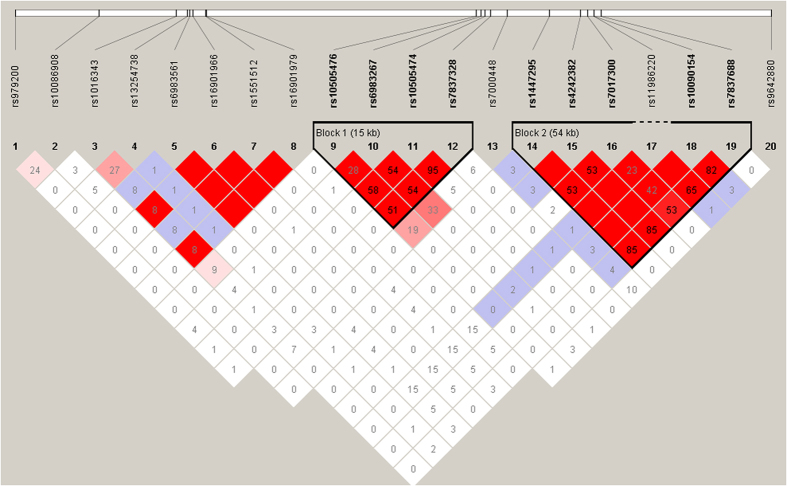
Moreover, although mechanisms by which 8q24 modulates the risk of PCa is poorly understood, mounting evidence from genetic association studies and fine-mapping informatics supports that 8q24 genetic variant may impart susceptibility to PCa in an ethnic-specific manner. 8q24 region is commonly subdivided into three regions on the basis of the local genetic characteristics of 8q24 and the fine-mapping study11. Region 1 (126.54–128.62 Mb) was initially identified by the original linkage and admixture studies in Icelandic families populations; and initial association studies indicated that this region might contribute to a higher incidence of PCa in Africa-American men than men of European ancestry7,8. Region 2 (128.14–128.28 Mb) harbors a 14-SNP haplotype that efficiently tags a relatively uncommon (2–4%) susceptibility variant in individuals of European descent, which happens to be very common (42%) in Africa-Amricans2,10. And region 3 (128.47–128.54 Mb) is defined as a recombination hot-spot among European Americans. There is little linkage disequilibrium among SNPs across the three regions. Although regions 1 and 3 are physically close to each other, they are separated by a recombination hotspot among individuals of European ancestry2. Hence, SNPs across all three neighboring regions seem to independently influence 8q24 signal transduction, and the combined effects of SNPs across regions closely follow a multiplicative model2,10. Human chromosome 8q24 contains three regions spanning 6000 kb, according to the dbSNP database (http://www.ncbi.nlm.nih.gov/SNP), in which more than 207 variants were reported. Twenty previously reported 8q24SNPs statistically significantly associated with PCa risk show various linkage disequilibrium (LD) with a range from low to high degree (Fig. 3). These SNPs can be divided into three blocks. For example, block1 included rs698267, rs10505476, rs10505474, and rs7837328, showing LD with r2 values ranging from 0.28 to 0.95. Block2 consisted of rs1447295, rs4242382, rs7017300, rs11986220, rs10090154, and 7837688, with LD (r2) varying from 0.23 to 1.0. Additionally, rs16901979 was found in high LD of 1.0 with some polymorphisms including rs6983561, rs16901966, and rs1551512. None of the variants resides within known genes, of which there are few across the regions of 8q24. It was predicted that those risk-associated variants could affect the regulation or transcription of a causal pseudogene or gene outside the region, including proto-oncogene MYC (located approximately 260 kb telomeric to region 1), FAM84B, TCF7L2, and POU5F121,44, but the biological mechanisms underlying these findings remain unclear. Another speculation that may help to explain the association is that some functional polymorphisms linked to those SNPs may account for the association. However, based on current understanding, none of these variants and their respectively linked-SNPs seems to be potentially functional. Therefore, the exact mechanisms underlying the observed association of the SNPs with PCa risk need more investigations.
Figure 3. Linkage disequilibrium in the 8q24 region for the previously reported polymorphisms.
Linkage disequilibrium coefficients (r2) between SNPs in the 8q24 region between 128.12 and 128.54 Mb.
8q24 was considered as a gene-free region, flanked by the FAM84B and MYC genes on the centromeric and telomeric ends, respectively. Although data based on epidemiologic studies have indicated the possible association between 8q24 variants and PCa risk, little is known about the mechanisms. There are significant differences in allele frequency distribution of the three selected SNPs among different ethnic-populations, indicating these 8q24 variant(s) may modulate PCa in an ethnic-specific manner. One study suggested that variants in this region may be a part of the cis-regulatory enhancer element for the MYC gene, with a long-range physical interaction with this gene in PCa4. For example, a recent report indicated that rs6983267 displayed an altered capacity of binding of transcription factor 7-like 2 (TCF7L2), thereby leading to a different physical interaction with MYC44. However, others failed to find clear association between rs6983267 genotype and MYC expression. Therefore, more powerful studies should be performed to further investigate the function of the risk- associated SNPs.
Up to now, several meta-analyses have investigated the associations between 8q24 polymorphisms and PCa risk45,46,47. And consistent findings were observed among the previously reported meta-analyses and the present study, indicating that 8q24-rs1447295, -rs16901979, and –rs6983267 polymorphisms might confer genetic susceptibility to PCa. Additionally, this meta-analysis was the first to further explore the associations by stratification analyses (ethnicity, source of control, and quality score), as compared to others. However, although significant associations were found in the stratification analyses by ethnicity, these findings warrant further validation in large, well-designed, multiethnic case-control studies.
Finally, some limitations in the meta-analysis should be addressed. First, relatively small samples were included for the assessment, especially for rs16901979 polymorphism. Second, the controls were not uniformly defined, which might result in obscure findings. Third, there was a significant heterogeneity in different ethnic groups; the heterogeneity may be partially attributed to the difference in individuals’ genetic backgrounds or the environment in which subjects live, since these genetic and environmental factors may modify the risk effects of studied SNP on cancer susceptibility. Fourth, due to inadequate information, we failed to control for possible confounders such as age, smoking, obesity, alcohol consumption, and other lifestyle risk factors, and our results were based on unadjusted estimates. Finally, the ethnic-specific effects of the studied SNPs on PCa risk were not adequately discussed due to lack of relevant publications.
In summary, this meta-analysis provided evidence of the association between 8q24 polymorphisms (i.e. rs1447295 C>A, rs16901979 C>A, and rs6983267 T>G) and PCa risk, suggesting that these 8q24 variants may be a low penetrance contributor to PCa. Considering that PCa is one of genetic and geographic related malignancies, more relevant SNPs, variants, SNP-induced gene structural changes, epigenetic changes, and interactions of SNP-SNP, gene-gene, and gene-environment should be addressed in future large multicentric studies, which should lead to better, comprehensive understanding of the association between the 8q24 Polymorphisms and PC risk.
Methods
Search strategy and selection criteria
A comprehensive literature search was carried out for publications investigating the association between 8q24 polymorphisms and PCa risk. We searched PubMed, Embase and China Biology Medicine (CBM) databases up to June 2014 using the following keywords, including “8q24”, “variant or polymorphism”, “cancer, carcinoma, or malignancy”, and “prostate disease”. Further limited searching strategy included “English and Chinese language publications” and “human species”. Abstracts alone and unpublished articles were not considered. Moreover, the reference lists of the retrieved articles and reviews were sought manually to find relevant original articles. Eligible Studies for this meta-analysis were required to meet all of the following criteria: (i) studies on associations of at least one of 8q24 polymorphisms (rs1447295 C>A, rs6983267 T>G, rs16901979 C>A) with PCa risk; (ii) enrolled patients had sporadic, primary and histologically confirmed PCa, and controls had no history of cancer or neoplasm; (iii) case-control or nested case-control studies; (iv) availability of genotype data of both case and control group to calculate the odds ratio (OR) with its 95% confidence interval (CI) and P value; and (v) genotype distribution in controls is complied with the Hardy-Weinberg equilibrium (HWE, P>0.05). All of the included studies were candidate-based association studies. If participants in studies overlapped, only the study with the largest sample size was included in the meta-analysis. The flow chart of identification the eligible studies was shown in Fig. 4, and the protocol was checked according to the PRISMA checklist (Supporting Information).
Figure 4. Flow chart of identification of included studies.
Assessment of study quality: Extended Quality Score
The quality of each included investigation was estimated using Extended Quality Score to limit the risk of introducing bias into meta-analyses or systematic reviews. Each article is defined as “high” or “poor” quality according to an extended-quality scale system by evaluating special characteristics of the participant groups and genotyping methods of the studies48. In this meta-analysis, two investigators appraised the quality of each investigation. If there was any disagreement, a third investigator (Jin-Hong Zhu) would mediate and a decision would be made by voting.
Data extraction
Two reviewers (Qiaoxin Li and Xia Liu) independently appraised and extracted data according to the inclusion criteria listed above. Disagreements were documented and resolved by full discussion with a third reviewer (Jin-Hong Zhu) until a consensus was reached. The following information were extracted from each eligible study: the first author’s surname, year of publication, country in which study was conducted, ethnicity of participants (categorized as Caucasians, Asians, and African descents), study design, sample size, source of control, P value of HWE test, genotyping methods, genotype counts of case and controls for three studied SNPs, and the clinical/pathological characteristics of cases. For studies involving subjects of different ethnic groups, data were extracted and split by ethnicity.
Statistical analysis
HWE in the control groups was assessed by the Pearson’s goodness-of-fit chi-square test, with a P value less than 0.05 indicating derivation from HWE, and population was regarded as suitable if more than one SNP agreed with HWE. The strength of association between 8q24 polymorphisms and PCa risk was estimated by calculating odds ratios (OR) and the corresponding 95% confidence intervals (CI). For each of studied SNP, we conducted comparison of the frequency of wild (W) and variant (V) alleles between PCa cases and controls (V vs. W). Moreover, pooled ORs were calculated under the following genetic models: homozygous (VV vs. WW), heterozygous (VW vs. WW), dominant (VV + VW vs. WW), and recessive (VV vs. VW + WW) models. Stratification analyses were performed by ethnicity, source of control, and quality score, but not by tumor stage, Gleason score, and PAS levels because of the small size of the eligible studies. For each genetic model, a χ2-based Q-test was performed to check the presence of between-study heterogeneity across all of the comparisons at a level of significance of 0.1049. In addition, the heterogeneity was also quantified with I2 statistics, which is independent of the number of studies included in the meta-analysis. I2 lies between 0% and 100% with higher values indicating a greater heterogeneity50. The fixed-effects model was chosen to calculate the combined OR in the absence of heterogeneity. Otherwise, a random-effects model was used to estimate the pooled OR. The significance of the pooled OR was determined using the Z test. Finally, false positive report probability (FPRP) was introduced as a multiple testing for the whole significant associations. The statistical power of the current study was calculated with an FPRP threshold of 0.2 was defined as true finding, i.e., the probability of a false positive result is <20%49. In sensitivity analysis, following the sequential exclusion of a single study at a time, the pooled estimates were recomputed to determine the stability of the results. Furthermore, potential publication bias was assessed by visual inspection of Begg’s funnel plots with an asymmetry plot suggested possible publication bias51. All statistical analyses were performed using STATA statistical software (version 11.0, StataCorp, College Station, TX) and SAS version 9.1 (SAS Institute, Cary, NC). Two-sided P values were adopted, and P <0.05 were considered statistically significant without further notification.
Additional Information
How to cite this article: li, Q. et al. Association of three 8q24 polymorphisms with prostate cancer susceptibility: evidence from a meta-analysis with 50,854 subjects. Sci. Rep. 5, 12069; doi: 10.1038/srep12069 (2015).
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (Grant No. 81460513).
Footnotes
Author Contributions All authors contributed significantly to this work. Q.L., X.L., R.H. and F.W. performed the research study and collected the data; Q.L., H.A. and W.Z. analyzed the data; J.Z. designed the research study; Q.L. and J.Z. wrote the paper. All authors reviewed the manuscript. In addition, all authors approved the final draft.
References
- Penney K. L. et al. Evaluation of 8q24 and 17q risk loci and prostate cancer mortality. Clin Cancer Res 15, 3223–30 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager M. et al. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet 39, 645–9 (2007). [DOI] [PubMed] [Google Scholar]
- Haiman C. A. et al. Multiple regions within 8q24 independently affect risk for prostate cancer. Nat Genet 39, 638–44 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman N. F., Aneas I. & Nobrega M. A. An 8q24 gene desert variant associated with prostate cancer risk confers differential in vivo activity to a MYC enhancer. Genome Res 20, 1191–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson J. et al. A study based on whole-genome sequencing yields a rare variant at 8q24 associated with prostate cancer. Nat Genet 44, 1326–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager M. et al. Identification of a new prostate cancer susceptibility locus on chromosome 8q24. Nat Genet 41, 1055–7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M. L. et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc Natl Acad Sci USA 103, 14068–73 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundadottir L. T. et al. A common variant associated with prostate cancer in European and African populations. Nat Genet 38, 652–8 (2006). [DOI] [PubMed] [Google Scholar]
- Gudmundsson J. et al. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet 39, 977–83 (2007). [DOI] [PubMed] [Google Scholar]
- Gudmundsson J. et al. Genome-wide association study identifies a second prostate cancer susceptibility variant at 8q24. Nat Genet 39, 631–7 (2007). [DOI] [PubMed] [Google Scholar]
- Witte J. S. Multiple prostate cancer risk variants on 8q24. Nat Genet 39, 579–80 (2007). [DOI] [PubMed] [Google Scholar]
- Salinas C. A. et al. Multiple independent genetic variants in the 8q24 region are associated with prostate cancer risk. Cancer Epidemiol Biomarkers Prev 17, 1203–13 (2008). [DOI] [PubMed] [Google Scholar]
- Fletcher O. et al. Association of genetic variants at 8q24 with breast cancer risk. Cancer Epidemiol Biomarkers Prev 17, 702–5 (2008). [DOI] [PubMed] [Google Scholar]
- Turnbull C. et al. Genome-wide association study identifies five new breast cancer susceptibility loci. Nat Genet 42, 504–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiemeney L. A. et al. Sequence variant on 8q24 confers susceptibility to urinary bladder cancer. Nat Genet 40, 1307–12 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanke B. W. et al. Genome-wide association scan identifies a colorectal cancer susceptibility locus on chromosome 8q24. Nat Genet 39, 989–94 (2007). [DOI] [PubMed] [Google Scholar]
- Park S. L. et al. Associations between variants of the 8q24 chromosome and nine smoking-related cancer sites. Cancer Epidemiol Biomarkers Prev 17, 3193–202 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoussaini M. et al. Multiple loci with different cancer specificities within the 8q24 gene desert. J Natl Cancer Inst 100, 962–6 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagk D. et al. Expression analysis of pancreatic cancer cell lines reveals association of enhanced gene transcription and genomic amplifications at the 8q22.1 and 8q24.22 loci. Oncol Rep 17, 399–407 (2007). [PubMed] [Google Scholar]
- Shete S. et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet 41, 899–904 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagopoulos I., Moller E., Collin A. & Mertens F. The POU5F1P1 pseudogene encodes a putative protein similar to POU5F1 isoform 1. Oncol Rep 20, 1029–33 (2008). [PubMed] [Google Scholar]
- Pomerantz M. M. et al. Evaluation of the 8q24 prostate cancer risk locus and MYC expression. Cancer Res 69, 5568–74 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadiyeh N. et al. 8q24 prostate, breast, and colon cancer risk loci show tissue-specific long-range interaction with MYC. Proc Natl Acad Sci USA 107, 9742–6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo J. et al. Long-range enhancers on 8q24 regulate c-Myc. Proc Natl Acad Sci USA 107, 3001–5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suuriniemi M. et al. Confirmation of a positive association between prostate cancer risk and a locus at chromosome 8q24. Cancer Epidemiol Biomarkers Prev 16, 809–14 (2007). [DOI] [PubMed] [Google Scholar]
- Schumacher F. R. et al. A common 8q24 variant in prostate and breast cancer from a large nested case-control study. Cancer Res 67, 2951–6 (2007). [DOI] [PubMed] [Google Scholar]
- Severi G. et al. The common variant rs1447295 on chromosome 8q24 and prostate cancer risk: results from an Australian population-based case-control study. Cancer Epidemiol Biomarkers Prev 16, 610–2 (2007). [DOI] [PubMed] [Google Scholar]
- Zheng S. L. et al. Association between two unlinked loci at 8q24 and prostate cancer risk among European Americans. J Natl Cancer Inst 99, 1525–33 (2007). [DOI] [PubMed] [Google Scholar]
- Terada N. et al. Association of genetic polymorphisms at 8q24 with the risk of prostate cancer in a Japanese population. Prostate 68, 1689–95 (2008). [DOI] [PubMed] [Google Scholar]
- Liu M. et al. Significance of common variants on human chromosome 8q24 in relation to the risk of prostate cancer in native Japanese men. BMC Genet 10, 37 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. et al. Common variants at 8q24 are associated with prostate cancer risk in Taiwanese men. Prostate 70, 502–7 (2010). [DOI] [PubMed] [Google Scholar]
- Benford M. L., VanCleave T. T., Lavender N. A., Kittles R. A. & Kidd L. R. 8q24 sequence variants in relation to prostate cancer risk among men of African descent: a case-control study. BMC Cancer 10, 334 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okobia M. N., Zmuda J. M., Ferrell R. E., Patrick A. L. & Bunker C. H. Chromosome 8q24 variants are associated with prostate cancer risk in a high risk population of African ancestry. Prostate 71, 1054–63 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeegers M. P. et al. Genetic marker polymorphisms on chromosome 8q24 and prostate cancer in the Dutch population: DG8S737 may not be the causative variant. Eur J Hum Genet 19, 118–20 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. et al. Risk loci on chromosome 8q24 are associated with prostate cancer in northern Chinese men. J Urol 187, 315–21 (2012). [DOI] [PubMed] [Google Scholar]
- Papanikolopoulou A. et al. The multi-cancer marker, rs6983267, located at region 3 of chromosome 8q24, is associated with prostate cancer in Greek patients but does not contribute to the aggressiveness of the disease. Clin Chem Lab Med 50, 379–85 (2012). [DOI] [PubMed] [Google Scholar]
- Joung J. Y. et al. Association of common variations of 8q24 with the risk of prostate cancer in Koreans and a review of the Asian population. BJU Int 110, E318–25 (2012). [DOI] [PubMed] [Google Scholar]
- Chan J. Y. et al. 8q24 and 17q prostate cancer susceptibility loci in a multiethnic Asian cohort. Urol Oncol 31, 1553–60 (2013). [DOI] [PubMed] [Google Scholar]
- Brankovic A. S. et al. Common variants at 8q24 are associated with prostate cancer risk in Serbian population. Pathol Oncol Res 19, 559–69 (2013). [DOI] [PubMed] [Google Scholar]
- San F. I. et al. Association of RNASEL and 8q24 variants with the presence and aggressiveness of hereditary and sporadic prostate cancer in a Hispanic population. J Cell Mol Med 18, 125–33 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A. et al. Global cancer statistics. CA Cancer J Clin 61, 69–90 (2011). [DOI] [PubMed] [Google Scholar]
- Bock C. H. et al. Results from a prostate cancer admixture mapping study in African-American men. Hum Genet 126, 637–42 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. C. et al. Germ-line mutations of the macrophage scavenger receptor 1 gene: association with prostate cancer risk in African-American men. Cancer Res 63, 3486–9 (2003). [PubMed] [Google Scholar]
- Pomerantz M. M. et al. The 8q24 cancer risk variant rs6983267 shows long-range interaction with MYC in colorectal cancer. Nat Genet 41, 882–4 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng I. et al. 8q24 and prostate cancer: association with advanced disease and meta-analysis. Eur J Hum Genet 16, 496–505 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisbin A. G. et al. Meta-analysis of 8q24 for seven cancers reveals a locus between NOV and ENPP2 associated with cancer development. BMC Med Genet 12, 156 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troutman S. M. et al. Racial disparities in the association between variants on 8q24 and prostate cancer: a systematic review and meta-analysis. Oncologist 17, 312–20 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J. et al. Association of MTHFR C677T and A1298C polymorphisms with non-Hodgkin lymphoma susceptibility: evidence from a meta-analysis. Sci Rep 4, 6159 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–88 (1986). [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–60 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–101 (1994). [PubMed] [Google Scholar]