Abstract
Mutations in the protein alpha-synuclein (SNCA) have been linked to Parkinson’s disease. We recently reported that non-mutated SNCA enhanced glucose uptake through the Gab1-PI3 kinase-Akt pathway and elucidated its effects on glucose regulation. Here, we examined the association of SNCA with insulin resistance (IR), a condition that is characterized by decreased tissue glucose uptake. Our observations include those from a population study as well as a SNCA-deficient mouse model, which had not previously been characterized in an IR scenario. In 1,152 patients, we found that serum SNCA levels were inversely correlated with IR indicators—body mass index, homeostatic model assessment for IR (HOMA-IR) and immunoreactive insulin (IRI)—and, to a lesser extent, with blood pressure and age. Additionally, SNCA-deficient mice displayed alterations in glucose and insulin responses during diet-induced IR. Moreover, during euglycemic clamp assessments, SNCA knock-out mice fed a high-fat diet (HFD) showed severe IR in adipose tissues and skeletal muscle. These findings provide new insights into IR and diabetes and point to SNCA as a potential candidate for further research.
Metabolic syndrome is one of the most common findings in the clinic and affects millions of patients worldwide, resulting in a substantial burden on healthcare systems around the globe1,2,3. This syndrome is characterized by the presence of several risk factors and conditions that can precipitate the appearance of metabolic disorders, including hypertension, type 2 diabetes mellitus, obesity and dyslipidemias4. One of these conditions is insulin resistance (IR), which may lead to type 2 diabetes mellitus3.
The detailed mechanism of IR has yet to be elucidated. Among the hypothesized contributing factors are cytotoxic effects of palmitate in peripheral tissues5, Toll-like receptor-4-dependent NF-kB activation6, glycation damage7, and mitochondria dysfunction8. The first hypothesized contributing factor (palmitate), however, has drawn the most attention from researchers, giving rise to novel therapies such as G protein-coupled receptor (GPCR)-targeted therapies (i.e., GPR-40 agonists)9.
Alpha-synuclein (SNCA) is a protein richly expressed in neurons and hematopoietic tissues, and is present in serum10,11,12,13,14,15. Intriguingly, SNCA seems to function in some of the above-mentioned mechanisms underlying IR. SNCA interacts with lipids such as palmitate as a free acid-binding protein (FABP)-like transporter16, prevents glyoxalase I expression and glycation damage in neuron cells17, and has numerous extracerebral functions. SNCA was recently linked to glucose regulation as well as negative insulin secretion through K-channel modulation in beta cells of the pancreas18. The latter indicates that SNCA may have a role in decreasing hyperinsulinemia, which is observed in conditions such as IR. We also previously reported the function of SNCA in glucose metabolism in vitro and in vivo through a G-protein-coupled-receptor heterodimer lipo-phosphatidic acid receptor (LPAR2)- CD9019; however, how SNCA affects insulin is unclear. This question was the subject of our investigation.
Interestingly, we observed a previously unreported SNCA profile related to IR in both humans and rodents.
Results
Serum SNCA and IR in humans
We measured serum SNCA levels in a transversal study of a Japanese population from medical checkups in 2006 (Tanno-Subetsu in Hokkaido, n = 1152). To investigate differences between the patients’ serum SNCA levels and their metabolic profile, we categorized the data into 4 groups according to the serum SNCA levels (Grade 1, 13.4 ± 0.4 ng/dL; Grade 2, 35.2 ± 0.3 ng/dL; Grade 3, 52.8 ± 0.3 ng/dL and Grade 4, 95.1 ± 1.6 ng/dL). The Grade 1 and Grade 4 groups were the lowest- and highest-serum groups, respectively. We compared the differences between these two groups, as well as those between the intermediate groups (Grade 2 and 3). Each prespecified group had a similar number of subjects and included data on anthropometric and metabolic patient profiles (Table 1). After a preliminary analysis, we detected significant differences between SNCA grades, especially between the Grade 1 (lowest) and Grade 4 (highest) groups for serum immunoreactive insulin levels (IRI), homeostatic model assessment for IR (HOMA-IR), hematocrit (Hct) and systolic blood pressure values (SBP) via ANOVA (p < 0.0001). Next, these variables were analyzed to investigate correlations with the serum SNCA concentration itself (and not the grades) to avoid artifacts in the data analysis. Univariate linear regression models were independently fit for each study variable. The dependent variable in the model was the plasma SNCA concentration (ng/dL). The results from these models are given in Table 2. The variables with a significant association with the dependent variable at the 0.20 level were included in the multivariate model; thus, all the variables were included. To assess collinearity, tolerance factors were obtained for each variable in the multivariate model. The variables with tolerance factors <0.10 were examined further. HOMA-IR, IRI, hemoglobin (Hb), and hematocrit (Hct) had tolerance factors <0.10, and standard error estimates were much larger in the multivariate model than the univariate models. Pearson correlation coefficients were obtained for the associations between the four variables. HOMA-IR and IRI were strongly correlated (r = 0.92), as were Hb and Hct (r = 0.96). Furthermore, multivariate models were examined to determine which of these variables to keep in the final multivariate model. HOMA-IR and Hb were excluded from the initial multivariate model. The parameter estimates for the initial multivariate model, with tolerance factors, are provided in Table 3. To determine the final multivariate model, a stepwise selection was used. Both the entry and keep criteria in the model were set to an alpha of 0.20. The results from the final multivariate model are displayed in Table 4. The variables included in the model were age, BMI, IRI, diastolic blood pressure (DBP), and Hct. There was an inverse relationship between the dependent variable and age, BMI, IRI, and DBP, and a positive relationship between the dependent variables and Hct. These results are consistent with the results of the correlation analysis.
Table 1. Anthropometric characteristics and metabolic profile in the studied population (n=1,152).
| Profile | SNCA Grade | P | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| N | 288 | 288 | 288 | 288 | |
| Serum SNCA | 13.4 ± 0.4 | 35.2 ± 0.3 | 52.8 ± 0.3 | 95.1 ± 1.6 | <0.0001* |
| Age | 64.9 ± 0.8 | 63.2 ± 0.8 | 62.8 ± 0.8 | 61.1 ± 0.8 | 0.0057* |
| Gender (Masculine) | 40.8% | 40.3% | 40.6% | 40.6% | 0.9995 |
| Height | 155.5 ± 9.3 | 155.6 ± 9.3 | 156.6 ± 8.9 | 157.4 ± 9.1 | 0.1043 |
| Weight | 58.9 ± 10.5 | 58.2 ± 11.3 | 58.4 ± 11.3 | 58.2 ± 11.1 | 0.633 |
| BMI | 24.3 ± 3.7 | 23.9 ± 3.8 | 23.6 ± 3.3 | 23.4 ± 3.5 | 0.011* |
| Abdominal Circ. | 87.0 ± 9.6 | 84.5 ± 10.0 | 85.0 ± 9.4 | 84.7 ± 9.8 | 0.126 |
| Glucose | 99.4 ± 20.1 | 96.9 ± 15.8 | 97.2 ± 19.4 | 96.2 ± 23.3 | 0.325 |
| HbA1c | 5.44 ± 0.66 | 5.33 ± 0.61 | 5.41 ± 0.65 | 5.36 ± 0.69 | 0.608 |
| IRI | 6.08 ± 3.9 | 5.36 ± 3.88 | 5.50 ± 4.62 | 4.77 ± 3.16 | <0.0001* |
| HOMA IR | 1.52 ± 1.0 | 1.35 ± 1.23 | 1.35 ± 1.26 | 1.16 ± 0.95 | <0.0001* |
| RBC | 442.4 ± 45.3 | 440.8 ± 47.6 | 450.6 ± 39.9 | 453.2 ± 40.3 | 0.0019* |
| Hb | 13.49 ± 1.4 | 13.45 ± 1.4 | 13.74 ± 1.2 | 13.80 ± 1.3 | 0.0079* |
| Hct | 41.4 ± 4.0 | 41.2 ± 4.0 | 42.1 ± 3.4 | 42.5 ± 3.5 | 0.0005* |
| WBC | 5.41 ± 0.08 | 5.36 ± 0.08 | 5.19 ± 0.09 | 5.28 ± 0.09 | 0.273 |
| Total Cholest | 202.4 ± 34.0 | 197.4 ± 35.9 | 201.7 ± 31.7 | 203.6 ± 32.4 | 0.243 |
| Triglycerides | 106.7 ± 50.6 | 111.3 ± 72.1 | 105.0 ± 74.8 | 101.7 ± 69.6 | 0.628 |
| HDL | 52.4 ± 15.4 | 53.0 ± 14.2 | 54.2 ± 13.6 | 55.6 ± 13.1 | 0.011 |
| SBP | 143.5 ± 22.9 | 138.9 ± 23.2 | 137.7 ± 23.3 | 134.9 ± 21.2 | <0.0001* |
| DBP | 78.6 ± 10.9 | 77.8 ± 12.6 | 76.3 ± 11.2 | 75.3 ± 10.7 | 0.0023* |
| TP | 7.32 ± 0.37 | 7.33 ± 0.39 | 7.34 ± 0.39 | 7.35 ± 0.39 | 0.0186* |
| Uric Acid | 5.33 ± 1.34 | 5.09 ± 1.25 | 5.14 ± 1.30 | 5.13 ± 1.36 | 0.1963 |
| BUN | 15.8 ± 4.7 | 15.4 ± 4.0 | 15.3 ± 4.3 | 15.3 ± 3.6 | 0.676 |
| Creatinine | 0.68 ± 0.20 | 0.66 ± 0.14 | 0.71 ± 0.63 | 0.66 ± 0.15 | 0.3078 |
| AST | 26.1 ± 10.2 | 27.9 ± 13.3 | 24.0 ± 8.2 | 24.0 ± 8.2 | 0.0413* |
| ALT | 23.8 ± 13.2 | 25.1 ± 17.2 | 22.4 ± 13.2 | 22.4 ± 13.3 | 0.1258 |
Serum SNCA, alpha synuclein serum level (ng/dL); Age (years); Height (cm); Weight (kg); BMI, Body Mass Index (kg/cm2); Abdominal Circ, abdominal circumference (cm); Glucose, fasting blood glucose (mg/dL); HbA1c, glycated hemoglobin (%); IRI, Immunoreactive insulin (μU/mL); HOMA IR, Homeostatic model assessment insulin resistance (HOMAIR Units); RBC, red blood cells count (X10,000 cells/μL); Hb, hemoglobin (g/dL); Hct, hematocrit, Hct (%); WBC, white blood cells count (X1,000 cells/μL); Total Cholest, total cholesterol (mg/dL); Triglycerides (mg/dL), HDL, high density lipoproteins concentration (mg/dL); SBP, systolic blood pressure (mmHg); DBP, diastolic blood pressure (mmHg); TP, total serum protein (g/dL); Uric acid (mg/dL); BUN, blood urea nitrogen (mg/dL); Creatinine (mg/dL); AST, aspartate aminotranferase (U/L); ALT, alanine aminotransferase (U/L). Significant differences were assesed by ANOVA test. ± SD, plus/minus standard deviation, *P < 0.0019.
Table 2. Univariate Linear Regression Model Parameter Estimates.
| Variable | Estimate (β) | StdErr | tValue | P-value |
|---|---|---|---|---|
| Age | −0.27775 | 0.07460 | −3.72 | 0.0002 |
| BMI | −0.95687 | 0.27199 | −3.52 | 0.0005 |
| HOMA IR | −4.85829 | 0.88828 | −5.47 | <0001 |
| IRI | −1.46830 | 0.25754 | −5.70 | <0001 |
| Glucose | −0.09380 | 0.04657 | −2.01 | 0.0442 |
| SBP | −0.17848 | 0.04333 | −4.12 | <0001 |
| DBP | −0.30613 | 0.08728 | −3.51 | 0.0005 |
| RBC | 0.07289 | 0.02294 | 3.18 | 0.0015 |
| Hb | 1.91436 | 0.72883 | 2.63 | 0.0087 |
| Hct | 0.95129 | 0.26084 | 3.65 | 0.0003 |
Dependent Variable: SNCA concentration (ng/dL).
Univariate linnear regression model for each study variable. HOMA- IR displayed high β values (−4.85, p < 0.0001) together with IRI (−1.46, p < 0.0001). Hb and Hct were also correlated with SNCA serum levels in a lower degree. SNCA, alpha synuclein; Age (years); BMI, Body Mass Index (kg/cm2); HOMA IR, Homeostatic model assessment for insulin resistance (HOMAIR Units); IRI, Immunoreactive insulin (μU/mL); Glucose, fasting blood glucose (mg/dL); SBP, systolic blood pressure (mmHg); DBP, diastolic blood pressure (mmHg); RBC, red blood cells count (X10,000 cells/μL); Hb, hemoglobin (g/dL); Hct, hematocrit, Hct (%). n = 1152.
Table 3. Initial Multivariate Linear Regression Model Parameter Estimates.
| Variable | Estimate (β) | StdErr | tValue | P-value | Tolerance | Variance Inflation |
|---|---|---|---|---|---|---|
| Intercept | 39.91342 | 13.53136 | 2.95 | 0.0032 | — | — |
| Age | −0.16249 | 0.08982 | −1.81 | 0.0707 | 0.66026 | 1.51455 |
| BMI | −0.38189 | 0.31258 | −1.22 | 0.2221 | 0.72602 | 1.37738 |
| IRI | −1.36687 | 0.28699 | −4.76 | <0001 | 0.77726 | 1.28656 |
| Glucose | −0.04843 | 0.04741 | −1.02 | 0.3072 | 0.91121 | 1.09744 |
| SBP | −0.01448 | 0.06661 | −0.22 | 0.8280 | 0.40433 | 2.47325 |
| DBP | −0.29349 | 0.12065 | −2.43 | 0.0151 | 0.49757 | 2.00978 |
| RBC | −0.00454 | 0.04609 | −0.10 | 0.9216 | 0.24473 | 4.08608 |
| Hct | 1.59311 | 0.51290 | 3.11 | 0.0019 | 0.25240 | 3.96189 |
Dependent Variable: SNCA concentration (ng/dL).
Parameter estimates for the initial multivariate model using IRI. HOMA-IR was excluded from this model to avoid collinearities. SNCA, alpha synuclein; Age (years); BMI, Body Mass Index (kg/cm2); IRI, Immunoreactive insulin (μU/mL); Glucose, fasting blood glucose (mg/dL); SBP, systolic blood pressure (mmHg); DBP, diastolic blood pressure; RBC, red blood cells count (X10,000 cells/μL); Hct, hematocrit, Hct (%); (mmHg). n = 1152.
Table 4. Final Multivariate Linear Regression Model Parameter Estimates.
| Variable | Estimate (β) | StdErr | tValue | P-value | Tolerance | Variance Inflation |
|---|---|---|---|---|---|---|
| Intercept | 37.50949 | 13.04383 | 2.88 | 0.0041 | — | — |
| Age | −0.18183 | 0.07438 | −2.44 | 0.0147 | 0.96132 | 1.04024 |
| BMI | −0.42207 | 0.30801 | −1.37 | 0.1709 | 0.74649 | 1.33960 |
| IRI | −1.39992 | 0.28442 | −4.92 | <0001 | 0.79013 | 1.26561 |
| DBP | −0.30748 | 0.09060 | −3.39 | 0.0007 | 0.88103 | 1.13504 |
| Hct | 1.52374 | 0.27512 | 5.54 | <0001 | 0.87582 | 1.14179 |
Dependent Variable: SNCA concentration (ng/dL).
Final multivariate linear regression model using IRI. In this model, stepwise selection was used. HOMA-IR was excluded from this model to avoid collinearities. Both the entry and keep criteria into the model was set at the alpha level of 0.20. SNCA, alpha synuclein; Age (years); BMI, Body Mass Index (kg/cm2); IRI, Immunoreactive insulin (μU/mL); Hct, hematocrit, Hct (%); DBP, diastolic blood pressure (mmHg). n = 1152.
Because there is clinical interest in the association between HOMA-IR and outcome (SNCA concentration), the multivariate models were fit but excluded IRI and Hb. The parameter estimates for the initial multivariate model, with tolerance factors, are provided in Table 5. A stepwise selection was used to create the final multivariate model. Both the entry and keep criteria in the model were set to an alpha of 0.20. The results from the final multivariate model are displayed in Table 6. The variables included in the model were age, BMI, HOMA-IR, DBP, and Hct. There was an inverse relationship between the dependent variable and age, BMI, HOMA-IR, and DBP, and a positive relationship between the dependent variables and Hct. These results are consistent with those of the correlation analysis.
Table 5. Initial Multivariate Linear Regression Model Parameter Estimates.
| Variable | Estimate (β) | StdErr | tValue | P-value | Tolerance | Variance Inflation |
|---|---|---|---|---|---|---|
| Intercept | 34.36649 | 13.91504 | 2.47 | 0.0137 | — | — |
| Age | −0.16930 | 0.08993 | −1.88 | 0.0600 | 0.66018 | 1.51474 |
| BMI | −0.45627 | 0.30940 | −1.47 | 0.1406 | 0.74261 | 1.34660 |
| HOMA IR | −4.95366 | 1.10339 | −4.49 | <0001 | 0.62547 | 1.59879 |
| Glucose | 0.03628 | 0.05330 | 0.68 | 0.4962 | 0.72269 | 1.38373 |
| SBP | −0.01575 | 0.06671 | −0.24 | 0.8134 | 0.40408 | 2.47477 |
| DBP | −0.29285 | 0.12082 | −2.42 | 0.0155 | 0.49728 | 2.01095 |
| RBC | −0.00632 | 0.04613 | −0.14 | 0.8911 | 0.24482 | 4.08458 |
| Hct | 1.58563 | 0.51345 | 3.09 | 0.0021 | 0.25241 | 3.96181 |
Dependent Variable: SNCA concentration (ng/dL).
Parameter estimates for the initial multivariate model using HOMA-IR. IRI was excluded from this model to avoid collinearities. SNCA, alpha synuclein; Age (years); BMI, Body Mass Index (kg/cm2); HOMA IR, Homeostatic model assessment for insulin resistance (HOMAIR Units); Glucose, fasting blood glucose (mg/dL); SBP, systolic blood pressure (mmHg); DBP, diastolic blood pressure; RBC, red blood cells count (X10,000 cells/μL); Hct, hematocrit, Hct (%); (mmHg). n = 1152.
Table 6. Final Multivariate Linear Regression Model Parameter Estimates.
| Variable | Estimate (β) | StdErr | tValue | P-value | Tolerance | Variance Inflation |
|---|---|---|---|---|---|---|
| Intercept | 36.22095 | 13.13491 | 2.76 | 0.0059 | — | — |
| Age | −0.16978 | 0.07445 | −2.28 | 0.0228 | 0.96098 | 1.04061 |
| BMI | −0.47702 | 0.30565 | −1.56 | 0.1189 | 0.75931 | 1.31698 |
| HOMA IR | −4.60500 | 0.97431 | −4.73 | <0001 | 0.80043 | 1.24932 |
| DBP | −0.31489 | 0.09063 | −3.47 | 0.0005 | 0.88188 | 1.13394 |
| Hct | 1.54805 | 0.27585 | 5.61 | <0001 | 0.87261 | 1.14598 |
Dependent Variable: SNCA concentration (ng/dL).
Final multivariate linear regression model using HOMA- IR In this model, stepwise selection was used. IRI was excluded from this model to avoid collinearities. Both the entry and keep criteria into the model was set at the alpha level of 0.20. SNCA, alpha synuclein; Age (years); BMI, Body Mass Index (kg/cm2); HOMA IR, Homeostatic model assessment for insulin resistance (HOMAIR Units); DBP, diastolic blood pressure (mmHg); Hct, hematocrit, Hct (%). n=1152.
We found that Hb and Hct are positively correlated with SNCA levels, confirming the latter’s contribution to systemic SNCA serum levels as previously suggested12. Additionally, we found an inverse correlation with IR indicators (body mass index, HOMA-IR, and IRI) and a weaker correlation with DBP and age. These findings suggest that serum SNCA plays an important role in human metabolic disease, especially in insulin-glucose metabolism. Therefore, we further investigated SNCA’s metabolic implications in relation to IR and glucose metabolism.
SNCAKO mice display impairment in glucose metabolism during diet-induced IR
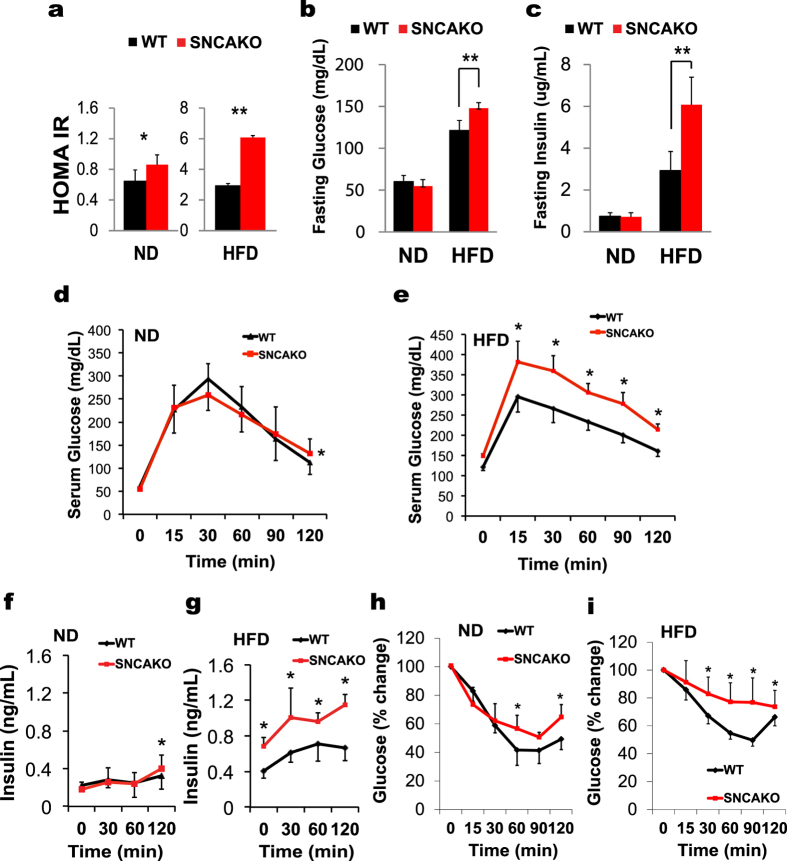
We used SNCA-deficient (SNCAKO) mice to perform the functional analysis of SNCA (Supplementary Fig. 1A and 1B). SNCAKO mice are viable and fertile, exhibit intact brain architecture, and have normal dopaminergic cell bodies, fibers and synapses20. We investigated the function of SNCA in Western diet-induced IR. We exposed SNCAKO mice to a high-fat diet (HFD). Under these conditions, the SNCAKO mice displayed a higher degree of IR compared with normal mice (WT) (C57/BL6), as indicated by an increased HOMA-IR index and higher levels of fasting blood glucose and serum insulin21,22,23,24 (Fig. 1A,C). Furthermore, SNCAKO mice displayed impairments in glucose and insulin responses as assessed via an intraperitoneal glucose tolerance test (ipGTT) and an intraperitoneal insulin tolerance test (ipITT), respectively. Additionally, SNCAKO mice presented increased insulinemia in ipGTT compared with their WT littermates, suggesting that SNCA plays an important role in glucose regulation and homeostasis during HFD-induced IR (Fig. 1D–I). Because SNCAKO demonstrated a poorer tissue insulin sensitivity profile during the ipITT and increased HOMA-IR, we decided to focus on IR in tissue. To address possible artifacts that may have affected our results, we performed all the experiments using animals with the same body weight and fat mass (Supplementary Fig. 1C and 1D).
Figure 1. SNCAKO mice display disturbances in glucose metabolism.
(a) Homeostatic model assessment index for insulin resistance (HOMA IR) in WT and SNCAKO mice after 5 weeks of a HFD. n = 10, each group. *P < 0.05 and **P < 0.01 via ANOVA. (b) Fasting blood glucose measurements in WT and SNCAKO mice fed a normal diet (ND) and a high-fat diet (HFD) for 5 weeks. n = 10, each group. (c) Fasting immunoreactive insulin measurements in WT and SNCAKO mice fed a ND or a HFD for 5 weeks. n = 10, each group. (d) Intraperitoneal glucose tolerance test (ipGTT, 2 g/kg) after 5 weeks of a ND in the WT and SNCAKO mice. n = 10, each group. (e) Intraperitoneal glucose tolerance test (ipGTT, 2 g/kg) after 5 weeks of a HFD in the WT and SNCAKO mice. n = 10, each group. (f) Serum insulin measurement (ELISA) during ipGTT in WT and SNCAKO mice fed a ND at 0, 30, 60 and 120 min. n = 10, each group.(g) Serum insulin measurement (ELISA) during ipGTT in WT and SNCAKO mice fed a HFD at 0, 30, 60 and 120 min. n = 10, each group. (h) Intraperitoneal insulin tolerance test (ipITT, 0.8 IU/kg) under a ND. n = 10, each group. (i) Intraperitoneal insulin tolerance test (ipITT, 0.8 IU/kg) after 5 weeks of a HFD. n = 10, each group. From (b) to (i): P < 0.05 was obtained for the area under the curve comparisons between the SNCAKO and WT groups (ANOVA test). In all the experiments: *P < 0.05; **P < 0.01.All histogram bars show the means ± s.d.
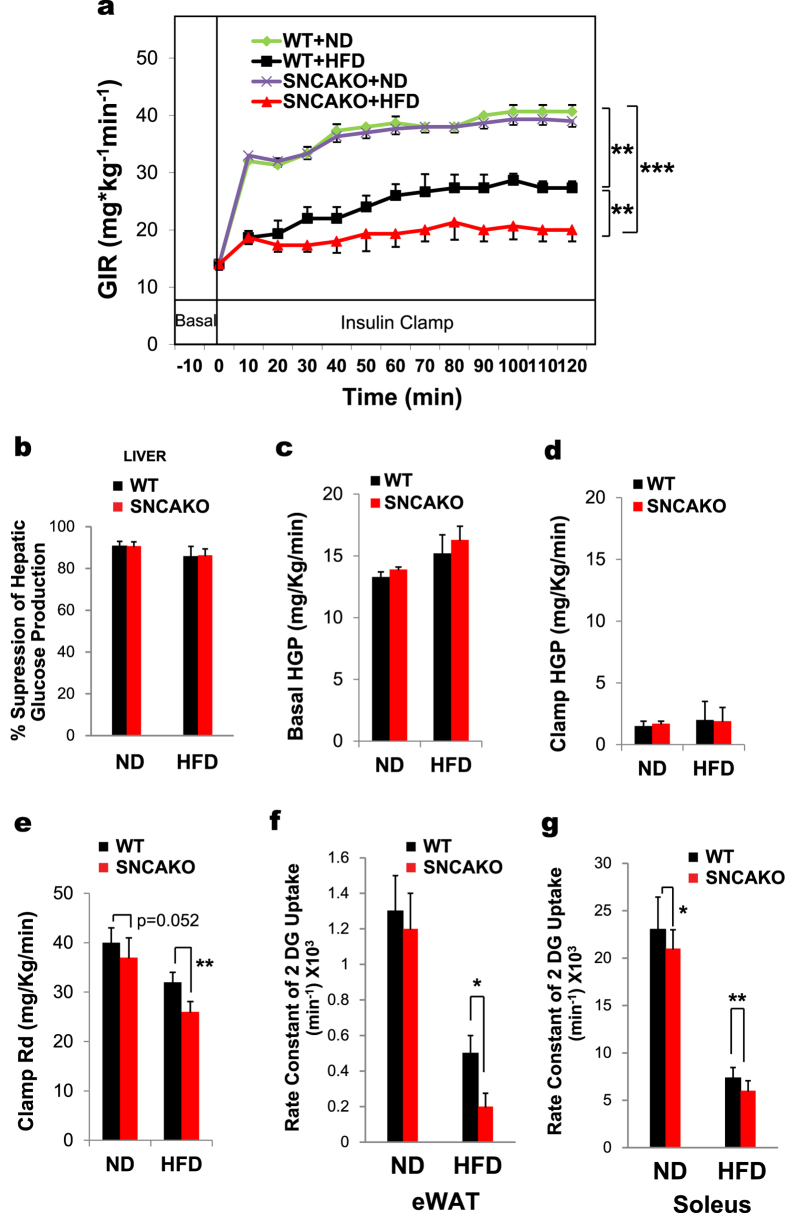
To determine whether SNCAKO mice are more sensitive to the development of IR under HFD, we applied hyperinsulinemic euglycemic clamps. Using this technique, we observed a significant increase in IR in the HFD-fed SNCAKO mice compared with the HFD-fed WT mice, with very modest changes (not statistically significant) between basal and insulin-hepatic glucose production (Fig. 2A,D). This IR was clearly noticeable in peripheral tissues, such as the adipose and skeletal tissues, of the SNCAKO mice (Fig. 2E,G). Taken together, our results indicate that SNCA plays an important role in diminishing insulin insensitivity in HFD-induced IR. Therefore, SNCA deletion results in a decreased tissue response to insulin; this is consistent with our initial findings in humans.
Figure 2. Hyperinsulinemic euglycemic clamp. SNCAKO mice display evident insulin resistance.
(a) Hyperinsulinemic euglycemic clamp (HEC) in WT and SNCAKO mice fed a ND or a HFD (after 5 weeks). n = 5 each group. GIR, glucose infusion rate (mg*kg−1min−1). (b) Percentage suppression of hepatic glucose production (HGP) in WT and SNCAKO mice fed a ND or a HFD (after 5 weeks) during the HEC. n = 5 each group. (c) Basal HGP in WT and SNCAKO mice fed a ND or a HFD (after 5 weeks). n = 5 each group. (d) Clamp HGP in WT and SNCAKO mice fed a ND and a HFD (after 5 weeks). n = 5 in each group. (e) Clamp rate of disappearance indicating peripheral insulin resistance, especially in the HFD group. WT and SNCAKO mice fed a ND or a HFD (after 5 weeks). n = 5 in each group. (f) Glucose uptake in white adipose tissues in WT and SNCAKO mice fed a ND or a HFD during the HEC. Rate of [3H] deoxyglucose uptake in eWAT (epididymal white adipose tissue). n = 5 in each group. (g) Glucose uptake in the skeletal muscle of WT and SNCAKO mice fed a ND or a HFD during the HEC. Rate of [3H] deoxyglucose uptake in the soleus muscle. n = 5 in each group. In (a), each of the group comparisons were made at the end of the HEC using the area under the curve and WT ND group as the baseline response. In all the experiments: **P < 0.05; ***P < 0.001 via ANOVA test and Bonferroni’s correction when appropriate. Graph bars show the means ± s.d.
Finally, we measured the serum SNCA levels in diabetic mice. Interestingly, the serum SNCA level was decreased in db/db mice and also decreased with age (10, 24 and 96 weeks old) in mice (Supplementary Fig. 2A and 2B), similarly to our results in humans (Supplementary Fig. 2C). These results indicate that declining serum SNCA levels with age may contribute to the development of IR or diabetes.
Discussion
This report elucidates the novel role of SNCA in IR. Ablation of SNCA exacerbates IR in tissues of rodents fed an HFD, and IR is also observed in patients with decreased SNCA serum levels. The higher the concentration of serum SNCA, the greater is the insulin sensitivity in both humans and rodents. SNCA demonstrated a clear benefit in terms of limiting IR in mice when exposed to HFD, as observed in our knock-out model and corroborated in the euglycemic clamp studies. The physiological function of glucose metabolism and regulation is to ensure effective energy substrate supplies for tissues. In the IR state, this mechanism is defective in transmitting signals to elicit sufficient energy substrate entry into the cell25,26. This status can be induced by exposure to saturated fats through a HFD27. Our report is the first to describe the use of SNCA knock-out mice for studying glucose metabolism in a pathological model (HFD IR model). We hypothesize that SNCA acts as a dynamic stress buffer to protect insulin signaling from saturated-fat-related IR and, additionally, as a promoter of glucose metabolism in tissues. In vitro evidence supports our hypothesis regarding lipids and indicates that SNCA could participate in the inhibition of fatty acid-dependent tissue toxicity because this protein can bind to lipids, has a high affinity for them and can interact with them through direct or indirect mechanisms28,29. Additionally, SNCA is important for glucose metabolism in tissues because it can elicit glucose uptake via the LPAR2-CD90 heterodimer (receptor), especially in adipose tissues and skeletal muscle19.
In humans, the SNCA serum levels and HOMA- IR or IRI were significantly and strongly correlated. Low levels of serum SNCA clearly displayed a higher HOMA- IR and insulin serum levels, which is congruent with the glucose metabolism profile of the SNCA knock-out mice. Interestingly, DBP, BMI and age were also correlated (modest correlation); this may indicate that SNCA is interacting with other risk factors of the metabolic disease together with HOMA-IR and IRI in humans. These SNCA interactions with conditions other than HOMA-IR and IRI require further research and will be the subject of future investigations by our research team.
The limitations of our population study include its transversal design. A prospective approach will give us a better understanding of the relationship between causality and serum SNCA over time as well as the cut-off points for normal serum SNCA levels. Additionally, because this study included only patients with Japanese ancestry, our findings cannot be generalized to other ethnicities. Another study in patients with metabolic disease or type 2 diabetes is needed to evaluate the possible associations between serum SNCA and disease severity or complications.
The strengths of our study include a large population, a broad spectrum of patient clinical characteristics, medical personnel with extensive experience in previous parallel clinical trials in the Tanno and Subetsu project, a comprehensive design (human and in vivo approaches) and a robust statistical analysis.
Currently, the narrow therapeutic arsenal against IR is a substantial challenge for physicians in attempts to reduce the risk of IR and/or type 2 diabetes onset in which tissue insulin response is greatly curtailed. As such, SNCA may provide new avenues to novel therapeutics in IR and diabetes.
Materials and Methods
Materials
We used recombinant SNCA (Atgen, South Korea) and insulin (Humulin-R100, Lilly, Japan). Anti-BSA (albumin) was purchased from Upstate Biotechnology (USA); all the other antibodies were purchased from Cell Signaling (USA).
Human study
This was an observational cross-sectional study performed with 1,152 patients residing in Tanno and Subetsu in Hokkaido, Japan, in 200630,31. The study was approved by Sapporo Medical University’s Institutional Review Board and in accordance with the Declaration of Helsinki. We included patients aged 20 years or more who were willing to participate in the study; they gave informed consent. We excluded severely sick patients with or without previous myocardial infarction or stroke as well as those with symptomatic cognitive disorders. Severely sick patients were defined as those who were in the terminal stages of chronic or acute diseases, such as type 2 diabetes mellitus (microvascular damage, i.e., retinopathy, diabetic foot, diabetic nephropathy), oncological diseases (solid or hematological tumors), sepsis, multiple organ dysfunction, cardiovascular disease, renal insufficiency or any other life-threatening disease that in the opinion of the principal investigator would have had a substantial impact on life expectancy, patient deambulation or ability to come to the clinic for medical checkups. These patients were excluded because they have very different pathophysiology that could interfere in the characterization of SNCA as a glucoregulator in a population study. The participating patients were enrolled consecutively in a prospective fashion; at the time of our investigation, this count was 1,152. A total of 1,152 patients were included and assessed for serum SNCA using the Human α-Synuclein Kit (Invitrogen, USA.). HOMA-IR was assessed in these patients using the following formula: Fasting glucose (mg/dL) × Immunoreactive insulin (μU/mL) / 405.
Animal procedures
The experiments were approved by the Ethical Committee for Animal Experiments of the Osaka University Graduate School of Medicine. All the in vivo experiments were performed in compliance with Osaka University’s Animal Facility regulations. We used mice of the same body weight for the group comparisons. Serum insulin (Morinaga, Japan.), glucagon (R&D, USA.) and cortisol (Cayman Chemical Company, USA.) were quantified using ELISA.
Methods
Immunoblotting
The samples were processed with SDS-PAGE and transferred to PVDF membranes. Then, the membranes were blocked in 5% skim milk and incubated with their respective antibody. We assessed chemiluminescence using an Image Quant LAS4000 mini (GE, USA).
Intraperitoneal glucose and insulin tolerance test (ipGTT and ipITT)
After 5 weeks of exposure to an HFD32 and after 8 hr of fasting, the mice were injected with glucose (2 g/kg) or insulin (0.8 IU/kg). Blood samples were extracted at 0, 15, 30, 60, 90 and 120 min.
Glucose clamp
After cardiac catheterization and mice habituation, the mice were fasted for 5–6 h. Then, under euglycemic conditions, the WT and SNCAKO mice were assessed using radiolabeled tracers. Blood and tissue sampling was performed as previously reported (see Supplementary Materials)33,34.
Statistical Analysis
The human data were analyzed using IBM SPSS Statistics 19. We used Stat View v5.0 for both the in vivo and in vitro data. We presented the data as the means ± s.d. We used Student’s t test and ANOVA for statistical significance.
Additional Information
How to cite this article: Rodriguez-Araujo, G. et al. Low alpha-synuclein levels in the blood are associated with insulin resistance. Sci. Rep. 5, 12081; doi: 10.1038/srep12081 (2015).
Supplementary Material
Acknowledgments
This project was supported by the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government (Monbukagakusho).
Footnotes
The Department of Clinical Gene Therapy at Osaka University is financially supported by Mitsubishi-Tanabe, AnGes MG, Novartis, Shionogi, Boeringher and Rohto. The Division of Vascular Medicine and Epigenetics is financially supported by Bayer.
Author Contributions G.R.A., H.N. and Y.K. designed the study; G.R.A., H.N. and Y.T. performed the experiments and analyzed the data. T.K., H.A., S.S., K.S., R.M. and H.R. also analyzed the data. All the authors discussed and agreed on the results. G.R.A. wrote the paper.
References
- Malik S. et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 110, 1245–1250 (2004). [DOI] [PubMed] [Google Scholar]
- Burton W. N., Chen C. Y., Li X., Schultz A. B. & Abrahamsson H. The association of self-reported employee physical activity with metabolic syndrome, health care costs, absenteeism, and presenteeism. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine. 56, 919–926 (2014). [DOI] [PubMed] [Google Scholar]
- Lebovitz H. E. Insulin resistance: definition and consequences. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology [and] German Diabetes Association. 109, S135–148 (2001). [DOI] [PubMed] [Google Scholar]
- Lusis A. J., Attie A. D. & Reue K. Metabolic syndrome: from epidemiology to systems biology. Nature reviews Genetics. 9, 819–830 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachek L. I., Musiyenko S. I., LeDoux S. P. & Wilson G. L. Palmitate induced mitochondrial deoxyribonucleic acid damage and apoptosis in l6 rat skeletal muscle cells. Endocrinology. 148, 293–299 (2007). [DOI] [PubMed] [Google Scholar]
- Shi H. et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. The Journal of clinical investigation. 116, 3015–3025 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone C. et al. Relationship between sRAGE and eotaxin-3 with CRP in hypertensive patients at high cardiovascular risk. Journal of nephrology. 26, 144–151 (2013). [DOI] [PubMed] [Google Scholar]
- Lee H. K. et al. Mitochondrial dysfunction and metabolic syndrome-looking for environmental factors. Biochimica et biophysica acta. 1800, 282–289 (2010). [DOI] [PubMed] [Google Scholar]
- Nagasumi K. et al. Overexpression of GPR40 in pancreatic beta-cells augments glucose-stimulated insulin secretion and improves glucose tolerance in normal and diabetic mice. Diabetes. 58, 1067–1076 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2, 492–501 (2001). [DOI] [PubMed] [Google Scholar]
- Duran R. et al. Plasma alpha-synuclein in patients with Parkinson’s disease with and without treatment. Mov Disord. 25, 489–493 (2010). [DOI] [PubMed] [Google Scholar]
- Barbour R. et al. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis. 5, 55–59 (2008). [DOI] [PubMed] [Google Scholar]
- Park S. M. et al. Evidence that alpha-synuclein functions as a negative regulator of Ca(++)-dependent alpha-granule release from human platelets. Blood. 100, 2506–2514 (2002). [DOI] [PubMed] [Google Scholar]
- Hong S., Lee H. K., Kim C. Y. & Seong G. J. Identification and localization of alpha-synuclein in human cornea. Korean J Ophthalmol. 22, 145–146 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer B. & Trenkwalder C. Neurochemical biomarkers in the differential diagnosis of movement disorders. Mov Disord. 24, 1411–1426 (2009). [DOI] [PubMed] [Google Scholar]
- Golovko M. Y., et al. Alpha-synuclein gene deletion decreases brain palmitate uptake and alters the palmitate metabolism in the absence of alpha-synuclein palmitate binding. Biochemistry. 44, 8251–8259 (2005). [DOI] [PubMed] [Google Scholar]
- Kurz A., et al. Alpha-synuclein deficiency leads to increased glyoxalase I expression and glycation stress. Cell Mol Life Sci. 68, 721–733 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X., et al. alpha-Synuclein binds the K(ATP) channel at insulin-secretory granules and inhibits insulin secretion. American journal of physiology Endocrinology and metabolism. 300, E276–286 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Araujo G. et al. Alpha-synuclein elicits glucose uptake and utilization in adipocytes through the Gab1/PI3K/Akt transduction pathway. Cell Mol Life Sci. 70, 1123–1133 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabin D. E., et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 22, 8797–8807 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace T. M., Levy J. C. & Matthews D. R. Use and abuse of HOMA modeling. Diabetes Care. 27, 1487–1495 (2004). [DOI] [PubMed] [Google Scholar]
- Herbach N. et al. Dominant-negative effects of a novel mutated Ins2 allele causes early-onset diabetes and severe beta-cell loss in Munich Ins2C95S mutant mice. Diabetes. 56, 1268–1276 (2007). [DOI] [PubMed] [Google Scholar]
- Mather K. Surrogate measures of insulin resistance: of rats, mice, and men. Am J Physiol Endocrinol Metab. 296, E398–399 (2009). [DOI] [PubMed] [Google Scholar]
- Stolarczyk E. et al. Improved insulin sensitivity despite increased visceral adiposity in mice deficient for the immune cell transcription factor T-bet. Cell metabolism. 17, 520–533 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel A. R. & Kahn C. R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 414, 799–806 (2001). [DOI] [PubMed] [Google Scholar]
- Taniguchi C. M., Emanuelli B. & Kahn C. R. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 7, 85–96 (2006). [DOI] [PubMed] [Google Scholar]
- Turner N., Cooney G. J., Kraegen E. W. & Bruce C. R. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J Endocrinol. 220, T61–79 (2014). [DOI] [PubMed] [Google Scholar]
- Haque F., Pandey A. P., Cambrea L. R., Rochet J. C. & Hovis J. S. Adsorption of alpha-synuclein on lipid bilayers: modulating the structure and stability of protein assemblies. J Phys Chem B. 114, 4070–4081 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnev P.A. et al. Alpha-synuclein lipid-dependent membrane binding and translocation through the alpha-hemolysin channel. Biophys J. 106, 556–565 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furugen M. et al. Matsuda-DeFronzo insulin sensitivity index is a better predictor than HOMA-IR of hypertension in Japanese: the Tanno-Sobetsu study. Journal of human hypertension. 26, 325–333 (2012). [DOI] [PubMed] [Google Scholar]
- Obara F., Saitoh S., Takagi S. & Shimamoto K. Influence of hypertension on the incidence of cardiovascular disease in two rural communities in Japan: the Tanno-Sobetsu [corrected] study. Hypertension research : official journal of the Japanese Society of Hypertension. 30, 677–682 (2007). [DOI] [PubMed] [Google Scholar]
- Winzell M. S. & Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 53, S215–219 (2004). [DOI] [PubMed] [Google Scholar]
- Shiuchi T. et al. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 10, 466–480 (2009). [DOI] [PubMed] [Google Scholar]
- Ayala J. E. et al. Hyperinsulinemic-euglycemic clamps in conscious, unrestrained mice. J Vis Exp 57, 3188 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.