A loss-of-function mutation in the single tomato DELLA gene PROCERA revealed DELLA-related phenotypes and facilitated the identification of DELLA-independent gibberellin responses.
Abstract
Gibberellin (GA) regulates plant development primarily by triggering the degradation/deactivation of the DELLA proteins. However, it remains unclear whether all GA responses are regulated by DELLAs. Tomato (Solanum lycopersicum) has a single DELLA gene named PROCERA (PRO), and its recessive pro allele exhibits constitutive GA activity but retains responsiveness to external GA. In the loss-of-function mutant proΔGRAS, all examined GA developmental responses were considerably enhanced relative to pro and a defect in seed desiccation tolerance was uncovered. As pro, but not proΔGRAS, elongation was promoted by GA treatment, pro may retain residual DELLA activity. In agreement with homeostatic feedback regulation of the GA biosynthetic pathway, we found that GA20oxidase1 expression was suppressed in proΔGRAS and was not affected by exogenous GA3. In contrast, expression of GA2oxidase4 was not affected by the elevated GA signaling in proΔGRAS but was strongly induced by exogenous GA3. Since a similar response was found in Arabidopsis thaliana plants with impaired activity of all five DELLA genes, we suggest that homeostatic GA responses are regulated by both DELLA-dependent and -independent pathways. Transcriptome analysis of GA-treated proΔGRAS leaves suggests that 5% of all GA-regulated genes in tomato are DELLA independent.
INTRODUCTION
The phytohormone gibberellin (GA) regulates numerous developmental processes throughout the plant life cycle, including seed germination, stem elongation, flowering, and fruit set (Yamaguchi, 2008). The signaling pathway from GA perception to transcriptional activation has been intensively studied over the past two decades and its major components have been identified. The nuclear DELLA proteins, a subgroup of the GRAS transcription factors family, suppress GA signaling (Locascio et al., 2013). GA binding to the soluble GIBBERELLIN INSENSITIVE DWARF1 (GID1) receptor triggers GID1 interaction with the DELLA proteins (Ueguchi-Tanaka et al., 2005; Nakajima et al., 2006; Griffiths et al., 2006), which then stimulates assembly of the DELLA proteins into an SCF E3 ubiquitin ligase complex via the GID2/SLEEPY1 F-box proteins. The SCF complex polyubiquitinates the DELLA proteins, targeting them for destruction by the 26S proteosome (Sasaki et al., 2003; Dill et al., 2004; Griffiths et al., 2006; Harberd et al., 2009; Hauvermale et al., 2012). GA, via GID1, can also reduce DELLA activity through a degradation-independent mechanism (Ariizumi et al., 2008, 2013; Ueguchi-Tanaka et al., 2008).
Despite the central role of DELLAs in GA signaling, the mechanism underlying this regulation is not fully understood. Several studies have shown that protein-protein interactions play a major role in DELLA function. DELLAs bind to various transcription factors and proteins affecting transcription, including PHYTOCHROME-INTERACTING FACTORs (PIFs), ALCATRAZ, MYC2, JASMONATE-ZIM-DOMAIN PROTEIN9, SCARECROW LIKE3 (SCL3), and TCP transcription factors (de Lucas et al., 2008; Feng et al., 2008; Arnaud et al., 2010; Gallego-Bartolomé et al., 2010; Hong et al., 2012; Yang et al., 2012; Hou et al., 2010; Zhang et al., 2011; Davière et al., 2014). The interaction between DELLA and PIFs, for example, suppresses the binding of the latter to target promoters and thus inhibits their activity. Although DELLAs lack a DNA binding domain, they possess transactivation properties (Hirano et al., 2012), and several studies have shown that DELLAs can act as coregulators when interacting with transcription factors and directly regulate gene expression (Zentella et al., 2007; Hirano et al., 2012; Yoshida et al., 2014).
The DELLA N-terminal region consists of the conserved DELLA and VHYNP motifs (Locascio et al., 2013). These motifs interact with the GID1 N-terminal arm to form the GID1-GA-DELLA complex (Murase et al., 2008). The C-terminal region of DELLAs consists of several distinct motifs comprising the GRAS domain. These motifs include two leucine heptad repeats (LHRI and LHRII) with putative nuclear localization signals, flanking a VHIID motif, forming the LHRI-VHIID-LHRII domain said to be involved in protein-protein interactions (Sun et al., 2012). Hirano et al. (2010) have shown that the SLENDER RICE1 (SLR1; the rice [Oryza sativa] DELLA protein) GRAS domain is also required for a stable interaction between DELLA and GID1. Recently, Sato et al. (2014) confirmed this observation and demonstrated an interaction between the purified SLR1 GRAS domain and GID1.
Arabidopsis thaliana has five DELLA proteins (Repressor of ga1-3 [RGA], GA-INSENSITIVE [GAI], RGA-LIKE1 [RGL1], RGL2, and RGL3), whereas rice, barley (Hordeum vulgare), and tomato (Solanum lycopersicum) each have only one, called SLR1, SLENDER1, and PROCERA (PRO), respectively (Ikeda et al., 2001; Chandler et al., 2002; Jasinski et al., 2008; Harberd et al., 2009). The one well-studied recessive tomato pro allele contains a point mutation within the VHIID domain (Val [V] to Glu [E] at position 273; Bassel et al., 2008). Creating a similar mutation in gai, an Arabidopsis gain-of-function DELLA allele, completely abolished its growth-suppressing activity (Jasinski et al., 2008), suggesting a loss-of-function allele. The pro phenotype resembles wild-type plants treated with GA and includes elongated internodes, thinner leaves, and reduced lobing of the main leaflets (George Jones, 1987; Jupe et al., 1988; Van Tuinen et al., 1999; Bassel et al., 2008; Jasinski et al., 2008). Antisense suppression of PRO also promoted GA responses, including pollination-independent ovary growth, resulting in parthenocarpic fruit formation (Martí et al., 2007).
In striking contrast with other plants with a single DELLA, such as barley and rice, pro plants respond to GA treatment and the pro mutation does not completely suppress chemicals or mutations that inhibit GA biosynthesis (George Jones, 1987; Jupe et al., 1988; Van Tuinen et al., 1999; Bassel et al., 2008; Jasinski et al., 2008; Fleishon et al., 2011). The responsiveness of pro to GA might be due to an incomplete loss of DELLA function (Van Tuinen et al., 1999) or due to the activity of a DELLA-independent response pathway (Fleishon et al., 2011).
While the central role of DELLA in the regulation of GA responses is indisputable, it is not yet clear if DELLA mediates all GA responses. Recently, Yano et al. (2015) have shown that GID1-DELLA is the sole mechanism for GA regulation of gene expression in rice aleurone cells. On the other hand, results from a number of studies support the existence of a DELLA-independent GA signaling pathway. Our earlier work in Arabidopsis has suggested the existence of a cytosolic, SPINDLY-dependent, DELLA-independent GA response pathway (Maymon et al., 2009). These findings stood in line with those reported by Cao et al. (2006), who demonstrated that some GA-regulated genes are not regulated by DELLA. Moreover, GA-induced increases in cytosolic calcium concentrations, detectable within ∼2 min of exposure to GA (Bush, 1996), have been suggested to occur too rapidly to be regulated by DELLA proteins, whose levels are only significantly reduced 5 to 10 min after GA treatment (Gubler et al., 2002). Furthermore, cytosolic activity of DELLA has never been detected, thereby challenging attempts to ascribe it a regulatory role in cytosol-emanating responses. Finally, application of GA to emasculated pistils of global (an Arabidopsis mutant that lacks the activities of all five DELLA proteins) resulted in significant promotion of their growth (Fuentes et al., 2012). This DELLA-independent response is mediated by the basic helix-loop-helix transcription factor SPATULA, which suppresses fruit growth. Despite these findings and other evidence of the existence of a DELLA-independent, GA response pathway, its significance remains unclear.
Here, we present a pro loss-of-function mutant tomato line named proΔGRAS. All examined GA-dependent developmental responses were much stronger in proΔGRAS than in pro. In addition, roles of PRO in seed desiccation tolerance and pollen tube elongation were uncovered. The presented results suggest that while GA regulation of tomato plant development is primarily DELLA dependent, ∼5% of all identified GA-regulated genes are DELLA independent. Our results indicate that feedback regulation of GA catabolism is at least partially DELLA independent.
RESULTS
Identification and Characterization of a pro Mutant
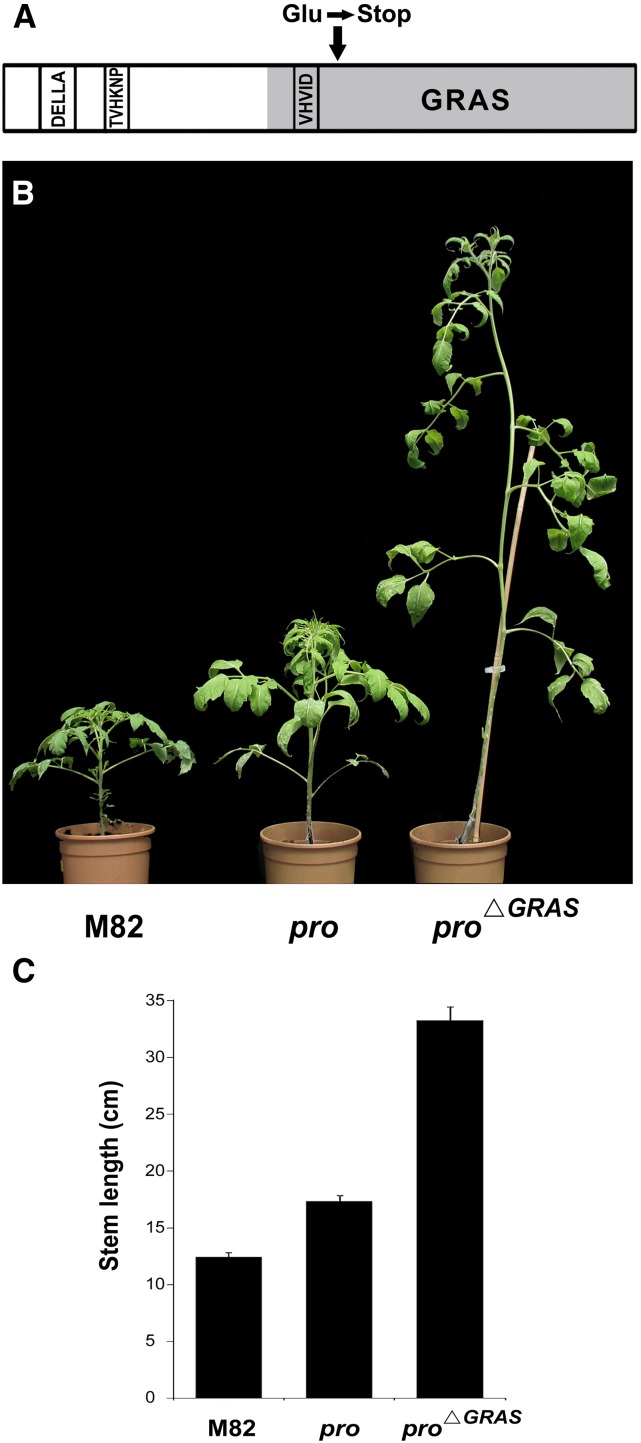
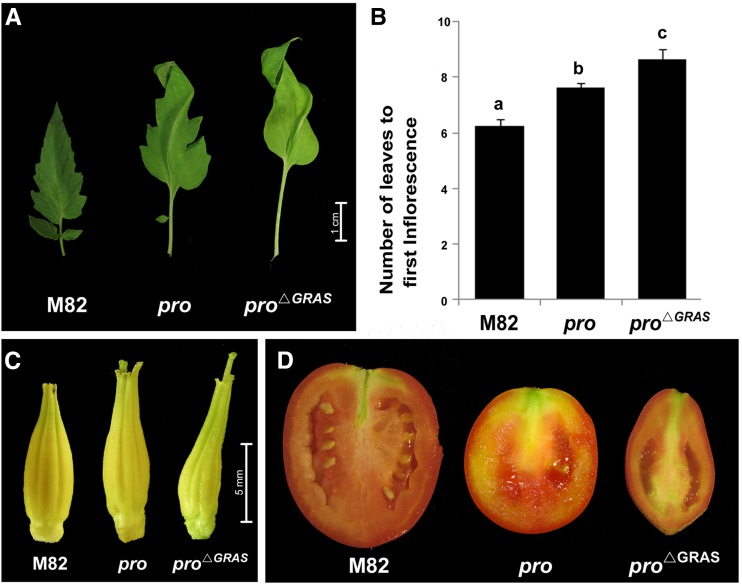
In a visual screen of a tomato activation tagging population, a slender elongated mutant was identified. This mutant population was produced in the dwarf Micro-Tom tomato background by a maize Ds transposon element containing an enhancer sequence (see Methods). Backcross analysis of the newly identified mutant showed a recessive mode of inheritance, suggesting a loss-of-function mutation. After introgressing the mutant into the M82 (SP+) background by four successive backcrosses, the homozygous progeny exhibited similar slender-elongated growth. Since the mutant phenotype resembled that of pro, we sequenced the PRO gene and found a mutation likely to be caused by excision of a transposon used for activation tagging. The mutation created a stop codon downstream to the VHIID domain (position 339, Glu to stop); thus, the allele was predicted to encode a truncated protein lacking most of the GRAS domain (Figure 1A; Supplemental Figure 1). These pro∆GRAS plants were extremely slender and tall compared with M82 and the pro mutant. Four-week-old pro∆GRAS plants were ∼3 times taller than M82 plants and twice as tall as M82 with pro introgressed into it (Figures 1B and 1C). The leaf phenotype of pro∆GRAS was also stronger than that of pro, with larger, smoother, and curlier leaflets that featured longer petioles lacking intercalary leaflets (Figure 2A). In addition, flowering time was delayed and first inflorescence emerged after the production of 8 to 10 leaves rather than 5 to 7 and 7 to 8 leaves in M82 and pro, respectively (Figure 2B). The stigmas of the pro∆GRAS pistils protruded above the staminal cone due to the long style (Figure 2C), and when fruits were made, they were all seedless, small, and oval (Figure 2D). Notably, the development of partenocarpic fruits in tomato can be triggered by constitutive GA signaling (Carrera et al., 2012).
Figure 1.
The pro∆GRAS Mutant.
(A) Schematic presentation of the PRO protein structure. The arrow indicates the position of the mutation in pro∆GRAS converting the amino acid Glu to a stop codon.
(B) Five-week-old M82, pro, and pro∆GRAS plants.
(C) The mean length (n = 12) ± se of the main stem of 4-week-old M82, pro, and pro∆GRAS plants.
Figure 2.
Phenotypic Characterization of pro∆GRAS .
(A) First leaflet of the fifth leaf in 5-week-old plants.
(B) Mean number of leaves to first inflorescence (n = 11 plants) ± se. Letters indicate significant differences, as determined by t test P < 0.05.
(C) M82, pro, and pro∆GRAS flowers before anthesis.
(D) M82, pro, and pro∆GRAS fruits.
Recently, new pro alleles were produced using a transcription activator-like effector nuclease (TALEN; Lor et al., 2014). pro∆TALEN-2 plants were similar to pro∆GRAS and had stronger defects than pro (Supplemental Figures 2A and 2B). This includes longer stem, simpler leaves with smoother leaflets, long styles, and production of small partenocarpic fruits. When pro, proTALEN_2, pro∆GRAS, and proTALEN_2/pro∆GRAS plants (Supplemental Figure 3) were grown side by side for 4 weeks and their phenotypes were compared, proTALEN_2/pro∆GRAS plants were indistinguishable from homozygous proTALEN_2 and pro∆GRAS plants (Supplemental Figure 2B), indicating that both are strong alleles that are likely null.
Loss of PRO Activity Affects Fertilization and Seed Set
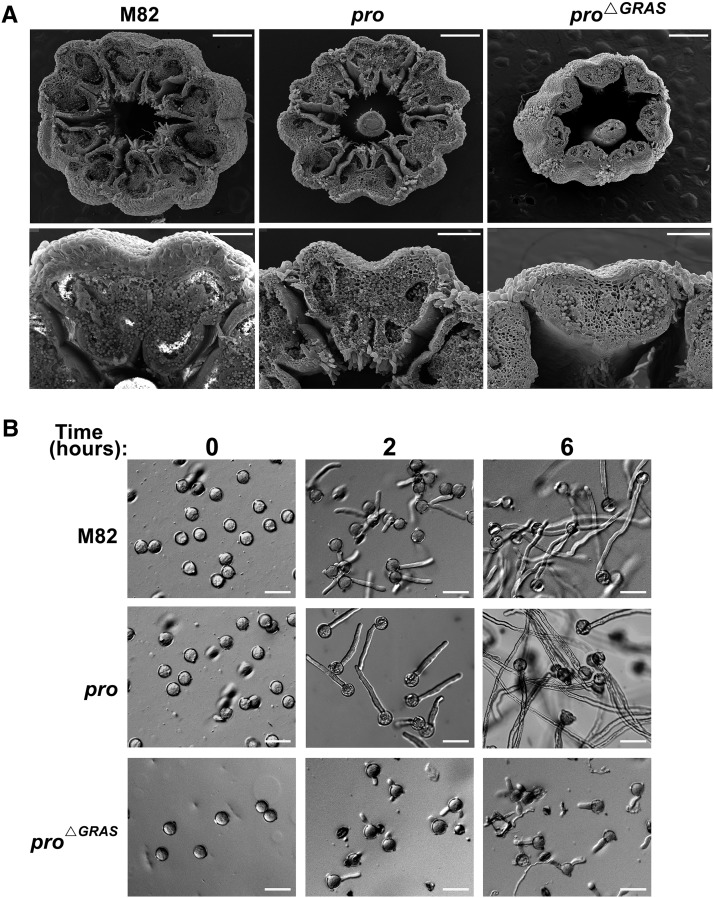
As lack of fertilization in pro∆GRAS and proTALEN_2 flowers could stem from the long styles that prevent self-pollination (Figure 2C), pro∆GRAS flowers were hand-pollinated with pro∆GRAS pollen. Fertilization was rarely observed, suggesting a physiological barrier that prevents the fertilization process. This differs from the pro mutant that exhibits facultative parthenocarpy (Carrera et al., 2012). Pollination of pro∆GRAS flowers with M82 pollen grains resulted in partial seed set (Supplemental Figure 4), suggesting that female gametophytes are fertile. Similar male sterility and female fertility were found in pro∆TALEN_2 (Supplemental Figure 4 and Supplemental Table 1). Scanning electron microscopy images revealed that pro∆GRAS anthers were thinner and smaller and contained fewer pollen grains compared with M82 and pro (Figure 3A). An in vitro pollen germination assay showed that pollen of M82, pro, and pro∆GRAS germinated; however, while M82 and pro pollen tubes continued to elongate during the 6 h of the experiment, pro∆GRAS pollen tubes stopped elongating shortly after germination (Figure 3B). This growth suppression of pro∆GRAS pollen tubes may explain the obligatory parthenocarpy observed in this mutant.
Figure 3.
The Effect of pro∆GRAS and pro on Anther Development, Pollen Production, and Pollen Tube Elongation.
(A) Scanning electron microscopy images of M82, pro, and pro∆GRAS anther cones and single anthers. Flowers were detached prior to anthesis and cut widthwise. Bars in the upper panels = 500 μm; bars in the lower panels = 250 μm.
(B) Real-time observation of in vitro germination of M82, pro, and pro∆GRAS pollen. Flowers were detached at anthesis and pollen was incubated in germination solution. Germination and tube elongation were monitored for 6 h using a light microscope. Bar = 50 μm.
Seed Viability and Segregation Distortion in Progenies of proΔGRAS/+ Plants
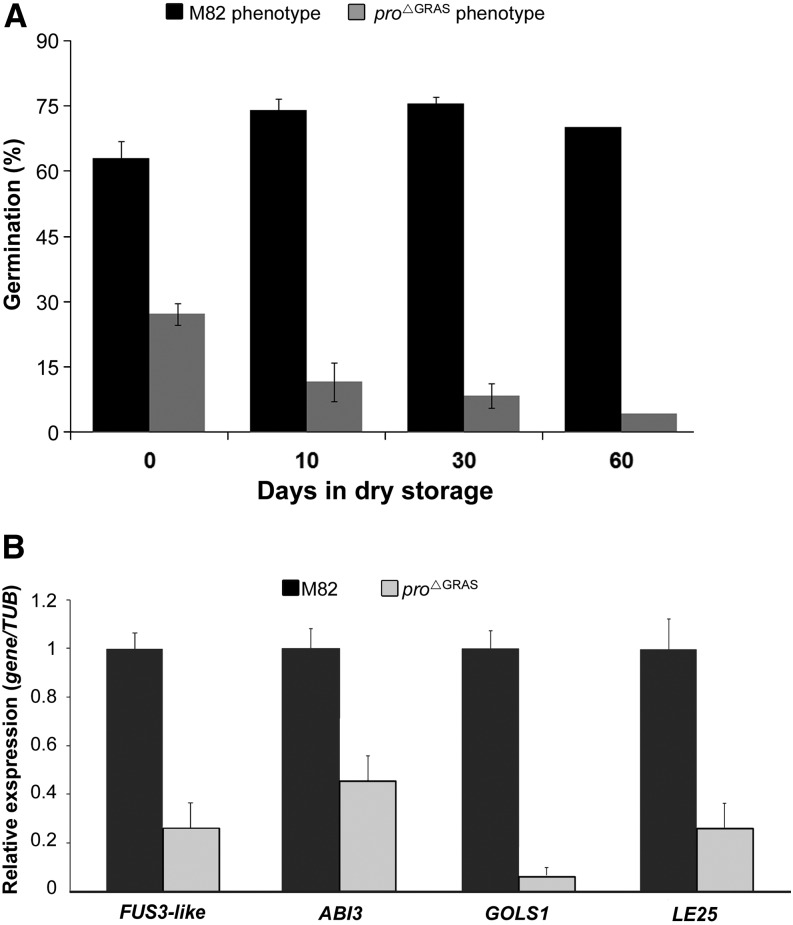
Since homozygous pro∆GRAS did not produce seeds, we had to use progenies of heterozygous plants to obtain homozygous plants. When sowing these seeds after a short period of storage (2 to 5 weeks of dry storage), ∼2 to 8% of the seedlings were homozygous, and not the 25% expected by the Mendelian segregation ratio (Supplemental Figure 5A). To test if PRO activity is required for embryo vitality or for embryo survival under dry storage conditions, we extracted seeds from red fruits and sowed them either immediately or after longer periods of dry storage. The expected ratio of 25% seedlings with a pro∆GRAS phenotype was obtained for fresh seeds. In contrast, only 8% of the seedlings from seeds that were stored for 10 d exhibited the pro∆GRAS phenotype, while ∼18% of the seeds did not germinate (Figure 4A). After 2 months of dry storage, only 5% of the seedlings exhibited the pro∆GRAS phenotype. These results led us to speculate that pro∆GRAS seeds are intolerant to desiccation. However, it should be mentioned that when seeds were sown, pro∆GRAS seedlings were the first to germinate, pointing at a promoting effect of the constitutive GA signaling on germination (Supplemental Figure 5B). To further examine this phenomenon, we conducted the same experiment with pro and proTALEN_2 seeds. Dry storage of pro seeds (5 months) did not affect their germination (Supplemental Figure 6A), while proTALEN_2 seeds, similar to pro∆GRAS, exhibited reduced germination after short periods of dry storage (Supplemental Figure 6B).
Figure 4.
pro∆GRAS Seeds Are Sensitive to Desiccation.
(A) Seeds were harvested from heterozygous pro∆GRAS fruits (after self-pollination) and sown immediately thereafter, or after different periods of dry storage. Values represent the percentages of germinating seedlings with M82 (M82 and heterozygous pro∆GRAS seedlings) or pro∆GRAS (homozygous pro∆GRAS seedlings) phenotypes from total number of seeds. Values are the average of three replicates; each contains 50 seeds ± se.
(B) qRT-PCR analyses of ABI3, FUS3-like, LE25, and GOLS expression in M82 and pro∆GRAS seeds. RNA was extracted from fresh M82 and pro∆GRAS homozygous seeds. Values are the average of three biological replicas ± se.
Abscisic Acid Responses in proΔGRAS Seeds
To understand how PRO promotes desiccation tolerance, we followed the expression of desiccation-related genes by quantitative RT-PCR (qRT-PCR) analysis of RNA extracted from both M82 and the scarce fresh homozygous pro∆GRAS seeds. To this end, we collected pollen from a large number of pro∆GRAS anthers and pollinated many pro∆GRAS flowers that eventually produced a few homozygous seeds. We analyzed the expression of the tomato ABA INSENSITIVE3 (ABI3), LATE EMBRYOGENESIS25 (LE25), and GALACTINOL SYNTHASE1 (GOLS1) genes, all of which are known to be regulated by abscisic acid (ABA) and to be involved in the acquisition of seed desiccation tolerance (Cohen and Bray, 1992; Downie et al., 2003; Bassel et al., 2006; To et al., 2006). In addition, we analyzed the expression of the tomato FUSCA3-like (FUS3-like) homolog, a major player in the acquisition of desiccation tolerance (To et al., 2006). All four genes exhibited significantly lower levels of expression in pro∆GRAS compared with M82 seeds (Figure 4B), suggesting that the machinery to induce desiccation tolerance is suppressed in pro∆GRAS seeds. Since ABA has a major role in the acquisition of desiccation tolerance during seed maturation (Ooms et al., 1993; Koornneef et al., 2002; Finkelstein et al., 2008), and DELLA positively regulates ABA accumulation via the transcriptional activation of XERICO, a RING-E3 ligase (Zentella et al., 2007; Ariizumi et al., 2013), we analyzed the expression of the tomato XERICO homolog in fresh pro∆GRAS seeds. XERICO-like expression was lower in pro∆GRAS compared with M82 seeds (Supplemental Figure 7), implying that the lack of desiccation tolerance in pro∆GRAS seeds may result from reduced ABA levels.
DELLA-Independent GA Responses
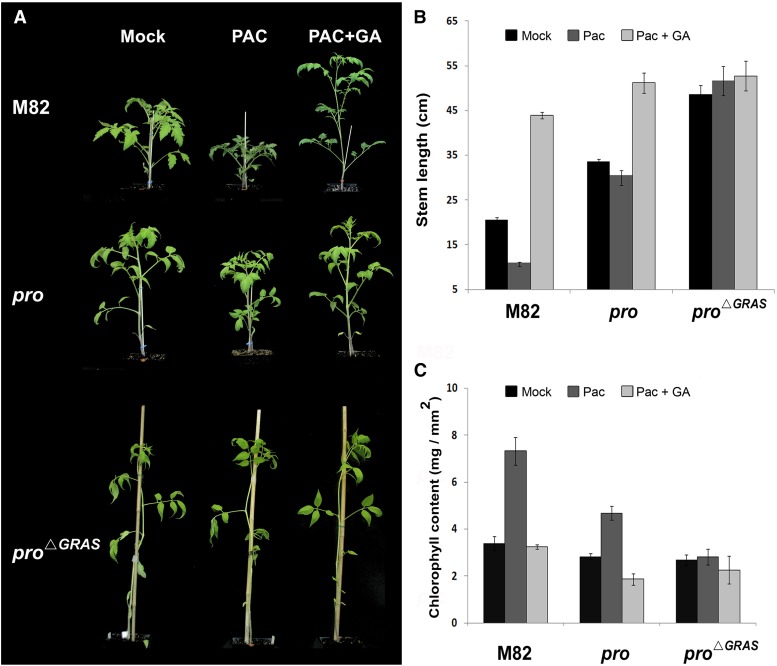
Our data suggest that the pro∆GRAS allele is much stronger than pro and may represent a null allele. Thus, we next tested whether the well-documented responsiveness of pro to GA (Van Tuinen et al., 1999) is due to a partial loss of DELLA function or due to the activity of a DELLA-independent GA signaling pathway (Fleishon et al., 2011). To this end, we first treated M82, pro, and pro∆GRAS seedlings with the GA biosynthesis inhibitor paclobutrazol (PAC), followed by application of GA3. PAC treatment of M82 and of the pro mutant suppressed stem elongation (Figures 5A and 5B), an effect that was reversed by application of GA3. However, PAC, GA3, or their sequential application did not alter elongation of pro∆GRAS or proTALEN_2 stems (Supplemental Figure 8). Likewise, chlorophyll content was elevated by PAC and reduced by GA3 in M82 and pro but not in pro∆GRAS leaves (Figure 5C). These results suggest that pro∆GRAS and proTALEN_2 plants are largely insensitive to GA, while pro plants retain some DELLA activity.
Figure 5.
pro∆GRAS Is Insensitive to PAC and GA.
(A) Six-week-old M82, pro, and pro∆GRAS plants were treated with 10 mg/L PAC three times a week, for 2 weeks (starting at two true leaves), followed by 2 weeks of GA3 application (100 μM, three times a week).
(B) Mean length ± se of the main stems of the plants treated as in (A) (n = 8 to 11 plants).
(C) Mean chlorophyll content ± se in the first leaflet of the forth leaf taken from 6-week-old plant treated as in (A) (n = 8).
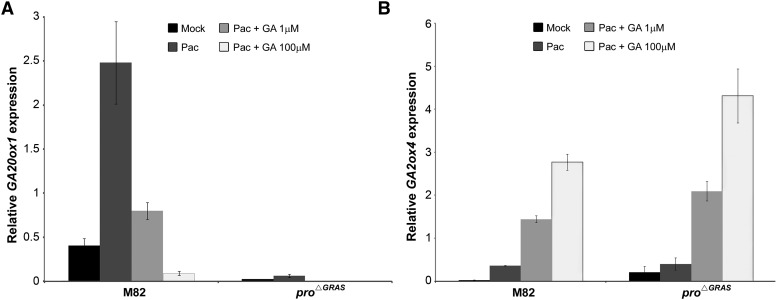
To examine the molecular responses of pro∆GRAS to GA, we compared the regulation of GA metabolism and catabolism genes by GA. GA homeostasis is regulated by a negative feedback loop, where high GA levels/signals suppress GA production via the inhibition of the GA biosynthetic gene GA20oxidase (GA20ox) and promote GA deactivation by the induction of the GA deactivation gene, GA2oxidase (GA2ox; Yamaguchi, 2008). M82 and pro∆GRAS seedlings were treated with PAC for 3 d and then treated with 0, 1, or 100 μM GA3. Three hours after the GA treatment, RNA was extracted from young leaves and the expression levels of GA20ox1 and GA2ox4 were analyzed by qRT-PCR. We would like to emphasis that the names of these and other tomato GA metabolism and catabolism genes do not necessarily reflect their relatedness to the Arabidopsis genes. The accession numbers of all the tomato genes used in this study can be found in Methods. As expected, GA20ox1 expression was promoted by PAC and suppressed by GA3 in M82 leaves. In agreement with the constitutive GA signaling and insensitivity to GA, GA20ox1 expression was extremely low in pro∆GRAS and neither affected by PAC nor by GA3 treatment (Figure 6A). GA2ox4 expression was low in mock-treated M82 and induced by GA3 treatment. However, the GA2ox4 expression level in pro∆GRAS remained low, similar to the level found in M82 leaves, indicating that it was not affected by the endogenous constitutive GA signal. Moreover, expression of this gene in pro∆GRAS was strongly induced by exogenous GA3 (Figure 6B). As these results were unexpected, the experiment was repeated six times and similar results were obtained (Supplemental Figure 9). However, it should be noted that in some experiments, the GA induction of GA2ox4 was stronger in pro∆GRAS than is M82, but not in others (Figure 6B versus Supplemental Figure 9). We next examined the impact of GA3 treatment of pro leaves on the expression of these two genes. GA20ox1 expression was low in mock-treated pro (due to the constitutive GA responses) but was further inhibited by treatment with 10 μM GA3 (Supplemental Figure 10), indicating partial PRO activity. GA2ox4 expression, on the other hand, was not affected by the constitutive GA signaling in pro but was induced by exogenous GA3 treatment. We next analyzed the expression levels of other GA2ox genes, GA2ox2 and GA2ox5. GA2ox2 expression was not altered by GA application to the wild-type M82; therefore, its expression was not examined in pro∆GRAS seedlings (Supplemental Figure 11A). The expression profile of GA2ox5, on the other hand, in response to GA was similar to that of GA2ox4, i.e., induced by exogenous GA3 in pro∆GRAS (Supplemental Figure 11B).
Figure 6.
Regulation of GA20ox and GA2ox Expression by GA in pro∆GRAS.
qRT-PCR analysis of GA20ox1 (A) and GA2ox4 (B) expression. Seedlings were treated with 10 mg/L PAC for 3 d, followed by one application of GA3 (1 or 100 μM). RNA was extracted from young leaves and analyzed. Values (gene-to-TUBULIN ratios) are means of three biological replicates ± se.
The strong induction of GA2ox4 by exogenous GA3 in pro∆GRAS combined with the lack of effect of the constitutive endogenous GA signaling in this mutant suggest a GA response that is DELLA independent. However, it should be noted that GA2ox4 did not respond to application of GA3 in M82 and proΔGRAS, without prior exposure to PAC, and the PAC treatment itself, typically weakly promoted expression. Similar results were found previously in rice (Huang et al., 2010).
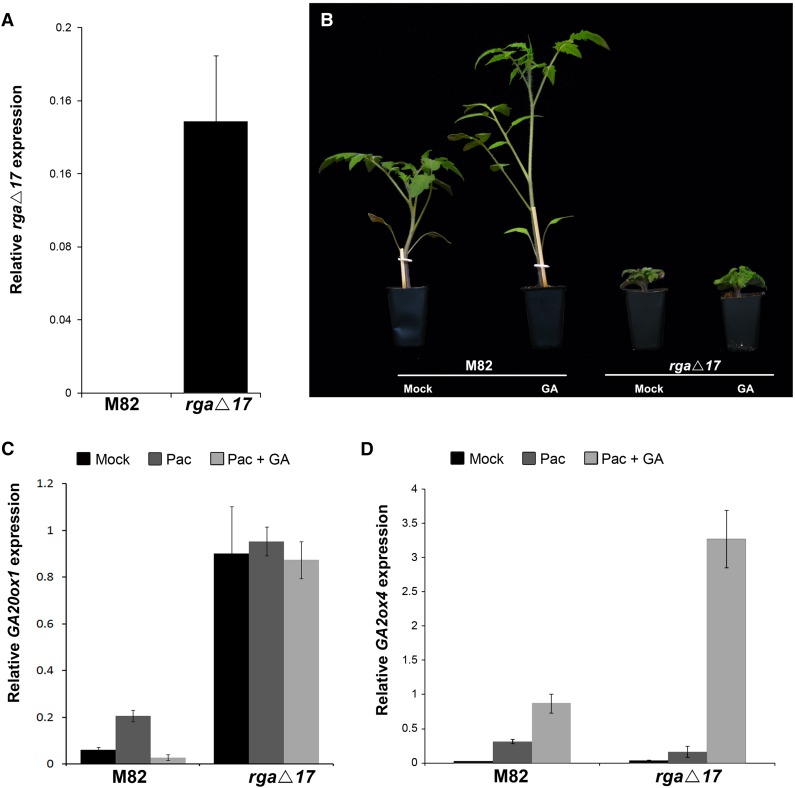
To further investigate this possible DELLA-independent GA response, we generated transgenic rga∆17 tomato plants (M82 background) overexpressing the Arabidopsis DELLA RGA lacking the DELLA domain (Dill et al., 2001). The 17-amino acid deletion in RGA inhibits the degradation of the protein in response to GA and, therefore, when overexpressed, constitutively suppresses GA responses (Dill et al., 2001). We used the Arabidopsis gene to bypass possible cosuppression. 35S:rga∆17 tomato lines with high rga∆17 expression levels (Figure 7A) and a severe dwarfism were self-pollinated and homozygous lines were generated. These lines also had small dark-green leaves, typical of tomato plants with reduced GA activity (Nir et al., 2014). Application of exogenous GA3 (100 μM) strongly promoted elongation of M82 plants, but had no effect on the elongation of rga∆17 plants, suggesting insensitivity to GA (Figure 7B).
Figure 7.
Regulation of Growth and Gene (GA20ox and GA2ox) Expression by GA in the Transgenic Tomato Overexpressing the Arabidopsis RGA∆17 Mutant Gene.
(A) qRT-PCR analysis of RGA∆17 expression in M82 and transgenic tomato plants. RNA was extracted from young leaves of the T2 generation. Values (gene-to-TUBULIN ratios) are means of three biological replicates ± se.
(B) M82 and transgenic RGA∆17 plants treated with 100 μM GA3 three times a week for 2 weeks.
(C) and (D) qRT-PCR analyses of GA20ox1 (C) and GA2ox4 (D) expression in tomato leaves treated with 10 mg/L PAC for 3 d or PAC for 3 d followed by one application of 100 μM GA3. Values (gene-to-TUBULIN ratios) are means of three biological replicates ± se.
We analyzed the expression of GA20ox1 and GA2ox4 in rga∆17 tomato plants, following PAC and GA treatments, as described above. As expected in cases of feedback regulation, GA20ox1 expression was high in the untreated RGA∆17 plants (Figure 7C). The expression of this gene was suppressed by GA3 treatment in M82 plants but was not affected in leaves of the transgenic line. On the other hand, GA2ox4 expression was induced by GA3 in both M82 and rga∆17 leaves (Figure 7D), again suggesting that GA regulates the tomato GA2ox4 via a DELLA-independent pathway. Notably, while in some experiments the induction of GA2ox4 by GA3 in rga∆17 leaves was stronger than that in M82 (Figure 7D), in other experiments, we found similar response to GA3 in the different lines (Supplemental Figure 12).
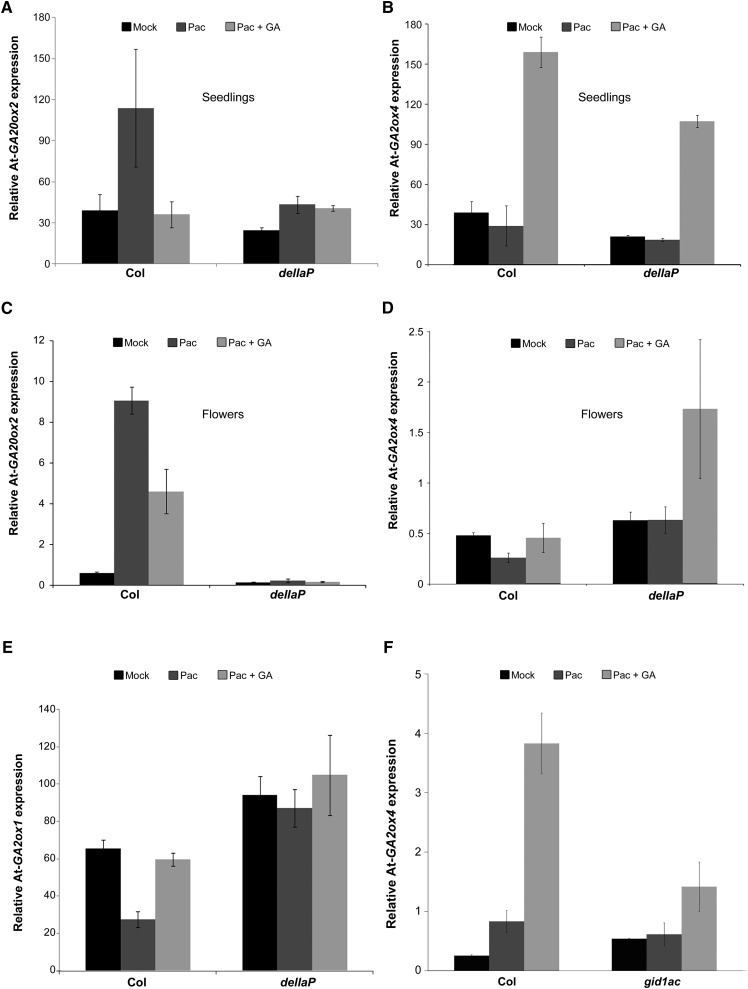
To examine whether feedback regulation of GA2ox by GA is DELLA independent in other species, we examined the Arabidopsis della pentuple mutant (dellaP; Park et al., 2013). The dellaP (rga-28, gai-t6, rgl1-SK62, rgl2-SK54, and rgl3-3) in the Columbia-0 (Col-0) background has impaired activity of all five DELLA genes and, therefore, as in pro∆GRAS, exhibits constitutive GA signaling. Wild-type Col-0 and dellaP seedlings were treated with PAC (5 mg/L) once a day for 3 d followed by a single GA3 application (10 μM). Three hours after the GA treatment, RNA was extracted from seedling shoots and analyzed for At-GA20ox2 and At-GA2ox4 expression. At-GA20ox2 exhibited normal feedback regulation in the wild type but expression was unaffected by PAC or GA in dellaP (Figure 8A). At-GA2ox4 was induced by GA3 in both the wild type and dellaP (Figure 8B). We also tested the response of these two genes to GA3 in flowers. To this end, seedlings were treated with PAC (5 mg/L) twice a week until flowering and then treated once with 10 μM GA3. Three hours after the GA treatment, RNA was extracted from the flowers and analyzed for At-GA20ox2 and At-GA2ox4 expression. While At-GA20ox2 exhibited normal feedback regulation in the wild type, in dellaP its basal expression was low and was unaffected by either PAC or GA (Figure 8C). At-GA2ox4 was not affected by the endogenous constitutive GA signaling in dellaP but was induced by GA3 in both the wild type and dellaP (Figure 8D). These results suggest that in Arabidopsis, the regulation of At-GA2ox4 by GA is also DELLA independent.
Figure 8.
Regulation of Arabidopsis GA20ox2, GA2ox4, and GA2ox1 Expression by GA in Arabidopsis.
(A) and (B) Seedlings of wild-type Col-0 and dellaP mutant Arabidopsis plants were treated with PAC (5 mg/L) once a day for 3 d followed by a single GA3 application (10 μM). Three hours after the GA treatment, RNA was extracted from the seedlings and analyzed by qRT-PCR for At-GA20ox2 (A) and At-GA2ox4 (B) expression.
(C) to (E) Plants (wild-type Col-0 and dellaP) were treated with PAC (5 mg/L) twice a week until flowering and then treated once with 10 μM GA3. Three hours after the GA treatment, RNA was extracted from the flowers and analyzed by qRT-PCR for At-GA20ox2 (C), At-GA2ox4 (D), and At-GA2ox1 (E).
(F) Wild type (Col-0) and gid1ac seedlings were treated with PAC (5 mg/L) once a day for 3 d followed by a single GA3 application (10 μM). Three hours after the GA treatment, RNA was extracted and analyzed (qRT-PCR) for At-GA2ox4 expression.
Values (gene-to-TUBULIN ratios) in (A) to (F) are means of three biological replicates ± se.
To further explore DELLA-independent GA responses in Arabidopsis, we examined the expression of At-GA2ox1 in the flowers. At-GA2ox1 behaved as expected of a DELLA-regulated gene, i.e., high expression in dellaP and lack of response to GA3 (Figure 8E). To examine whether the activation of At-GA2ox4 by GA is initiated by the GA receptor GID1, we treated wild-type and gid1ac (loss of two out of the three GID1 receptor genes; Griffiths et al., 2006) seedlings with PAC (5 mg/L) once a day for 3 d followed by a single GA3 application (10 µM). Three hours after the GA treatment, RNA was extracted and analyzed for At-GA2ox4 expression. The lack of GID1a and GID1c activity significantly reduced the response of At-GA2ox4 to GA3 (Figure 8F), suggesting that this DELLA-independent GA response is initiated by GA binding to the GID1 receptors. The observed weak response of At-GA2ox4 to GA3 in gid1ac was probably mediated by GID1b.
Global Analysis of DELLA-Independent GA Responses
To understand the scope of DELLA-independent GA-regulated genes, deep sequencing (RNA-seq) was performed to RNA samples extracted from GA-treated M82 and pro∆GRAS plants. M82 and pro∆GRAS seedlings were treated with PAC (10 mg/L) once a day for 3 d followed by a single GA3 application (100 μM). Three hours after the GA treatment, young leaves were collected, RNA was extracted, and cDNA libraries were sequenced by Illumina HiSequation 2500. A total of eight samples were analyzed, and each treatment had two biological replicates. TopHat was used to align the reads to the tomato genome SL2.50 (Trapnell et al., 2009). Counts of aligned reads per gene were obtained using HTSeq-count (Anders et al., 2015), and the DESeq2 package was used to identify genes that were differentially expressed between PAC and PAC + GA3 treated leaves. Using a 2-fold increase or decrease cutoff (adjusted P value for multiple comparisons ≤0.05), we identified 81 GA-upregulated and 15 GA-downregulated genes (Tables 1 and 2; Supplemental Table 2). The majority of these genes were DELLA dependent, i.e., their expression was unaffected by GA3 in pro∆GRAS. These include some well-characterized GA-regulated genes, such as GA20ox, GID1, SCL, GAST1, and EXPANSINS (Shi et al., 1992; Chen et al., 2001; Zentella et al., 2007). Five of the GA-regulated genes (four upregulated and one downregulated) were DELLA-independent, i.e., they were similarly induced or suppressed by GA3 in M82 and pro∆GRAS (Table 3). It should be noted that in this experiment, all GA2ox genes were expressed at low levels and none of them was affected significantly by GA3 in M82 or pro∆GRAS. To confirm the results, we analyzed the expression of the identified GA-regulated DELLA-independent genes, Solyc07g064600.2 (encoding Endoribonuclease) and Solyc09g008670.2 (encoding Thr ammonia lyase) by qRT-PCR. The results confirm those of the RNA-seq and show that GA induces both in a DELLA-independent manner (Supplemental Figure 13).
Table 1. GA Upregulated, DELLA-Dependent Genes (Fold Change > 4).
| SolyC Locus | Description | Mean Paca | Mean Pac+GAa | Fold Changeb | Adj. P Valuec |
|---|---|---|---|---|---|
| Solyc05g007950.2 | Ribonuclease T2 | 68 | 1068 | 15.78 | 5.62E-08 |
| Solyc12g010800.1 | BZIP transcription factor | 10 | 106 | 10.29 | 7.15E-06 |
| Solyc03g025380.2 | Peroxidase | 24 | 243 | 9.98 | 0.000267 |
| Solyc03g005320.2 | 3-Ketoacyl-CoA synthase | 60 | 379 | 6.36 | 1.64E-06 |
| Solyc01g110630.2 | Auxin-induced SAUR-like | 18 | 108 | 5.95 | 0.000945 |
| Solyc04g017720.2 | GAST1 | 55 | 312 | 5.71 | 1.22E-07 |
| Solyc12g056250.1 | Glutathione S-transferase | 341 | 1917 | 5.62 | 2.76E-06 |
| Solyc07g062710.2 | BZIP transcription factor | 64 | 342 | 5.38 | 6.02E-08 |
| Solyc04g081790.2 | GDSL esterase/lipase | 54 | 290 | 5.38 | 6.02E-08 |
| Solyc04g016190.1 | Glucosyltransferase | 87 | 465 | 5.33 | 0.001001 |
| Solyc03g097170.2 | Cinnamoyl-CoA reductase | 140 | 739 | 5.29 | 8.31E-07 |
| Solyc03g078090.2 | Pectinesterase | 19 | 89 | 4.80 | 0.033812 |
| Solyc10g005210.2 | Methyladenine glycosylase | 42 | 195 | 4.60 | 1.64E-06 |
| Solyc10g011730.2 | Arabinogalactan peptide | 49 | 219 | 4.49 | 3.75E-05 |
| Solyc03g006100.2 | Receptor-like kinase, RLK | 144 | 633 | 4.41 | 0.000322 |
| Solyc08g075210.1 | Acyltransferase-like protein | 60 | 259 | 4.31 | 0.011786 |
| Solyc03g114710.2 | Glucosyltransferase | 33 | 141 | 4.30 | 0.00217 |
| Solyc10g052530.1 | Auxin-responsive protein | 544 | 2301 | 4.28 | 0.00546 |
| Solyc11g069960.1 | Receptor-like kinase, RLK | 32 | 137 | 4.22 | 0.000267 |
| Solyc04g081870.2 | Expansin | 467 | 1964 | 4.20 | 1.98E-07 |
| Solyc02g088100.2 | Expansin | 297 | 1232 | 4.15 | 0.000293 |
| Solyc07g008560.2 | Purple acid phosphatase | 25 | 103 | 4.04 | 0.010653 |
Mean value of two biological replicates.
Fold change is the ratio mean Pac + GA/mean Pac.
Corrected P values were calculated using the Benjamini and Hochberg (1995) false discovery rate approach.
Table 2. GA-Downregulated, DELLA-Dependent Genes.
| SolyC Locus | Description | Mean Paca | Mean Pac+GAa | Fold Changeb | Adj. P Valuec |
|---|---|---|---|---|---|
| Solyc06g035530.2 | Gibberellin 20-oxidase-2 | 79 | 7 | −11.18 | 2.67E-05 |
| Solyc03g006880.2 | Gibberellin 20-oxidase-1 | 1244 | 122 | −10.18 | 0.003222 |
| Solyc01g008910.2 | Scarecrow-like | 123 | 15 | −8.31 | 4.17E-05 |
| Solyc03g119530.2 | LOB domain protein 42 | 88 | 20 | −4.45 | 0.001399 |
| Solyc12g099900.1 | GRAS family | 298 | 68 | −4.38 | 6.06E-06 |
| Solyc09g009520.2 | Hydrolase α/β fold | 110 | 26 | −4.32 | 0.00961 |
| Solyc09g009220.2 | Unknown protein | 128 | 30 | −4.20 | 0.005171 |
| Solyc12g095750.1 | Auxin efflux carrier | 116 | 29 | −3.99 | 0.015785 |
| Solyc06g008870.2 | GID1-like GA receptor | 661 | 174 | −3.81 | 8.98E-05 |
| Solyc06g067950.2 | Acyl-protein thioesterase | 135 | 39 | −3.50 | 0.048555 |
| Solyc01g095580.2 | GH3 family protein | 1357 | 432 | −3.14 | 0.000576 |
| Solyc02g080510.1 | Unknown protein | 340 | 117 | −2.91 | 0.017273 |
| Solyc09g075590.1 | Unknown protein | 339 | 130 | −2.61 | 0.045166 |
| Solyc05g006420.2 | ARR3 | 2685 | 1182 | −2.27 | 0.013313 |
Mean value of two biological replicates.
Corrected P values were calculated using the Benjamini and Hochberg (1995) false discovery rate approach.
Table 3. GA-Regulated, DELLA-Independent Genes.
| SolyC Locus | Description | Fold Changea | Adj. P Valueb | Fold Changea | Adj. P Valueb |
|---|---|---|---|---|---|
| M82 |
pro∆GRAS |
||||
| Solyc07g064600.2 | Endoribonuclease L-PSP | 3.20 | 0.013414 | 11.88 | 7.53E-13 |
| Solyc09g083440.2 | Proteinase inhibitor I | 4.22 | 0.00546 | 5.73 | 0.000413 |
| Solyc09g008670.2 | Threonine ammonia-lyase | 4.14 | 0.000293 | 4.03 | 0.001424 |
| Solyc03g121270.2 | IAA-amino acid hydrolase | 2.54 | 0.015785 | 3.37 | 0.00038 |
| Solyc03g117280.2 | Unknown protein | −2.21 | 0.014012 | −2.40 | 0.01025 |
Fold change is the ratio mean Pac + GA/mean Pac [for fold change <1, the value is presented as: −1(Pac + GA/Pac)].
Corrected P values were calculated using the Benjamini and Hochberg (1995) false discovery rate approach.
DISCUSSION
The tomato genome contains a single DELLA gene, named PRO, and a pro mutant has been extensively characterized (George Jones, 1987; Jupe et al., 1988; Van Tuinen et al., 1999; Jasinski et al., 2008; Bassel et al., 2008; Carrera et al., 2012). pro exhibits constitutive GA activity but retains some responsiveness to the hormone, either due to incomplete loss of DELLA activity (Van Tuinen et al., 1999) or due to activity of a DELLA-independent GA response pathway (Fleishon et al., 2011). Here, we describe pro mutants, proΔGRAS and proTALEN_2 (Lor et al., 2014) that are likely null or close to null alleles. Our study suggests that the responsiveness of the “classic” pro mutant to GA is due to residual DELLA activity but also uncovers DELLA-independent GA responses.
The phenotype of proΔGRAS and proTALEN_2 plants resembles that of tomato plants treated with high doses of GA. In tomato, exogenous GA application has a dramatic effect on stem elongation. In Arabidopsis, on the other hand, application of GA or lack of DELLA activity has only a mild effect on final stem length (King et al., 2001). A strong effect is found only when the hormone is applied to GA-deficient mutants. This difference between Arabidopsis and tomato may be due to differences in basal levels of endogenous active GAs. A rapid stem elongation (bolting) in Arabidopsis occurs after the floral transition and is associated with a dramatic increase in GA level (Eriksson et al., 2006). Thus, GA activity may be saturated and the loss of DELLA or addition of exogenous GA has only a mild effect. On the other hand, the tomato stem elongates slowly but continuously throughout the life of the plant. It is possible that this slow elongation requires intermediate GA levels, below saturation; therefore, loss of PRO activity or application of high GA doses has a dramatic effect on stem elongation.
All analyzed GA-related phenotypes were more severe in proΔGRAS and proTALEN_2 plants than in pro, suggesting that pro is a “leaky” mutant, as previously proposed (Van Tuinen et al., 1999). While pro exhibits facultative partenocarpy (Carrera et al., 2012), proΔGRAS and proTALEN_2 did not produce seeds even after hand-pollination, suggesting obligatory partenocarpy. Previous studies suggested that the facultative partenocarpy of pro is due to the longer style, which prevents self-pollination (Bassel et al., 2008; Carrera et al., 2012). While the proΔGRAS and proTALEN_2 styles are longer than that of pro, it cannot explain the obligatory partenocarpy. Since pollination of proΔGRAS and proTALEN_2 flowers with M82 pollen resulted in an almost normal seed set, the lack of fertilization in homozygous proΔGRAS or proTALEN_2 flowers is probably due to male sterility. An in vitro pollen germination assay showed that the elongation of proΔGRAS pollen tube, but not that of pro, is arrested shortly after germination. Previous studies in Arabidopsis and rice suggested that while GA is required for pollen tube elongation, GA concentrations higher than optimal inhibit this process (Singh et al., 2002; Chhun et al., 2007). This can explain why proΔGRAS but not pro, inhibited pollen tube elongation. The suppression of pollen tube elongation in proΔGRAS is probably not a cell-autonomous effect. If it was, homozygous seeds would not be obtained by self-pollination of heterozygous plants, since haploid proΔGRAS pollen would not elongate to fertilize the proΔGRAS egg cells. Thus, it is possible that the effect of proΔGRAS on the ability of the pollen cells to elongate is via the supporting tissues, the connective and tapetum cell layers. Indeed, scanning electron microscopy analysis showed malformation of these tissues in proΔGRAS.
Tomato seeds can be considered “orthodox” seeds (Angelovici et al., 2010), since they can tolerate desiccation and can be stored in a dry state for years (Priestley et al., 1985). Our results show that homozygous proΔGRAS and proTALEN_2 seeds lose their ability to germinate shortly after harvest and cannot survive even short periods (days) of dry storage. Analysis of desiccation tolerance-related genes (ABI3, FUS3, LE25, and GOLS) in proΔGRAS seeds revealed reduced expression levels, suggesting that PRO is required for activation of the machinery that acquire tolerance. The germination of pro seeds, on the other hand, was not affected by long dry storage, suggesting that residual DELLA activity is sufficient to acquire desiccation tolerance.
ABA plays a major role in the acquisition of desiccation tolerance as well in the induction of dormancy during the late stages of seed maturation (Ooms et al., 1993; Koornneef et al., 2002; Finkelstein et al., 2008). Previous studies have shown that DELLA regulates ABA synthesis in seeds via the transcriptional activation of the RING ubiquitin E3 ligase XERICO, an inducer of ABA synthesis (Zentella et al., 2007; Piskurewicz et al., 2008; Ariizumi et al., 2013). We found reduced expression of the putative tomato homolog of XERICO in proΔGRAS seeds, suggesting that PRO increases desiccation tolerance by promoting ABA synthesis. Although desiccation tolerance is tightly associated with dormancy and both are regulated by ABA, previous studies linked DELLA activity in seeds with dormancy only (Lee et al., 2010; Ariizumi et al., 2013). Our results suggest that the loss of PRO activity suppresses both processes; while homozygous proΔGRAS seeds had reduced desiccation tolerance, they germinated much faster than M82 seeds, suggesting weaker dormancy.
It is possible that the loss of seed viability during dry storage prevented the identification of strong pro alleles in all previous tomato mutant screenings. It is also possible that the Micro-Tom background, which has a mutation in the DWARF (D) gene, allowed the identification of this allele in our screening. D encodes a P450 protein involved in brassinosteroid biosynthesis (Bishop et al., 1999). Since GA and brassinosteroids act synergistically (Bai et al., 2012), and the response to GA in Micro-Tom partially depends on brassinosteroids (Martí et al., 2006), it is possible the GA responses are partially suppressed in Micro-Tom, improving seed tolerance to desiccation.
Our results suggest that the reported, relatively strong response of pro to GA (Van Tuinen et al., 1999) is due to the “leaky” nature of the pro allele and not due to the activity of an alternative GA signaling pathway. In parallel, while the null mutants proΔGRAS and proTALEN_2 exhibited insensitivity of growth to GA and PAC, a DELLA-independent GA response in proΔGRAS plants, namely, the feedback regulation of GA catabolism, was discovered. As expected, the expression level of GA20ox1 was lower in proΔGRAS than in M82 and was not affected by GA or PAC treatments. On the other hand, the expression of GA2ox4 and GA2ox5 was unexpectedly low in proΔGRAS and was strongly induced by GA3. These findings suggest that GA2ox4 and GA2ox5 do not respond to the endogenous constitutive GA signaling produced by the loss of PRO, but rather, are induced by exogenous GA treatment. In addition, although transgenic tomato plants overexpressing the Arabidopsis gain-of-function DELLA protein RGA∆17 were insensitive to GA in terms of growth, GA2ox4 was strongly induced by GA3 treatment in these plants. In summary, these findings suggest that expression of tomato GA2ox4 is activated by GA via a DELLA-independent pathway. Similar results were found in the Arabidopsis dellaP mutant. While At-GA2ox1 behaved as expected, i.e., exhibited high levels of expression in dellaP and insensitivity to GA3 treatment, the expression of At-GA2ox4 was strongly induced by exogenous GA3 in this mutant. While numerous studies have shown that the expression of GA20ox is suppressed, and that of GA2ox, is promoted by GA (Yamaguchi, 2008), Zentella et al. (2007) suggested that At-GA20ox genes, but not At-GA2ox, are regulated directly by DELLA. The mechanism by which GA promotes GA2ox expression in a DELLA-independent manner is yet unknown, but our results imply that GA binding to the GID1 receptor is required. High GA activity increases plant susceptibility to various biotic and abiotic stresses (Achard et al., 2006, 2008; Nir et al., 2014) and therefore can be destructive to plants. Thus, it is possible that both DELLA-dependent and -independent induction of GA catabolism by increased GA signal evolved to ensure efficient regulation of GA homeostasis.
Our results suggest that ∼5% of all tomato GA-regulated genes are DELLA-independent (Tables 1 to 3). Similarly, Cao et al. (2006) suggested that only a portion of the GA-regulated genes in Arabidopsis are DELLA dependent. In tomato, the strongest DELLA-independent induction by GA was on a ribonuclease (RNase) gene (Solyc05g007950.2, 15-fold change). A previous study in barley aleurone identified RNase as a GA-induced gene (Rogers and Rogers, 1999). Tomato GA-regulated genes include homologs of well-characterized Arabidopsis genes: GA downregulated, such as GA20ox, GID1, and SCL, and GA upregulated genes, such as GASA-like (GAST1) and EXPANSIN (Shi et al., 1992; Chen et al., 2001; Zentella et al., 2007). Thus, while many “classic” GA-associated genes are common to distantly related plants, others, e.g., bZIP transcription factor (Solyc12g010800.1, 10-fold induction), may represent tomato-specific GA responses. For the five DELLA-independent genes, we were not able to find a common theme that characterizes their specific regulation.
In summary, this work presents new tomato DELLA loss-of-function mutants. Phenotypic, physiological, and molecular analyses of these pro mutants uncovered DELLA-regulated processes and identified GA-regulated, DELLA-independent responses, providing a powerful tool to study GA physiology and the role of DELLA in plant biology.
METHODS
Plant Materials and Growth Conditions
Tomato (Solanum lycopersicum) plants were in the M82 background (SP+). The recessive pro∆GRAS allele was isolated from an activation-tagging population of Micro-Tom, mutagenized with an Ac/Ds system carrying a 4×35S enhancer element in the Ds transposon (MacAlister et al., 2012). The pro∆GRAS line used in this study was backcrossed with M82 (SP+) plants four times. pro (Bassel et al., 2008) and proTALEN (Lor et al., 2014) were in the M82 (SP+) background. Plants were grown in a greenhouse under 24/20°C (day/night) at natural daylength conditions. Arabidopsis thaliana plants were grown in a growth room under controlled temperature (22°C) and long-day (16 h light/8 h dark) conditions. The Arabidopsis DELLA pentuple mutant (dellaP; Park et al., 2013) and gid1ac double mutant (Griffiths et al., 2006) were in the Col-0 background. Tomato seeds were harvested from ripe fruits, incubated with 10% sucrose overnight at 37°C, and then treated with 1% sodium hypochlorite followed by 1% Na3PO4. Seeds were stored dry at room temperature.
Molecular Cloning/Constructs and Plant Transformation
The RGA∆17 coding sequence (Zentella et al., 2007) was fused to the 5′ of the enhanced GFP coding sequence, in a KpnI site. The GFP-RGA∆17 fusion was inserted to a pART7 plasmid downstream of the 35S promoter, into XhoI and BamHI sites, to create 35S:GFP-RGA∆17. The construct was subcloned into the pART27 binary vector and was introduced into Agrobacterium tumefaciens strain GV3101 by electroporation. The construct was transferred to S. lycopersicum variety M82 cotyledons, using the transformation and regeneration methods described by McCormick (1991). Kanamycin-resistant T0 plants were grown in the greenhouse, and three independent transgenic lines were selected and self-pollinated to generate homozygous transgenic lines. All primer sequences are presented in Supplemental Table 3.
Hormone Treatments
Tomato seedlings with two true leaves were sprayed with PAC (10 mg/L) three times a week for 2 weeks, followed by GA3 (Sigma-Aldrich) application (100 μM), throughout the experiment. For the analysis of GA biosynthesis gene expression, young tomato seedlings were sprayed for 3 d with PAC (10 mg/L) and on the fourth day, immersed in 10 or 100 μM GA3 for 30 min. Leaves were collected after 3 h and RNA was extracted. Arabidopsis seedlings were treated with PAC (5 mg/L) once a day for 3 d followed by a single GA3 application (10 μM) or twice a week with PAC (5 mg/L) until flowering and then immersed in 10 μM GA3 for 30 min. Seedlings or flowers were collected 3 h after the GA treatments and RNA was extracted.
Chlorophyll Measurements
Chlorophyll was extracted from fresh leaves in acetone (100%) and spectrophotometrically measured at 645 and 663 nm (Arnon, 1949). Chlorophyll concentrations were calculated using the formula: (20.2 × A645 + 8.02 × A663)/cm2.
RNA Extraction and cDNA Synthesis
Total RNA was isolated using the GHC-phenol chloroform method: Frozen tissues were ground and resuspended in guanidine HCl and then phenol/chloroform was added. Samples were mixed by vortexing for 30 s and after 30 min were centrifuged at 4°C for 45 min. Ethanol (100%) and 1 M acetic acid were added, and the samples were mixed and stored overnight at −80°C. NaAc (3 M) was added and samples were washed with cold 70% ethanol. For the synthesis of cDNA, we used SuperScript II reverse transcriptase (Invitrogen) and 3 μg of total RNA, according to the manufacturer’s instructions.
qRT-PCR Analyses
qRT-PCR analysis was performed using the Absolute Blue qPCR SYBR Green ROX Mix (AB-4162/B) kit (Thermo Fisher Scientific). Reactions were performed using a Rotor-Gene 6000 cycler (Corbett Research). A standard curve was obtained for each gene using dilutions of a cDNA sample. Each gene was quantified using Corbett Research Rotor-Gene software. At least three independent technical repeats were performed for each cDNA sample. Relative expression of each sample was calculated by dividing the expression level of the analyzed gene by that of TUBULIN. Gene-to-TUBULIN ratios were then averaged. All primer sequences are presented in Supplemental Table 3.
Library Construction and Sequencing
Total RNA (0.5 μg) was processed using the TruSeq RNA Sample Preparation Kit v2 protocol (Illumina). Libraries were evaluated by Qubit and TapeStation. Sequencing libraries were constructed with barcodes to allow multiplexing of eight samples on one lane. Twenty to twenty-five million single-end 60-bp reads were sequenced per sample on an Illumina HiSequation 2500 V4 instrument.
Sequence Data Analysis
TopHat (v2.0.10) was used to align the reads to the tomato genome sequence SL2.50 (downloaded from the Sol genomics network http://solgenomics.net/organism/Solanum_lycopersicum/genome) (Trapnell et al., 2009). The percentage of the reads that were aligned uniquely to the genome was between 85 and 91%. Counting reads on ITAG2.4 genes (downloaded from Sol genomics network) was done with HTSeq-count (version 0.6.1p1) (Anders et al., 2015). Differential analysis was performed using DESeq2 (1.6.3) (Anders and Huber, 2010). Raw P values were adjusted for multiple testing using the procedure of Benjamini and Hochberg (1995). Genes with a false discovery rate of <0.05 and fold changes >2 were regarded as differentially expressed genes.
Expression data were submitted to the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/; accession number GSE68018).
Genotyping proTALEN_2/proΔGRAS Seedlings
DNA was extracted from cotyledons of progenies of the proTALEN_2 × pro∆GRAS crosses that exhibited elongated hypocotyls using the DNeasy Plant Mini Kit (Qiagen). To identify the proTALEN_2 allele, the forward primer proTALEN_tF1 and reverse primer proTAELN_tR1 (Supplemental Table 3) were used to amplify the region encompassing the proTALEN_2 deletion site (Lor et al., 2014). Each PCR reaction used 50 ng of genomic DNA template in a 50-μL volume using ExTaq polymerase (Clontech). Thermocycler conditions were set according to the manufacturer’s recommendations with the annealing temperature set for 55°C and elongation time set for 1 min. proTALEN_2 PCR amplicons were digested with Sm1I, which cuts the wild-type sequence but not the proTALEN_2 mutant sequence, and 10 μL of the digestion was run on a 0.8% agarose gel. To identify the pro∆GRAS allele, we designed derived cleaved amplified polymorphic sequence (Neff et al., 1998) primers pro∆GRAS_dF1 and pro∆GRAS_dR1 using dCAPs Finder 2.0 (http://helix.wustl.edu/dcaps). The resulting primers produce a wild-type PRO amplicon that is digested with PvuII to produce 302- and 27-bp products, while the pro∆GRAS amplicon is resistant to digestion. PCR reaction mixes and conditions are similar to the proTALEN_2 PCR conditions except for the anneal temperature that was set at 65°C. pro∆GRAS PCR amplicons were digested with PvuII and separated on 1.5% agarose gel.
Microscopy
Samples for scanning electron microscopy were immersed in increasing concentrations of ethanol (25% up to 100%) and critical-point dried with liquid carbon dioxide in a CPD 750 (Bio-Rad), sputter-coated with gold, and photographed with a Jeol scanning electron microscope (JSM-5410 LV).
In Vitro Pollen Germination Assay
Flowers were detached at anthesis and shaken with a pollen buzzer into a microfuge tube containing germination solution (100 g L−1 sucrose, 40% polyethylene glycol 4000, 0.01 M HEPES, pH 6, 2 mM boric acid, 2 mM calcium nitrate, 2 mM magnesium sulfate, and 1 mM potassium nitrate); tubes were shaken well to release the pollen grains. The final solution with the pollen grains was transferred to a slide covered with glass slip and sealed with grease. Germination and tube elongation were monitored for 6 h under a light microscope.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL libraries under the following accession numbers: PROCERA, Solyc11g011260; ABI3, Solyc06g083590; GOLS1, Solyc01g100830.1.1; LE25, Solyc10g078770.1.1; FUS3-like, Solyc02g094460.1.1; XERICO-like, Solyc07g045190.1.1; GA2ox2, Solyc07g056670.2.1; GA2OX4, Solyc07g061720.2.1; GA2OX5, Solyc07g061730.2.1; GA20OX1, Solyc03g006880.2.1; At-GA20OX2, AT5G51810.1; At-GA2OX1, AT1G78440.1; At-GA2OX4, AT1G47990.1; At-RGA, AT2G01570.1; ENDORIBONUCLEASE, Solyc07g064600.2; THREONINE AMMONIA LYASE, Solyc09g008670.2. In addition, sequence data and their sources are provided in Tables 1 to 3 and Supplemental Table 2.
Supplemental Data
Supplemental Figure 1. Sequence alignment of PRO from M82, pro, and pro∆GRAS.
Supplemental Figure 2. Phenotypic characterization of proTALEN_2 and pro∆GRAS/proTALEN_2 plants.
Supplemental Figure 3. Genotyping of pro∆GRAS/proTALEN_2 plants shown in Supplemental Figure 2B.
Supplemental Figure 4. Seed set in tomato fruits following hand-pollination of pro∆GRAS and proTALEN_2 emasculated flowers with M82 pollen grains.
Supplemental Figure 5. pro∆GRAS seeds are sensitive to desiccation and have weak dormancy.
Supplemental Figure 6. proTALEN_2 but not pro seeds are sensitive to desiccation.
Supplemental Figure 7. qRT-PCR expression analysis of the putative XERICO gene in tomato.
Supplemental Figure 8. pro∆GRAS and proTALEN_2 are insensitive to PAC and GA3.
Supplemental Figure 9. Regulation of GA2ox4 expression by GA in pro∆GRAS.
Supplemental Figure 10. Expression analyses of GA20ox1 and GA2ox4 in M82 and pro.
Supplemental Figure 11. Expression analyses of GA2ox2 and GA2ox5 expression.
Supplemental Figure 12. Expression analyses of GA2ox4 in M82 and rgaΔ17 leaves.
Supplemental Figure 13. Expression analyses (qRT-PCR) of Solyc07g064600.2 (Endoribonuclease) and Solyc09g008670.2 (Thr ammonia lyase) in M82 and pro∆GRAS leaves.
Supplemental Table 1. proTALEN_2 plants are male, but not female, sterile.
Supplemental Table 2. Complete list of GA upregulated genes.
Supplemental Table 3. Primers used in this study.
Supplementary Material
Acknowledgments
We thank Gilgi Friedlander (INCPM unit, Weizmann Institute of Science) for help with the bioinformatic analysis. We thank Naomi Ori for valuable suggestions that improved the article. This research was supported by research grants from the U.S.–Israel Binational Agriculture Research and Development fund to D.W. and N.E.O. (Grant 4429-11) and by the I-CORE Program of the Planning and Budgeting Committee and The Israel Science Foundation (Grant 757/12) to D.W., A.A., and Y.E. The work in the laboratory of A. A. was supported by European Research Council (ERC) project SAMIT (Framework Programme 7).
AUTHOR CONTRIBUTIONS
S.L., V.S.L., N.E.O., Y.E., and D.W. designed the research. S.L., I.N., and V.S.L. performed the research. A.A. contributed new tools. S.L., V.S.L., N.E.O., Y.E., and D.W. analyzed data. S.L., A.A., N.E.O., Y.E., and D.W. wrote the article.
Glossary
- GA
gibberellin
- qRT-PCR
quantitative RT-PCR
- ABA
abscisic acid
- PAC
paclobutrazol
- Col-0
Columbia-0
References
- Achard P., Cheng H., De Grauwe L., Decat J., Schoutteten H., Moritz T., Van Der Straeten D., Peng J., Harberd N.P. (2006). Integration of plant responses to environmentally activated phytohormonal signals. Science 311: 91–94. [DOI] [PubMed] [Google Scholar]
- Achard P., Renou J.P., Berthomé R., Harberd N.P., Genschik P. (2008). Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 18: 656–660. [DOI] [PubMed] [Google Scholar]
- Anders S., Huber W. (2010). Differential expression analysis for sequence count data. Genome Biol. 11: R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders, S., Pyl, T.P., and Huber, W. (2015). HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169. [DOI] [PMC free article] [PubMed]
- Angelovici R., Galili G., Fernie A.R., Fait A. (2010). Seed desiccation: a bridge between maturation and germination. Trends Plant Sci. 15: 211–218. [DOI] [PubMed] [Google Scholar]
- Ariizumi T., Murase K., Sun T.-P., Steber C.M. (2008). Proteolysis-independent downregulation of DELLA repression in Arabidopsis by the gibberellin receptor GIBBERELLIN INSENSITIVE DWARF1. Plant Cell 20: 2447–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariizumi T., Hauvermale A.L., Nelson S.K., Hanada A., Yamaguchi S., Steber C.M. (2013). Lifting DELLA repression of Arabidopsis seed germination by nonproteolytic gibberellin signaling. Plant Physiol. 162: 2125–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud N., Girin T., Sorefan K., Fuentes S., Wood T.A., Lawrenson T., Sablowski R., Østergaard L. (2010). Gibberellins control fruit patterning in Arabidopsis thaliana. Genes Dev. 24: 2127–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D.I. (1949). Cooper enzymes in isolated chloroplasts polyhenoloxidase in beta vulgaris. Plant Physiol. 24: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M.Y., Shang J.X., Oh E., Fan M., Bai Y., Zentella R., Sun T.P., Wang Z.Y. (2012). Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat. Cell Biol. 14: 810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassel G.W., Mullen R.T., Bewley J.D. (2006). ABI3 expression ceases following, but not during, germination of tomato and Arabidopsis seeds. J. Exp. Bot. 57: 1291–1297. [DOI] [PubMed] [Google Scholar]
- Bassel G.W., Mullen R.T., Bewley J.D. (2008). Procera is a putative DELLA mutant in tomato (Solanum lycopersicum): effects on the seed and vegetative plant. J. Exp. Bot. 59: 585–593. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57: 289–300. [Google Scholar]
- Bishop G.J., Nomura T., Yokota T., Harrison K., Noguchi T., Fujioka S., Takatsuto S., Jones J.D.G., Kamiya Y. (1999). The tomato DWARF enzyme catalyses C-6 oxidation in brassinosteroid biosynthesis. Proc. Natl. Acad. Sci. USA 96: 1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D. (1996). Effects of gibberellic acid and environmental factors on cytosolic calcium in wheat aleurone cells. Planta 199: 89–99. [Google Scholar]
- Cao D., Cheng H., Wu W., Soo H.M., Peng J. (2006). Gibberellin mobilizes distinct DELLA-dependent transcriptomes to regulate seed germination and floral development in Arabidopsis. Plant Physiol. 142: 509–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera E., Ruiz-Rivero O., Peres L.E.P., Atares A., Garcia-Martinez J.L. (2012). Characterization of the procera tomato mutant shows novel functions of the SlDELLA protein in the control of flower morphology, cell division and expansion, and the auxin-signaling pathway during fruit-set and development. Plant Physiol. 160: 1581–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler P.M., Marion-Poll A., Ellis M., Gubler F. (2002). Mutants at the Slender1 locus of barley cv Himalaya. Molecular and physiological characterization. Plant Physiol. 129: 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Dahal P., Bradford K.J. (2001). Two tomato expansin genes show divergent expression and localization in embryos during seed development and germination. Plant Physiol. 127: 928–936. [PMC free article] [PubMed] [Google Scholar]
- Chhun T., Aya K., Asano K., Yamamoto E., Morinaka Y., Watanabe M., Kitano H., Ashikari M., Matsuoka M., Ueguchi-Tanaka M. (2007). Gibberellin regulates pollen viability and pollen tube growth in rice. Plant Cell 19: 3876–3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Bray E.A. (1992). Nucleotide sequence of an ABA-induced tomato gene that is expressed in wilted vegetative organs and developing seeds. Plant Mol. Biol. 18: 411–413. [DOI] [PubMed] [Google Scholar]
- Davière J.-M., Wild M., Regnault T., Baumberger N., Eisler H., Genschik P., Achard P. (2014). Class I TCP-DELLA interactions in inflorescence shoot apex determine plant height. Curr. Biol. 24: 1923–1928. [DOI] [PubMed] [Google Scholar]
- de Lucas M., Davière J.M., Rodríguez-Falcón M., Pontin M., Iglesias-Pedraz J.M., Lorrain S., Fankhauser C., Blázquez M.A., Titarenko E., Prat S. (2008). A molecular framework for light and gibberellin control of cell elongation. Nature 451: 480–484. [DOI] [PubMed] [Google Scholar]
- Dill A., Jung H.S., Sun T.P. (2001). The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc. Natl. Acad. Sci. USA 98: 14162–14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill A., Thomas S.G., Hu J., Steber C.M., Sun T.P. (2004). The Arabidopsis F-box protein SLEEPY1 targets gibberellin signaling repressors for gibberellin-induced degradation. Plant Cell 16: 1392–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie B., Gurusinghe S., Dahal P., Thacker R.R., Snyder J.C., Nonogaki H., Yim K., Fukanaga K., Alvarado V., Bradford K.J. (2003). Expression of a GALACTINOL SYNTHASE gene in tomato seeds is up-regulated before maturation desiccation and again after imbibition whenever radicle protrusion is prevented. Plant Physiol. 131: 1347–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S., Böhlenius H., Moritz T., Nilsson O. (2006). GA4 is the active gibberellin in the regulation of LEAFY transcription and Arabidopsis floral initiation. Plant Cell 18: 2172–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., et al. (2008). Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451: 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R., Reeves W., Ariizumi T., Steber C. (2008). Molecular aspects of seed dormancy. Annu. Rev. Plant Biol. 59: 387–415. [DOI] [PubMed] [Google Scholar]
- Fleishon S., Shani E., Ori N., Weiss D. (2011). Negative reciprocal interactions between gibberellin and cytokinin in tomato. New Phytol. 190: 609–617. [DOI] [PubMed] [Google Scholar]
- Fuentes S., Ljung K., Sorefan K., Alvey E., Harberd N.P., Østergaard L. (2012). Fruit growth in Arabidopsis occurs via DELLA-dependent and DELLA-independent gibberellin responses. Plant Cell 24: 3982–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego-Bartolomé J., Minguet E.G., Marín J.A., Prat S., Blázquez M.A., Alabadí D. (2010). Transcriptional diversification and functional conservation between DELLA proteins in Arabidopsis. Mol. Biol. Evol. 27: 1247–1256. [DOI] [PubMed] [Google Scholar]
- George Jones M. (1987). Gibberellins and the procera mutant of tomato. Planta 172: 280–284. [DOI] [PubMed] [Google Scholar]
- Griffiths J., Murase K., Rieu I., Zentella R., Zhang Z.-L., Powers S.J., Gong F., Phillips A.L., Hedden P., Sun T.P., Thomas S.G. (2006). Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18: 3399–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler F., Chandler P.M., White R.G., Llewellyn D.J., Jacobsen J.V. (2002). Gibberellin signaling in barley aleurone cells. Control of SLN1 and GAMYB expression. Plant Physiol. 129: 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harberd N.P., Belfield E., Yasumura Y. (2009). The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: how an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell 21: 1328–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauvermale A.L., Ariizumi T., Steber C.M. (2012). Gibberellin signaling: a theme and variations on DELLA repression. Plant Physiol. 160: 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K., Asano K., Tsuji H., Kawamura M., Mori H., Kitano H., Ueguchi-Tanaka M., Matsuoka M. (2010). Characterization of the molecular mechanism underlying gibberellin perception complex formation in rice. Plant Cell 22: 2680–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K., Kouketu E., Katoh H., Aya K., Ueguchi-Tanaka M., Matsuoka M. (2012). The suppressive function of the rice DELLA protein SLR1 is dependent on its transcriptional activation activity. Plant J. 71: 443–453. [DOI] [PubMed] [Google Scholar]
- Hong G.-J., Xue X.-Y., Mao Y.-B., Wang L.-J., Chen X.-Y. (2012). Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 24: 2635–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X., Lee L.Y.C., Xia K., Yan Y., Yu H. (2010). DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev. Cell 19: 884–894. [DOI] [PubMed] [Google Scholar]
- Huang J., Tang D., Shen Y., Qin B., Hong L., You A., Li M., Wang X., Yu H., Gu M., Cheng Z. (2010). Activation of gibberellin 2-oxidase 6 decreases active gibberellin levels and creates a dominant semi-dwarf phenotype in rice (Oryza sativa L.). J. Genet. Genomics 37: 23–36. [DOI] [PubMed] [Google Scholar]
- Ikeda A., Ueguchi-Tanaka M., Sonoda Y., Kitano H., Koshioka M., Futsuhara Y., Matsuoka M., Yamaguchi J. (2001). slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell 13: 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski S., Tattersall A., Piazza P., Hay A., Martinez-Garcia J.F., Schmitz G., Theres K., McCormick S., Tsiantis M. (2008). PROCERA encodes a DELLA protein that mediates control of dissected leaf form in tomato. Plant J. 56: 603–612. [DOI] [PubMed] [Google Scholar]
- Jupe S.C., Causton D.R., Scott I.M. (1988). Cellular basis of the effects of gibberellin and the pro gene on stem growth in tomato. Planta 174: 106–111. [DOI] [PubMed] [Google Scholar]
- King K.E., Moritz T., Harberd N.P. (2001). Gibberellins are not required for normal stem growth in Arabidopsis thaliana in the absence of GAI and RGA. Genetics 159: 767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M., Bentsink L., Hilhorst H. (2002). Seed dormancy and germination. Curr. Opin. Plant Biol. 5: 33–36. [DOI] [PubMed] [Google Scholar]
- Lee K.P., Piskurewicz U., Turecková V., Strnad M., Lopez-Molina L. (2010). A seed coat bedding assay shows that RGL2-dependent release of abscisic acid by the endosperm controls embryo growth in Arabidopsis dormant seeds. Proc. Natl. Acad. Sci. USA 107: 19108–19113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locascio A., Blázquez M.A., Alabadí D. (2013). Genomic analysis of DELLA protein activity. Plant Cell Physiol. 54: 1229–1237. [DOI] [PubMed] [Google Scholar]
- Lor V.S., Starker C.G., Voytas D.F., Weiss D., Olszewski N.E. (2014). Targeted mutagenesis of the tomato PROCERA gene using custom TALENs. Plant Physiol. 166: 1288–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlister C.A., Park S.J., Jiang K., Marcel F., Bendahmane A., Izkovich Y., Eshed Y., Lippman Z.B. (2012). Synchronization of the flowering transition by the tomato TERMINATING FLOWER gene. Nat. Genet. 44: 1393–1398. [DOI] [PubMed] [Google Scholar]
- Martí C., Orzáez D., Ellul P., Moreno V., Carbonell J., Granell A. (2007). Silencing of DELLA induces facultative parthenocarpy in tomato fruits. Plant J. 52: 865–876. [DOI] [PubMed] [Google Scholar]
- Martí E., Gisbert C., Bishop G.J., Dixon M.S., García-Martínez J.L. (2006). Genetic and physiological characterization of tomato cv. Micro-Tom. J. Exp. Bot. 57: 2037–2047. [DOI] [PubMed] [Google Scholar]
- Maymon I., Greenboim-Wainberg Y., Sagiv S., Kieber J.J., Moshelion M., Olszewski N., Weiss D. (2009). Cytosolic activity of SPINDLY implies the existence of a DELLA-independent gibberellin-response pathway. Plant J. 58: 979–988. [DOI] [PubMed] [Google Scholar]
- McCormick S. (1991). Transformation of tomato with Agrobacterium tumefaciens. In Plant Tissue Culture Manual, Linclsey H., ed (Dordrecht, The Netherlands: Kluwer Academic Publishers; ), pp. 1–9. [Google Scholar]
- Murase K., Hirano Y., Sun T.P., Hakoshima T. (2008). Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature 456: 459–463. [DOI] [PubMed] [Google Scholar]
- Nakajima M., et al. (2006). Identification and characterization of Arabidopsis gibberellin receptors. Plant J. 46: 880–889. [DOI] [PubMed] [Google Scholar]
- Neff M.M., Neff J.D., Chory J., Pepper A.E. (1998). dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: experimental applications in Arabidopsis thaliana genetics. Plant J. 14: 387–392. [DOI] [PubMed] [Google Scholar]
- Nir I., Moshelion M., Weiss D. (2014). The Arabidopsis gibberellin methyl transferase 1 suppresses gibberellin activity, reduces whole-plant transpiration and promotes drought tolerance in transgenic tomato. Plant Cell Environ. 37: 113–123. [DOI] [PubMed] [Google Scholar]
- Ooms J., Leon-Kloosterziel K.M., Bartels D., Koornneef M., Karssen C.M. (1993). Acquisition of desiccation tolerance and longevity in seeds of Arabidopsis thaliana. Plant Physiol. 102: 1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Nguyen K.T., Park E., Jeon J.-S., Choi G. (2013). DELLA proteins and their interacting RING finger proteins repress gibberellin responses by binding to the promoters of a subset of gibberellin-responsive genes in Arabidopsis. Plant Cell 25: 927–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskurewicz U., Jikumaru Y., Kinoshita N., Nambara E., Kamiya Y., Lopez-Molina L. (2008). The gibberellic acid signaling repressor RGL2 inhibits Arabidopsis seed germination by stimulating abscisic acid synthesis and ABI5 activity. Plant Cell 20: 2729–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestley D.A., Cullinan V.I., Wolfe B.J. (1985). Differences in seed longevity at the species level. Plant Cell Environ. 8: 557–562. [Google Scholar]
- Rogers S.W., Rogers J.C. (1999). Cloning and characterization of a gibberellin-induced RNase expressed in barley aleurone cells. Plant Physiol. 119: 1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A., Itoh H., Gomi K., Ueguchi-Tanaka M., Ishiyama K., Kobayashi M., Jeong D.-H., An G., Kitano H., Ashikari M., Matsuoka M. (2003). Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 299: 1896–1898. [DOI] [PubMed] [Google Scholar]
- Sato T., Miyanoiri Y., Takeda M., Naoe Y., Mitani R., Hirano K., Takehara S., Kainosho M., Matsuoka M., Ueguchi-Tanaka M., Kato H. (2014). Expression and purification of a GRAS domain of SLR1, the rice DELLA protein. Protein Expr. Purif. 95: 248–258. [DOI] [PubMed] [Google Scholar]
- Shi L., Gast R.T., Gopalraj M., Olszewski N.E. (1992). Characterization of a shoot-specific, GA3- and ABA-regulated gene from tomato. Plant J. 2: 153–159. [PubMed] [Google Scholar]
- Singh D.P., Jermakow A.M., Swain S.M. (2002). Gibberellins are required for seed development and pollen tube growth in Arabidopsis. Plant Cell 14: 3133–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Jones W.T., Rikkerink E.H.A. (2012). GRAS proteins: the versatile roles of intrinsically disordered proteins in plant signalling. Biochem. J. 442: 1–12. [DOI] [PubMed] [Google Scholar]
- To, A., Valon, C., Savino, G., Guilleminot, J., and Devic, M. (2006). A network of local and redundant gene regulation governs Arabidopsis seed maturation. Plant Cell 18: 1642–1651. [DOI] [PMC free article] [PubMed]
- Trapnell C., Pachter L., Salzberg S.L. (2009). TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M., Ashikari M., Nakajima M., Itoh H., Katoh E., Kobayashi M., Chow T.Y., Hsing Y.I., Kitano H., Yamaguchi I., Matsuoka M. (2005). GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437: 693–698. [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M., Hirano K., Hasegawa Y., Kitano H., Matsuoka M. (2008). Release of the repressive activity of rice DELLA protein SLR1 by gibberellin does not require SLR1 degradation in the gid2 mutant. Plant Cell 20: 2437–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tuinen A., Peters A.H.L.J., Kendrick R.E., Zeevaart J.A.D., Koornneef M. (1999). Characterisation of the procera mutant of tomato and the interaction of gibberellins with end-of-day far-red light treatments. Physiol. Plant. 106: 121–128. [Google Scholar]
- Yamaguchi S. (2008). Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 59: 225–251. [DOI] [PubMed] [Google Scholar]
- Yang D.L., et al. (2012). Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. USA 109: E1192–E1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K., Aya K., Hirano K., Ordonio R.L., Ueguchi-Tanaka M., Matsuoka M. (2015). Comprehensive gene expression analysis of rice aleurone cells: probing the existence of an alternative gibberellin receptor. Plant Physiol. 167: 531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., et al. (2014). DELLA protein functions as a transcriptional activator through the DNA binding of the indeterminate domain family proteins. Proc. Natl. Acad. Sci. USA 111: 7861–7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentella R., Zhang Z.-L., Park M., Thomas S.G., Endo A., Murase K., Fleet C.M., Jikumaru Y., Nambara E., Kamiya Y., Sun T.-P. (2007). Global analysis of DELLA direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 19: 3037–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.-L., Ogawa M., Fleet C.M., Zentella R., Hu J., Heo J.-O., Lim J., Kamiya Y., Yamaguchi S., Sun T.P. (2011). SCARECROW-LIKE 3 promotes gibberellin signaling by antagonizing master growth repressor DELLA in Arabidopsis. Proc. Natl. Acad. Sci. USA 108: 2160–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.