The bHLH subgroup IIIe factors MYC2, MYC3, and MYC4 redundantly meditate JA-induced leaf senescence; the subgroup IIId factors bHLH03, bHLH13, bHLH14, and bHLH17 antagonize the subgroup IIIe factors.
Abstract
Plants initiate leaf senescence to relocate nutrients and energy from aging leaves to developing tissues or storage organs for growth, reproduction, and defense. Leaf senescence, the final stage of leaf development, is regulated by various environmental stresses, developmental cues, and endogenous hormone signals. Jasmonate (JA), a lipid-derived phytohormone essential for plant defense and plant development, serves as an important endogenous signal to activate senescence-associated gene expression and induce leaf senescence. This study revealed one of the mechanisms underlying JA-induced leaf senescence: antagonistic interactions of the bHLH subgroup IIIe factors MYC2, MYC3, and MYC4 with the bHLH subgroup IIId factors bHLH03, bHLH13, bHLH14, and bHLH17. We showed that MYC2, MYC3, and MYC4 function redundantly to activate JA-induced leaf senescence. MYC2 binds to and activates the promoter of its target gene SAG29 (SENESCENCE-ASSOCIATED GENE29) to activate JA-induced leaf senescence. Interestingly, plants have evolved an elaborate feedback regulation mechanism to modulate JA-induced leaf senescence: The bHLH subgroup IIId factors (bHLH03, bHLH13, bHLH14, and bHLH17) bind to the promoter of SAG29 and repress its expression to attenuate MYC2/MYC3/MYC4-activated JA-induced leaf senescence. The antagonistic regulation by activators and repressors would mediate JA-induced leaf senescence at proper level suitable for plant survival in fluctuating environmental conditions.
INTRODUCTION
To cope with environmental stresses and survive in the fluctuating environment, plants trigger leaf senescence to relocate nutrients and energy from aging leaves to developing tissues or storage organs, by which plants save and utilize the limited nutrients and energy for growth, reproduction, and defense. Leaf senescence, the last stage of leaf development, is accompanied by rapid loss of chlorophyll (Hortensteiner, 2006), decrease of photochemical efficiency, increase of membrane ion leakage (Woo et al., 2001), activation of senescence-associated genes (such as SENESCENCE-ASSOCIATED GENE12 [SAG12], SAG13, SAG29, SAG113, and SENESCENCE4 [SEN4]) (Lohman et al., 1994; Gan and Amasino, 1995; Park et al., 1998; Weaver et al., 1998; Miller et al., 1999), and repression of photosynthetic genes (such as CHLOROPHYLL A/B BINDING PROTEIN1 [CAB1] and RIBULOSE BISPHOSPHATE CARBOXYLASE SMALL CHAIN [RBCS]) (Park et al., 1998; Weaver et al., 1998). The progression of leaf senescence is stimulated by darkness, drought, and pathogen infection and other diverse environmental stresses and is controlled by developmental cues (such as age) (Woo et al., 2001) and various endogenous phytohormone signals, including jasmonate (Lim et al., 2007; Zhang and Zhou, 2013).
Jasmonates (JAs), a new class of lipid-derived plant hormones (Browse, 2009; Wasternack and Hause, 2013), regulate diverse plant defense responses and various developmental processes (Browse, 2009; Rowe et al., 2010; De Geyter et al., 2012; Zheng et al., 2012; Hu et al., 2013; Song et al., 2013a, 2014a; Huang et al., 2014). The JA signal is perceived by CORONATINE INSENSITIVE1 (COI1) (Xie et al., 1998; Yan et al., 2009; Rowe et al., 2010; Sheard et al., 2010), which subsequently recruits the JA ZIM-domain (JAZ) repressors (Chini et al., 2007; Thines et al., 2007; Yan et al., 2007; Pauwels et al., 2010) for ubiquitination and degradation, and activates various downstream transcription factors essential for respective JA responses, including the IIIe bHLH transcription factors (MYC2, MYC3, and MYC4) (Cheng et al., 2011; Fernández-Calvo et al., 2011; Niu et al., 2011), the IIId bHLH factors (bHLH03/JAM3, bHLH13/JAM2, bHLH14, and bHLH17/JAM1) (Nakata et al., 2013; Sasaki-Sekimoto et al., 2013; Song et al., 2013b; Fonseca et al., 2014), the R2R3-MYB transcription factors (MYB21 and MYB24) (Song et al., 2011), and the WD-repeat/bHLH/MYB transcription complex (Qi et al., 2011, 2014).
Previous studies revealed that JA induces leaf senescence and regulates expression of various senescence-associated genes in diverse plant species (Reinbothe et al., 2009), including Artemisia absinthium (Ueda and Kato, 1980), maize (Zea mays; Yan et al., 2012), rice (Oryza sativa; Lee et al., 2015), and Arabidopsis thaliana (He et al., 2002; Shan et al., 2011). Repression of JA biosynthesis decreases expression of senescence-related genes and delays leaf senescence. The Arabidopsis TEOSINTE BRANCHED/CYCLOIDEA/PCF (TCP) transcription factor TCP4 controls JA biosynthesis via directly activating the JA biosynthetic gene LIPOXYGENASE2, while miR319 targets TCP4 to repress JA biosynthesis and delay leaf senescence (Schommer et al., 2008). However, it is so far unknown which key signaling components in JA pathway activate JA-induced leaf senescence. In this study, we demonstrate that the bHLH subgroup IIIe factors MYC2, MYC3, and MYC4 are key signaling components, which function redundantly to activate JA-induced leaf senescence. Furthermore, we show that MYC2 binds to the promoter of its target gene SAG29 to activate expression of SAG29. Moreover, we reveal that the bHLH subgroup IIId factors bHLH03, bHLH13, bHLH14, and bHLH17 bind to the promoter of SAG29 and repress the MYC2-activated expression of SAG29 to attenuate JA-induced leaf senescence. The antagonistic regulation by the IIIe bHLH activators (MYC2, MYC3, and MYC4) and the IIId bHLH repressors (bHLH03/JAM3, bHLH13/JAM2, bHLH14, and bHLH17) would mediate JA-induced leaf senescence at suitable level, which ensures plants can properly respond to diverse environmental stresses and development cues.
RESULTS
The IIIe bHLH Factors MYC2, MYC3, and MYC4 Redundantly Activate JA-Induced Leaf Senescence
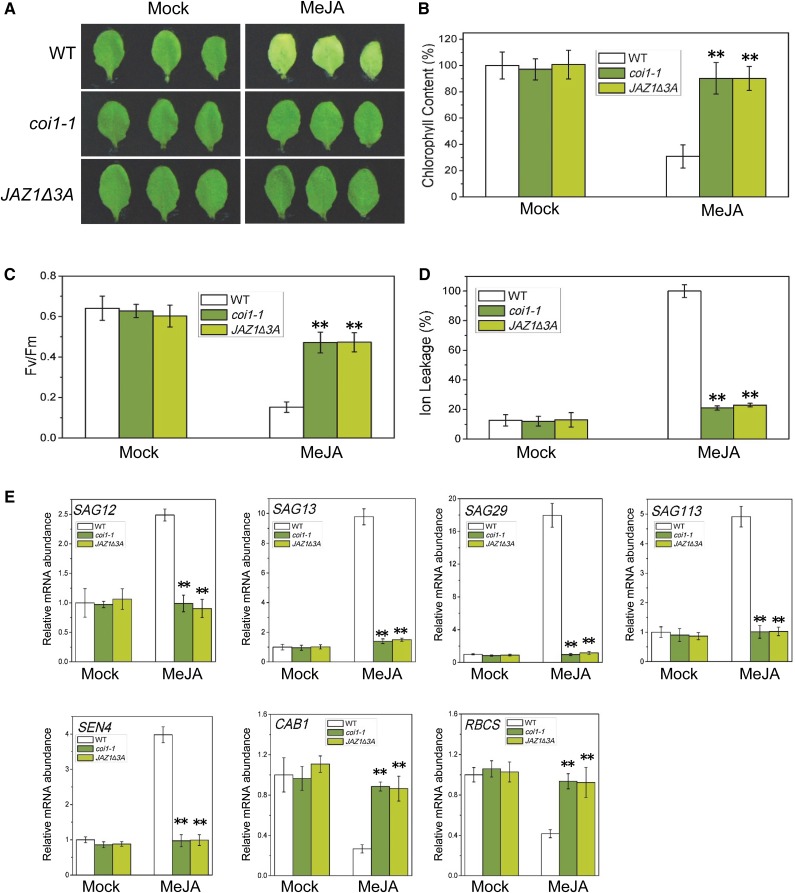
To identify which key signaling components activate JA-induced leaf senescence, we comprehensively investigated JA-induced leaf senescence in various mutants of the JA signaling pathway. Consistent with previous observation (Shan et al., 2011), the coi1-1 mutant abolished JA-induced leaf senescence and exhibited a stay-green phenotype (Figure 1A). Furthermore, we observed that the JAZ1Δ3A transgenic plant, in which the overexpressed JAZ1Δ3A does not contain the Jas domain and dominantly represses JA responses probably by forming stable dimers with the native JAZ proteins (Thines et al., 2007; Chung and Howe, 2009), also clearly blocked the JA-induced leaf senescence and displayed a stay-green phenotype (Figure 1A). In JA-treated wild type, the chlorophyll was rapidly lost (Figure 1B), the photochemical efficiency of photosystem II (PSII) (indicated by Fv/Fm) was clearly decreased (Figure 1C), the membrane ion leakage was obviously increased (Figure 1D), the expression of representative senescence-induced senescence-associated genes (such as SAG12, SAG13, SAG29, SAG113, and SEN4)was significantly induced, and the expression of senescence-reduced photosynthetic genes (such as CAB1 and RBCS) was clearly reduced (Figure 1E). On the other hand, coi1-1 and JAZ1Δ3A abolished the JA-decreased chlorophyll content and photochemical efficiency, JA-induced membrane ion leakage, and JA-regulated expression of senescence-associated genes (SAG12, SAG13, SAG29, SAG113, and SEN4) and photosynthetic genes (CAB1 and RBCS) (Figures 1B to 1E). Taken together (Figure 1), these results demonstrate that JA induces leaf senescence in a COI1-JAZ dependent manner.
Figure 1.
JA Induces Leaf Senescence in a COI1-JAZ-Dependent Manner.
(A) The senescence phenotype of leaves detached from 3-week-old Arabidopsis Col-0 (WT), coi1-1, and JAZ1Δ3A and treated without (Mock) or with 100 μM MeJA in the dark for 6 d.
(B) to (D) Chlorophyll content (B), photochemical efficiency (Fv/Fm) of PSII (C), and membrane ion leakage (D) in the detached leaves with the same treatment as shown in (A). The maximum value of the chlorophyll contents in (B) is 2.09 mg/g fresh weight (FW). Data are means (±sd) of three biological replicates. Asterisks represent Student’s t test significance between wild-type and coi1-1 or JAZ1Δ3A samples (**P < 0.01).
(E) Quantitative real-time PCR analysis for senescence-associated genes (SAG12, SAG13, SAG29, SAG113, and SEN4) and photosynthetic genes (CAB1 and RBCS) of the detached leaves with the same treatment as shown in (A). The y axis is the relative mRNA abundance of the detected genes. The mRNA abundance of the detected gene in mock-treated wild type with ACTIN8 as the internal control was set to 1. Data are means (±sd) of three biological replicates. Asterisks represent Student’s t test significance between wild-type and coi1-1 or JAZ1Δ3A samples (**P < 0.01).
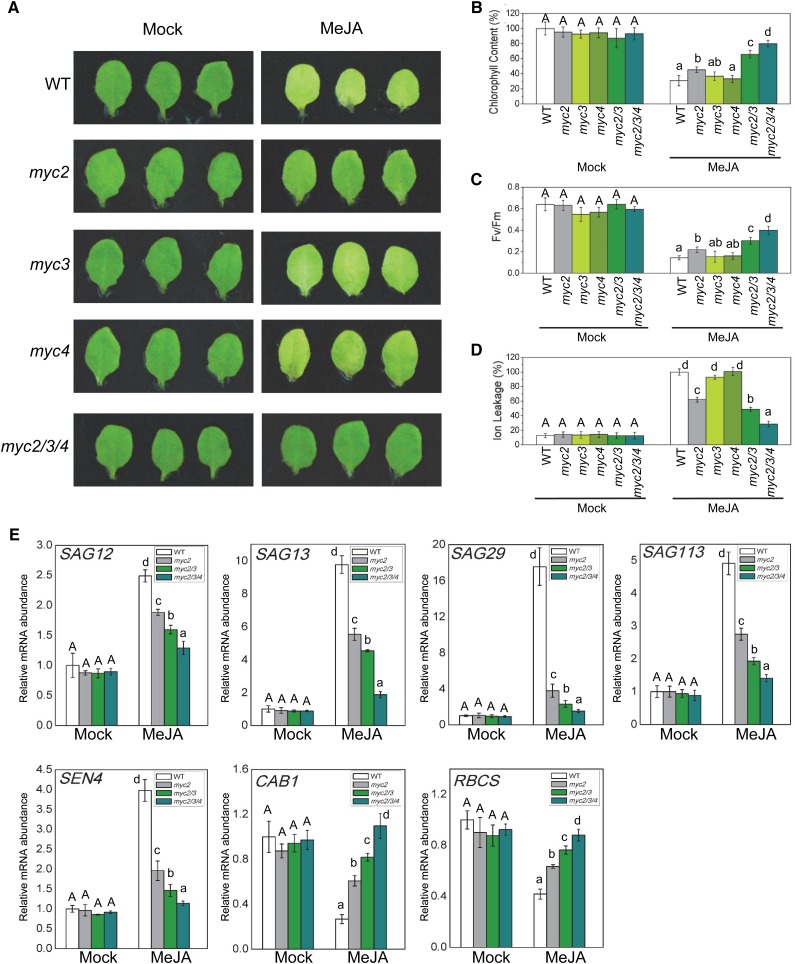
Previous studies showed that the R2R3-MYB transcription factors (MYB21 and MYB24) (Song et al., 2011), the WD-repeat/bHLH/MYB complex (Qi et al., 2011), and the IIIe bHLH factors (MYC2, MYC3, and MYC4) (Cheng et al., 2011; Fernández-Calvo et al., 2011; Niu et al., 2011) act as transcription activators to interact with JAZ proteins and regulate JA responses. Here, we examined whether these transcription activators regulated leaf senescence. As shown in Figure 2 and Supplemental Figure 1, the JA-treated myc2 single mutant exhibited a mild stay-green phenotype (Figure 2A), while mutations in other tested mutants of the transcription activators did not obviously alter JA-induced leaf senescence (Supplemental Figure 1). Compared with the wild type, myc2 contained higher chlorophyll contents, showed higher photochemical efficiency, and exhibited decreased membrane ion leakage in response to JA treatment (Figures 2B to 2D). These results indicate that MYC2 plays a role in activating JA-induced leaf senescence.
Figure 2.
MYC2, MYC3, and MYC4 Function Redundantly to Mediate JA-Induced Leaf Senescence.
(A) The senescence phenotype of leaves detached from 3-week-old Arabidopsis Col-0 (WT), myc2, myc3, myc4, and myc2 myc3 myc4 (myc2/3/4) plants treated without (Mock) or with 100 μM MeJA in the dark for 6 d.
(B) to (D) Chlorophyll content (B), photochemical efficiency (Fv/Fm) of PSII (C), and membrane ion leakage (D) of the detached leaves with the same treatment as shown in (A). The maximum value of the chlorophyll contents in (B) is 2.07 mg/g FW. Data are means (±sd) of three biological replicates. Different letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05). Capital letters compare with each other, and lowercase letters compare with each other.
(E) Quantitative real-time PCR analysis for senescence-associated genes (SAG12, SAG13, SAG29, SAG113, and SEN4) and photosynthetic genes (CAB1 and RBCS) of the leaves that were detached from 3-week-old Arabidopsis Col-0, myc2, myc2 myc3 (myc2/3), and myc2 myc3 myc4 (myc2/3/4) and treated without (Mock) or with 100 μM MeJA in the dark for 6 d. The y axis is the relative mRNA abundance of the detected genes. The mRNA abundance of the detected gene in mock-treated wild type with ACTIN8 as the internal control was set to 1. Data are means (±sd) of three biological replicates. Different letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05). Capital letters compare with each other, and lowercase letters compare with each other.
As MYC2, MYC3, and MYC4 belong to the IIIe subgroup bHLH family and show high similarity at the amino acid level (Heim et al., 2003), we further investigated whether they shared redundancy in activating JA-induced leaf senescence. As shown in Figure 2, compared with the single mutants myc2 and myc3, the JA-treated myc2 myc3 double mutants exhibited higher chlorophyll content and photochemical efficiency, lower membrane ion leakage, and repression of JA-regulated expression of senescence-associated genes (SAG12, SAG13, SAG29, SAG113, and SEN4) and photosynthetic genes (CAB1 and RBCS) (Figures 2B to 2E). Moreover, the JA-treated myc2 myc3 myc4 triple mutant, similar to coi1-1 (Figure 1), abolished JA-induced leaf senescence and exhibited a clear stay-green phenotype, as indicated by the highest chlorophyll content and photochemical efficiency, and the lowest membrane ion leakage (Figures 2B to 2D). Consistent with this, the JA-induced expression of SAG12, SAG13, SAG29, SAG113, and SEN4 and the JA-repressed expression of CAB1 and RBCS were severely abolished in the triple mutant (Figure 2E). Taken together, these results demonstrate that MYC2, MYC3, and MYC4 exhibit redundant function in activation of JA-induced leaf senescence.
MYC2 Binds to and Activates the Promoter of SAG29
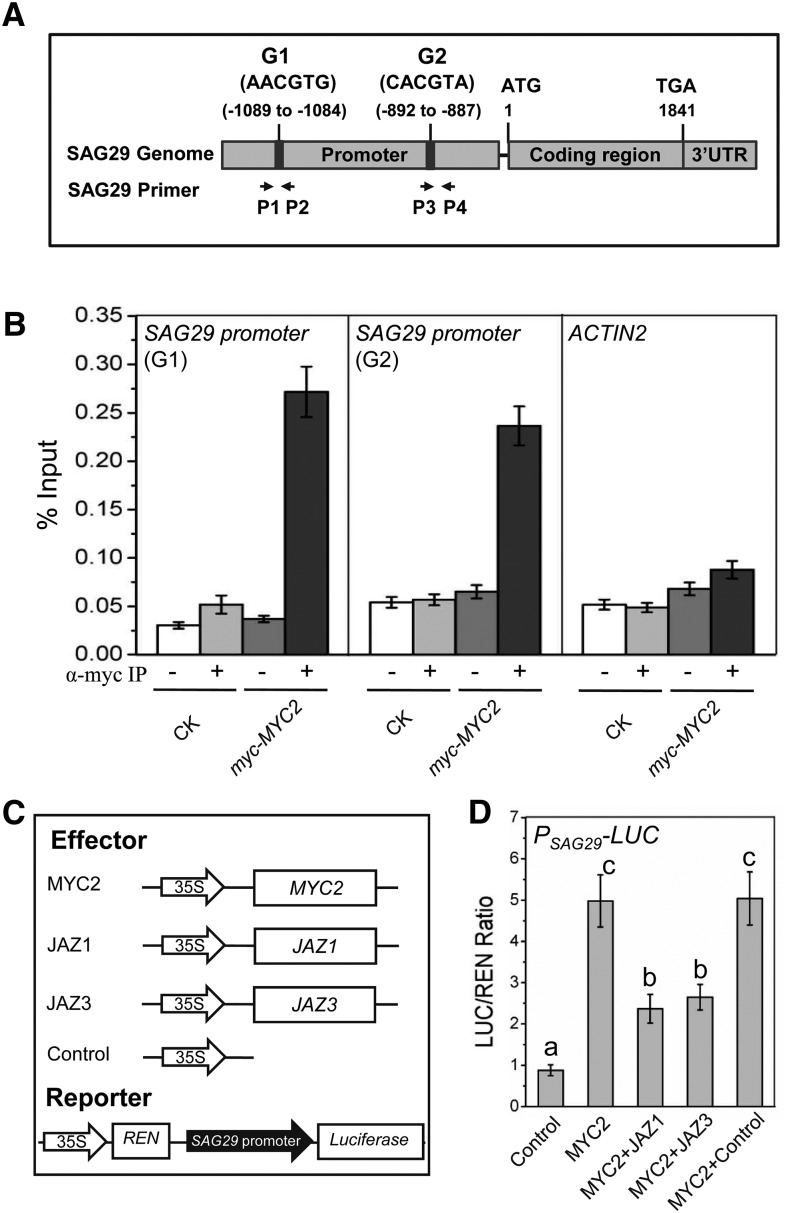
Previous studies demonstrated that MYC2, MYC3, and MYC4 bind to G-box (CACGTG) and G-box-like motifs in the promoter of target genes to activate target gene expression (Lorenzo et al., 2004; Fernández-Calvo et al., 2011). We further investigated whether MYC2 directly binds to promoter of the senescence-induced senescence-associated gene SAG29 and activates the transcription of SAG29 (Quirino et al., 1999), which encodes a member of the SWEET glucoside transporter family and enhances senescence (Seo et al., 2011). Our promoter analysis showed that there are two G-box-like motifs (AACGTG and CACGTA) in the promoter region of SAG29 (Figure 3A). We further used the myc-fused MYC2 (myc-MYC2) transgenic plant for chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR) analysis. As shown in Figure 3B, the two G-box-like motifs were specifically detected in ChIP-qPCR analysis, suggesting that MYC2 directly binds to the promoter of SAG29.
Figure 3.
MYC2 Binds to the Promoter and Activates the Expression of SAG29.
(A) Schematic diagram of the genome structure of the SAG29 gene. G1 and G2 denote G-box-like motif in the promoter of SAG29. Numbers indicate the nucleotide sites relative to the start code (ATG). Arrows indicate the primer pairs (P1/P2 and P3/P4) used to detect the DNA fragments in ChIP-qPCR assays.
(B) ChIP-qPCR analysis of the in vivo binding of myc-MYC2 to the promoter of SAG29. Chromatin from the Col-0 wild-type control (CK) and the myc-MYC2 transgenic plants was immunoprecipitated without (−) or with anti-myc antibody (+). Primer pairs indicated in (A) were used to quantify the enrichment of the promoter fragment containing G-box-like motifs (AACGTG and CACGTA) by MYC2 with quantitative real-time PCR. ACTIN2 was used as a negative control. Data are means (±sd) of three biological replicates
(C) The schematic diagram shows the constructs used in the transient expression assays of (D).
(D) Transient expression assays show that the SAG29 promoter can be activated by MYC2, and the activation of SAG29 promoter by MYC2 is suppressed by JAZ1 or JAZ3. The PSAG29-LUC reporter was cotransformed with the indicated constructs. The LUC/REN ratio represents the PSAG29-LUC activity relative to the internal control (REN activity driven by 35S promoter). Data are means (±sd) of three biological replicates. Lowercase letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05).
To investigate whether MYC2 activates the expression of SAG29, we performed Arabidopsis protoplast transfection-based transient expression assay. The promoter of SAG29 was fused with Luciferase (LUC) to generate the PSAG29-LUC reporter, and MYC2 driven by 35S promoter was used as an effector (Figure 3C). As shown in Figure 3D, coexpression of MYC2 dramatically activated PSAG29-LUC activity, whereas mutations in the two G-box-like motifs severely attenuated the activation of MYC2 on the SAG29 promoter (Supplemental Figure 2), demonstrating that MYC2 binds to the G-box-like motifs of the SAG29 promoter to activate SAG29 expression. Moreover, we also observed that coexpression of JAZ1 and JAZ3, two representative JAZs that interact with and repress MYC2 (Chini et al., 2007; Thines et al., 2007), significantly attenuated the MYC2-activated PSAG29-LUC activity (Figure 3D). These results demonstrate that MYC2 directly binds to the G-box-like motifs of the SAG29 promoter to activate its expression, while JAZ proteins interact with and repress MYC2.
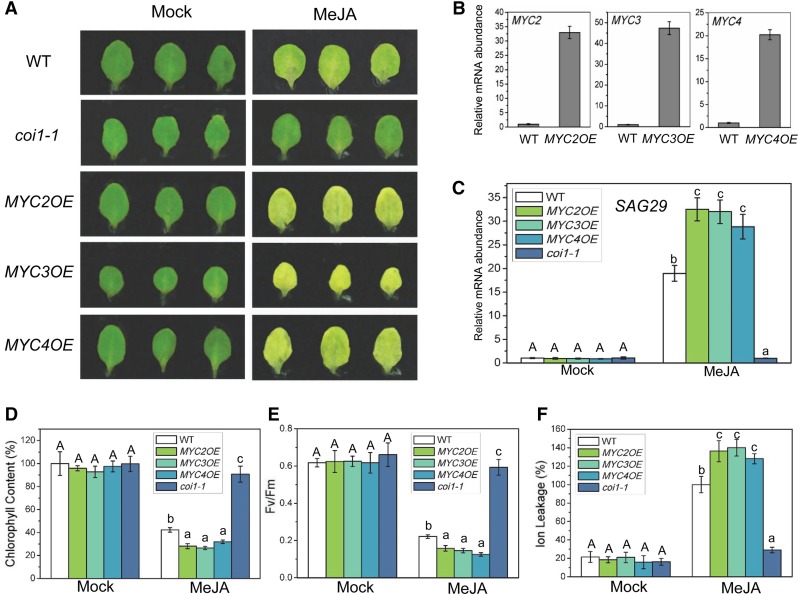
To further examine whether the IIIe bHLH factors MYC2, MYC3, and MYC4 act as activators in planta to activate the target gene SAG29 and promote JA-induced leaf senescence, we generated transgenic plants that overexpress these three IIIe bHLH factors. As shown in Figure 4, the transgenic plants overexpressing MYC2, MYC3, or MYC4 exhibited a precocious leaf senescence phenotype in response to JA treatment. SAG29 was highly expressed in the JA-treated overexpression lines (Figure 4C). Consistent with the enhanced leaf yellowing phenotype, we also observed lower chlorophyll content and photochemical efficiency as well as higher membrane ion leakage in these JA-treated overexpression lines (Figures 4D to 4F). Consistent with the observation of the myc2 myc3 myc4 mutant (Figure 2), analysis of these overexpression lines further demonstrate that MYC2, MYC3, and MYC4 act as activators to enhance the expression of the target gene SAG29 and accelerate JA-induced leaf senescence in planta (Figure 4).
Figure 4.
Overexpression of MYC2, MYC3, and MYC4 Accelerates JA-Induced Leaf Senescence.
(A) The senescence phenotype of leaves that were detached from Arabidopsis Col-0 (WT), coi1-1, and transgenic line with overexpression of MYC2 (MYC2OE), MYC3 (MYC3OE), or MYC4 (MYC4OE) and treated without (Mock) or with 100 μM MeJA in the dark for 5 d.
(B) Quantitative real-time PCR analysis for MYC2, MYC3, or MYC4 in 2-week-old seedlings of wild-type and transgenic plants overexpressing MYC2, MYC3, or MYC4, respectively. The y axis is the relative mRNA abundance of MYC2, MYC3, or MYC4, and the mRNA abundance of MYC2, MYC3, or MYC4 in the wild type with ACTIN8 as the internal control was set to 1. Data are means (±sd) of three biological replicates.
(C) to (F) Quantitative real-time PCR analysis for SAG29 (C), chlorophyll content (D), photochemical efficiency (Fv/Fm) of PSII membrane (E), and ion leakage (F) of the detached leaves with the same treatment as shown in (A). The y axis in (C) is the relative mRNA abundance of SAG29, and the mRNA abundance of SAG29 in mock-treated wild type with ACTIN8 as the internal control was set to 1. The maximum value of the chlorophyll contents in (D) is 2.05 mg/g FW. Data are means (±sd) of three biological replicates. Different letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05). Capital letters compare with each other, and lowercase letters compare with each other.
Taken together (Figures 2 to 4), this genetic, physiological, and molecular evidence suggests that MYC2, MYC3, and MYC4 bind to and activate the promoter of the senescence-associated genes (e.g., SAG29) to enhance JA-induced leaf senescence.
The IIId bHLH Factors bHLH03, bHLH13, bHLH14, and bHLH17 Function Redundantly in Repression of JA-Induced Leaf Senescence
Previous studies have revealed that the IIId bHLH factors (bHLH03, bHLH13, bHLH14, and bHLH17) act as transcription repressors to interact with JAZ proteins and repress JA responses (Nakata et al., 2013; Sasaki-Sekimoto et al., 2013; Song et al., 2013b; Fonseca et al., 2014). We further investigated whether these IIId bHLH factors regulate JA-induced leaf senescence.
As shown in Supplemental Figures 3 and 4, the single mutants of IIId bHLH factor (bhlh03, bhlh13, bhlh14, or bhlh17) showed similar JA-induced senescence phenotypes to the wild type, as indicated by leaf yellowing, chlorophyll content, photochemical efficiency, membrane ion leakage, and the JA-regulated expression of senescence-associated genes (SAG12, SAG13, SAG29, SAG113, and SEN4) and photosynthetic genes (CAB1 and RBCS).
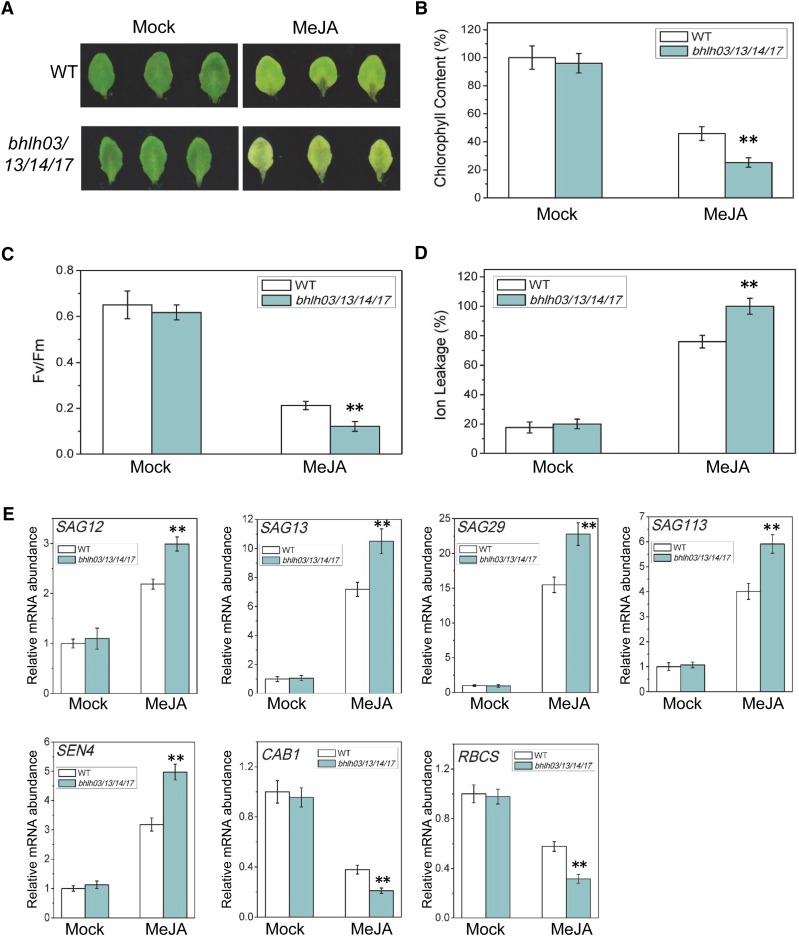
However, the JA-induced leaf senescence was clearly enhanced in the bhlh03 bhlh13 bhlh14 bhlh17 quadruple mutant (Figure 5A). The JA-treated quadruple mutant contained the reduced chlorophyll content and photochemical efficiency and the increased membrane ion leakage (Figures 5B to 5D). Furthermore, the expression of senescence-associated genes (SAG12, SAG13, SAG29, SAG113, and SEN4) was increased, while the expression of photosynthetic genes (CAB1 and RBCS) was decreased in the JA-treated quadruple mutant compared with that in the wild type (Figure 5E). Taken together (Figure 5; Supplemental Figures 3 and 4), our results demonstrate that the IIId bHLH factors bHLH03, bHLH13, bHLH14, and bHLH17 exhibit redundant functions in repression of JA-induced leaf senescence.
Figure 5.
Loss of Function of the IIId bHLH Factors Accelerates JA-Induced Leaf Senescence.
(A) The senescence phenotype of leaves that were detached from 3-week-old Arabidopsis Col-0 (WT) and the bhlh03 bhlh13 bhlh14 bhlh17 (bhlh03/13/14/17) quadruple mutant of the IIId bHLH factors (bHLH03, bHLH13, bHLH14, and bHLH17) and treated without (Mock) or with 100 μM MeJA in the dark for 5 d.
(B) to (E) Chlorophyll content (B), photochemical efficiency (Fv/Fm) of PSII (C), membrane ion leakage (D), and quantitative real-time PCR analysis for senescence-associated genes (SAG12, SAG13, SAG29, SAG113, and SEN4) and photosynthetic genes (CAB1 and RBCS) (E) of the detached leaves with the same treatment as shown in (A). The maximum value of the chlorophyll contents in (B) is 2.10 mg/g FW. The y axis in (E) is the relative mRNA abundance of the detected genes, and the mRNA abundance of the detected gene in mock-treated wild type with ACTIN8 as the internal control was set to 1. Data are means (±sd) of three biological replicates. Asterisks represent Student’s t test significance between wild-type and bhlh03 bhlh13 bhlh14 bhlh17 samples (**P < 0.01).
bHLH03 Binds to the Promoter of SAG29 to Repress the MYC2-Activated SAG29 Expression
We previously showed that the IIId bHLH factors (bHLH03, bHLH13, bHLH14, and bHLH17) act as transcription repressors to antagonize transcription activator MYC2 in regulating JA responses via competitive interaction with the mutual target genes, including TYROSINE AMINOTRANSFERASE and TYROSINE AMINOTRANSFERASE1 (Song et al., 2013b). Here, we investigated whether the IIId bHLH factors antagonize MYC2/MYC3/MYC4 through binding to the promoter of SAG29.
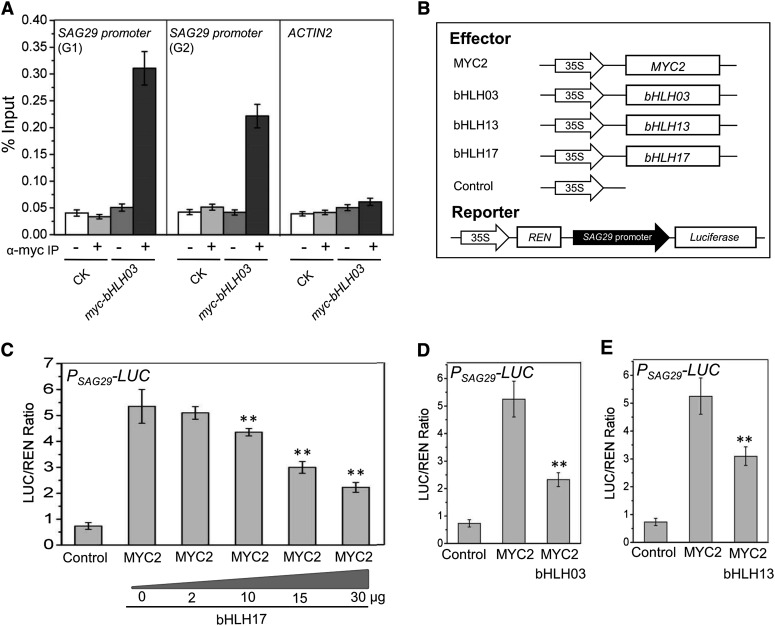
We used the myc-fused bHLH03 transgenic plants to perform ChIP-qPCR analysis. The two G-box-like motifs in the promoter of SAG29 were specifically detected in the ChIP-qPCR assay (Figure 6A), suggesting that bHLH03 binds to the promoter of SAG29. Moreover, our Arabidopsis protoplast transient expression assays showed that MYC2 dramatically activated the LUC activity driven by the SAG29 promoter (PSAG29-LUC); however, bHLH17 obviously suppressed the MYC2-activated PSAG29-LUC activity in a dosage-dependent manner (Figures 6B and 6C), and bHLH03 or bHLH13 also attenuated the MYC2-activated PSAG29-LUC activity (Figures 6B, 6D, and 6E), demonstrating that the IIId bHLH factors bind to the promoter of SAG29 to repress the MYC2-activated SAG29 expression. Taken together (Figures 5 and 6), our results suggest that the IIId bHLH factors function as transcription repressors to antagonize MYC2, MYC3, and MYC4 via binding to the promoter of their mutual target gene SAG29.
Figure 6.
The IIId bHLH Factors Bind to the SAG29 Promoter to Antagonize Its Activation by MYC2.
(A) ChIP-PCR assay of the in vivo binding of myc-bHLH03 to the promoter of SAG29. Chromatin from the Col-0 wild-type control (CK) and myc-bHLH03 transgenic plant was immunoprecipitated without (−) or with anti-myc antibody (+). Primers indicated in Figure 3A were used to quantify the enrichment of the promoter fragments (G1 and G2 as shown in Figure 6A) by bHLH03 with quantitative real-time PCR. ACTIN2 was used as a negative control. Data are means (±sd) of three biological replicates.
(B) Schematic diagram shows the constructs used in (C) to (E).
(C) Transient transcriptional activity assays show that activation of PSAG29-LUC activity by MYC2 is repressed by coexpression of bHLH17 in a dosage-dependent manner. The 0, 2, 10, 15, or 30 μg of bHLH17 plasmid DNA were used for cotransformation with MYC2. Data are means (±sd) of three biological replicates. Asterisks represent Student’s t test significance between MYC2 and MYC2 plus bHLH17 (**P < 0.01).
(D) and (E) Transient transcriptional activity assays show that activation of PSAG29-LUC activity by MYC2 is repressed by coexpression of bHLH03 (D) or bHLH13 (E). Data are means (±sd) of three biological replicates. Asterisks represent Student’s t test significance between MYC2 and MYC2 plus bHLH03 (D) or bHLH13 (E) (**P < 0.01).
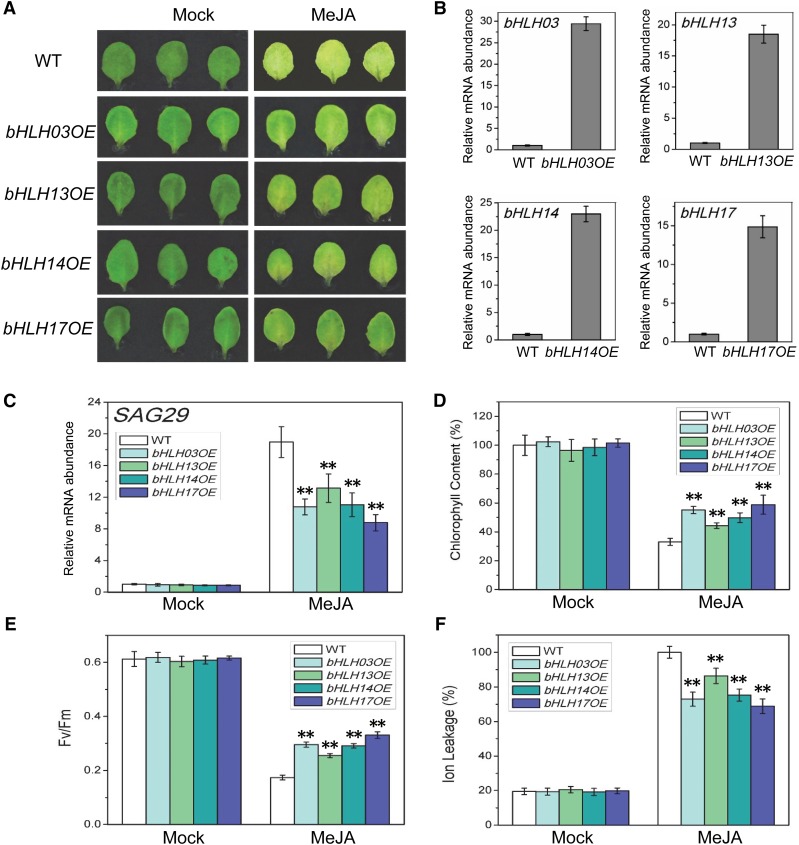
To further examine whether the IIId bHLH factors bHLH03, bHLH13, bHLH14, and bHLH17 act as repressors in planta to inhibit SAG29 expression and suppress JA-induced leaf senescence, we generated transgenic plants overexpressing the IIId bHLH factors. As shown in Figure 7, the transgenic plants with overexpression of bHLH03, bHLH13, bHLH14, or bHLH17 exhibited a reduced leaf senescence phenotype in response to JA treatment. SAG29 expression was obviously repressed in the JA-treated transgenic plants (Figure 7C). Consistent with the stay-green leaf phenotype, we also observed higher chlorophyll contents and photochemical efficiency and lower membrane ion leakage in these JA-treated transgenic plants (Figures 7D to 7F). In support of the observation with the bhlh03 bhlh13 bhlh14 bhlh17 mutant (Figure 5), analysis of these overexpression lines further demonstrated that bHLH03, bHLH13, bHLH14, and bHLH17 act as repressors to inhibit the expression of the target gene SAG29 and attenuate JA-induced leaf senescence in planta.
Figure 7.
Overexpression of the IIId bHLH Transcription Repressors Attenuates JA-Induced Leaf Senescence.
(A) The senescence phenotype of leaves that were detached from Arabidopsis Col-0 (WT), transgenic plant overexpressing the IIId bHLH transcription repressors bHLH03 (bHLH03OE), bHLH13 (bHLH13OE), bHLH14 (bHLH14OE), or bHLH17 (bHLH17OE) and treated without (Mock) or with 100 μM MeJA in the dark for 6 d.
(B) Quantitative real-time PCR analysis for bHLH03, bHLH13, bHLH14, or bHLH17 in 2-week-old seedlings of wild-type and transgenic plants overexpressing bHLH03, bHLH13, bHLH14, or bHLH17, respectively. The y axis is the relative mRNA abundance of bHLH03, bHLH13, bHLH14, or bHLH17, and the mRNA abundance of bHLH03, bHLH13, bHLH14, or bHLH17 in wild-type with ACTIN8 as the internal control was set to 1. Data are means (±sd) of three biological replicates.
(C) to (F) Quantitative real-time PCR analysis for SAG29 (C), chlorophyll content (D), photochemical efficiency (Fv/Fm) of PSII (E), and membrane ion leakage (F) of the detached leaves with the same treatment as shown in (A). The y axis in (C) is the relative mRNA abundance of SAG29, and the mRNA abundance of SAG29 in mock-treated wild type with ACTIN8 as the internal control was set to 1. The maximum value of the chlorophyll contents in (D) is 2.05 mg/g FW. Data are means (±sd) of three biological replicates. Asterisks represent Student’s t test significance between wild-type and bHLH03OE, bHLH13OE, bHLH14OE, or bHLH17OE samples (**P < 0.01).
The IIId bHLH Factors Repress Leaf Senescence via Attenuation of MYC2/MYC3/MYC4-Activated Leaf Senescence
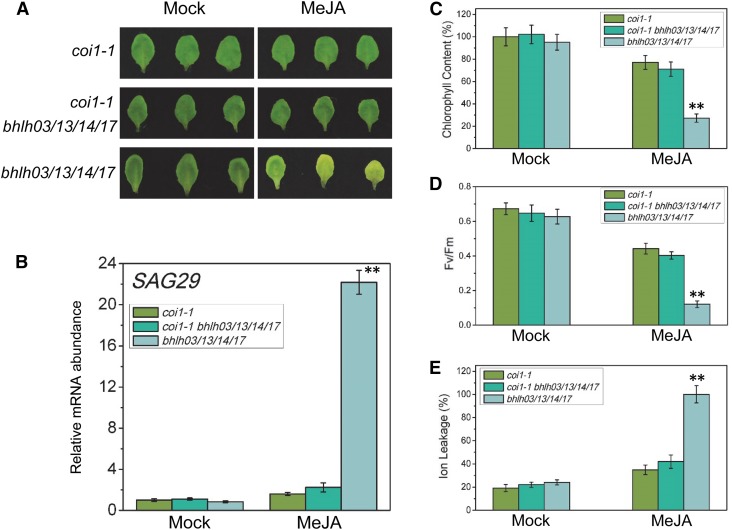
Our in vitro assays demonstrate that the IIId bHLH factors (bHLH03, bHLH13, bHLH14, and bHLH17) repress JA-induced leaf senescence via repression of the MYC2 activation on their mutual target genes, such as SAG29 (Figure 6); therefore, mutations of the IIId bHLH factors would prevent their repression of MYC2-activated SAG29 expression to enhance JA-induced leaf senescence in the bhlh03 bhlh13 bhlh14 bhlh17 quadruple mutant (Figure 5). Our in vitro data indicate that, without the transcription activator MYC2 (and MYC3 as well as MYC4), mutations of the transcription repressors (bHLH03, bHLH13, bHLH14, and bHLH17) would not enhance the MYC2-activated SAG29 expression or the JA-induced leaf senescence. To verify this speculation, we generated the coi1-1 bhlh03 bhlh13 bhlh14 bhlh17 quintuple mutant and the myc2 myc3 myc4 bhlh03 bhlh13 bhlh14 bhlh17 septuple mutant to examine whether mutations of the IIId bHLH factors could enhance the JA-induced leaf senescence in the mutant background of coi1-1 or myc2 myc3 myc4.
As expected, the bhlh03 bhlh13 bhlh14 bhlh17 mutant exhibited an obvious leaf yellowing phenotype (Figures 5A, 8A, and 9A). The coi1-1 mutant in which JAZ proteins accumulate to high levels to repress MYC2, MYC3, and MYC4 (Chini et al., 2007; Thines et al., 2007; Fernández-Calvo et al., 2011; Niu et al., 2011) and the myc2 myc3 myc4 mutant disrupted JA-induced expression of their target genes (such as SAG29), abolished JA-induced leaf senescence, and showed a stay-green phenotype under JA treatment (Figures 1A, 2A, 8A, and 9A).
Figure 8.
Mutation of the IIId bHLH Factors Cannot Restore the Leaf Senescence Phenotype in coi1-1.
(A) The senescence phenotype of leaves detached from Arabidopsis coi1-1, the bhlh03 bhlh13 bhlh14 bhlh17 (bhlh03/13/14/17) quadruple mutant of the IIId bHLH factors in the wild type, and the coi1-1 bhlh03 bhlh13 bhlh14 bhlh17 (coi1-1 bhlh03/13/14/17) quintuple mutant and treated without (Mock) or with 100 μM MeJA in the dark for 5 d.
(B) to (E) Quantitative real-time PCR analysis for SAG29 (B), chlorophyll content (C), photochemical efficiency (Fv/Fm) of PSII (D), and membrane ion leakage (E) of the detached leaves with the same treatment as shown in (A). The y axis in (B) is the relative mRNA abundance of SAG29, and the mRNA abundance of SAG29 in mock-treated coi1-1 with ACTIN8 as the internal control was set to 1. The maximum value of the chlorophyll contents in (C) is 2.10 mg/g FW. Data are means (±sd) of three biological replicates. Asterisks represent Student’s t test significance compared with coi1-1 mutant (**P < 0.01).
Figure 9.
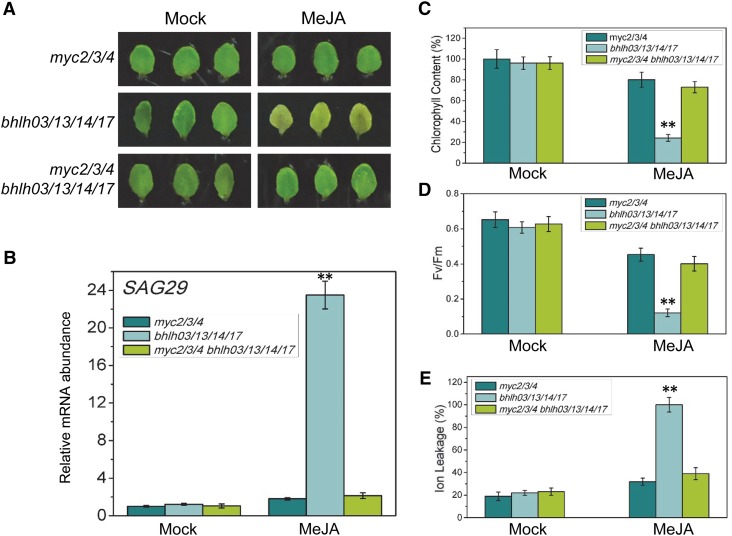
Loss of the IIId bHLH Factors Is Unable to Accelerate the Leaf Senescence of the myc2 myc3 myc4 Triple Mutants.
(A) The senescence phenotype of leaves detached from the Arabidopsis myc2 myc3 myc4 triple mutant (myc2/3/4), the bhlh03 bhlh13 bhlh14 bhlh17 (bhlh03/13/14/17) quadruple mutant of the IIId bHLH factors, and the myc2 myc3 myc4 bhlh03 bhlh13 bhlh14 bhlh17 (myc2/3/4 bhlh03/13/14/17) septuple mutant and treated without (Mock) or with 100 μM MeJA in the dark for 5 d.
(B) to (E) Quantitative real-time PCR analysis for SAG29 with ACTIN8 as the internal control (B), chlorophyll content (C), photochemical efficiency (Fv/Fm) of PSII (D), and membrane ion leakage (E) of the detached leaves with the same treatment as shown in (A). The y axis is (B) is the relative mRNA abundance of SAG29, and the mRNA abundance of SAG29 in mock-treated myc2 myc3 myc4 with ACTIN8 as the internal control was set to 1. The maximum value of the chlorophyll contents in (C) is 2.12 mg/g FW. Data are means (±sd) of three biological replicates. Asterisks represent Student’s t test significance compared with the myc2 myc3 myc4 triple mutant (**P < 0.01).
The coi1-1 bhlh03 bhlh13 bhlh14 bhlh17 and myc2 myc3 myc4 bhlh03 bhlh13 bhlh14 bhlh17 exhibited similar stay-green phenotype with coi1-1and myc2 myc3 myc4 (Figures 8A and 9A). Measurement of chlorophyll content, photochemical efficiency, and membrane ion leakage and analysis of senescence-associated genes and photosynthetic genes demonstrate that mutations of the IIId bHLH factors are unable to enhance JA-induced leaf senescence in mutant background of coi1-1 and myc2 myc3 myc4 (Figures 8B to 8E and 9B to 9E; Supplemental Figures 5 and 6).
Together with the in vitro data (Figures 8 and 9), these results reveal that the IIId bHLH factors repress leaf senescence via attenuation of the MYC2/MYC3/MYC4-activated leaf senescence.
DISCUSSION
The IIIe bHLH factors MYC2, MYC3, and MYC4 are direct targets of JAZ repressors (Cheng et al., 2011; Fernández-Calvo et al., 2011; Niu et al., 2011) and function redundantly to regulate JA-inhibitory root growth (Cheng et al., 2011; Fernández-Calvo et al., 2011; Niu et al., 2011), hook formation (Song et al., 2014b; Zhang et al., 2014), JA-induced glucosinolate biosynthesis (Schweizer et al., 2013), defense against insect attack (Fernández-Calvo et al., 2011), and resistance to pathogens (Fernández-Calvo et al., 2011). In this study, we reveal a function for MYC2, MYC3, and MYC4 in regulation of senescence. We showed that the IIIe bHLH factors MYC2, MYC3, and MYC4 function redundantly to activate JA-induced leaf senescence. Interestingly, we observed that plants have evolved an elaborate feedback regulation mechanism on JA-induced leaf senescence: JA simultaneously derepresses the JAZ-repressed IIId bHLH factors (bHLH03, bHLH13, bHLH14, and bHLH17), which act as repressors to suppress the MYC2/MYC3/MYC4-activated JA-induced leaf senescence. The antagonistic regulation by the transcription activators and the transcription repressors determines the senescence output.
JA induced expression of SAG29 in a MYC2/MYC3/MYC4-dependent manner (Figure 2E). Similarly, JA-induced expression of many other senescence-associated genes, such as SAG12, SAG13, SAG113, and SEN4 (Figures 1E and 2E) (Park et al., 1998; Weaver et al., 1998; Shan et al., 2011). SAG29 acts as a direct target of MYC2/MYC3/MYC4 (Figures 2 and 3), but it is unknown whether other senescence-associated genes also serve as the target genes. Although potential MYC2-targeted G-box (or G-box-like) motifs exist in the promoters of these genes, further experiment is required to examine whether these genes act as direct targets of MYC2/MYC3/MYC4. In addition to these JA-induced genes, JA represses expression of many photosynthetic genes, such as CAB1, RBCS (Figure 1E), and RCA (Shan et al., 2011), and the mechanism for JA repression of these genes remains to be elucidated.
Many plant hormones act as endogenous signals to regulate senescence. JA, salicylic acid (SA), gibberellin (GA), ethylene (ET), and abscisic acid (ABA) promote senescence (Pourtau et al., 2004; Li et al., 2013; Zhang et al., 2013; Chen et al., 2014; Yang et al., 2014), while auxin delays senescence (Kim et al., 2011). Current studies showed that JA interacts with SA and auxin in regulating senescence: SA antagonizes JA to induce the expression of WRKY70/WRKY53, which promote/repress natural senescence (Li et al., 2004; Miao and Zentgraf, 2007; Ulker et al., 2007; Xie et al., 2014). Auxin inhibits JA-induced leaf senescence via derepression of the IAA29-repressed WRKY57, which attenuates leaf senescence (Jiang et al., 2014). Previous studies revealed that crosstalk of JA with GA, ET, and ABA mediates various plant developmental processes and defense responses: JA and ET antagonistically regulate apical hook curvature (Song et al., 2014b; Zhang et al., 2014) but collaboratively control resistance to necrotrophic pathogens (Zhu et al., 2011). JA and GA antagonistically regulate growth and defense (Hou et al., 2010; Yang et al., 2012), while coordinately controlling stamen and trichome development (Cheng et al., 2009; Qi et al., 2014). JA and ABA additively induce anthocyanin accumulation (Loreti et al., 2008) but antagonistically regulate defense (Melotto et al., 2006). However, the crosstalk of JA with ET, GA, or ABA in leaf senescence remains unclear.
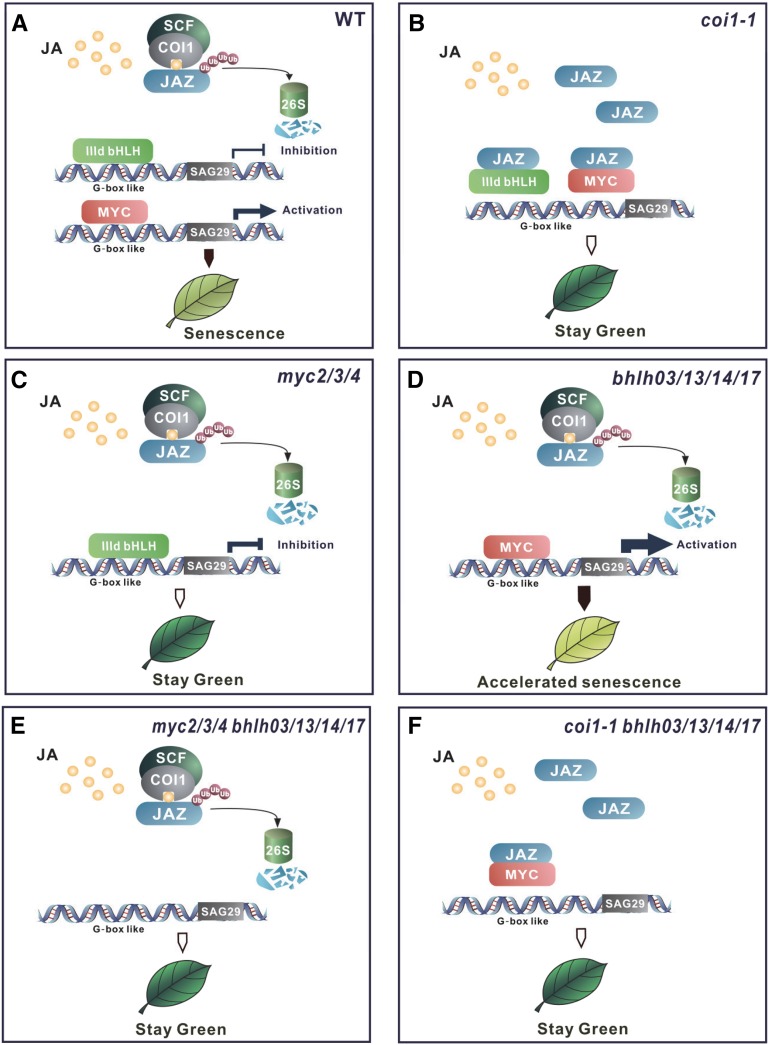
Plants face dynamic environments during their lifetimes. JA is known as a vital hormone in optimizing resource distribution and adjusting plant responses upon internal signals or external cues (Hou et al., 2010; Kazan and Manners, 2012; Wild et al., 2012; Yang et al., 2012). After being triggered by environmental stresses or developmental signals, plants biosynthesize JA, which stimulates JA signal transduction to induce leaf senescence: JA induces degradation of JAZ proteins via the COI1-26S proteasome pathway, thereby releasing MYCs (MYC2, MYC3, and MYC4), which bind to and activate the promoters of senescence-associated genes (such as SAG29) to promote leaf senescence (Figure 10). Meanwhile, the IIId bHLH transcription repressors (bHLH03, bHLH13, bHLH14, and bHLH17) are released to repress senescence-associated genes and antagonize the MYC-activated JA-induced leaf senescence (Figure 10). The MYCs and the IIId bHLH factors also antagonistically regulate root growth, anthocyanin accumulation, and defense against insects (Fernández-Calvo et al., 2011; Schweizer et al., 2013; Song et al., 2013b). The antagonistic function between activators and repressors determines the outputs of growth, secondary metabolism, defense, and senescence, and dynamically modulate progression for plant survival according to the changing environment.
Figure 10.
A Model for JA-Induced Leaf Senescence.
JAZ proteins interact with and repress the bHLH transcription activators MYC2, MYC3, and MYC4 (indicated as MYC) and the IIId bHLH transcription repressors bHLH03, bHLH13, bHLH14, and bHLH17 (indicated as IIId bHLH). COI1 perceives JA and recruits JAZ proteins for degradation via 26S proteasome to release MYC and IIId bHLH, which bind to the promoter of senescence-associated genes (such as SAG29). MYC activates SAG29 expression to promote leaf senescence, while IIId bHLH represses MYC2-activated SAG29 expression to attenuate JA-induced leaf senescence. In JA-treated wild-type plant (A), the balance between the MYC activation function and the IIId bHLH repression function determines the senescence outcome as the leaf yellowing phenotype. When MYC is fully inhibited by JAZ proteins in coi1-1 (B) and coi1 bhlh03 bhlh13 bhlh14 bhlh17 (coi1-1bhlh03/13/14/17) (E) or when MYCs are mutated in myc2 myc3 myc4 (myc2/3/4) (C) and myc2 myc3 myc4 bhlh03 bhlh13 bhlh14 bhlh17 (myc2/3/4bhlh03/13/14/17) (F), JA failed to induce the MYC-activated SAG29 expression, leading to stay-green phenotype. In the JA-treated bhlh03 bhlh13 bhlh14 bhlh17 (bhlh03/13/14/17) quadruple mutant (D), the IIId bHLH repression on MYCs is lost, which enhances MYC-activated SAG29 expression, leading to the accelerated leaf senescence.
METHODS
Plant Materials and Growth Conditions
The Arabidopsis thaliana Columbia-0 (Col-0) wild type and mutants coi1-1 (Xie et al., 1998), JAZ1Δ3A (Thines et al., 2007), myc2-2 (Boter et al., 2004), myc3 (Fernández-Calvo et al., 2011), myc4 (Fernández-Calvo et al., 2011), myb21 myb24 (Cheng et al., 2009), gl3 egl3 tt8 (Qi et al., 2011), bhlh03, bhlh13, bhlh14, bhlh17, and bhlh3 bhlh13 bhlh14 bhlh17 (Song et al., 2013b), and myc-bHLH3 (Song et al., 2013b) were described previously. The higher order mutants myc2-2 myc3, myc2-2 myc3 myc4, coi1-1 bhlh3 bhlh13 bhlh14 bhlh17, and bhlh3 bhlh13 bhlh14 bhlh17 myc2 myc3 myc4 (bhlh03/13/14/17 myc2/3/4) were generated through genetic crosses. The Arabidopsis seeds were sterilized with 20% bleach for 10 min, plated on Murashige and Skoog medium (Sigma-Aldrich), stored at 4°C in the dark for 3 d, and transferred to a growth room with a light/dark photoperiod of 16 h (20 to 24°C)/8 h (16 to 19°C).
Leaf Senescence Assay and Measurements of Chlorophyll Content, Membrane Ion Leakage, and Photochemical Efficiency
The third and fourth rosette leaves from 3-week-old plants were floated on 3 mL of distilled water supplied with mock or 100 μM methyl-jasmonate (MeJA) and kept in the dark at 22°C for 5 or 6 d. For chlorophyll extraction, the leaves after treatment were incubated in 80% acetone (v/v) in the dark. Absorbance was measured at 647 and 664 nm, and the chlorophyll contents were calculated as previously described (Seltmann and Berger, 2013) and expressed as a percentage of the chlorophyll content of Col-0 wild type treated with mock.
For measurement of membrane ion leakage, about six leaves for each treatment were incubated in deionized water with gentle shaking overnight, and the conductivity of the incubated solution was measured before (C1) and after (C2) boiling for 10 min with an electroconductivity meter. The membrane ion leakage rate is presented as the ratio of C1:C2. For the photosynthetic efficiency, the leaves were incubated in the dark for 15 min and the Fv/Fm of each leaf was measured with a pulse amplitude modulation fluorometer.
Protoplast Transient Expression Assay
For transient expression assay, an ∼1629-bp SAG29 promoter was inserted into the pGreenII 0800-LUC vector to generate the PSAG29-LUC reporter construct (Hellens et al., 2005). The coding sequences of MYC2, JAZ1, JAZ3, bHLH03, bHLH13, and bHLH17 were inserted into pGreenII 62-SK vector under the control of the 35S promoter. For the mutation analysis of the SAG29 promoter, two G-box-like motifs (AACGTG and CACGTA) in the SAG29 promoter were simultaneously mutated to AAAAAA for generation of SAG29 promoter variants (SAG29proG1m, SAG29proG2m, and SAG29proG1G2m) as shown in Supplemental Figure 2. The variants of the SAG29 promoter with mutation in G-box-like motifs were also respectively inserted into the pGreenII 0800-LUC vector to generate the reporter constructs. All primers used for these constructs are listed in Supplemental Table 1. After protoplast preparation and subsequent transfection (Yoo et al., 2007), firefly LUC and renilla luciferase (REN) activities were measured using the Dual-Luciferase Reporter Assay System (Promega), and the LUC/REN ratio was presented.
Quantitative Real-Time PCR
For Figures 1E, 2E, 4C, 5E, 7C, 8B, and 9B and Supplemental Figures 4 to 6, leaves from 3-weeks-old plants were detached, treated with mock or 100 μM MeJA under dark for 5 or 6 d (as indicated in the figure legends), and collected for RNA expression and subsequent reverse transcription. For Figures 4B and 7B, 2-week-old seedlings were collected for RNA extraction and subsequent reverse transcription. Real-time PCR analysis was performed with the RealMasterMix (SYBR Green I) (Takara) using the ABi7500 quantitative real-time PCR system as described previously (Qi et al., 2014). ACTIN8 was used as the internal control. The primers for real-time PCR analysis are presented in Supplemental Table 2.
ChIP Assays
For the ChIP assay, 2-week-old seedlings of myc-bHLH03 (Song et al., 2013b), myc-MYC2, and Col-0 seedlings were used as materials. The ChIP experiment was performed as described previously (Saleh et al., 2008) with anti-MYC antibody and protein G agarose beads (Abmart). The enrichment of specific motifs in the promoter was measured by the %input method with quantitative real-time PCR analysis (Yamaguchi et al., 2014). The promoter of ACTIN2 was used as a negative control. Primers for the ChIP assays are listed in Supplemental Table 2.
Generation of Transgenic Plants
To generate Arabidopsis transgenic plants overexpressing MYC2, MYC3, MYC4, bHLH03, bHLH13, bHLH14, and bHLH17, the coding sequences of MYC3, MYC4, bHLH03, bHLH13, bHLH14, and bHLH17 were amplified and cloned into pCAMBIA1300 vector under control of 35S promoter, and Agrobacterium tumefaciens containing the pCAMBIA1300 vectors with MYC3, MYC4, bHLH03, bHLH13, or bHLH17 under the control of the 35S promoter or myc-MYC2 driven by 35S promoter (Zhai et al., 2013) was introduced into Arabidopsis Col-0 using the Agrobacterium-mediated floral dip method.
Accession Numbers
The Arabidopsis Genome Initiative numbers for the genes mentioned in this article are as follows: MYC2 (AT1G32640), MYC3 (AT5G46760), MYC4 (AT4G17880), COI1 (AT2G39940), JAZ1 (AT1G19180), JAZ3 (AT3G17860), bHLH03 (AT4G16430), bHLH13 (AT1G01260), bHLH14 (AT4G00870), bHLH17 (AT2G46510), MYB21 (AT3G27810), MYB24 (AT5G40350), TT8 (AT4G09820), EGL3 (AT1G63650), GL3 (AT5G41315), SAG29 (AT5G13170), SAG12 (AT5G45890), SAG13 (AT2G29350), SAG113 (AT5G59220), SEN4 (AT4G30270), CAB1 (AT1G29930), RBCS (AT1G67090), ACTIN8(AT1G49240), and ACTIN2 (AT3G18780).
Supplemental Data
Supplemental Figure 1. TT8, GL3, EGL3, MYB21, and MYB24 are not involved in JA-induced leaf senescence.
Supplemental Figure 2. MYC2 activates the SAG29 promoter via G-box like motifs in Arabidopsis protoplast transient expression assays.
Supplemental Figure 3. JA-induced leaf senescence is not obviously altered in the bhlh03, bhlh13, bhlh14, and bhlh17 single mutants.
Supplemental Figure 4. Mutation of single IIId bHLHs does not alter expression of senescence-related genes.
Supplemental Figure 5. Mutation of the IIId bHLHs could not recover the expression of senescence-related genes in coi1-1.
Supplemental Figure 6. Loss of the IIId bHLH factors is unable to accelerate the JA-regulated expression of senescence-related genes in the myc2 myc3 myc4 triple mutants.
Supplemental Table 1. Primers used for vector construction.
Supplemental Table 2. Primers used for quantitative real-time PCR analysis.
Supplementary Material
Acknowledgments
We thank John Browse, Roberto Solano, Hongwei Guo, and Chuanyou Li for materials, Shuang Han, Lanxiang Wang, and Chengyuan Xie for technical support. This work was supported by the National Natural Science Foundation of China (31230008, 91417302, 31421001, and 31400254) and the Ministry of Science and Technology (973 Program 2011CB915404).
AUTHOR CONTRIBUTIONS
T.Q., S.S., and D.X. designed the research. T.Q., J.W, H.H., B.L., H.G., and S.S. performed research. T.Q., J.W., Y.L., S.S., and D.X. analyzed data. T.Q., S.S., and D.X. wrote the article.
Glossary
- JA
jasmonate
- PSII
photosystem II
- ChIP-qPCR
chromatin immunoprecipitation-quantitative PCR
- SA
salicylic acid
- GA
gibberellin
- ET
ethylene
- ABA
abscisic acid
- Col-0
Columbia-0
- MeJA
methyl-jasmonate
- FW
fresh weight
References
- Boter M., Ruíz-Rivero O., Abdeen A., Prat S. (2004). Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes Dev. 18: 1577–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J. (2009). Jasmonate passes muster: a receptor and targets for the defense hormone. Annu. Rev. Plant Biol. 60: 183–205. [DOI] [PubMed] [Google Scholar]
- Chen M., Maodzeka A., Zhou L., Ali E., Wang Z., Jiang L. (2014). Removal of DELLA repression promotes leaf senescence in Arabidopsis. Plant Sci. 219-220: 26–34. [DOI] [PubMed] [Google Scholar]
- Cheng H., Song S., Xiao L., Soo H.M., Cheng Z., Xie D., Peng J. (2009). Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis. PLoS Genet. 5: e1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z., Sun L., Qi T., Zhang B., Peng W., Liu Y., Xie D. (2011). The bHLH transcription factor MYC3 interacts with the Jasmonate ZIM-domain proteins to mediate jasmonate response in Arabidopsis. Mol. Plant 4: 279–288. [DOI] [PubMed] [Google Scholar]
- Chini A., Fonseca S., Fernández G., Adie B., Chico J.M., Lorenzo O., García-Casado G., López-Vidriero I., Lozano F.M., Ponce M.R., Micol J.L., Solano R. (2007). The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671. [DOI] [PubMed] [Google Scholar]
- Chung H.S., Howe G.A. (2009). A critical role for the TIFY motif in repression of jasmonate signaling by a stabilized splice variant of the JASMONATE ZIM-domain protein JAZ10 in Arabidopsis. Plant Cell 21: 131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geyter N., Gholami A., Goormachtig S., Goossens A. (2012). Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci. 17: 349–359. [DOI] [PubMed] [Google Scholar]
- Fernández-Calvo P., et al. (2011). The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 23: 701–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca S., Fernández-Calvo P., Fernández G.M., Díez-Díaz M., Gimenez-Ibanez S., López-Vidriero I., Godoy M., Fernández-Barbero G., Van Leene J., De Jaeger G., Franco-Zorrilla J.M., Solano R. (2014). bHLH003, bHLH013 and bHLH017 are new targets of JAZ repressors negatively regulating JA responses. PLoS ONE 9: e86182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan S., Amasino R.M. (1995). Inhibition of leaf senescence by autoregulated production of cytokinin. Science 270: 1986–1988. [DOI] [PubMed] [Google Scholar]
- He Y., Fukushige H., Hildebrand D.F., Gan S. (2002). Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 128: 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim M.A., Jakoby M., Werber M., Martin C., Weisshaar B., Bailey P.C. (2003). The basic helix-loop-helix transcription factor family in plants: a genome-wide study of protein structure and functional diversity. Mol. Biol. Evol. 20: 735–747. [DOI] [PubMed] [Google Scholar]
- Hellens R.P., Allan A.C., Friel E.N., Bolitho K., Grafton K., Templeton M.D., Karunairetnam S., Gleave A.P., Laing W.A. (2005). Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortensteiner, S. (2006). Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 57: 55–77. [DOI] [PubMed] [Google Scholar]
- Hou X., Lee L.Y., Xia K., Yan Y., Yu H. (2010). DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev. Cell 19: 884–894. [DOI] [PubMed] [Google Scholar]
- Hu P., Zhou W., Cheng Z., Fan M., Wang L., Xie D. (2013). JAV1 controls jasmonate-regulated plant defense. Mol. Cell 50: 504–515. [DOI] [PubMed] [Google Scholar]
- Huang H., Wang C., Tian H., Sun Y., Xie D., Song S. (2014). Amino acid substitutions of GLY98, LEU245 and GLU543 in COI1 distinctively affect jasmonate-regulated male fertility in Arabidopsis. Sci China Life Sci 57: 145–154. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Liang G., Yang S., Yu D. (2014). Arabidopsis WRKY57 functions as a node of convergence for jasmonic acid- and auxin-mediated signaling in jasmonic acid-induced leaf senescence. Plant Cell 26: 230–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan K., Manners J.M. (2012). JAZ repressors and the orchestration of phytohormone crosstalk. Trends Plant Sci. 17: 22–31. [DOI] [PubMed] [Google Scholar]
- Kim J.I., Murphy A.S., Baek D., Lee S.-W., Yun D.-J., Bressan R.A., Narasimhan M.L. (2011). YUCCA6 over-expression demonstrates auxin function in delaying leaf senescence in Arabidopsis thaliana. J. Exp. Bot. 62: 3981–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Sakuraba Y., Lee T., Kim K.W., An G., Lee H.Y., Paek N.C. (2015). Mutation of Oryza sativa CORONATINE INSENSITIVE 1b (OsCOI1b) delays leaf senescence. J. Integr. Plant Biol. 57: 562–576. [DOI] [PubMed] [Google Scholar]
- Li J., Brader G., Palva E.T. (2004). The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 16: 319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Peng J., Wen X., Guo H. (2013). Ethylene-insensitive3 is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. Plant Cell 25: 3311–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, P.O., Kim, H.J., and Nam, H.G. (2007). Leaf senescence. Annu. Rev. Plant Biol. 58: pp. 115–136. [DOI] [PubMed] [Google Scholar]
- Lohman K.N., Gan S.S., John M.C., Amasino R.M. (1994). Molecular analysis of natural leaf senescence in Arabidopsis thaliana. Physiol. Plant. 92: 322–328. [Google Scholar]
- Lorenzo O., Chico J.M., Sánchez-Serrano J.J., Solano R. (2004). JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16: 1938–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreti E., Povero G., Novi G., Solfanelli C., Alpi A., Perata P. (2008). Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol. 179: 1004–1016. [DOI] [PubMed] [Google Scholar]
- Melotto M., Underwood W., Koczan J., Nomura K., He S.Y. (2006). Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980. [DOI] [PubMed] [Google Scholar]
- Miao Y., Zentgraf U. (2007). The antagonist function of Arabidopsis WRKY53 and ESR/ESP in leaf senescence is modulated by the jasmonic and salicylic acid equilibrium. Plant Cell 19: 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.D., Arteca R.N., Pell E.J. (1999). Senescence-associated gene expression during ozone-induced leaf senescence in Arabidopsis. Plant Physiol. 120: 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata M., Mitsuda N., Herde M., Koo A.J., Moreno J.E., Suzuki K., Howe G.A., Ohme-Takagi M. (2013). A bHLH-type transcription factor, ABA-INDUCIBLE BHLH-TYPE TRANSCRIPTION FACTOR/JA-ASSOCIATED MYC2-LIKE1, acts as a repressor to negatively regulate jasmonate signaling in Arabidopsis. Plant Cell 25: 1641–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y., Figueroa P., Browse J. (2011). Characterization of JAZ-interacting bHLH transcription factors that regulate jasmonate responses in Arabidopsis. J. Exp. Bot. 62: 2143–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.H., Oh S.A., Kim Y.H., Woo H.R., Nam H.G. (1998). Differential expression of senescence-associated mRNAs during leaf senescence induced by different senescence-inducing factors in Arabidopsis. Plant Mol. Biol. 37: 445–454. [DOI] [PubMed] [Google Scholar]
- Pauwels L., et al. (2010). NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464: 788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtau N., Marès M., Purdy S., Quentin N., Ruël A., Wingler A. (2004). Interactions of abscisic acid and sugar signalling in the regulation of leaf senescence. Planta 219: 765–772. [DOI] [PubMed] [Google Scholar]
- Qi T., Huang H., Wu D., Yan J., Qi Y., Song S., Xie D. (2014). Arabidopsis DELLA and JAZ proteins bind the WD-repeat/bHLH/MYB complex to modulate gibberellin and jasmonate signaling synergy. Plant Cell 26: 1118–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi T., Song S., Ren Q., Wu D., Huang H., Chen Y., Fan M., Peng W., Ren C., Xie D. (2011). The Jasmonate-ZIM-domain proteins interact with the WD-Repeat/bHLH/MYB complexes to regulate Jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell 23: 1795–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirino B.F., Normanly J., Amasino R.M. (1999). Diverse range of gene activity during Arabidopsis thaliana leaf senescence includes pathogen-independent induction of defense-related genes. Plant Mol. Biol. 40: 267–278. [DOI] [PubMed] [Google Scholar]
- Reinbothe C., Springer A., Samol I., Reinbothe S. (2009). Plant oxylipins: role of jasmonic acid during programmed cell death, defence and leaf senescence. FEBS J. 276: 4666–4681. [DOI] [PubMed] [Google Scholar]
- Rowe H.C., Walley J.W., Corwin J., Chan E.K., Dehesh K., Kliebenstein D.J. (2010). Deficiencies in jasmonate-mediated plant defense reveal quantitative variation in Botrytis cinerea pathogenesis. PLoS Pathog. 6: e1000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A., Alvarez-Venegas R., Avramova Z. (2008). An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat. Protoc. 3: 1018–1025. [DOI] [PubMed] [Google Scholar]
- Sasaki-Sekimoto Y., Jikumaru Y., Obayashi T., Saito H., Masuda S., Kamiya Y., Ohta H., Shirasu K. (2013). Basic helix-loop-helix transcription factors JASMONATE-ASSOCIATED MYC2-LIKE1 (JAM1), JAM2, and JAM3 are negative regulators of jasmonate responses in Arabidopsis. Plant Physiol. 163: 291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schommer C., Palatnik J.F., Aggarwal P., Chételat A., Cubas P., Farmer E.E., Nath U., Weigel D. (2008). Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biol. 6: e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer F., Fernández-Calvo P., Zander M., Diez-Diaz M., Fonseca S., Glauser G., Lewsey M.G., Ecker J.R., Solano R., Reymond P. (2013). Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 25: 3117–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltmann M.A., Berger S. (2013). Phenotyping jasmonate regulation of senescence. Methods Mol. Biol. 1011: 3–11. [DOI] [PubMed] [Google Scholar]
- Seo P.J., Park J.M., Kang S.K., Kim S.G., Park C.M. (2011). An Arabidopsis senescence-associated protein SAG29 regulates cell viability under high salinity. Planta 233: 189–200. [DOI] [PubMed] [Google Scholar]
- Shan X., Wang J., Chua L., Jiang D., Peng W., Xie D. (2011). The role of Arabidopsis Rubisco activase in jasmonate-induced leaf senescence. Plant Physiol. 155: 751–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheard L.B., et al. (2010). Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468: 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Qi T., Huang H., Xie D. (2013a). Regulation of stamen development by coordinated actions of jasmonate, auxin, and gibberellin in Arabidopsis. Mol. Plant 6: 1065–1073. [DOI] [PubMed] [Google Scholar]
- Song S., Qi T., Wasternack C., Xie D. (2014a). Jasmonate signaling and crosstalk with gibberellin and ethylene. Curr. Opin. Plant Biol. 21: 112–119. [DOI] [PubMed] [Google Scholar]
- Song S., Qi T., Fan M., Zhang X., Gao H., Huang H., Wu D., Guo H., Xie D. (2013b). The bHLH subgroup IIId factors negatively regulate jasmonate-mediated plant defense and development. PLoS Genet. 9: e1003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Qi T., Huang H., Ren Q., Wu D., Chang C., Peng W., Liu Y., Peng J., Xie D. (2011). The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect Jasmonate-regulated stamen development in Arabidopsis. Plant Cell 23: 1000–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Huang H., Gao H., Wang J., Wu D., Liu X., Yang S., Zhai Q., Li C., Qi T., Xie D. (2014b). Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between jasmonate and ethylene signaling in Arabidopsis. Plant Cell 26: 263–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines B., Katsir L., Melotto M., Niu Y., Mandaokar A., Liu G., Nomura K., He S.Y., Howe G.A., Browse J. (2007). JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448: 661–665. [DOI] [PubMed] [Google Scholar]
- Ueda J., Kato J. (1980). Isolation and identification of a senescence-promoting substance from wormwood (Artemisia absinthium L.). Plant Physiol. 66: 246–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulker B., Shahid Mukhtar M., Somssich I.E. (2007). The WRKY70 transcription factor of Arabidopsis influences both the plant senescence and defense signaling pathways. Planta 226: 125–137. [DOI] [PubMed] [Google Scholar]
- Wasternack C., Hause B. (2013). Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. (Lond.) 111: 1021–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver L.M., Gan S., Quirino B., Amasino R.M. (1998). A comparison of the expression patterns of several senescence-associated genes in response to stress and hormone treatment. Plant Mol. Biol. 37: 455–469. [DOI] [PubMed] [Google Scholar]
- Wild M., Davière J.M., Cheminant S., Regnault T., Baumberger N., Heintz D., Baltz R., Genschik P., Achard P. (2012). The Arabidopsis DELLA RGA-LIKE3 is a direct target of MYC2 and modulates jasmonate signaling responses. Plant Cell 24: 3307–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo H.R., Chung K.M., Park J.H., Oh S.A., Ahn T., Hong S.H., Jang S.K., Nam H.G. (2001). ORE9, an F-box protein that regulates leaf senescence in Arabidopsis. Plant Cell 13: 1779–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D.X., Feys B.F., James S., Nieto-Rostro M., Turner J.G. (1998). COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280: 1091–1094. [DOI] [PubMed] [Google Scholar]
- Xie Y., Huhn K., Brandt R., Potschin M., Bieker S., Straub D., Doll J., Drechsler T., Zentgraf U., Wenkel S. (2014). REVOLUTA and WRKY53 connect early and late leaf development in Arabidopsis. Development 141: 4772–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N., Winter C.M., Wu M.F., Kwon C.S., William D.A., Wagner D. (2014). Protocols: Chromatin immunoprecipitation from Arabidopsis tissues. The Arabidopsis Book 12: e0170, doi/10.1199/tab.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Christensen S., Isakeit T., Engelberth J., Meeley R., Hayward A., Emery R.J., Kolomiets M.V. (2012). Disruption of OPR7 and OPR8 reveals the versatile functions of jasmonic acid in maize development and defense. Plant Cell 24: 1420–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Stolz S., Chételat A., Reymond P., Pagni M., Dubugnon L., Farmer E.E. (2007). A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19: 2470–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Zhang C., Gu M., Bai Z., Zhang W., Qi T., Cheng Z., Peng W., Luo H., Nan F., Wang Z., Xie D. (2009). The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21: 2220–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.L., et al. (2012). Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. USA 109: E1192–E1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Worley E., Udvardi M. (2014). A NAP-AAO3 regulatory module promotes chlorophyll degradation via ABA biosynthesis in Arabidopsis leaves. Plant Cell 26: 4862–4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.D., Cho Y.H., Sheen J. (2007). Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2: 1565–1572. [DOI] [PubMed] [Google Scholar]
- Zhai Q., Yan L., Tan D., Chen R., Sun J., Gao L., Dong M.Q., Wang Y., Li C. (2013). Phosphorylation-coupled proteolysis of the transcription factor MYC2 is important for jasmonate-signaled plant immunity. PLoS Genet. 9: e1003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhou C. (2013). Signal transduction in leaf senescence. Plant Mol. Biol. 82: 539–545. [DOI] [PubMed] [Google Scholar]
- Zhang K., Halitschke R., Yin C., Liu C.-J., Gan S.-S. (2013). Salicylic acid 3-hydroxylase regulates Arabidopsis leaf longevity by mediating salicylic acid catabolism. Proc. Natl. Acad. Sci. USA 110: 14807–14812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhu Z., An F., Hao D., Li P., Song J., Yi C., Guo H. (2014). Jasmonate-activated MYC2 represses ETHYLENE INSENSITIVE3 activity to antagonize ethylene-promoted apical hook formation in Arabidopsis. Plant Cell 26: 1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X.Y., Spivey N.W., Zeng W., Liu P.P., Fu Z.Q., Klessig D.F., He S.Y., Dong X. (2012). Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe 11: 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., et al. (2011). Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc. Natl. Acad. Sci. USA 108: 12539–12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.