Abstract
STUDY QUESTION
Does intrauterine biosynthesis of estrogen play an important role in early pregnancy by altering the function of uterine natural killer (uNK) cells?
SUMMARY ANSWER
Estrogens directly regulate the function of human uNK cells by increasing uNK cell migration and secretion of uNK cell-derived chemokine (C-C motif) ligand 2 (CCL2) that critically facilitates uNK-mediated angiogenesis.
WHAT IS KNOWN ALREADY
uNK cells are a phenotypically distinct population of tissue-resident immune cells that regulate vascular remodelling within the endometrium and decidua. Recently we discovered that decidualisation of human endometrial stromal cells results in the generation of an estrogen-rich microenvironment in areas of decidualised endometrium. We hypothesize that intrauterine biosynthesis of estrogens plays an important role in early pregnancy by altering the function of uNK cells.
STUDY DESIGN, SIZE, DURATION
This laboratory-based study used primary human uNK cells which were isolated from first trimester human decidua (n = 32).
PARTICIPANTS/MATERIALS, SETTING, METHODS
Primary uNK cells were isolated from first trimester human decidua using magnetic cell sorting. The impact of estrogens on uNK cell function was assessed. Isolated uNK cells were treated with estrone (E1, 10−8 M) or estradiol (E2, 10−8 M) alone or in combination with the anti-estrogen ICI 182 780 (ICI, 10−6 M). uNK cell motility was assessed by transwell migration assay and time-lapse microscopy. Expression of chemokine receptors was assessed by quantitative PCR (qPCR) and immunohistochemistry, and angiogenic factors were assessed by qPCR and cytokine array. Concentrations of CCL2 in supernatants were measured by enzyme-linked immunosorbent assay. Angiogenesis was assessed in a human endometrial endothelial cell network formation assay.
MAIN RESULTS AND THE ROLE OF CHANCE
Treatment with either E1 or E2 increased uNK cell migration (P = 0.0092 and P = 0.0063, respectively) compared with control. Co-administration of the anti-estrogen ICI blocked the effects of E1 and E2 on cell migration. Concentrations of C-X-C chemokine receptor type 4 (CXCR4) mRNA in uNK cells were increased by E2 treatment. The network formation assay revealed that conditioned media from uNK cells treated with E2 significantly increased human endometrial endothelial cell (HEEC) angiogenesis (P = 0.0029 versus control). Analysis of media from uNK cells treated with E2 using an antibody array identified CCL2 as the most abundant cytokine. Validation assays confirmed concentrations of CCL2 mRNA and protein were increased by E2 in uNK cells (P < 0.05 versus controls). Compared with the control, recombinant human CCL2 was found to increase HEEC network formation (P < 0.05) and neutralization of CCL2 in uNK conditioned media significantly decreased E2-dependent uNK-mediated network formation (P = 0.0006).
LIMITATIONS, REASONS FOR CAUTION
Our results are based on in vitro responses of primary human cells and we cannot be certain that similar mechanisms occur in vivo in humans. Primary human uNK cells were isolated from first trimester decidua at a range of gestations (8–12 weeks), which may be a source of variation. Primary human uNK cells from non-pregnant endometrium were not assessed and therefore the responses of uNK cells to E2 treatment described in this study may be distinct to uNK cells from first trimester decidua.
WIDER IMPLICATIONS OF THE FINDINGS
E2 is an essential regulator of reproductive competence. This study demonstrates a critical role for E2 in regulating cellular cross-talk within the endometrium during early pregnancy. We provide the first evidence that E2 directly regulates the function of human uNK cells by altering uNK cell migration and the secretion of uNK-derived angiogenic factors. We describe a novel mechanism of estrogen-dependent secretion of CCL2 which critically mediates uNK-dependent endometrial angiogenesis. Dysregulation of uNK cell function has been implicated in the aetiology of early implantation disorders and disorders of pregnancy. These novel findings provide unique insight into the regulation of uNK cell activity during the establishment of pregnancy in women and highlight key processes which may be targeted in future therapeutic strategies.
STUDY FUNDING/COMPETING INTEREST(S)
Studies undertaken in the authors' laboratory were supported by MRC Programme Grant G1100356/1 to P.T.K.S. The authors have no conflicts of interest to disclose.
Keywords: uterine natural killer cell, estrogen receptor β, estradiol, angiogenesis, chemokine (C-C motif) ligand 2 CCL2
Introduction
The endometrium is a complex multicellular tissue that undergoes dynamic remodelling in order to establish a microenvironment capable of supporting a pregnancy. During the establishment of pregnancy endometrial remodelling is characterized by three key processes; influx of uterine natural killer (uNK) cells, decidualisation (differentiation) of stromal fibroblasts and remodelling of the endometrial vasculature. Coordinated regulation of these processes is critical for pregnancy success.
uNK cells are a phenotypically distinct population of tissue-resident immune cells that are abundant in secretory phase endometrium and first trimester decidua (King et al., 1991). uNK cell numbers increase from Day 22 of the standardized 28 day cycle (Russell et al., 2013), coincident with increasing levels of progesterone, increasing to be become the predominant leukocyte population in first trimester decidua. The mechanisms that control accumulation and localization of uNK cells in the uterus remain unresolved although there is evidence to suggest that in situ proliferation, and recruitment and differentiation of NK cell precursors and/or haematopoietic stem cells may contribute to the rapid increase in cell numbers in the endometrium during the establishment of pregnancy (King et al., 1991; Jones et al., 2004; Sentman et al., 2004; Kitaya et al., 2005, 2007; Wu et al., 2005; Carlino et al., 2008; Kane et al., 2009; Vacca et al., 2011). Although uNK cells do not express progesterone receptors (Henderson et al., 2003), progesterone indirectly promotes accumulation and differentiation of uNK cells in the endometrium. For example, incubation with progesterone increases synthesis and secretion of interleukin (IL)-15 from human endometrial stromal cells in vitro (Okada et al., 2000) and treatment of women with the selective progesterone receptor modulator Asoprisnil results in a striking reduction in both IL15 mRNA and the number of CD56+ uNK cells detected in non-pregnant endometrium (Wilkens et al., 2013).
During the mid-secretory phase, decidualisation (differentiation) of the stromal cells is first detected adjacent to the spiral arteries in the upper (functional) layer of the endometrium in response to the rising levels of ovarian-derived progesterone. Decidualisation then spreads ‘wave-like’ throughout the tissue and if pregnancy ensues the cells remain in situ within the decidua of early pregnancy (reviewed in (Gellersen and Brosens, 2014)). Notably, decidualisation stimulates endometrial stromal cells to secrete a number of growth factors and cytokines that are key regulators of immune cell function and vascular development during endometrial remodelling. Recently we discovered that decidualisation of human endometrial stromal cells also results in biosynthesis of estrogens which we believe may be important in regulating early pregnancy tissue remodelling (Gibson et al., 2013). We described expression of the estrogen biosynthetic enzyme aromatase in both first trimester human decidua and in decidualised human endometrial stromal cells. In in vitro assays we detected secretion of significant (nM) concentrations of both estrone (E1) and estradiol (E2), from decidualised stromal cells (Gibson et al., 2013). Given that human uNK cells are immunopositive for estrogen receptor (ER)β in tissue sections (Henderson et al., 2003), these new data prompted us to explore whether the uNK cell population could be responding to local concentrations of bio-available estrogens in decidualised endometrium in early pregnancy.
To date there is only limited evidence to suggest that uNK cells may be directly regulated by estrogens. DeLoia et al. reported that in women who had received an artificial hormonal regimen to mimic the secretory phase (progesterone and E2), increased numbers of uNK cells were detected in endometrial biopsies when circulating estrogen concentrations were increased (DeLoia et al., 2002) but whether the effects of estrogens on uNK cells were direct or indirect was not explored. Most of the data generated to explore the function of uNK cells in early pregnancy events has relied upon studies in mouse models including recent reports that uNK cells play a role in regulating uterine vascular remodelling in early pregnancy (Croy et al., 2012; Hofmann et al., 2014) and reviewed in (Lima et al., 2014). However, as the uNK cells in mouse implantation sites are reported to be devoid of ER mRNAs (Borzychowski et al., 2003), we are only able to gain a greater understanding of uNK cell regulation by estrogens and their potential regulatory role in human pregnancy by performing studies using primary human uNK cells.
Isolated human uNK cells are reported to secrete angiogenic factors such as vascular endothelial growth factor (VEGF) and placental growth factor (PLGF) which promote angiogenesis in vitro and in vivo (Hanna et al., 2006; Wallace et al., 2012). In women, histological studies have revealed that uNK cells accumulate around spiral arterioles and are abundant in perivascular sites in both late secretory phase endometrium and in early pregnancy decidua (Bulmer et al., 1991). There is convincing evidence that uNK cells regulate vascular remodelling during trophoblast invasion and placentation (Lash et al., 2010, 2011; Wallace et al., 2012). However, there is also accumulating evidence that in early pregnancy spiral artery modifications in the decidua can occur independently of cellular interactions with trophoblast cells (Craven et al., 1998). For example, Histological studies have demonstrated that changes in spiral arteries, such as loss of vascular smooth muscle cells and endothelial cell breaks, are associated with maternal uNK cells but not fetal extravillous trophoblasts (Craven et al., 1998; Smith et al., 2009). Studies utilizing co-cultures of placental villi and decidual explants have demonstrated that decidual macrophages and uNK cells initiate early stages of vascular remodelling (Hazan et al., 2010). In addition, recent data support a role for uNK cells in promoting the early stages of endometrial vascular remodelling through secretion of angiogenic growth factors such as angiopoietin-2 (ANGPT2) (Robson et al., 2012). Gestational differences in the secretion of angiogenic growth factors from uNK cells purified and maintained for 48 h in culture have been reported. For example, uNK cells recovered at 8–10 weeks gestation secreted more of the angiogenic growth factors VEGF-C and ANGPT2 than uNK cells recovered at 12–14 weeks gestation (Lash et al., 2006). Taken together these data suggest human uNK cells play a key role in directly regulating vascular remodelling during early pregnancy.
Disorders of pregnancy are often associated with impaired vascular remodelling and some studies have suggested that this may be associated with alterations in uNK cell number and function although the data are conflicting. For example, pre-eclampsia (PE) is a complex syndrome with a multifactorial aetiology characterized by vascular pathology and associated with defects in uterine spiral arteries. The associated role of uNK cells in spiral artery modifications has led researchers to speculate that uNK cells may be dysregulated in PE although this is still to be fully elucidated. Although some studies report that decreased numbers of uNK cells are associated with PE and fetal growth restriction (Williams et al., 2009; Lockwood et al., 2013), Bachmayer et al. reported increased uNK cells in term placental tissues from women with PE (Bachmayer et al., 2006). uNK cell density is also reported to be proportional to endometrial angiogenesis in women with recurrent reproductive failure (Quenby et al., 2009). It has been proposed that an increase in the number of uNK cells in the stroma underlying the luminal epithelium of the endometrium of women during the secretory phase can be used as a diagnostic test to guide therapies for women suffering from infertility (Tang et al., 2013). However, a recent meta-analysis that considered 22 studies found no significant difference in the percentage of uNK cells between fertile and infertile women and concluded that NK cell analysis or immune cell therapy should only be considered in the context of clinical research until further studies were conducted (Seshadri and Sunkara, 2014).
In conclusion, it is generally accepted that coordinated cellular cross-talk between decidualised stromal cells, uNK cells and the endometrial vasculature is critical for appropriate regulation of endometrial remodelling in early pregnancy. Despite an association between dysregulation of uNK function and disorders of pregnancy, the fundamental mechanisms underlying normal regulation of human uNK cell function remain unresolved. In the present study we tested the hypothesis that estrogens produced by decidualised stromal cells (Gibson et al., 2013) can have a direct impact on the function of uNK cells. We assessed the impact of estrogens on human uNK cell motility and investigated whether E2 could modulate changes in vascular function by directly inducing changes in production of secreted factors from uNK cells.
Materials and Methods
Isolation of uNK cells
Decidual samples (n = 32) were obtained from women requesting surgical termination of pregnancy procedure with a mean gestation of 10 weeks (range 8–12 weeks, Supplementary Table SI) dated according to the woman's reported last menstrual period. All women had an ultrasound scan to confirm viability of pregnancy and gestational age; none were over 12 weeks gestation. Local ethical committee approval was granted and written informed patient consent was obtained prior to tissue collection by a dedicated research nurse. (Ethical approval held by HODC; LREC/05/51104/12 and LREC/10/51402/59). Primary human uNK cells were isolated from human first trimester decidua as described previously (Kane et al., 2009) using magnetic separation and the MACS® system (Miltenyi Biotech, Germany). uNK cells were isolated following CD3 depletion and CD56 positive selection using the appropriate antibody-coated magnetic microbeads. Isolated uNK cells were immuno-phenotyped using flow cytometry (Supplementary Fig. S1 and Supplementary Table SII).
uNK cell migration assay
Migration of uNK cells was assessed using a modified transwell migration assay. Isolated uNK cells were treated with vehicle control (dimethyl sulfoxide, DMSO) or either 10−8 M E1 or 10−8 M E2, alone or in combination with 10−6 M ICI 182 780 (ICI) for 1 h prior to assay (n = 6 per group). Following treatment, cells were pelleted and resuspended in culture media (Phenol red-free RPMI 1640 plus 10% charcoal stripped fetal calf serum, Invitrogen) prior to migration assay. Cell culture plates were coated with a 1.6 mg/ml collagen gel (GIBCO/Invitrogen, A10644-01); serum-free, Phenol red-free RPMI 1640 media was added to the well on top of the collagen matrix. Cell culture plates were collagen coated prior to assay to provide an adherent surface for attachment of migrated uNK cells. Cell culture inserts with a 5 µm membrane pore (Costar/Corning, 3421, NY, USA) were then placed into the well into which the uNK suspension was added (2 × 105 cells per insert). Cells were treated in duplicate and left in the incubator to migrate for 1 h. Migrated cells were counted by imaging 8 random fields using Axiovert 200 Inverted Fluorescent Microscope (Zeiss) and the mean number of migrated cells was calculated for each treatment. The fold change compared with the vehicle control was plotted.
Immunofluorescence
Expression of CD56, CXCR4 and C-X-C motif chemokine 12 (CXCL12) in sections of human decidua (chosen because they have a very high abundance of CD56+ cells) was determined by immunohistochemistry using standard protocols and tyramide signal amplification detection system (Perkin Elmer). Primary antibodies; CXCL12 (mouse monoclonal antibody; R&D systems MAB350), CXCR4 (mouse monoclonal antibody; R&D systems MAB172) and CD56 (mouse monoclonal antibody; Zymed 18-0152) were incubated overnight at 4°C. Fluorescent images were examined using Zeiss LSM 510 Meta-Confocal microscope.
uNK cell chemokinesis
The chemokinesis of uNK cells was assessed using Ibidi µ-Slide Chemotaxis3D chamber slides (Ibidi, 80 326, supplied by Thistle Scientific Ltd, Uddingston, UK). Isolated uNK cells were suspended in a collagen matrix (as above) and the response to E2 treatment was measured using time-lapse microscopy. Chamber slides were set up containing serum-free phenol red-free RPMI 1640 media in both reservoirs and uNK cells were treated ±E2. Cells were imaged every 5 min for 4 h (n = 30 per treatment) using an Axiovert 200 Inverted Fluorescent Microscope (Zeiss). Data were analysed using ImageJ (manual cell tracking plug-in) and chemotaxis and migration tool software (Ibidi).
Network formation assay
The formation of networks by telomerase immortalized human endometrial endothelial cells (HEEC, gifted from Yale University, New Haven, CT, USA; (Schatz et al., 2000)) was assessed as described previously (Greaves et al., 2013). HEECs were derived from primary human endometrial endothelial cells as described in Schatz et al. (2000) and immortalized as described in Krikun et al. (2005). HEEC express classic endothelial cell markers, such as von Willebrand factor (VwF) and platelet/endothelial cell adhesion molecule 1 (PECAM1, CD31), and transcriptional analysis by microarray confirmed 93% identity with parent primary endothelial cells (Krikun et al., 2005); prior to use in the assays described in this paper expression of CD31 and VwF was reconfirmed as described previously (Greaves et al., 2013).
Isolated uNK cells were cultured at a density of 1 × 106 cells per/ml and treated with 10−8 M E2, 10−6 M ICI or a combination of E2 and ICI for 24 h (n = 10 per group). Following treatment, cells were pelleted and the supernatant, uNK cell conditioned media (CM), were stored at −80°C until assay. uNK CM was thawed immediately prior to assay and warmed to 37°C. uNK CM or phenol red-free RPMI 1640 media containing recombinant human chemokine (C-C motif) ligand 2 (CCL2) (RnD; Cat #279-MC-010) was added to the bottom chambers. Where appropriate, the pure anti-estrogen ICI was added into the upper chamber for 1 h before the addition of uNK-CM. Neutralization of CCL2 was achieved by incubating treatment media with a monoclonal antibody to CCL2 (mAb-CCL2; Monoclonal Mouse IgG1 Clone # 24822, RnD; Cat # MAB279) for 1 h prior to assay. HEEC were incubated for 16 h at 37°C to allow formation of networks. Network formation was visualized using an Axiovert 200 Inverted Fluorescent Microscope and analysed as described previously (Greaves et al., 2013). Network formation was quantified as fold change relative to comparator.
Angiogenesis RT2 profiler PCR array
RT2 profiler PCR arrays (SABiosciences, Frederick, MD, USA) were utilized to analyse a targeted panel of 84 genes related to angiogenesis. uNK cells were isolated from three patients and treated ±E2 for 2 h. Total RNA was extracted from uNK cells using a Qiagen RNAeasy kit (Qiagen, UK) according to manufacturer's instructions and retrotranscribed using a RT2 single strand cDNA synthesis kit (SAB Biosciences, Frederick, MD, USA).
PCR array data analysis was performed using the SABiosciences web portal according to the ΔΔCt method (http://www.SABiosciences.com/pcrarrayanalysis.php).
Expression of mRNAs encoding C-X-C chemokine receptor type 4 (CXCR4), Interleukin-15 receptor alpha (IL15RA), interferon gamma (IFNG) and CCL2 was assessed by quantitative PCR (qPCR) according to standard protocols. Samples were quantified using the comparative ΔΔCt method with ribosomal 18S as internal control reference gene. The primers/probes are given in Supplementary Table SIII. PCR primers were designed using the Roche Universal Probe Library Assay Design Center. Primer efficiency was between 90 and 110% and amplicon sizes were confirmed (Supplementary Fig. S2).
Cytokine array
The relative abundance of 60 cytokines in uNK CM was assessed using Raybiotech Human Cytokine Array (Cat# AAH Cyt-6) and visualized using the Odyssey® system (LiCor). Relative cytokine expression levels were determined by assessing signal intensities using Odyssey application software.
Milliplex assay
The concentration of CCL2 in uNK CM (n = 5 per group) was quantified using a customized singleplex bead enzyme-linked immunosorbent assay (HCYTOMAG-60 K, Millipore, Schwalbach, Germany) according to manufacturer's instructions. Standards and samples were measured in duplicate using a Bio-Plex 200 HTF machine and analysed using BioPlex Manager Software (Version 5, BioRad).
Statistical analysis
Statistical analysis was performed using Graphpad prism (GraphPad Software, Inc., La Jolla CA, USA). Student's t-test was used to determine significance between treatments in data that were normally distributed. Non-parametric testing was utilized where sample sizes were insufficient to confirm normality of data distribution. Mann–Whitney test was used to assess differences between treatments. Where data were analysed as fold change, significance was tested using one-sample t-test and a theoretical mean of 1. Criterion for significance was P < 0.05. All data are presented as mean ± SEM.
Results
Estrogens promote uNK cell motility
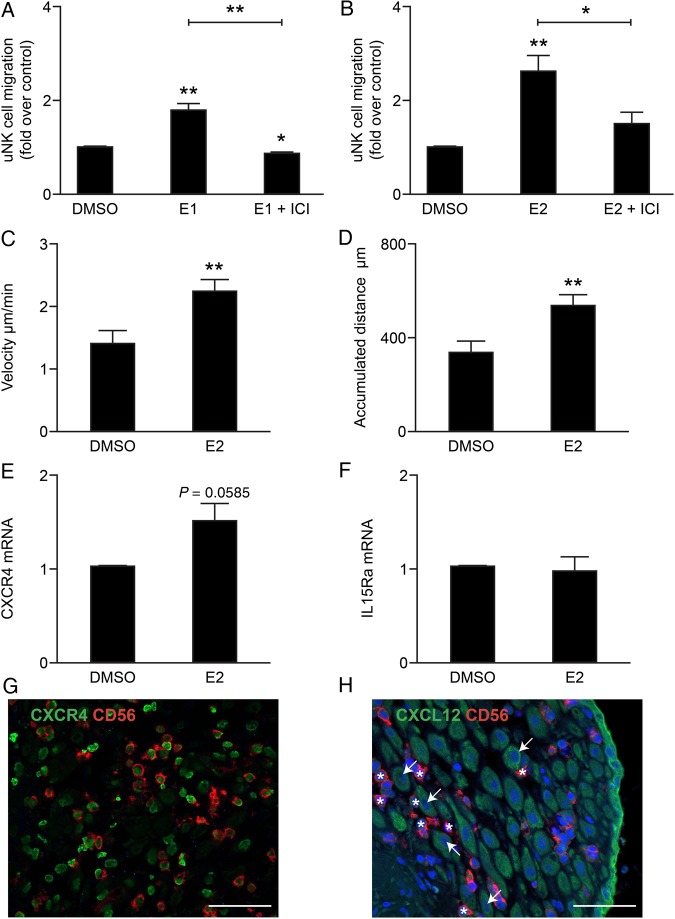
In agreement with results of tissue immunohistochemistry (Henderson et al., 2003) uNK cells freshly isolated from first trimester decidua (determined to be >93% pure; Supplementary Fig. S1) were shown to express ERβ mRNA and protein but were immunonegative for ERα (Supplementary Fig. S3). To determine whether estrogens could have a direct impact on uNK cell motility, cells were treated with either E1 (10−8 M) or E2 (10−8 M) in the presence or absence of the anti-estrogen ICI (fulvestrant, 10−6 M). Treatment with E1 or E2 significantly increased uNK cell migration (Fig. 1A and B; n = 6 per treatment, P = 0.0092 and P = 0.0063, respectively) and co-administration of ICI blocked the effect of either E1 (P = 0.0022) or E2 (P = 0.026).
Figure 1.
Estrogens promote uNK cell migration and chemokinesis. Uterine natural killer (uNK) cells were treated in duplicate with estrone (E1) or estradiol (E2) alone or in combination with the anti-estrogen ICI 182, 780 (ICI). Relative fold change responses were compared with vehicle control (dimethyl sulfoxide, DMSO). (A) E1 significantly increased uNK cell migration by ∼1.8-fold (n = 6, One-sample t-test, P = 0.0092), co-administration of ICI blocked E1-mediated increased migration (Mann–Whitney test, P = 0.0022). (B) E2 significantly increased uNK cell migration 2.6-fold (n = 6, One-sample t-test, P = 0.0063), co-administration of ICI blocked E2-mediated increased migration (Mann–Whitney test, P = 0.0260). uNK cell motility in cells treated with vehicle control (DMSO, n = 30 cells) or E2 (n = 30 cells) was assessed using time-lapse video microscopy (Supplementary Videos S1 and S2). (C) E2 treatment significantly increased uNK cell velocity (µm/min) compared with vehicle control (Student's t-test, P = 0.0016). (D) E2 treatment significantly increased the accumulated uNK cell distance (µm) compared with vehicle control (Student's t-test, P = 0.0016). The expression of chemokine receptors in uNK cells in response to E2 was assessed by quantitative PCR (qPCR); fold change compared with vehicle control was determined. (E) E2 tended to increase concentrations of mRNAs encoding C-X-C chemokine receptor type 4 (CXCR4) (n = 5, One-sample t-test, P = 0.0585). (F) IL15RA mRNA was unchanged. The expression of the uNK chemoattractant C-X-C motif chemokine 12 (CXCL12) and its receptor CXCR4 were assessed in first trimester human decidua by immunohistochemistry. (G) Positive staining for CXCR4 (green) was detected in CD56-positive uNK cells (red staining). (H) CXCL12 did not co-localize with CD56-positive uNK cells (asterisks); however, positive staining was detected in decidualised stromal cells (arrows) and epithelial cells (green staining) in decidua. Nuclear counterstain DAPI (blue staining). Scale bar 50 µm. *P < 0.05, **P < 0.01.
To complement and extend these studies, uNK cell motility was recorded using live cell imaging and time-lapse microscopy (Supplementary Videos S1 and S2). When cell movement was analysed using the chemotaxis and migration tool software (Ibidi) an increase in cellular activity was readily apparent following exposure to E2. Chemokinesis (non-directed cell movement) was measured: E2 treatment (10−8 M) was found to significantly increase both the velocity and the accumulated distance travelled by uNK cells (Fig. 1C and D, P < 0.01, n = 30 per treatment) compared with control (DMSO).
During decidualisation endometrial stromal cells secrete estrogens (Gibson et al., 2013) as well as uNK chemoattractants such as CXCL12 and IL-15 (Kitaya et al., 2005; Wu et al., 2005). To investigate whether E2 could increase responsiveness of uNK cells to chemoattractants, the expression of the CXCL12 receptor (CXCR4) and the IL-15 receptor alpha (IL15Ra) was assessed in uNK cells by qPCR. E2 treatment up-regulated concentrations of mRNAs encoding CXCR4 (Fig. 1E, P = 0.0585, n = 5) while concentrations of IL15RA were unchanged by E2 (Fig. 1F). Consistent with previous flow cytometry studies (Wu et al., 2005), fluorescent immunohistochemistry analysis of human decidua revealed that CD56+ uNK cells were CXCR4 positive (Fig. 1G). Furthermore, uNK cells (asterisks) appeared to accumulate near to CXCL12-positive decidualised stromal cells (white arrows, Fig. 1H). Interestingly, CXCR4 was immunolocalised to the nuclei of CD56+ cells, consistent with nuclear re-localization as a result of CXCL12 stimulation (Wang et al., 2009).
Estradiol promotes uNK-mediated angiogenesis
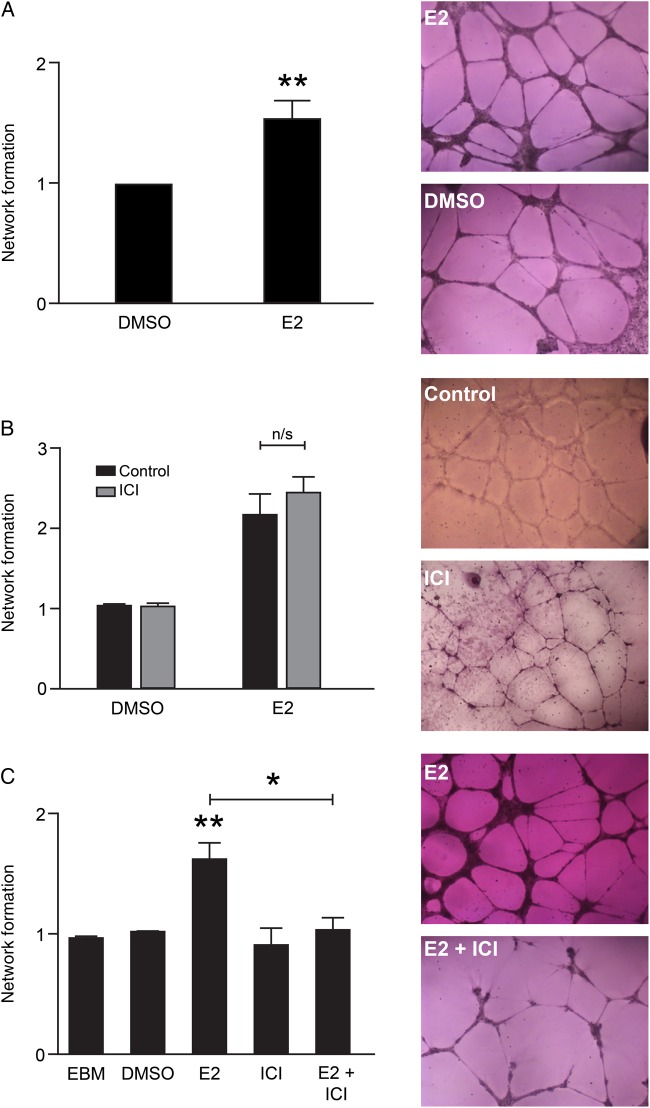
uNK cells are reported to secrete angiogenic factors and to promote angiogenesis in vitro and in vivo (Hanna et al., 2006). Recent reports suggest uterine artery remodelling and angiogenesis both occur in early pregnancy (Lima et al., 2014). We investigated the effect of E2 on uNK-mediated angiogenesis using an established in vitro assay (Greaves et al., 2013) of endothelial network formation which is considered a morphometric assay that models the reorganization stage of angiogenesis. HEEC network formation was assessed in response to CM from uNK cells treated with E2 (10−8 M), the anti-estrogen ICI (10−6 M) or E2 in combination with ICI. CM from uNK cells treated with E2 significantly increased network formation compared with control (Fig. 2A, P = 0.0029, n = 10). We have previously reported that HEEC can respond to estrogens via activation of ERβ (Greaves et al., 2013); therefore we blocked any indirect stimulation of HEEC by E2 present in CM by pre-treating HEEC with the anti-estrogen ICI (10−6 M). Pretreatment with ICI did not affect HEEC network formation in response to uNK CM (Fig. 2B, n = 3). Finally, we confirmed that the secretion of pro-angiogenic factors from uNK cells in response to E2 was receptor mediated, as CM from uNK cells treated with E2+ICI did not increase HEEC network formation (Fig. 2C, P = 0.0136, n = 7).
Figure 2.
Estradiol promotes uNK-mediated network formation in endometrial endothelial cells via uNK estrogen receptor. Human endometrial endothelial cell (HEEC) network formation was assessed in response to uNK conditioned media (CM). Network formation was quantified as fold change relative to vehicle control. (A) CM from uNK cells treated with E2 significantly increased network formation in HEECs (n = 10, One-sample t-test, P = 0.0029). (B) Blocking of HEEC estrogen receptor (ER)β with ICI did not affect uNK-mediated increased network formation (n/s: not significant, n = 3, Mann–Whitney test). (C) CM from uNK cells treated with ICI or uNK cells treated with E2 in combination with ICI (E2+ICI) had no significant impact on network formation. CM from uNK cells treated with E2 significantly increased network formation relative to all treatments including endothelial basal growth media (EBM, P = 0.002). CM from uNK cells co-treated with E2 and ICI significantly decreased network formation compared with CM from uNK cells treated with E2 alone (n = 7, Mann–Whitney test, P = 0.0136). *P < 0.05, **P < 0.01. Images were captured at ×5 magnification using the Axiovert 200 Inverted Fluorescent Microscope.
Targeted gene analysis reveals regulation of cytokines in uNK cells
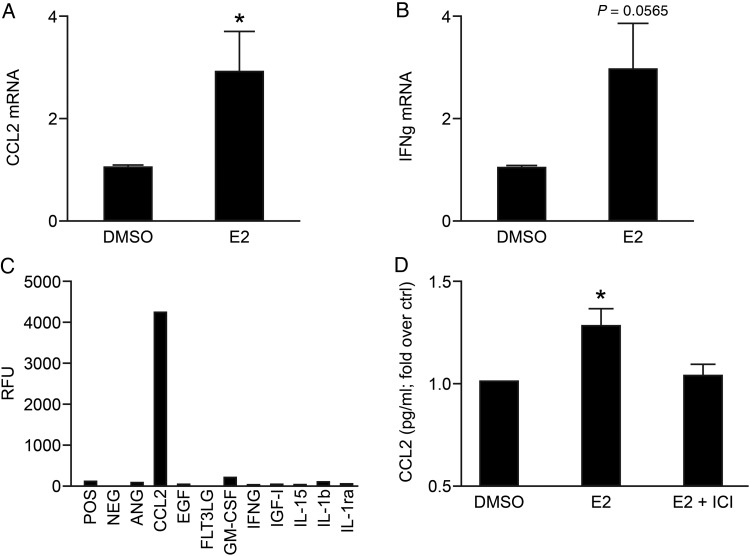
To investigate whether treatment with E2 had an impact on expression of genes known to play a role in angiogenesis, mRNAs expressed by uNK cells were assessed using a targeted PCR array (SAB biosciences). The identity of mRNAs with altered expression is summarized in Table I. Messenger RNAs encoded by VEGFC and PLGF, previously reported to be uNK-derived angiogenic factors (Hanna et al., 2006), were not detected and VEGFA expression was not affected by E2 treatment (Table I, n = 3). CCL2 and IFNG were selected for follow-up validation as these were the top two targets that exhibited greatest increased regulation in response to E2. Targeted analyses of IFNG and CCL2 were validated in a separate larger sample set of E2-treated uNK cells using qPCR and were increased in response to 10−8 M E2 (Fig. 3A; P = 0.0394, n = 10 and Fig. 3B; P = 0.0565, n = 9, respectively).
Table I.
Differential expression of angiogenesis genes in uterine natural killer (uNK) cells treated with estradiol (E2).
| Gene of interest | Fold change | P-value |
|---|---|---|
| VEGFA | 1.007 | 0.756185 |
| MMP9 | 1.4272 | 0.150482 |
| PECAM1 | 1.7144 | 0.29493 |
| MMP2 | 1.7916 | 0.066499 |
| CXCL10 | 1.9562 | 0.446108 |
| TIMP1 | 2.6924 | 0.105164 |
| CCL2 | 2.8044 | 0.128538 |
| IFNG | 4.4089 | 0.108815 |
Top targets that were subsequently validated are listed in bold.
uNK cells were treated with control (dimethyl sulfoxide) or E2 for 2 h (n = 3) and the differential expression of angiogenesis genes was determined. PCR array data analysis was performed using the SABiosciences web portal according to the ΔΔCt method (http://www.SABiosciences.com/pcrarrayanalysis.php) and gene expression expressed as fold change relative to vehicle control. The P-values are calculated based on a Student's t-test of the values for each gene in the control group and treatment groups. Angiogenesis genes that were differentially up-regulated in response to E2 are shown. Expression of vascular endothelial growth factor A (VEGFA) is also shown for comparison.
MMP9, matrix metallopeptidase 9; PECAM1, platelet/endothelial cell adhesion molecule 1; MMP2, matrix metallopeptidase 2; CXCL10, chemokine (C-X-C motif) ligand 10; TIMP1, TIMP metallopeptidase inhibitor 1; CCL2, chemokine (C-C motif) ligand 2; IFNG, interferon gamma.
Figure 3.
E2-dependent regulation of angiogenic factors in uNK cells. The top two targets identified by PCR array; chemokine (C-C motif) ligand 2 (CCL2) and interferon gamma (IFNγ), were selected for follow-up validation by qPCR. Fold change compared with vehicle control was determined. (A) E2 significantly increased expression of CCL2 mRNA (n = 10, One-sample t-test, P = 0.0394) compared with control (DMSO). (B) Concentrations of mRNAs encoding IFNG tended to be increased by E2 treatment (P = 0.0565, n = 9). (C) Cytokines/growth factors in CM from uNK cells treated with E2 were assessed by cytokine array; levels of IFNγ were low/undetectable, CCL2 was abundant. (D) Concentrations of CCL2 were assessed by Milliplex bead assay. Concentrations of CCL2 were significantly increased in CM from uNK cells treated with E2 (n = 5, One-sample t-test, P = 0.0408 versus control). *P < 0.05, **P < 0.01.
To assess if changes in gene transcription were reflected in alterations in secretion of pro-angiogenic factors the relative abundance of cytokines/growth factors in uNK-E2 CM was assessed using a Human Cytokine Array. Levels of IFNG were low but CCL2 was abundant in uNK-E2 CM compared with other cytokines/growth factors (Fig. 3C). The concentration of CCL2 was quantified by Milliplex bead assay. Concentrations of CCL2 (mean concentration 0.1 ng/ml) were significantly increased in CM from E2-treated uNK cells (Fig 3D, P = 0.0408, n = 5).
E2-dependent secretion of CCL2 promotes uNK-mediated angiogenesis
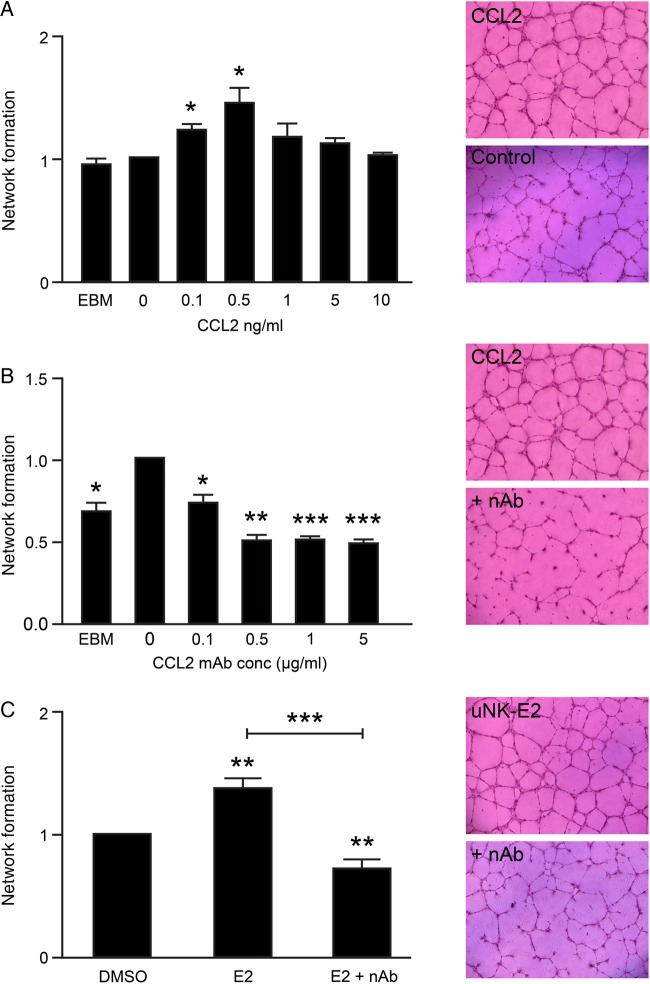
To investigate whether CCL2 could directly promote endometrial angiogenesis, the impact of recombinant human CCL2 (rCCL2) on network formation by HEEC was assessed. A biphasic dose-dependent increase in HEEC network formation was detected in response to rCCL2 that was significant at 0.1 ng/ml (Fig. 4A, P = 0.0348, n = 4) and 0.5 ng/ml (Fig 4A, P = 0.0470, n = 4) but inhibited at concentrations ≥5 ng/ml. Interestingly, the concentration of CCL2 detected in uNK CM (0.1 ng/ml) was comparable to the dose of rCCL2 which produced a significant response. The capacity for increasing concentrations of a monoclonal antibody to CCL2 (mAb-CCL2) to neutralize rCCL2-mediated network formation was tested against 0.5 ng/ml rCCL2. Incubation with mAb-CCL2 for 1 h prior to assay was sufficient to significantly inhibit network formation at mAb-CCL2 concentrations of 0.1 µg/ml (P = 0.0174, n = 4) and greater (Fig. 4B, 0.5 µg/ml; P = 0.0026, 1 and 5 µg/ml; P = 0.0005, n = 4).
Figure 4.
E2-dependent secretion of CCL2 regulates uNK-mediated angiogenesis. HEEC network formation was assessed in response to recombinant human CCL2 (rCCL2). (A) Network formation was significantly increased in response to 0.1 ng/ml rCCL2 (n = 4, One-sample t-test, P = 0.0348) and maximal in response to 0.5 ng/ml (n = 4, One-sample t-test, P = 0.047). (B) HEEC were incubated with 0.5 ng/ml rCCL2 and increasing concentrations of a monoclonal antibody to CCL2 (mAb-CCL2). HEEC network formation was assessed relative to 0 µg/ml mAb-CCL2. Neutralization of rCCL2 with mAb-CCL2 significantly inhibited HEEC network formation at concentrations of 0.1 µg/ml (P = 0.0174) and greater (n = 4, One-sample t-test, 0.5 µg/ml; P = 0.0026, 1 and 5 µg/ml; P = 0.0005). (C) uNK-E2 CM was incubated with 0.5 µg/ml mAb-CCL2 and HEEC network formation was assessed relative to control uNK CM (DMSO). Neutralization of CCL2 significantly reduced HEEC network formation (n = 8, One-sample t-test, P = 0.0069) and reversed uNK-E2-mediated increased network formation (n = 8, Mann–Whitney, P = 0.0006). *P < 0.05, **P < 0.01, ***P < 0.001. Images were captured at ×5 magnification using the Axiovert 200 Inverted Fluorescent Microscope.
To confirm whether CCL2 was the key E2-dependent factor secreted by uNK cells that could induce changes in vascular endothelial cells, CM from uNK cells treated with E2 was incubated with 0.5 µg/ml mAb-CCL2 before it was used to assess HEEC network formation. Neutralization of CCL2 resulted in a significant decrease in E2-dependent uNK-mediated network formation (Fig. 4C, P = 0.0006, n = 8).
Discussion
This study demonstrates that estrogens directly regulate the bioactivity of human uNK cells. Although there has been evidence to suggest uNK cells can be regulated indirectly by sex steroid hormones such as progesterone, this study provides the first evidence that estrogens are direct mediators of uNK cell function. Collectively, our novel findings indicate that estrogens increase uNK cell migration and responsiveness to chemoattractants. We report evidence that secretion of CCL2 from uNK cells is ER-dependent and that CCL2 critically facilitates uNK-mediated endometrial angiogenesis. These results support our hypothesis that estrogens synthesized within endometrial tissue promote regulation of uNK cells, leading to ER-dependent secretion of pro-angiogenic factors that promote vascular remodelling.
Understanding the dynamic changes that take place in the endometrium during the establishment of pregnancy is challenging. Our results provide new insights into the regulation of human endometrial remodelling and highlight the importance of cellular cross-talk between decidualised stromal cells, uNK cells and the endometrial vasculature. Decidualisation of endometrial stromal cells is a progressive process characterized by a time-dependent change in the ratios of secreted estrogens (E1 and E2). We have previously demonstrated that primary endometrial stromal cells induced to decidualise in vitro initially secrete a high E1:E2 ratio, followed by a progressive increase in E2 such that the concentration of E1 is equivalent to that of E2 when cells are fully transformed (Gibson et al., 2013). Interestingly, in the present study, similar molar concentrations of E1 and E2 were able to significantly increase the numbers of migrating uNK cells, a finding that was unexpected as E1 is considered to be a much ‘weaker’ estrogen than E2. However these assumptions about the activity of E1 are based on results reporting a lower binding affinity for ER and 10-fold lower activation of estrogen response element-driven reporter constructs in cell lines than E2 (Kuiper et al., 1998; Legler et al., 2002). Notably, the E1- and E2-driven increases in uNK cell migration were both abrogated by treatment with the pure ER antagonist ICI (Wakeling et al., 1991). The novel finding that E1 increases uNK migration may indicate a role for E1 in maintaining tissue-resident uNK cells during the secretory phase, while increasing concentrations of E2 that occur with decidualisation and establishment of pregnancy (Gibson et al., 2013) may enhance accumulation of uNK cells in decidua and influence recruitment of uNK cells within the endometrium in early pregnancy (Fig. 5; 1 and 2).
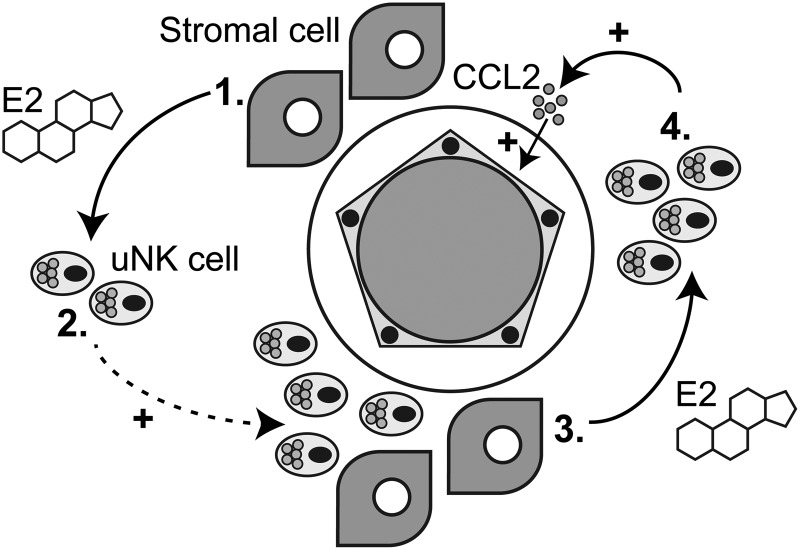
Figure 5.
Summary: Regulation of endometrial function in early pregnancy. Stromal cells and uNK cells interact with endometrial arterioles to promote vascular remodelling. 1. In the decidua, stromal cells secrete estrogens such as E1 and E2. 2. E2 increases uNK cell motility and migration both directly and by increasing the expression of the chemokine receptor CXCR4. This action of E2 may promote localization/recruitment of uNK cells in the perivascular niche. 3. E2 acts directly on uNK cells to promote secretion of pro-angiogenic factors including CCL2. 4. ER-dependent secretion of CCL2 promotes endothelial cell angiogenesis and modulation of vascular function.
Decidualised endometrial stromal cells secrete uNK chemoattractants such as CXCL12 (Kitaya et al., 2005; Wu et al., 2005). The expression and secretion of CXCL12 in endometrial stromal cells is reported to be promoted by E2 but suppressed by progesterone (Glace et al., 2009). Thus, areas of endometrium with increased bioavailability of E2 may have increased concentrations of CXCL12. In the present study, immunofluorescence highlighted the close association of CXCL12-positive stromal cells and CXCR4-positive uNK cells within human decidua and revealed that CXCR4 was immunolocalised to the nuclei of uNK cells (indicative of receptor activation) (Wang et al., 2009). In addition, E2 treatment increased concentrations of CXCR4 mRNA in isolated uNK cells, which suggests that E2 could enhance responsiveness of uNK cells to CXCL12. This may be of increasing importance in early pregnancy as primary human trophoblast cells also secrete CXCL12 (Wu et al., 2005). E2 may therefore influence the recruitment of uNK cells to invading trophoblast cells by altering CXCR4 expression and this may impact on uNK cell activity during the establishment of the materno–fetal interface. Collectively, our new findings provide the first evidence that the bioavailability of estrogens within the decidua could increase uNK cell migration, motility and responsiveness to chemoattractants.
uNK cells promote vascular remodelling during the establishment of pregnancy. The current study investigated the impact of E2 on expression of angiogenic factors in uNK cells. We found that CCL2 mRNA and protein concentrations were increased by E2 treatment in uNK cells in an ER-dependent manner (Fig. 5; 3 and 4). Transcriptional analysis of uNK cells did not reveal E2-dependent changes in angiogenic factors previously associated with uNK cells such as VEGFA and IFNG. In addition, VEGFC and PLGF were not detected by PCR array. This may indicate limited sensitivity of the PCR array or that the secreted proteins are more easily detectable than mRNA transcripts for these genes which may be subject to rapid mRNA turnover. CM from uNK cells contained a number of angiogenic factors all of which may contribute to regulation of the vasculature. We speculate that E2 may modulate production of only a limited subset of angiogenic factors, such as CCL2, which may act in addition to, or in synergism with, other uNK cell-derived factors, such as VEGFA, that are not influenced by the bioavailability of E2. However, blocking E2 action in uNK cells or neutralizing CCL2 abrogated the increases in network formation in our model system consistent with CCL2 being a key target for E2-dependent regulation in uNK cells.
We have previously demonstrated that CCL2 is immunolocalised to perivascular cells in first trimester human decidua (Jones et al., 1997); however, in vitro studies suggest that both progestins and E2 inhibit CCL2 expression in human endometrial stromal cells (Arici et al., 1999). This may indicate that E2-stimulated uNK cells may be the principal source of CCL2 in the endometrial microenvironment in early pregnancy. uNK cells are reported to express chemokine (C-C motif) receptor 2 (CCR2), the CCL2 receptor, and CCL2 is reported to decrease apoptosis and increase proliferation of decidual leukocytes (the majority of which are uNK cells) (He et al., 2012). Thus, CCL2 secreted from uNK cells in response to E2 may have an autocrine impact on uNK cell function and promote persistence/survival in the decidua.
Previous studies investigating the effect of uNK cells on angiogenesis have used human umbilical vein endothelial cells (HUVEC) in network formation assays; however, we and others have reported that endothelial cells from different vascular beds have distinct characteristics and responses (Schatz et al., 2000; Greaves et al., 2013). To the best of our knowledge the results in the current study represent the first in vitro evidence of the impact of uNK cells on endothelial cells from human endometrium (HEEC). Interestingly, while E2 is an established regulator of network formation in HUVEC (Morales et al., 1995), recent studies using an in vitro model suggest that direct stimulation of human endometrial endothelial cells with E2 has no significant effect on HEEC network formation (Greaves et al., 2013). We speculate that indirect stimulation of the vasculature by E2, relayed via uNK cells, may prevent excessive activation of angiogenesis and lead to controlled, site-specific vascular remodelling in areas of the tissue that have high local bioavailability of E2 and associated accumulation of uNK cells.
Tissue-resident uNK cells are a highly dynamic cell population that varies throughout pregnancy. Gestation-dependent changes in killer-cell immunoglobulin-like (KIR) receptor repertoire have been reported in uNK cells isolated from first trimester decidua from 6 to 12 weeks of gestation (Sharkey et al., 2008; Marlin et al., 2012). This may be particularly important in early pregnancy as changes in NK receptor expression are reported to alter secretion of growth factors and cytokines from uNK cells which in turn may impact on the regulation of endometrial remodelling (Hanna et al., 2006; Sharkey et al., 2008). Consistent with this, functional activity of uNK cells is reported to vary with gestational age. Trophoblast invasion is stimulated by factors secreted from uNK cells from 12 to 14 weeks gestation but not 8–10 weeks gestation and secretion of angiogenic growth factors from uNK cells decreases with gestational age (Lash et al., 2006, 2010). In the present study, uNK cells were isolated from human decidua between 8 and 12 weeks gestation (Supplementary Table SI) which may be a source of variation within our results. Interestingly, despite the variation in gestation the observed responses to E2 were consistent for all end-points which may indicate that E2 regulates uNK cell function independent of gestational age; however, further studies would be required to determine this. Transcriptional profiling suggests that human uNK cells from non-pregnant endometrium are distinct from uNK cells isolated from decidua (Kopcow et al., 2010). In our study we did not assess the impact on uNK cells isolated from non-pregnant endometrium due to the limitations of obtaining a sufficient yield of uNK cells from endometrial pipelle biopsies. However, as decidualisation of stromal cells begins in the mid-secretory phase, further studies are warranted to investigate whether the functional effects of E2 on decidual uNK cells are replicated in uNK cells from non-pregnant endometrium.
In summary, the present study provides mechanistic insight and critical new evidence modelling the physiological regulation of human uNK cell function and cell–cell interactions within the uterine microenvironment. Given our new data, it is conceivable that defects in estrogen-dependent processes may influence uNK cell function and hence targeting estrogen action could provide a new therapeutic strategy.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org.
Authors' roles
D.A.G. designed and carried out experimental work and wrote the manuscript. E.G. carried out experimental work. H.O.D.C. designed the work and revised the manuscript. P.T.K.S. designed the work, wrote and revised the manuscript.
Funding
Studies undertaken in the authors' laboratory were supported by MRC Programme Grant G1100356/1 to P.T.K.S. Funding to pay the Open Access publication charges for this article was provided by MRC programme Grant G1100356/i to P.T.K.S.
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
We thank Arantza Esnal, Ronan McLaughlin and members of PTKS group for technical assistance. We thank Catherine Murray and Sharon McPherson for patient recruitment and collection of tissues and Ronnie Grant for preparation of figures. We are very grateful to Dr Fiona Cousins for proof reading and to Prof. Steven Yellon and Prof. Jane Norman for critical feedback on the manuscript.
References
- Arici A, Senturk LM, Seli E, Bahtiyar MO, Kim G. Regulation of monocyte chemotactic protein-1 expression in human endometrial stromal cells by estrogen and progesterone. Biol Reprod 1999;61:85–90. [DOI] [PubMed] [Google Scholar]
- Bachmayer N, Rafik Hamad R, Liszka L, Bremme K, Sverremark-Ekstrom E. Aberrant uterine natural killer (NK)-cell expression and altered placental and serum levels of the NK-cell promoting cytokine interleukin-12 in pre-eclampsia. Am J Reprod Immunol 2006;56:292–301. [DOI] [PubMed] [Google Scholar]
- Borzychowski AM, Chantakru S, Minhas K, Paffaro VA, Yamada AT, He H, Korach KS, Croy BA. Functional analysis of murine uterine natural killer cells genetically devoid of oestrogen receptors. Placenta 2003;24:403–411. [DOI] [PubMed] [Google Scholar]
- Bulmer JN, Morrison L, Longfellow M, Ritson A, Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum Reprod 1991;6:791–798. [DOI] [PubMed] [Google Scholar]
- Carlino C, Stabile H, Morrone S, Bulla R, Soriani A, Agostinis C, Bossi F, Mocci C, Sarazani F, Tedesco F, et al. Recruitment of circulating NK cells through decidual tissues: a possible mechanism controlling NK cell accumulation in the uterus during early pregnancy. Blood 2008;111:3108–3115. [DOI] [PubMed] [Google Scholar]
- Craven CM, Morgan T, Ward K. Decidual spiral artery remodelling begins before cellular interaction with cytotrophoblasts. Placenta 1998;19:241–252. [DOI] [PubMed] [Google Scholar]
- Croy BA, Chen Z, Hofmann AP, Lord EM, Sedlacek AL, Gerber SA. Imaging of vascular development in early mouse decidua and its association with leukocytes and trophoblasts. Biol Reprod 2012;87:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLoia JA, Stewart-Akers AM, Brekosky J, Kubik CJ. Effects of exogenous estrogen on uterine leukocyte recruitment. Fertil Steril 2002;77:548–554. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev 2014;35:851–905. [DOI] [PubMed] [Google Scholar]
- Gibson DA, McInnes KJ, Critchley HO, Saunders PT. Endometrial Intracrinology—generation of an estrogen-dominated microenvironment in the secretory phase of women. J Clin Endocrinol Metab 2013;98:E1802–E1806. [DOI] [PubMed] [Google Scholar]
- Glace L, Grygielko ET, Boyle R, Wang Q, Laping NJ, Sulpizio AC, Bray JD. Estrogen-induced stromal cell-derived factor-1 (SDF-1/Cxcl12) expression is repressed by progesterone and by Selective Estrogen Receptor Modulators via estrogen receptor alpha in rat uterine cells and tissues. Steroids 2009;74:1015–1024. [DOI] [PubMed] [Google Scholar]
- Greaves E, Collins F, Critchley HO, Saunders PT. ERbeta-dependent effects on uterine endothelial cells are cell specific and mediated via Sp1. Hum Reprod 2013;28:2490–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006;12:1065–1074. [DOI] [PubMed] [Google Scholar]
- Hazan AD, Smith SD, Jones RL, Whittle W, Lye SJ, Dunk CE. Vascular-leukocyte interactions: mechanisms of human decidual spiral artery remodeling in vitro. Am J Pathol 2010;177:1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y-Y, He X-J, Guo P-F, Du M-R, Shao J, Li M-Q, Li D-J. The decidual stromal cells-secreted CCL2 induces and maintains decidual leukocytes into Th2 bias in human early pregnancy. Clin Immunol 2012;145:161–173. [DOI] [PubMed] [Google Scholar]
- Henderson TA, Saunders PT, Moffett-King A, Groome NP, Critchley HO. Steroid receptor expression in uterine natural killer cells. J Clin Endocrinol Metab 2003;88:440–449. [DOI] [PubMed] [Google Scholar]
- Hofmann AP, Gerber SA, Croy BA. Uterine natural killer cells pace early development of mouse decidua basalis. Mol Hum Reprod 2014;20:66–76. [DOI] [PubMed] [Google Scholar]
- Jones RL, Kelly RW, Critchley HO. Chemokine and cyclooxygenase-2 expression in human endometrium coincides with leukocyte accumulation. Hum Reprod 1997;12:1300–1306. [DOI] [PubMed] [Google Scholar]
- Jones RL, Hannan NJ, Kaitu'u TJ, Zhang J, Salamonsen LA. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab 2004;89:6155–6167. [DOI] [PubMed] [Google Scholar]
- Kane N, Kelly R, Saunders PT, Critchley HO. Proliferation of uterine natural killer cells is induced by human chorionic gonadotropin and mediated via the mannose receptor. Endocrinology 2009;150:2882–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Balendran N, Wooding P, Carter NP, Loke YW. CD3- leukocytes present in the human uterus during early placentation: phenotypic and morphologic characterization of the CD56++ population. Dev Immunol 1991;1:169–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaya K, Yamaguchi T, Honjo H. Central role of interleukin-15 in postovulatory recruitment of peripheral blood CD16(-) natural killer cells into human endometrium. J Clin Endocrinol Metab 2005;90:2932–2940. [DOI] [PubMed] [Google Scholar]
- Kitaya K, Yamaguchi T, Yasuo T, Okubo T, Honjo H. Post-ovulatory rise of endometrial CD16(-) natural killer cells: in situ proliferation of residual cells or selective recruitment from circulating peripheral blood? J Reprod Immunol 2007;76:45–53. [DOI] [PubMed] [Google Scholar]
- Kopcow HD, Eriksson M, Mselle TF, Damrauer SM, Wira CR, Sentman CL, Strominger JL. Human decidual NK cells from gravid uteri and NK cells from cycling endometrium are distinct NK cell subsets. Placenta 2010;31:334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikun G, Mor G, Huang J, Schatz F, Lockwood CJ. Metalloproteinase expression by control and telomerase immortalized human endometrial endothelial cells. Histol Histopathol 2005;20:719–724. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998;139:4252–4263. [DOI] [PubMed] [Google Scholar]
- Lash GE, Schiessl B, Kirkley M, Innes BA, Cooper A, Searle RF, Robson SC, Bulmer JN. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukoc Biol 2006;80:572–580. [DOI] [PubMed] [Google Scholar]
- Lash GE, Otun HA, Innes BA, Percival K, Searle RF, Robson SC, Bulmer JN. Regulation of extravillous trophoblast invasion by uterine natural killer cells is dependent on gestational age. Hum Reprod 2010;25:1137–1145. [DOI] [PubMed] [Google Scholar]
- Lash GE, Naruse K, Robson A, Innes BA, Searle RF, Robson SC, Bulmer JN. Interaction between uterine natural killer cells and extravillous trophoblast cells: effect on cytokine and angiogenic growth factor production. Hum Reprod 2011;26:2289–2295. [DOI] [PubMed] [Google Scholar]
- Legler J, Zeinstra LM, Schuitemaker F, Lanser PH, Bogerd J, Brouwer A, Vethaak AD, De Voogt P, Murk AJ, Van der Burg B. Comparison of in vivo and in vitro reporter gene assays for short-term screening of estrogenic activity. Environ Sci Technol 2002;36:4410–4415. [DOI] [PubMed] [Google Scholar]
- Lima PD, Zhang J, Dunk C, Lye SJ, Croy BA. Leukocyte driven-decidual angiogenesis in early pregnancy. Cell Mol Immunol 2014;11:522–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood CJ, Huang SJ, Chen CP, Huang Y, Xu J, Faramarzi S, Kayisli O, Kayisli U, Koopman L, Smedts D, et al. Decidual cell regulation of natural killer cell-recruiting chemokines: implications for the pathogenesis and prediction of preeclampsia. Am J Pathol 2013;183:841–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin R, Duriez M, Berkane N, de Truchis C, Madec Y, Rey-Cuille MA, Cummings JS, Cannou C, Quillay H, Barre-Sinoussi F, et al. Dynamic shift from CD85j/ILT-2 to NKG2D NK receptor expression pattern on human decidual NK during the first trimester of pregnancy. PLoS One 2012;7:e30017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales DE, McGowan KA, Grant DS, Maheshwari S, Bhartiya D, Cid MC, Kleinman HK, Schnaper HW. Estrogen promotes angiogenic activity in human umbilical vein endothelial cells in vitro and in a murine model. Circulation 1995;91:755–763. [DOI] [PubMed] [Google Scholar]
- Okada H, Nakajima T, Sanezumi M, Ikuta A, Yasuda K, Kanzaki H. Progesterone enhances interleukin-15 production in human endometrial stromal cells in vitro. J Clin Endocrinol Metab 2000;85:4765–4770. [DOI] [PubMed] [Google Scholar]
- Quenby S, Nik H, Innes B, Lash G, Turner M, Drury J, Bulmer J. Uterine natural killer cells and angiogenesis in recurrent reproductive failure. Hum Reprod 2009;24:45–54. [DOI] [PubMed] [Google Scholar]
- Robson A, Harris LK, Innes BA, Lash GE, Aljunaidy MM, Aplin JD, Baker PN, Robson SC, Bulmer JN. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J 2012;26:4876–4885. [DOI] [PubMed] [Google Scholar]
- Russell P, Sacks G, Tremellen K, Gee A. The distribution of immune cells and macrophages in the endometrium of women with recurrent reproductive failure. III: Further observations and reference ranges. Pathology 2013;45:393–401. [DOI] [PubMed] [Google Scholar]
- Schatz F, Soderland C, Hendricks-Munoz KD, Gerrets RP, Lockwood CJ. Human endometrial endothelial cells: isolation, characterization, and inflammatory-mediated expression of tissue factor and type 1 plasminogen activator inhibitor. Biol Reprod 2000;62:691–697. [DOI] [PubMed] [Google Scholar]
- Sentman CL, Meadows SK, Wira CR, Eriksson M. Recruitment of uterine NK cells: induction of CXC chemokine ligands 10 and 11 in human endometrium by estradiol and progesterone. J Immunol 2004;173:6760–6766. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Sunkara SK. Natural killer cells in female infertility and recurrent miscarriage: a systematic review and meta-analysis. Hum Reprod Update 2014;20:429–438. [DOI] [PubMed] [Google Scholar]
- Sharkey AM, Gardner L, Hiby S, Farrell L, Apps R, Masters L, Goodridge J, Lathbury L, Stewart CA, Verma S, et al. Killer Ig-like receptor expression in uterine NK cells is biased toward recognition of HLA-C and alters with gestational age. J Immunol 2008;181:39–46. [DOI] [PubMed] [Google Scholar]
- Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol 2009;174:1959–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AW, Alfirevic Z, Turner MA, Drury JA, Small R, Quenby S. A feasibility trial of screening women with idiopathic recurrent miscarriage for high uterine natural killer cell density and randomizing to prednisolone or placebo when pregnant. Hum Reprod 2013;28:1743–1752. [DOI] [PubMed] [Google Scholar]
- Vacca P, Vitale C, Montaldo E, Conte R, Cantoni C, Fulcheri E, Darretta V, Moretta L, Mingari MC. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc Natl Acad Sci USA 2011;108:2402–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res 1991;51:3867–3873. [PubMed] [Google Scholar]
- Wallace AE, Fraser R, Cartwright JE. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum Reprod Update 2012;18:458–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang Z, Yang B, Yang Q, Sun Y. CXCR4 nuclear localization follows binding of its ligand SDF-1 and occurs in metastatic but not primary renal cell carcinoma. Oncol Rep 2009;22:1333–1339. [DOI] [PubMed] [Google Scholar]
- Wilkens J, Male V, Ghazal P, Forster T, Gibson DA, Williams AR, Brito-Mutunayagam SL, Craigon M, Lourenco P, Cameron IT, et al. Uterine NK cells regulate endometrial bleeding in women and are suppressed by the progesterone receptor modulator asoprisnil. J Immunol 2013;191:2226–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PJ, Bulmer JN, Searle RF, Innes BA, Robson SC. Altered decidual leucocyte populations in the placental bed in pre-eclampsia and foetal growth restriction: a comparison with late normal pregnancy. Reproduction 2009;138:177–184. [DOI] [PubMed] [Google Scholar]
- Wu X, Jin LP, Yuan MM, Zhu Y, Wang MY, Li DJ. Human first-trimester trophoblast cells recruit CD56brightCD16- NK cells into decidua by way of expressing and secreting of CXCL12/stromal cell-derived factor 1. J Immunol 2005;175:61–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.